-
Turfgrass is an economically, environmentally, recreationally, and aesthetically important part of urban landscapes[1]. With the demand of rapid urbanization, turfgrass coverage has increased worldwide[2]. Grown in diverse climates and environments, turfgrass often suffers from various abiotic stresses such as heat, cold, drought, and salinity, resulting in the decline of grass quality, growth and development, and other functional attributes[3,4]. Therefore, improvement of turfgrass stress tolerance has been a crucial concern, and a better understanding of mechanisms of turfgrass stress tolerance is of great significance for developing stress tolerant germplasm through breeding and biotechnology.
Proline is a multifunctional amino acid. It is highly accumulated in plants under various stress conditions, which can confer stress tolerance[5,6]. Proline often exists widely in plants in a free state. As a signal, it plays a key role in regulating gene expression and some metabolic processes[7]. There are two ways for proline biosynthesis to occur in plants: the glutamate pathway or the arginine pathway[7]. As shown in Fig. 1, glutamate (Glu) is reduced to glutamate semialdehyde (GSA) by the bifunctional enzyme Δ1-pyrroline-carboxylate synthetase (P5CS), then GSA is converted to pyrroline-5-carboxylate (P5C), and P5C is further reduced to proline by P5C reductase (P5CR). Ornithine (Orn) produces GSA through ornithine δ-aminotransferase (OAT).
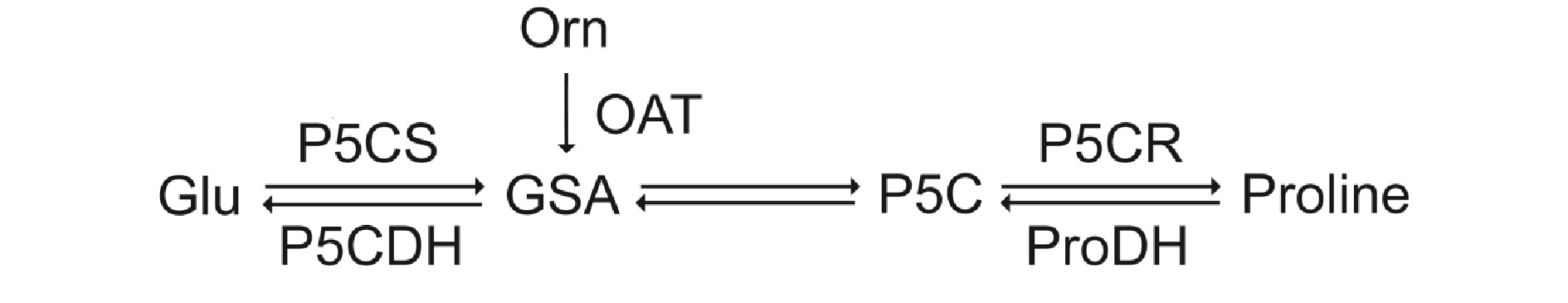
Figure 1.
Proline metabolic pathways in plants: the glutamate pathway. GSA, glutamic-γ-semialdehyde; P5C, Δ1-pyrroline-5-carboxylate; P5CS, Δ1-pyrroline-5-carboxylate synthetase; OAT, ornithine aminotransferase; P5CR, Δ1-pyrroline-5-carboxylate reductase; P5CDH. pyrroline-5-carboxylate dehydrogenase; ProDH, proline dehydrogenase.
To date, proline accumulation and expression of genes for proline metabolism have been documented in some turfgrass species in response to stress conditions[8−11]. However, there is no review on the current research status of proline associated with turfgrass stress tolerance. This review summarizes proline research including turfgrass species, types of abiotic stresses, and proline functions. Information provided by this review could be valuable for future studies into the role of proline in regulating turfgrass abiotic tolerance.
-
The role of proline in regulating stress responses has been studied in both cool-season turfgrasses such as Kentucky bluegrass (Poa pratensis), tall fescue (Festuca arundinacea), perennial ryegrass (Lolium perenne), and bentgrass (Agrostis spp.) and warm-season turfgrasses such as bermudagrass (Cynodon dactylon) and zoysiagrass (Zoysia spp.). The keywords TS = (turfgrass* OR lawngrass* OR grass*) AND 'abiotic stress' AND 'proline' was searched. The literature was from the past 10 years from 2013−2022, and the selected language was English. After initial searching, the literature were obtained relevant to proline and abiotic stress in turfgrass. Next, by checking the title and keywords. Finally, reading the full text. From 2013 to 2022, there were close to 100 publications related to proline under abiotic stresses including 22 in bentgrass and 20 in perennial ryegrass, followed by 15 in Kentucky bluegrass, 13 in bermudagrass, 12 in tall fescue, and nine in zoysiagrass (Fig. 2). There were a few publications (ranging from one to five) for other turfgrass species including centipedegrass (Eremochloa ophiuroides), seashore paspalum (Paspalum vaginatum), St. Augustinegrass (Stenotaphrum secundatum) and buffalograss (Bouteloua dactyloides).
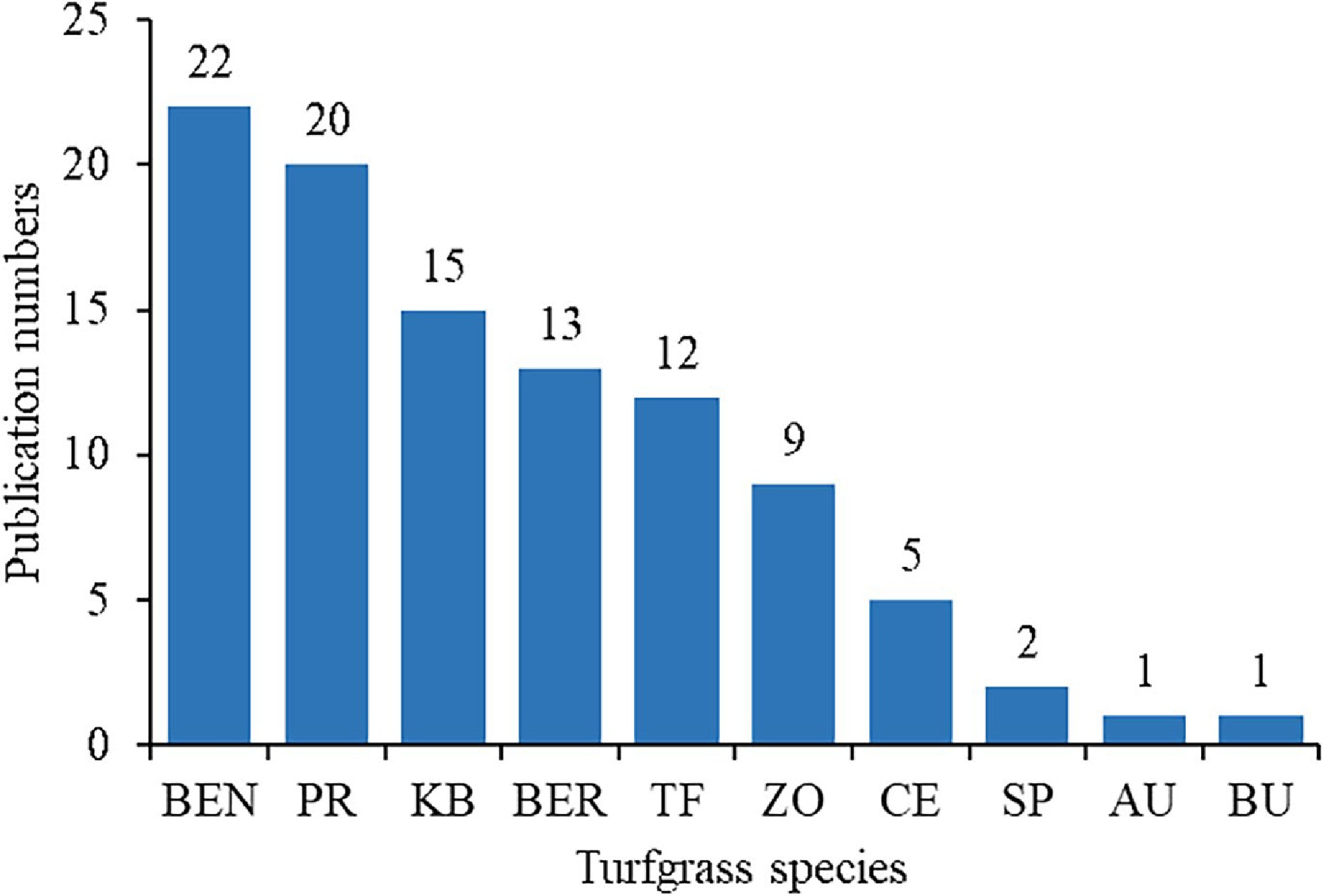
Figure 2.
The number of publications in relation to proline in different turfgrass species under stress conditions since 2013. BEN, Bentgrass; PR, Perennial ryegrass; KB, Kentucky bluegrass; BER, Bermudagrass; TF, Tall fescue; ZO, Zoysiagrass; CE, Centipedegrass; SP, Seashore paspalum; AU, St. Augustinegrass; BU, Buffalograss.
Among the various abiotic stresses, most studies on proline in the past decade were found to deal with drought, salt, cold and heat stresses (Fig. 3). However, there was only one literature report on effects of proline on photoinhibition stress tolerance of turfgrass.
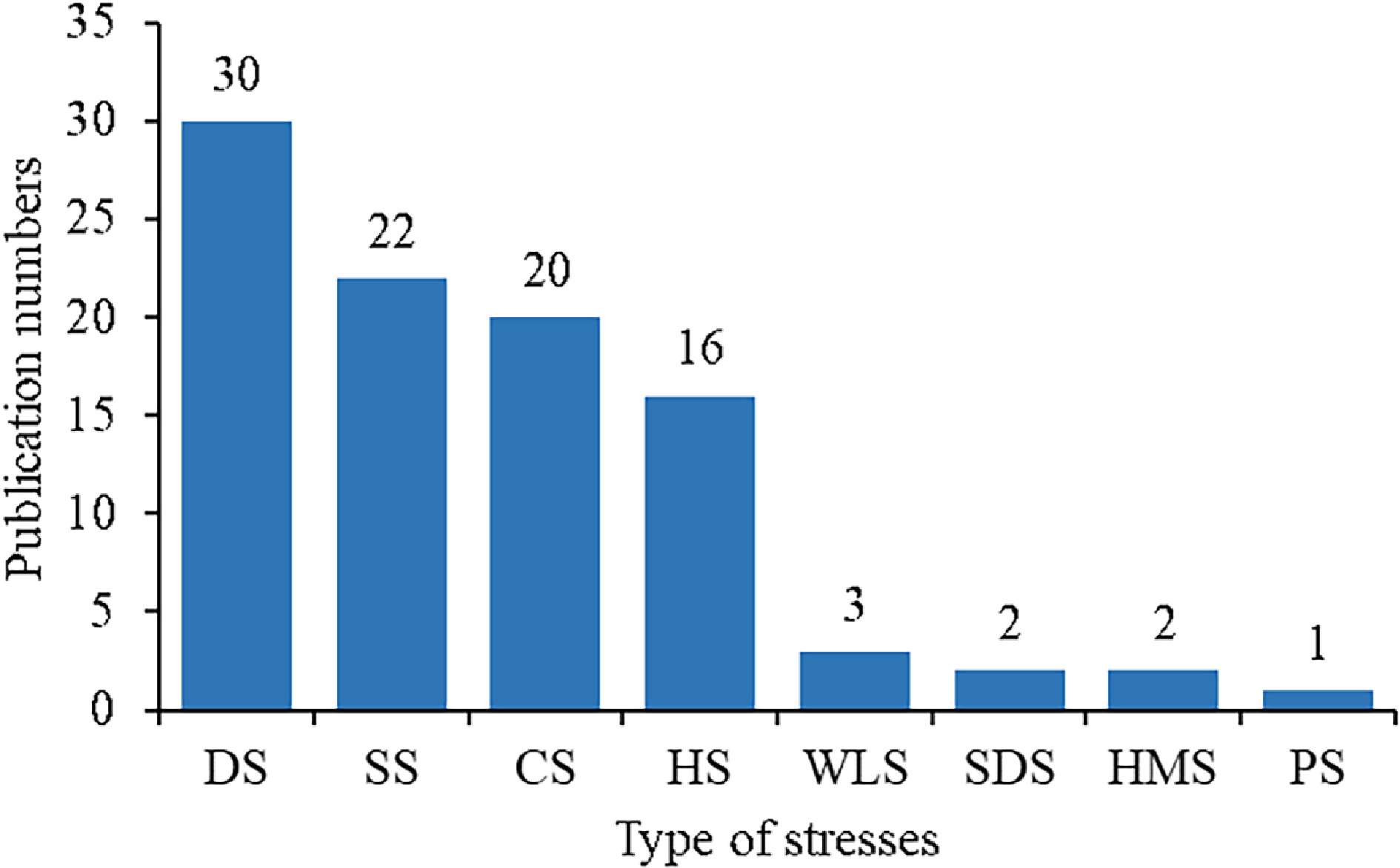
Figure 3.
The number of publications on turfgrasses under different stresses since 2013. The abbreviations on the x- axis from left to right represent drought (DS), salt (SS), cold (CS), heat (HS), waterlogging (WLS), sulfur dioxide (SDS), heavy metal (HMS), and photoinhibition (PS), respectively.
A network model was created by analyzing keywords in literature extracted from the Web of Science Database in the past 10 years (Fig. 4). The most frequently used keyword was 'lipid peroxidation', and the others were 'freezing tolerance', 'abiotic stress', 'gene expression', 'plant', etc. The lines between keywords in the model showed their correlations, which could indicate the past research hotspots of proline regulation in turfgrass responses to abiotic stresses. It showed that 'lipid peroxidation', 'various abiotic stresses' and 'gene expression' were the most popular keywords for proline related research reports in turfgrass species.
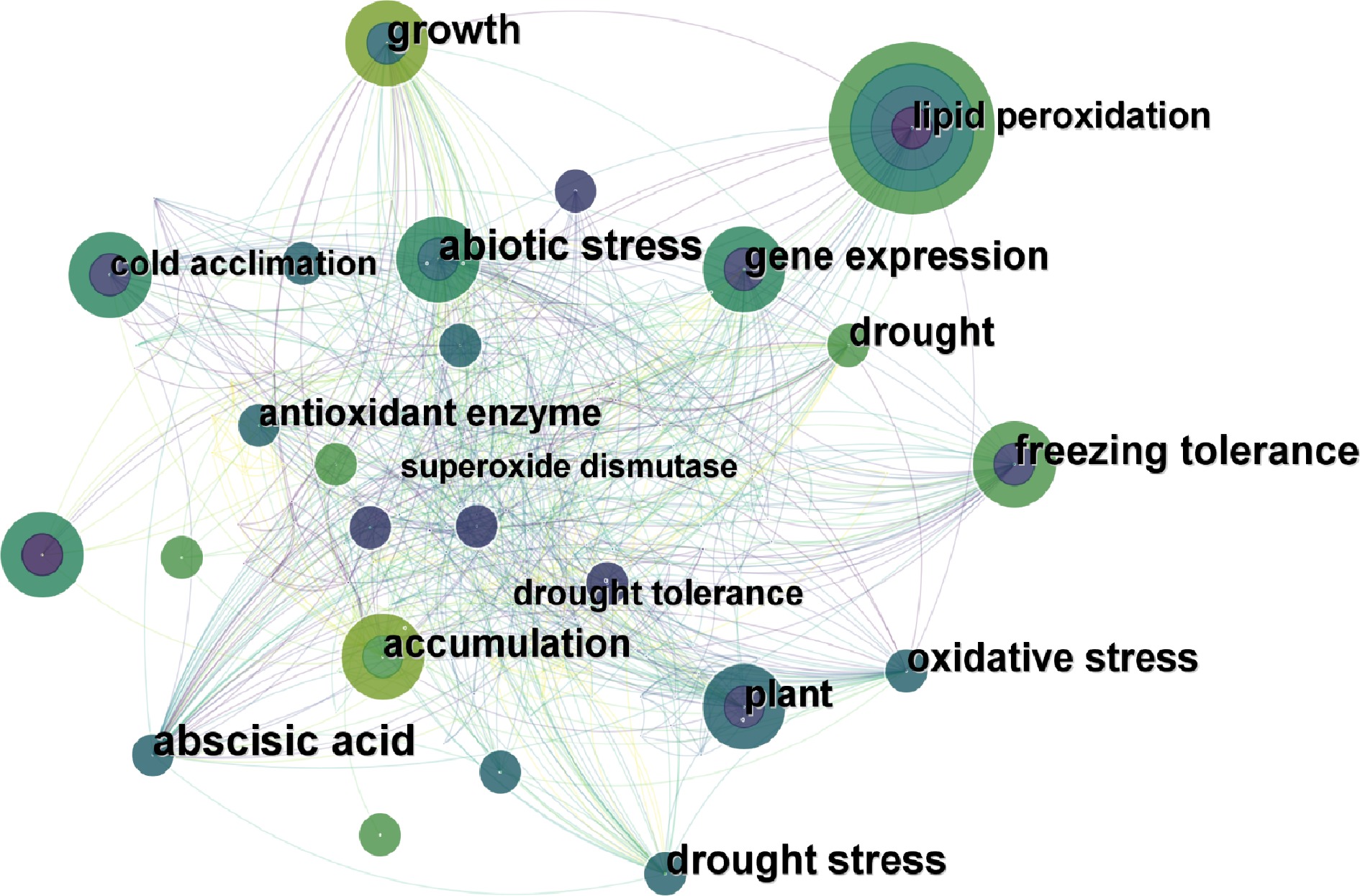
Figure 4.
The analysis of keywords on the research of proline in turfgrass response to abiotic stress (created by authors using CiteSpace). Each circle represents a keyword: the larger the circle, the higher the frequency of the keyword. The lines between the circles represent the relationship between keywords.
-
Proline is considered to have many unique functions in regulating homeostasis in plant tolerance to harsh environments, for example, as an essential amino acid is also a vital osmotic compound to maintain cellular homeostasis in plants[12,13], and as the molecular chaperon it is able to maintain the protein integrity and enhance the activities of different enzymes for preventing oxidative burst in plants by bringing concentrations of reactive oxygen species (ROS) within normal ranges[14]. Numerous studies have reported proline as an antioxidant suggesting its roles as ROS scavenger and singlet oxygen quencher[15,16]. In addition, it has been confirmed that proline is a metal chelator[17,18] and a signaling molecule in plants under adverse stresses[19−21]. Although proline metabolism has been studied in turfgrass species[5], the functional roles of proline in regulating turfgrass tolerance to abiotic stresses are not well understood.
Drought stress
-
Drought is a limiting factor for agricultural production worldwide. Proline is the most well-known osmotic protective substance. A large amount of evidence has shown that the accumulation of proline is positively correlated with drought tolerance in plants[4,22−25]. Mild drought stress (60% container capacity) induced proline accumulation and improved antioxidant metabolism in creeping bentgrass[26]. The proline, hydrogen peroxide and total ascorbate contents of Agropyron cristatum, A. intermedium, Festuca ovina, Festuca arundinaceae, Cynodon dactylon, Bromus inermis, and B. confinis, sources of low-maintenance turfgrasses for semi-arid regions, increased under drought[27]. Drought stress decreased tall fescue quality, relative water content (RWC), leaf indole-3-acetic acid and cytokinin-zeatin riboside (ZR) contents, and increased proline and abscisic acid (ABA) content, but the tolerant cultivar 'Van Gogh' had greater turfgrass quality rating, RWC, proline, ABA, and ZR content compared to the sensitive 'AST7002' under drought stress (26% container capacity)[28]. Lolium-Festuca complex genotype 'INT-40' showed a higher tolerance to field water deficit and had more denser root growth and more osmotic active compounds accumulated in the shoots, such as proline, trehalose and oligosaccharide[29]. Activities of antioxidant enzymes superoxide dismutase (SOD) and ascorbate peroxidase (APX) increased accompanied with increasing accumulation of proline in tall fescue cultivars 'Pixie' and 'Mini-mustang' under drought stress[30].
Exogenous application of proline or other molecules that affect proline content may influence turfgrass stress tolerance[31−43]. Exogenous application of the spermidine (Spd) improved drought stress tolerance of creeping bentgrass by upregulating proline biosynthesis pathway related genes P5CS and P5CR[44]. Foliar spray of γ-Aminobutyric acid (GABA) and proline alone or in combination improved creeping bentgrass dark green color index and stolon length, but proline treatment alone resulted in higher RWC, indicating that GABA or proline plays a role in creeping bentgrass tolerance to drought stress[31]. Also in creeping bentgrass, plants treated with spermine (Spm) increased nitrogen and proline metabolism, maintained tricarboxylic acid cycle, and enhanced chlorophyll content, photosynthesis, water use efficiency, and cell membrane stability[32]. Exogenous application of plant growth-promoting rhizobacteria isolated from the rhizosphere of Haloxylon ammodendron increased drought tolerance by promoting accumulation of proline, activities of the antioxidant enzymes catalase (CAT) and peroxidase (POD), photosynthetic capacity and RWC of perennial ryegrass[33]. Additionally, the application of exogenous substances such as hydrogen sulfide, abscisic acid, nitrogenous nutrition, silicon or nitric oxide (NO) can further improve the accumulation of proline, associated with high content of chlorophyll, total soluble phenols and glycine betaine in turfgrass species against drought stress[33−42]. However, accumulation of proline does not always occur in grass plants under drought stress. For example, exogenous application of 0.5 mM salicylic acid improved zoysiagrass drought tolerance by enhancing the net photosynthetic rate and antioxidant enzyme activities but decreasing proline content and lipid peroxidation compared to the controls[43]. Application of myo-inositol (1 mM) promoted the accumulation of water soluble carbohydrates but decreased drought-induced free proline in leaves of creeping bentgrass, suggesting that the contribution of water soluble carbohydrate to osmotic adjustment under drought stress was greater than that of proline[40].
SAGIPT41 transgenic bentgrass (IPT encoding isopentenyl transferase) showed better drought tolerance, with higher turfgrass quality, leaf RWC, and OA as well as more soluble proline, sugars, betaine and spermine found in transgenic plants than the control plants[45]. Transgenic tobacco overexpressing proline biosynthesis gene LpP5CS of perennial ryegrass had higher proline content and survival rate after drought treatment[6]. Transcriptome analysis of Kentucky bluegrass showed that two proline dehydrogenase unigenes were down regulated, which could be an advantage to slow the rate of proline degradation after application of ethephon (200 mg·L−1) under drought stress[41]. In brief, the results suggested a role of proline in promoting drought tolerance in turfgrass species.
Salt stress
-
Salt stress is one of the major abiotic stresses in many regions of the world. High salt concentration inhibits plant growth and reduces chlorophyll content, photosystem II photochemical efficiency (Fv/Fm) and K+ content, and increases Na+ accumulation[46−48]. The salt tolerant centipedegrass had lower growth inhibition and showed increased proline content and antioxidant enzyme activities than the salt sensitive centipedegrass[46]. When eight C3 turfgrass species were exposed to increasing salt concentration, proline content was positively correlated with salt tolerance, but negatively correlated with salt avoidance, suggesting that variations of salt tolerance among species was caused by the difference in proline content[49]. The proline concentration increased significantly in both a salt-sensitive Kentucky bluegrass and salt-tolerant tall fescue exposed to increasing salt concentration, but tall fescue had less accumulation of Na+ and Cl− and higher total soluble sugar than Kentucky bluegrass[48]. The results indicated that accumulating sugars other than proline mainly contributed to salt tolerance of tall fescue. Proline and glycine betaine content also increased with increasing salt concentration in four warm-season turfgrasses including St. Augustinegrass, manila grasses (Zoysia matrella), seashore paspalum and bermudagrass, suggesting a role of proline in cellular protection[47]. A comparative study of salt tolerance of creeping bentgrass (A. stolonifera ) 'Penncross' and rough bentgrass (A. scabra) 'NTAS' showed that the salt tolerant 'NTAS' maintained higher soluble sugar, proline, and glycine betaine accumulations, contributing to higher osmotic adjustment[50].
The application of some exogenous substances can reduce salt stress injury by increasing proline accumulation. Application of Spd alleviated the reduction of chlorophyll content and K+/Na+ ratio, increased the levels of proline, endogenous Spd, Spm and the activities of ornithine decarboxylase and S-adenosylmethionine decarboxylase and reduced salt injury (200 mM) in Kentucky bluegrass[51]. Exogenous 24-Epibrassinolide (EBR) treatment enhanced the activities of antioxidant enzymes, decreased the content of electrolyte leakage (EL), photosynthetic rate, malondialdehyde (MDA) and hydrogen peroxide (H2O2), and increased the RWC, proline, soluble sugar and soluble protein in leaves of perennial ryegrass under salt stress[52,53]. These results indicated that EBR could improve salt tolerance of perennial ryegrass by enhancing osmotic regulation and antioxidant defense system[52,53]. Foliar spray of GABA alleviated the growth inhibition, increased proline concentration, reduced Na+/ K+ ratio, and significantly increased POD and SOD activities of perennial ryegrass[54]. Foliar application of Si maintained leaf chlorophyll and RWC content, increased shoot length and shoot number, reduced Na+ concentration, but decreased proline content of tall fescue, perennial ryegrass and bermudagrass at all salinity levels, suggesting that other mechanisms than proline accumulation play a role in osmotic regulation[55].
Under salt stress (255 mM NaCl), the proline biosynthesis gene PrP5CS1 encoding pyrroline-5-carboxylate synthetase was significantly induced in perennial ryegrass leaves, and the up-regulated level of PrP5CS1 in the salt-tolerant cultivar 'Overdrive' was higher than that in sensitive cultivar 'Pizzazz'; at the same time, PrP5CS2 was significantly induced in 'Overdrive' but inhibited in 'Pizzazz'[56]. Transgenic tobacco over-expressing the proline-biosynthesis gene LpP5CS (encoding pyrroline-5-carboxylate synthetase) exhibited higher salt tolerance than the control[6]. The results indicate that salt tolerance of perennial ryegrass may be directly and positively correlated with proline metabolism, and that LpP5CS could be a candidate gene for genetic improvement of salt tolerance. The proline biosynthesis gene LpP5CS were up-regulated after treatment with salt (200 mM NaCl) in roots, stems and leaves of perennial ryegrass[6]. The drought and salt tolerance gene (DST) encoding a C2H2 zinc finger transcription factor negatively regulated salt tolerance in rice (Oryza sativa)[57]. Silencing the OsDST gene enhanced salt tolerance of perennial ryegrass, with higher leaf RWC and lower EL, MDA and H2O2 and proline content found in transgenic plants[58]. Collectively, proline plays a role in molecular regulation salt tolerance of turfgrass species.
Temperature stress
-
High and low temperatures are among the main environmental factors that influence plant growth, production and distribution. The high temperature range usually refers to the temperature at which plant growth begins to be inhibited, usually 5−10 °C above the ambient level, however, low temperature injury includes chilling stress (temperature above 0 °C) and freezing stress (temperature below 0 °C)[8]. High temperatures decreased chlorophyll content, Fv/Fm, and RWC, while water-soluble carbohydrates, proline, H2O2, MDA, and EL gradually increased in four cultivars of creeping bentgrass[59,60]. Foliar application of GABA, proline, or N significantly increased creeping bentgrass quality, chlorophyll content and Fv/Fm, and inhibited the activity of chlorophyll degrading enzymes to alleviate leaf senescence[60]. In addition, the levels of plant endogenous proline, GABA, glutamic acid and aspartate were significantly higher after exogenous proline (10 mM) application than those of the control[61], suggesting that proline may regulate 3-phosphoglycerate, GABA shunt, oxaloacetate, secondary metabolism, and pyruvate metabolic pathways to enhance heat tolerance of creeping bentgrass[61]. Significant differences in proline accumulation were found among different ecotypes of Kentucky bluegrass, perennial ryegrass and tall fescue under heat stress, but heat resistant varieties had higher proline content than that less resistant varieties, and this was also accompanied by higher growth rate, tiller number, and antioxidant activities[62−65]. Transcriptome analysis showed that up-regulation of genes involved in oxidative protection, proline biosynthesis, lipolysis, hemicellulose, and lignin biosynthesis were detected in heat-tolerant rough bentgrass compared to heat-sensitive creeping bentgrass[66]. The results indicate a positive role of proline in heat tolerance of turfgrass species, although proline accumulation was not always increased in plants with strong heat tolerance[67].
Freezing stress (−8 °C) increased proline content, sugar, and antioxidants in tall fescue[68]. Cold acclimation (5 °C) resulted in increased proline content in both cold tolerant and cold sensitive varieties of perennial ryegrass, compared to a non-acclimated control, while there were significant differences in proline content between cold tolerant and sensitive varieties[69]. Proline accumulated in creeping bentgrass, Kentucky bluegrass, and perennial ryegrass during cold acclimation improved the content of soluble phenols by regulating the pentose phosphate pathway related to proline, thereby enhancing antioxidant activity and the cold tolerance of the three plants[70]. However, the concentration of proline was generally low and similar between annual bluegrass (Poa annua) and creeping bentgrasss species throughout deacclimation (7 °C), but total soluble sugars, mainly high molecular weight (HMW) fructans, accumulated in each species/ecotypes with higher levels measured in creeping bentgrasss[71]. Perennial grasses often decrease capacity of cold tolerance upon deacclimation, and the low level of proline during deacclimation indicated less cold hardiness plants. Furthermore, transgenic zoysiagrass plants, expressing oat phytochrome A (PhyA) or a hyperactive mutant phytochrome A (S599A), showed a marked increase in proline content compared to non-transgenic control lines. The PhyA lines had approximately 42% increased proline accumulation, expressing S599A showed around 88% increased proline levels compared to the non-transgenic control lines when subjected to cold stress (10 °C)[72]. The results showed that increased proline accumulation was strongly associated with cold tolerance. Transcriptomic analyses reveal that proline synthesis pathways and photosynthesis pathways genes were involved in the regulation of cold response in bermudagrass and zoysiagrass[73]. In Kentucky bluegrass and manila grass, 'proline synthesis process' and 'proline transport' pathways were enriched under cold stress, with many genes in the proline synthesis and degradation pathways up-regulated, such as P5CS, P5CR and P5CD indicating that both the biosynthesis and degradation of proline were activated by cold stress[74−76]. DREB (dehydration-responsive element binding) is induced by cold stress, and overexpression of zoysiagrass DREB gene in Arabidopsis thaliana moderately increased the levels of proline and soluble sugar, and improved plant tolerance to high and low temperature stress[77]. Overexpression of the DREB-binding factor PpCBF3 from a cold-tolerant Kentucky bluegrass increased plant growth and survival of Arabidopsis thaliana at extremely low temperatures (4 °C) by protecting photosynthetic components and cell membrane structure, up-regulating proline synthesis and inhibiting ROS formation[78]. The results suggest a connection of proline with the expression of key genes in protecting leaf damage under cold stress. The ICE1 gene is a regulator of cold-induced transcriptional changes in Arabidopsis thaliana, and Korean lawngrass (Zoysia japonica) overexpressing AtICE1 showed increased proline level, higher activities of SOD and POD, and decreased MDA content compared with the wild type under cold stress (4 °C)[79]. The associations of proline with improved cold tolerance were also found in transgenic creeping bentgrass plants overexpressing a Picea wilsonii dehydrin gene (PicW) and tobacco plants overexpressing the creeping bentgrass AstEXP1 gene[80,81].
Other abiotic stresses
-
In addition to these stresses mentioned above, sulfur dioxide (SO2), light and heavy metal stresses can also cause serious damage to turf plants. SO2 is the main air pollutant at present. When nine varieties of bermudagrass were exposed to SO2, the SO2 resistant varieties exhibited higher content of soluble sugar, chlorophyll a and proline than that of sensitive varieties, indicating that the tolerance of bermudagrass to SO2 was closely related to proline[82]. Moreover, lower levels of ROS, MDA and EL, and increased antioxidant enzyme activity and proline content were also observed in SO2 tolerant sheep fescue (Festuca ovina) and perennial ryegrass[83].
Low-light can significantly reduce the quality of the turfgrass, and cause a serious delay in the growth and development of plants[84]. Proline accumulated in tall fescue leaves and roots under low light, and the content of proline was further enhanced by Ca2+ application, suggesting that exogenous Ca2+ promoted the ability of tall fescue to cope with low light stress by restoring various physiological mechanisms including increases in proline content[84].
The unreasonable discharge of industrial wastewater and waste gas lead to the gradual accumulation of heavy metals such as Cu and Cd in the soil. In perennial ryegrass, Cd stress increased the level of lipid peroxidation and triggered the activity of antioxidant enzymes, and the input of exogenous phosphorus increased the levels of proline and cysteine, partially alleviating the effect of lipid peroxidation, while the absorption of Cd was decreased by increasing phytochelatins[85]. Under heavy metal stress, transgenic zoysia and bentgrass plants carrying S599A-PhyA (oat phytochrome A) showed increased aboveground and underground biomass, antioxidant enzyme activities, chlorophyll and proline contents, and decreased H2O2 levels compared with the control[86]. The results proved a connection between proline and heavy metal tolerance. Overall, as an important metabolite, proline plays multifunctional roles in facilitating turfgrass tolerance to various abiotic stresses.
-
Research into turfgrass has shown that proline accumulation plays highly beneficial roles in promoting turfgrass stress tolerance. The mechanisms of proline for enhancing turfgrass stress tolerance mainly involve osmotolerance for maintaining cellular homeostasis, preventing oxidative injury, and acting as a signaling molecule in gene expression. Much research is still required for a deeper and complete understanding of the functions of proline in response to abiotic stresses in both cool-season and warm-season turfgrasses. The complex morph-physiological and molecular network associated with proline accumulation should be explored more extensively for further genetic engineering of proline metabolism in turfgrasses aimed at improving abiotic stress tolerance.
This research was funded by National Natural Science Foundation of China (No. 31971772; 32001407), China Postdoctoral Science Foundation (2022MD723773) and Heilongjiang Postdoctoral Fund (LBH-Z21009).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Jiang J, Guo Z, Sun X, Jiang Y, Xie F, et al. 2023. Role of proline in regulating turfgrass tolerance to abiotic stress. Grass Research 3:2 doi: 10.48130/GR-2023-0002
Role of proline in regulating turfgrass tolerance to abiotic stress
- Received: 21 October 2022
- Accepted: 30 January 2023
- Published online: 15 February 2023
Abstract: Turfgrasses used on golf courses, sports fields and in urban landscapes have significant ecological and economic value. Various abiotic stresses are crucial in limiting the growth and development of turfgrass. As an important amino acid metabolite, proline can act as a signal to trigger plant responses to stress. Proline accumulation is not only a stress signal, but also alleviates plant damage by maintaining photosynthesis and the activities of antioxidant enzymes and the levels of non-enzymatic antioxidant compounds, thus reducing reactive oxygen species content, and regulating osmosis. A better understanding of mechanisms of proline response to abiotic stress in turfgrass is vital for the development of stress-tolerant germplasm. This review summarizes research progress into the role of proline in regulating growth and physiological and molecular adaptations to abiotic stress, with emphasis on drought, salt and temperature tolerance.
-
Key words:
- Turfgrass /
- Proline /
- Abiotic stress












