-
Heat stress is one of the crucial environmental stresses limiting plant growth and development, and ultimately influencing productivity and quality of many plants including turfgrass species[1, 2]. Under heat stress, the unfavorable disorders in fundamental metabolic processes often occur in turfgrass species, such as disturbance in homeostasis of reactive oxygen species (ROS)[3, 4]. ROS are the by-products of enzymatic reactions, including superoxide anion (
${\text{O}^-_2} $ In order to resist oxidative injuries, plants have evolved a complex antioxidant defense system to scavenge and detoxify the excessive accumulation of ROS to maintain the relative steady balance between generation and elimination of ROS. The defense mechanism is composed of both non-enzymatic and enzymatic systems[9]. Non-enzymatic systems (ascorbic acid, glutathione, and nonprotein amino acids, etc.) are one of an important component of antioxidant defense systems[10]. Acting as electron donors, antioxidants directly or indirectly transform toxic ROS into less harmful oxidized products, and modulate redox homeostasis[11]. Enzymatic system involves several antioxidant enzymes, including superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), and ascorbate peroxidase (APX)[9]. The first line of defense in ROS-scavenging enzyme is SOD that catalyzes O2− to H2O2 and molecular oxygen (O2)[11]. Based on different metal co-factors, SOD could be distinguished into three classes including Cu/ZnSOD, MnSOD, and FeSOD, which have various subcellular localization[12]. CAT, POD, and the ascorbate-glutathione cycle further convert H2O2 into H2O and O2[13]. Ascorbate-glutathione cycle including two antioxidants (ascorbic acid and glutathione), as well as four important enzymes (APX, GR, DHAR, and MDHAR), plays a key role in detoxifying the excess H2O2[14, 15]. However, redox homeostasis is still destroyed when plants are exposed to prolonged heat stress[16, 17]. Therefore, maintaining the stability of antioxidant defense system is essential for enhancing heat tolerance in plants, especially in cool-season grass species[18, 19].
As a cool-season turfgrass, creeping bentgrass (Agrostis stolonifera) is widely used in golf greens and other high-quality turfs due to its fine texture and superior tolerance to low mowing height[20]. However, creeping bentgrass is sensitive to heat stress, and likely to be injured by heat stress during the summer season[21, 22]. Thus, it is an urgent need for turf managers to promote heat tolerance in creeping bentgrass. Previous studies have demonstrated that foliar application of plant growth regulators was an effective and convenient strategy to alleviate stress-induced damages[23, 24]. Chitosan is a natural, biocompatible, and biodegradable polysaccharide produced from chitin[25]. Chitosan has nucleophilic behavior, which allows it to form multifunction via structural chemical modifications[26]. Exogenous chitosan with the appropriate concentration and method could greatly mitigate harmful effects of abiotic stresses on plants. Under salt stress, chitosan application led to a significant improvement in salt tolerance through promoting plant growth, and enhancing the expression and activation of alternative oxidase in maize (Zea mays) seedlings[27]. Under drought stress, chitosan effectively alleviated drought stress in association with the altered antioxidant metabolism and various metabolic processes in white clover (Trifolium repens)[28], bermudagrass (Cynodon transvaalensis × C. dactylon)[29], and creeping bentgrass[30]. Under heat stress, foliar application of 200 and 800 mg∙L−1 chitosan had positive effect on mitigating heat damage in kimchi cabbage (Brassica rapa ssp. Pekinensis)[31] and cotton (Gossypium hirsutum)[32], respectively. Exogenous 100 mg∙L−1 chitosan also improved heat tolerance in creeping bentgrass through regulating chlorophyll metabolism, antioxidant defense, and the heat shock pathway[33]. In our previous study, foliar application of chitosan significantly enhanced heat tolerance in creeping bentgrass by inhibiting the decline in photosynthesis and maintaining cell membrane stability[34]. Despite present knowledge, chitosan-induced regulating mechanism on antioxidant defense system has not been well understood in creeping bentgrass under heat stress. Therefore, the objective of this study was to elucidate whether chitosan-promoted thermotolerance associated with the antioxidant defense system under long-term heat stress, including the content of antioxidants, activities of antioxidant enzymes, as well as the expression level of genes encoding antioxidant enzymes. Our findings would be expected to provide a new understanding for chitosan-induced promotion on heat tolerance in perennial turfgrass species.
-
Creeping bentgrass seeds (A. stolonifera cv. 'Penn A-4') were sown in polyvinyl chloride tubes (25-cm height and 10-cm diameter) filled with sand. Plants were grown for 2 months in a greenhouse at 25/20 °C (day/night) and 14 h photoperiod[34]. During establishment, plants were irrigated daily, fertilized once a week with water-soluble fertilizer (Scotts Miracle-Gro Company, USA), and trimmed every 2 d in order to maintain a canopy height of 4−5 cm. Plants were moved to a growth chamber (XBQH-1, Jinan Xubang, Jinan, Shandong Province, China) with temperature of 25/20 °C (day/night), 14 h photoperiod, and 60% relative humidity. Plants were pre-acclimated to the environment of growth chambers for one week before treatments.
Experimental design and treatments
-
After acclimation, the two-month-old plants were divided into four groups as four treatments: (1) control + H2O, foliar application with 10 mL deionized water under non-stressed condition (25/20 °C, day/night); (2) control + chitosan, foliar application with 10 mL chitosan under non-stressed condition (25/20 °C, day/night); (3) heat + H2O, foliar application with 10 mL deionized water under heat stress (38/28 °C, day/night); (4) heat + chitosan, foliar application with 10 mL chitosan under heat stress (38/28 °C, day/night). During 42 d of treatments, plants were sprayed with either deionized water or chitosan every 7 d. For chitosan treatment, chitosan concentration was set as 100 mg∙L−1 based on our previous study in creeping bentgrass[34]. This experiment was arranged in a completely randomized design with four biological replicates (four tubes) for each treatment. Each tube was rearranged daily to reduce impacts of the environment.
Turf quality and staining of ROS
-
Turf quality is a common parameter of overall turf performance rated on a scale of 1 (lowest) to 9 (best) based on texture, color, uniformity, and density[35]. A rating of 1 represented plants that were completely dead with brown leaves, and a rating of 9 indicated plants that were healthy with green and dense turf canopy. Turf quality at the minimal acceptable level was rated a 6.
The presence of hydrogen peroxide (H2O2) and superoxide (
${\text{O}^-_2} $ ${\text{O}^-_2} $ Non-enzymatic antioxidant content and antioxidant enzyme activity measurements
-
Glutathione content and ascorbic acid content in leaves of creeping bentgrass at 0, 21, and 42 d of treatments were determined using kits (Nanjing Yurun Biotechnology Co., Ltd., Jiangsu, China; Suzhou Comin Biotechnology Co., Ltd., Jiangsu, China). Antioxidant enzyme activity in leaves was measured using the previously described method[36, 37] with some modifications. Fresh leaf tissues (0.35 g) at 0, 21, and 42 d of treatments were sampled, then stored at −80 °C to conduct further analysis. Leaves were ground with liquid nitrogen to a fine powder, then extracted with 4 mL of cold extraction buffer (50 mM potassium phosphate, 1 mM ethylenediaminetetraacetic acid, 1% polyvinylpyrrolidone, pH 7.8). The extraction solution was centrifuged at 15,000 g for 30 min at 4 °C and the supernatant was collected for determination of antioxidant enzyme activity. SOD activity was determined based on the rate of NBT reduction in absorbance at 560 nm. CAT, POD, APX, DHAR, MDHAR, and GR activities were assayed following the increase or decrease in absorbance at 240, 470, 290, 265, 340, and 340 nm, respectively. All enzyme activities were determined using a spectrophotometer (Ultrospec 2100 pro, Biochrom Ltd., Cambridge, UK) and expressed on the basis of protein content, which was measured according to Bradford's method[38].
Gene expression analysis of antioxidant enzyme in leaves
-
The transcription levels of selected gene encoding antioxidant enzyme in leave tissue at 0, 21, and 42 d of treatments were detected by real-time quantitative polymerase chain reaction (qRT-PCR). Total RNA was isolated from leaves with using a FlaPure Plant Total RNA Extraction Kit (Genesand, Beijing, China) according to the manufacturer’s instructions. Determination in RNA concentration was conducted by Tecan Infinite 200 pro (Grödig, Austria). The RNA was reverse-transcribed to cDNA by using MonScript™ RTIII Super Mix with dsDNase (Two-Step) (Monad, Wuhan, China).
For detecting transcript levels of genes in heat-treated and chitosan-treated conditions, Roche LightCycler480 II machine (Roche Diagnostic, Rotkreuz, Switzerland) and MonAmp™ ChemoHS qPCR Mix (Monad, Wuhan, China) were used for performing qRT-PCR. Primer sequences of SOD (AsFeSOD, AsCu/ZnSOD, AsMnSOD), CAT (AsCATA, AsCATB, AsCATC), POD (AsPerox4), APX (AsAPX1, AsAPX2, AsAPX3, AsAPX4, AsAPX5, AsAPX6, AsAPX8), GR (AsGR1, AsGR2), DHAR (AsDHAR), MDHAR (AsMDHAR) genes, and reference gene (ACT2) are provided in Table 1[39]. The specificity of each primer pair was confirmed by analyzing melting curve in qRT-PCR. PCR condition for all genes was set on the basis of the following parameters: 95 °C at 10 min, 15 s at 95 °C (40 cycles of denaturation), annealing for 15 s at 60 °C, and extending for 20 s at 72 °C. The relative expression level between interest genes and reference gene was to calculate according to 2−ΔΔCᴛ method[40].
Table 1. Primer sequences used in the experiment.
Gene name Forward primer (5’-3’) Reverse primer (5’-3’) AsFeSOD TGCTCGTCTGTCATCCTTGT GGTTGGGTTTGGCTTGTCTT AsCu/ZnSOD AATGTGACAGCTGGAGTGGA CCCTTGCCAAGATCATCAGC AsMnSOD AGGAACCAGGTTTGCTCCTT GATGAATGCAGAGGGTGCTG AsCATA TACTCCGACGACAAGATGCT TTCTTGAATCCGCACTTGGG AsCATB AGTGGATTCCAGGGACAGTG GACCATCGATGCAGATCACG AsCATC CCTGGCTGCTTGAAGTTGTT ACTTCCCGTCCAGGTTTGAT AsPerox4 GATGTTGCCCATCTTGACCA ACTACAGCAACCTCCTGTCC AsAPX1 CTCCTACGCCGATCTCTACC TGCCGAAGACTTGCCTTAGA AsAPX2 GGAGAGAGGACAAGCCTGAG AAACCCATCTGAGCGGAGAA AsAPX3 TACATCGCGGAGATCGAGAG GATCTTGAGCCCTGCATTGG AsAPX4 CTGCAACTACTCCAGCAAGC CACAAGAACTGGTGGTGCAA AsAPX5 GCGGCTTAGTCAAGGAGTTG CGACGAGATGGTCTCTGACA AsAPX6 CAAGTCTCTGCATGGAACGG CCATACTTTGCTGCTGCCAT AsAPX8 TCCTTGTCATCAAGGCCCAT CACAGCTCCTGAGCAATGTC AsDHAR TGCGTGAACTCTATCGCTCT GAGCGTGCAGCTCCATTATT AsGR1 TCCTCCGCAGTCCACATATC GTTAGGGTTTGGAGGGTGGT AsGR2 CACACGGCGAAACACATACT AGAATCACAGCACGTTTCGG AsMDHAR GCACGTACTGGGTCAAAGAC TTCATATGTTGGCGGCGAAG ACT2 CCTTTTCCAGCCATCTTTCA GAGGTCCTTCCTGATATCCA Statistical analysis
-
Data were analyzed using the SPSS statistics software (SPSS 21.0; SPSS Inc., Chicago, IL, USA). The means ± standard error (SE) was calculated for all measured parameters in column charts. ANOVA analysis and Duncan multiple comparison were applied to determine significant differences between mean values for each parameter at the probability level of 0.05.
-
Heat stress led to a significant decrease in turf quality at 21 and 42 d of treatments, and increased accumulation of both H2O2 and
${\text{O}^-_2} $ ${\text{O}^-_2} $ 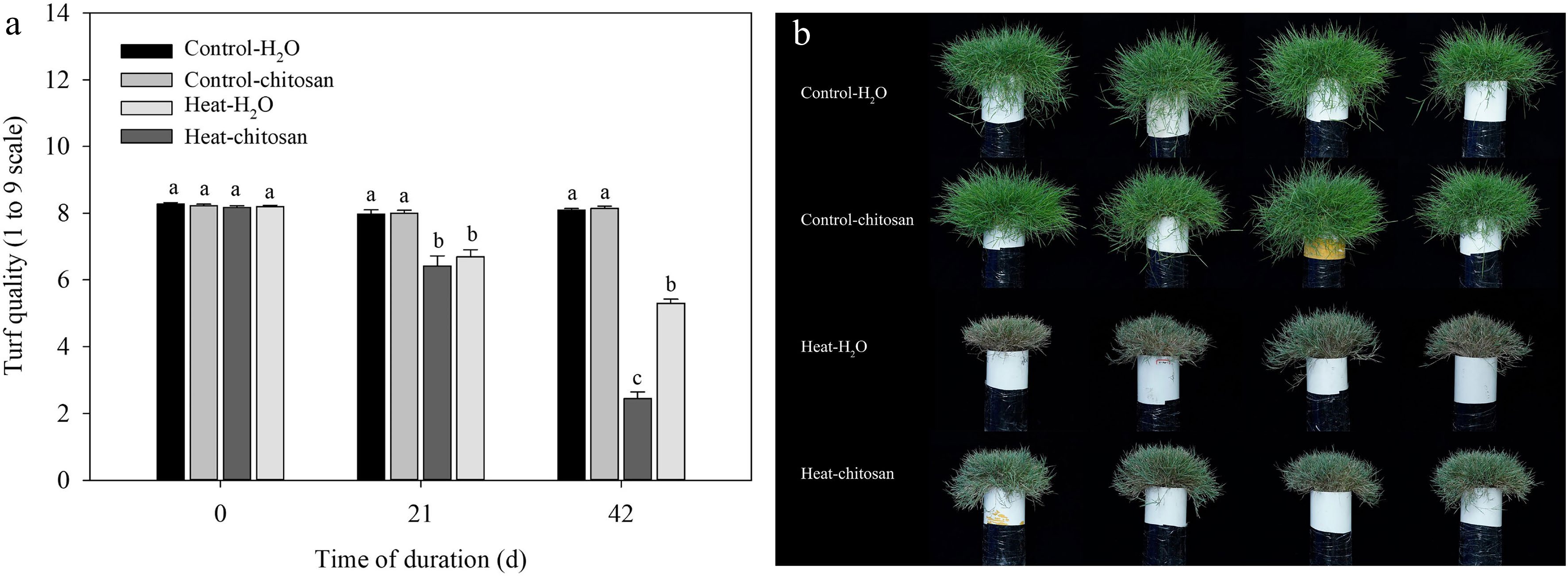
Figure 1.
Effects of heat stress and exogenous chitosan on (a) turf quality and (b) shoot phenotype in creeping bentgrass exposed to non-stressed control and heat stress. Control + H2O, foliar application with deionized water under non-stressed condition (25/20 °C, day/night); Control + chitosan, foliar application with 100 mg∙L−1 chitosan under non-stressed condition (25/20 °C, day/night); Heat + H2O, foliar application with deionized water under heat stress (38/28 °C, day/night); Heat + chitosan, foliar application with 100 mg∙L−1 chitosan under heat stress (38/28 °C, day/night). Shoot phenotype was taken at 42 d of treatments. Different lowercase letters represent significant difference between different treatments during the experimental period (P ≤ 0.05). Error bars represent standard error (SE).
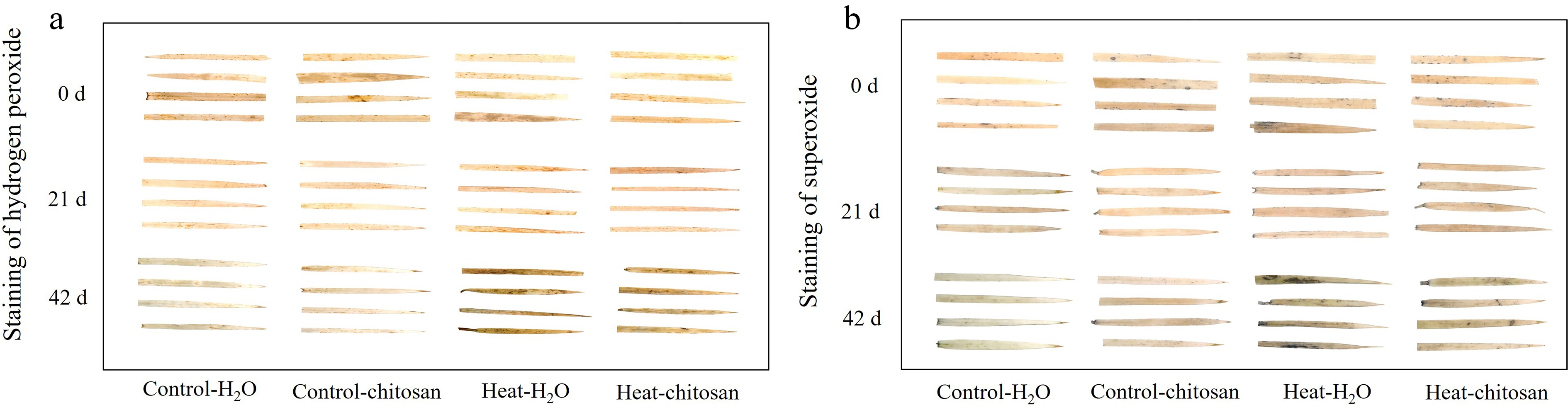
Figure 2.
Effects of heat stress and exogenous chitosan on staining of (a) hydrogen peroxide and (b) superoxide in creeping bentgrass. Control + H2O, foliar application with deionized water under non-stressed condition (25/20 °C, day/night); Control + chitosan, foliar application with 100 mg∙L−1 chitosan under non-stressed condition (25/20 °C, day/night); Heat + H2O, foliar application with deionized water under heat stress (38/28 °C, day/night); Heat + chitosan, foliar application with 100 mg∙L−1 chitosan under heat stress (38/28 °C, day/night).
Non-enzymatic antioxidant content
-
At 21 and 42 d under heat stress, chitosan-treated plants had 54% and 32% higher ascorbic acid content than untreated plants, respectively (Fig. 3a). Plants with or without chitosan under heat stress exhibited an obviously decrease in glutathione content at 21 and 42 d compared with non-stressed control (Fig. 3b). Chitosan-treated plants had higher glutathione content than untreated plants at 21 d of heat stress, but were not observed the significant difference in glutathione content at 42 d of heat treatment.
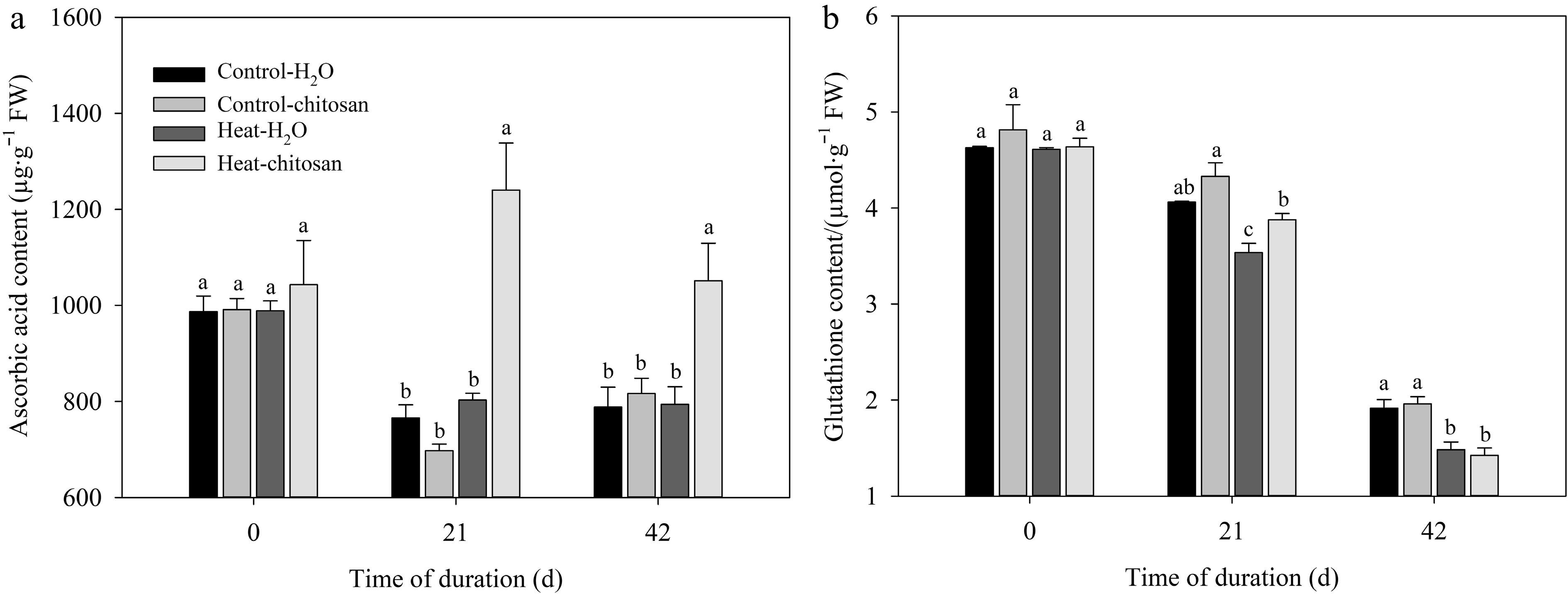
Figure 3.
Effects of heat stress and exogenous chitosan on content of (a) ascorbic acid and (b) glutathione in creeping bentgrass. Control + H2O, foliar application with deionized water under non-stressed condition (25/20 °C, day/night); Control + chitosan, foliar application with 100 mg∙L−1 chitosan under non-stressed condition (25/20 °C, day/night); Heat + H2O, foliar application with deionized water under heat stress (38/28 °C, day/night); Heat + chitosan, foliar application with 100 mg∙L−1 chitosan under heat stress (38/28 °C, day/night). Different lowercase letters represent significant difference between different treatments during the experimental period (P ≤ 0.05). Error bars represent standard error (SE).
Antioxidant enzyme activity
-
At 21 d of non-stressed treatment, exogenous chitosan caused 38% and 48% significant increase in activities of SOD and POD, respectively, compared with untreated plants (Fig. 4a, c). At 21 d of treatments, heat stress significantly decreased POD activity compared with plants under non-stressed condition (Fig. 4c). At 42 d, activities of SOD, CAT, and POD increased significantly in response to heat stress compared with plants under non-stressed condition, but no significant difference in activities of SOD and CAT was found between plants with or without chitosan (Fig. 4a−c). Foliar application of chitosan had 30% higher POD activity than untreated plants at 42 d of heat stress.
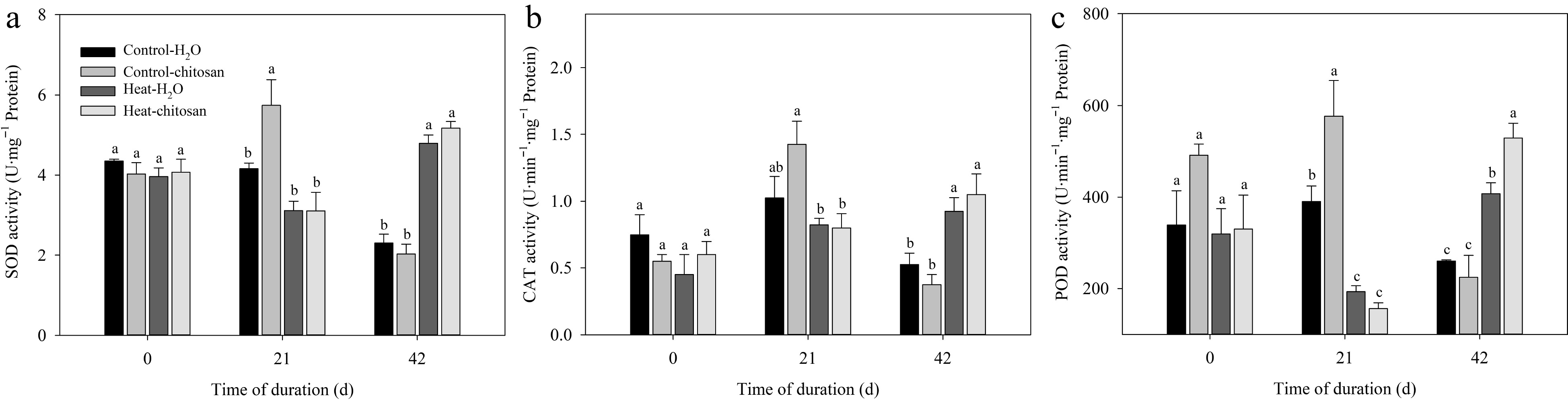
Figure 4.
Effects of heat stress and exogenous chitosan on activities of (a) SOD, (b) CAT, and (c) POD in creeping bentgrass. Control + H2O, foliar application with deionized water under non-stressed condition (25/20 °C, day/night); Control + chitosan, foliar application with 100 mg∙L−1 chitosan under non-stressed condition (25/20 °C, day/night); Heat + H2O, foliar application with deionized water under heat stress (38/28 °C, day/night); Heat + chitosan, foliar application with 100 mg∙L−1 chitosan under heat stress (38/28 °C, day/night). Different lowercase letters represent significant difference between different treatments during the experimental period (P ≤ 0.05). Error bars represent standard error (SE).
At 21 d of non-stressed condition, exogenous chitosan significantly increased APX activity compared with plants without chitosan. Chitosan-treated plants had 58% higher increase in APX activity than untreated plants at 42 d of heat stress (Fig. 5a). Heat stress significantly increased activities of both GR and DHAR compared with non-stressed control at 21 and 42 d. Plants applied with chitosan demonstrated significant enhancement in GR and DHAR activities compared with untreated plants at 42 d under heat stress (Fig. 5b, c). Exogenous chitosan significantly increased MDHAR activity by 94% compared with untreated plants at 21 d of heat stress (Fig. 5d), but there was no significant difference in MDHAR activity between chitosan-treated and untreated plants at 42 d of heat stress.
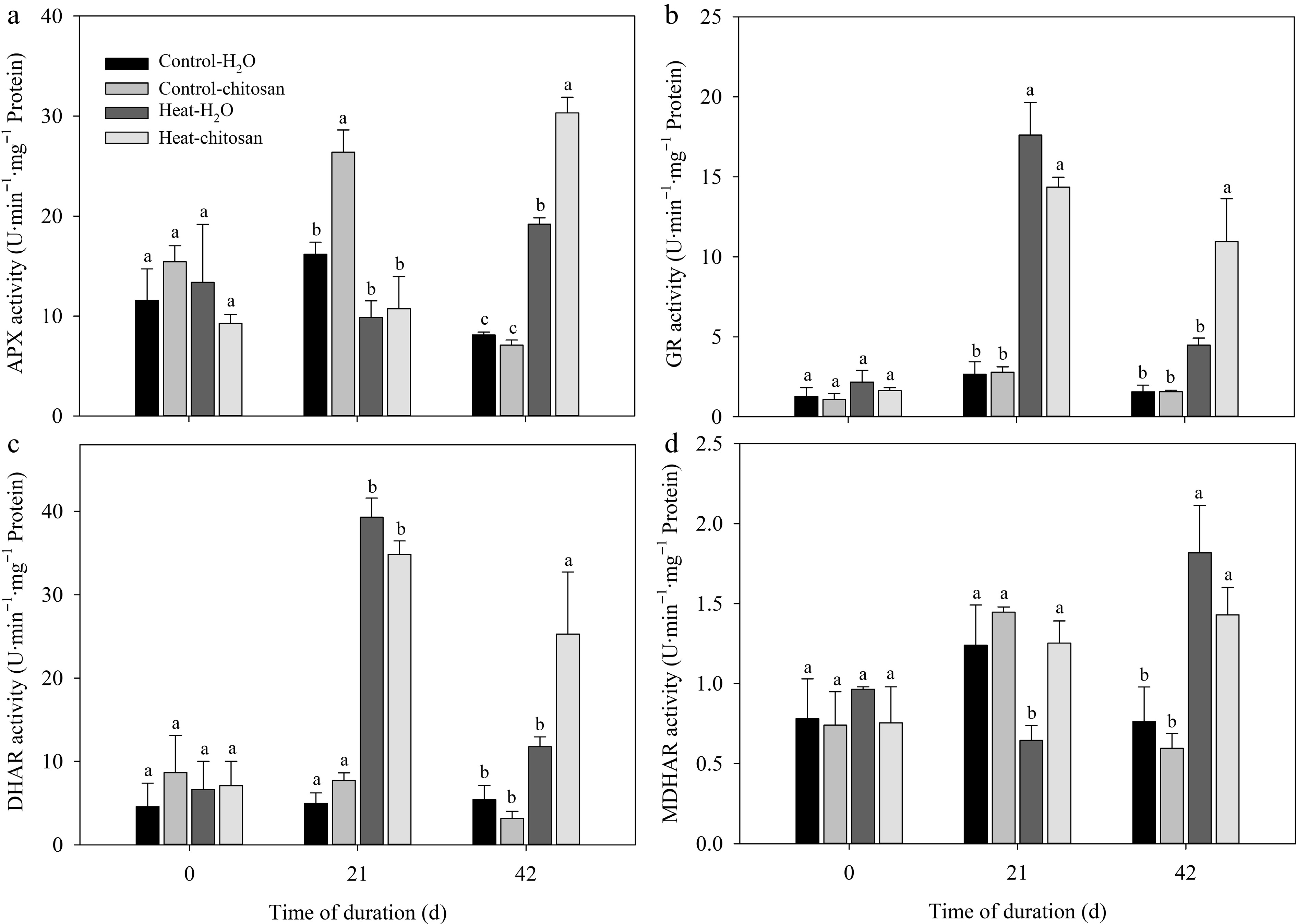
Figure 5.
Effects of heat stress and exogenous chitosan on activities of (a) APX, (b) GR, (c) DHAR, and (d) MDHAR in creeping bentgrass. Control + H2O, foliar application with deionized water under non-stressed condition (25/2 °C, day/night); Control + chitosan, foliar application with 100 mg∙L−1 chitosan under non-stressed condition (25/20 °C, day/night); Heat + H2O, foliar application with deionized water under heat stress (38/28 °C, day/night); Heat + chitosan, foliar application with 100 mg∙L−1 chitosan under heat stress (38/28 °C, day/night). Different lowercase letters represent significant difference between different treatments during the experimental period (P ≤ 0.05). Error bars represent standard error (SE).
Gene expression of antioxidant enzyme
-
The expression levels of SOD (AsFeSOD, AsCu/ZnSOD, and AsMnSOD), CAT (AsCATA, AsCATB, and AsCATC), and POD (AsPerox4) genes in leaves were measured in this experiment (Figs 6 & 7). For SOD genes, expression levels of both AsCu/ZnSOD and AsMnSOD were down-regulated and up-regulated significantly compared with non-stressed control at 21 d and 42 d, respectively (Fig. 6b, c). Chitosan-treated plants showed 1.1-fold higher expression level in AsCu/ZnSOD than untreated plants at 42 d of heat stress (Fig. 6b). At 42 d of heat stress, exogenous chitosan significantly up-regulated the expression of AsCATB by 82% compared with untreated plants, but without significant effects on AsCATA and AsCATC genes (Fig. 7a−c). Chitosan-treated plants had 1.2-fold higher expression levels of AsPerox4 than that without chitosan at 42 d of heat stress (Fig. 7d).
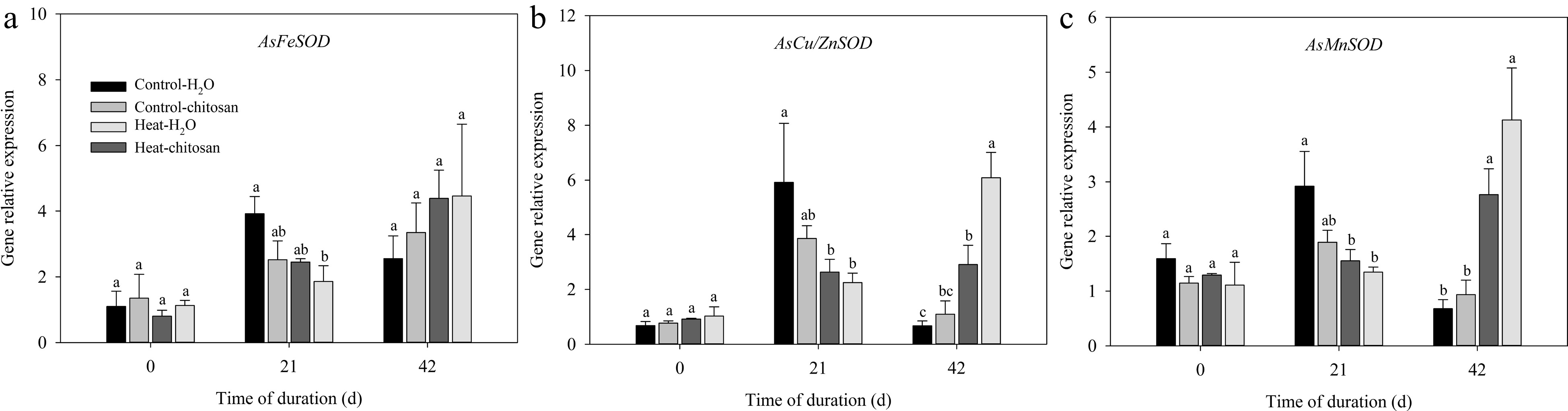
Figure 6.
Effects of heat stress and exogenous chitosan on expression levels of (a) AsFeSOD, (b) AsCu/ZnSOD, and (c) AsMnSOD in creeping bentgrass. Control + H2O, foliar application with deionized water under non-stressed condition (25/20 °C, day/night); Control + chitosan, foliar application with 100 mg∙L−1 chitosan under non-stressed condition (25/20 °C, day/night); Heat + H2O, foliar application with deionized water under heat stress (38/28 °C, day/night); Heat + chitosan, foliar application with 100 mg∙L−1 chitosan under heat stress (38/28 °C, day/night). Different lowercase letters represent significant difference between different treatments during the experimental period (P ≤ 0.05). Error bars represent standard error (SE).
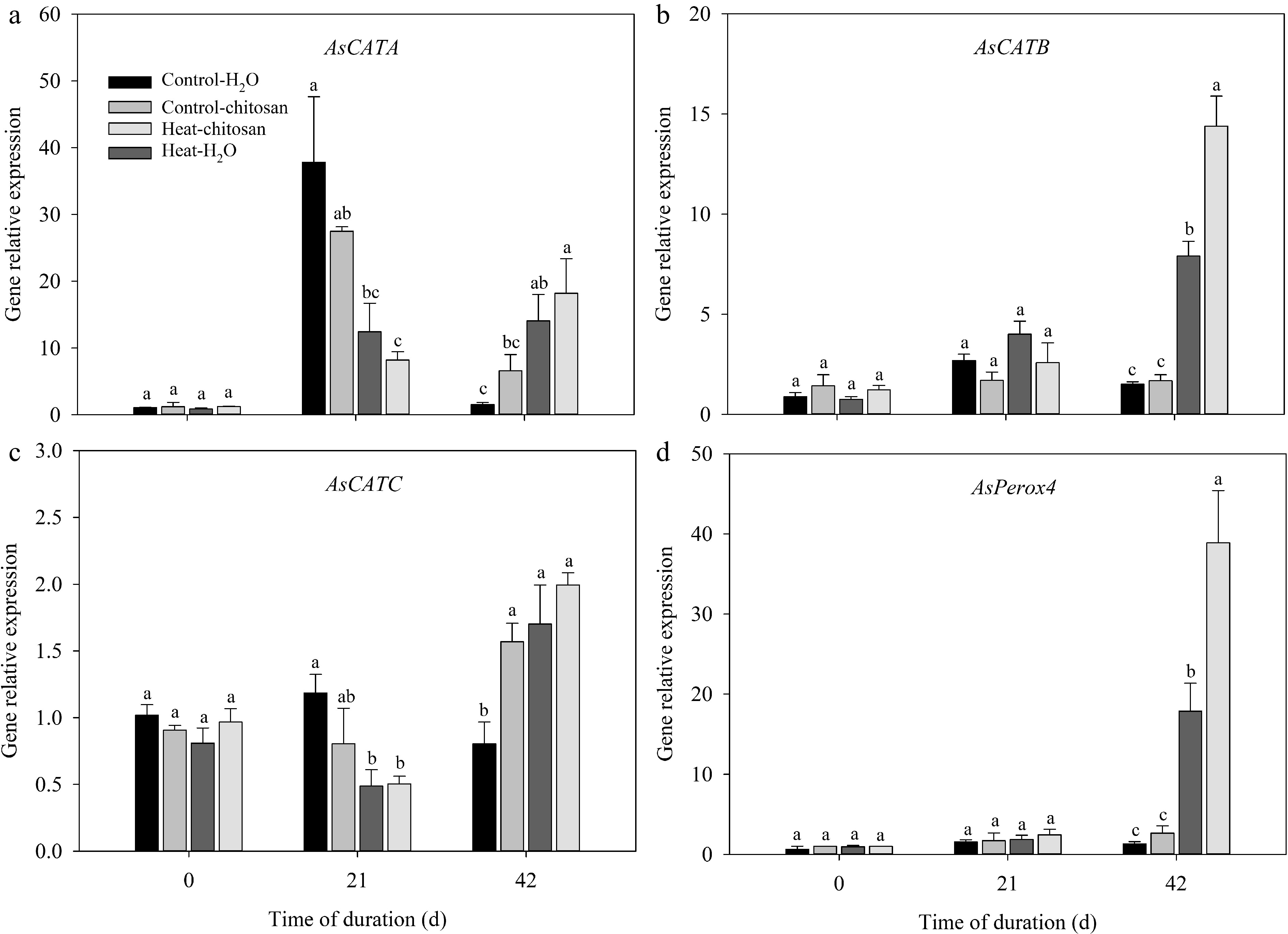
Figure 7.
Effects of heat stress and exogenous chitosan on expression levels of (a) AsCATA, (b) AsCATB, (c) AsCATC, and (d) AsPerox4 in creeping bentgrass. Control + H2O, foliar application with deionized water under non-stressed condition (25/20 °C, day/night); Control + chitosan, foliar application with 100 mg∙L−1 chitosan under non-stressed condition (25/20 °C, day/night); Heat + H2O, foliar application with deionized water under heat stress (38/28 °C, day/night); Heat + chitosan, foliar application with 100 mg∙L−1 chitosan under heat stress (38/28 °C, day/night). Different lowercase letters represent significant difference between different treatments during the experimental period (P ≤ 0.05). Error bars represent standard error (SE).
The transcript levels of APX, GR, DHAR, and MDHAR genes related to ascorbate-glutathione cycle were also quantified (Figs 8 & 9). Under normal condition, exogenous chitosan significantly up-regulated AsAPX1 compared with untreated plants at 21 and 42 d. The expression level of AsAPX1 in chitosan-treated plants also higher than untreated plants at 21 d of heat stress (Fig. 8a). At 42 d of heat stress, expressions of different APX genes were significantly up-regulated to certain extent due to foliar application of chitosan, including AsAPX2, AsAPX3, AsAPX4, AsAPX6, and AsAPX8, which demonstrated 1.4-fold, 1.2-fold, 1.0-fold, 0.8-fold, and 1.1-fold higher than untreated plants, respectively (Fig. 8b−d, f, g). At 42 d of treatments, transcript levels of both AsGR1 and AsMDHAR were significantly up-regulated compared with non-stressed control in response to heat stress, however, there was no significant difference in the expression of these genes between chitosan-treated and untreated plants under heat stress (Fig. 9a, d). Chitosan-treated plants showed 2.0-fold and 1.4-fold increase in expression level of both AsGR2 and AsDHAR, respectively, compared with untreated plants at 42 d of heat stress (Fig. 9b, c).
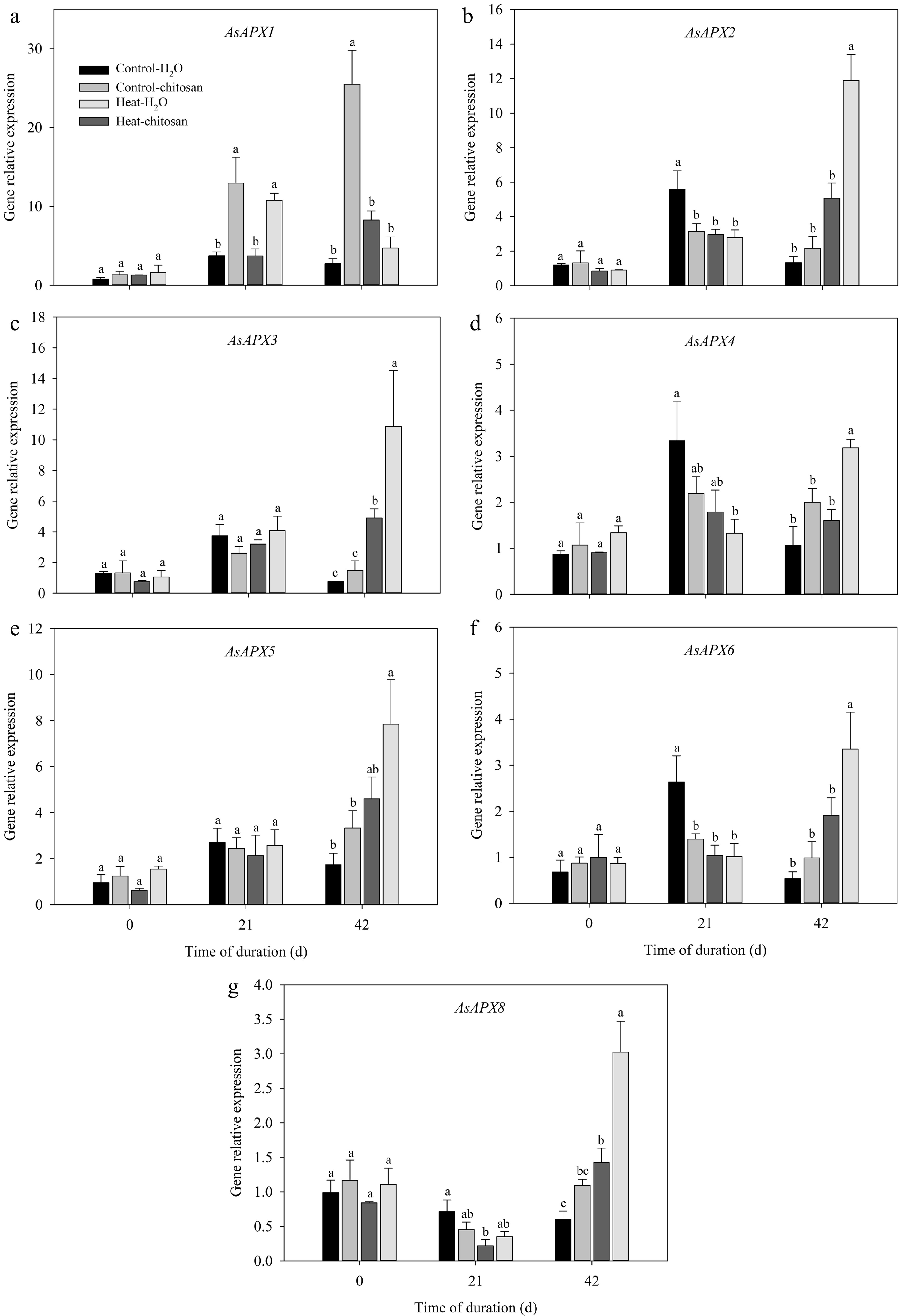
Figure 8.
Effects of heat stress and exogenous chitosan on expression levels of (a) AsAPX1, (b) AsAPX2, (c) AsAPX3, (d) AsAPX4, (e) AsAPX5, (f) AsAPX6, and (g) AsAPX8 in creeping bentgrass. Control + H2O, foliar application with deionized water under non-stressed condition (25/20 °C, day/night); Control + chitosan, foliar application with 100 mg∙L−1 chitosan under non-stressed condition (25/20 °C, day/night); Heat + H2O, foliar application with deionized water under heat stress (38/28 °C, day/night); Heat + chitosan, foliar application with 100 mg∙L−1 chitosan under heat stress (38/28 °C, day/night). Different lowercase letters represent significant difference between different treatments during the experimental period (P ≤ 0.05). Error bars represent standard error (SE).
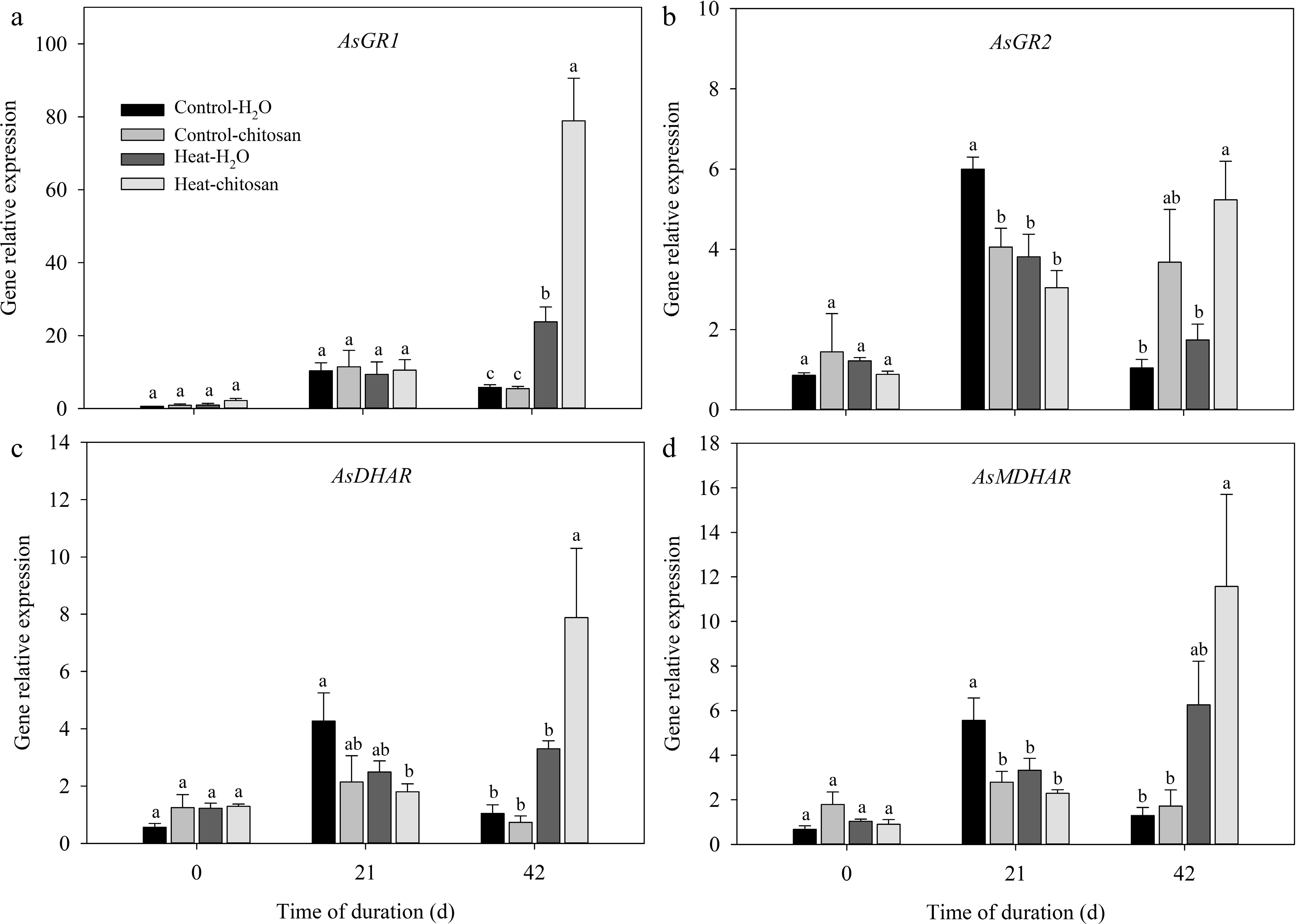
Figure 9.
Effects of heat stress and exogenous chitosan on expression levels of (a) AsGR1, (b) AsGR2, (c) AsDHAR, and (d) AsMDHAR in creeping bentgrass. Control + H2O, foliar application with deionized water under non-stressed condition (25/20 °C, day/night); Control + chitosan, foliar application with 100 mg∙L−1 chitosan under non-stressed condition (25/20 °C, day/night); Heat + H2O, foliar application with deionized water under heat stress (38/28 °C, day/night); Heat + chitosan, foliar application with 100 mg∙L−1 chitosan under heat stress (38/28 °C, day/night). Different lowercase letters represent significant difference between different treatments during the experimental period (P ≤ 0.05). Error bars represent standard error (SE).
-
It is generally known that the application of plant growth regulators is an effective method to enhance thermotolerance, which might be associated with changes in various physiological and biochemical processes under heat stress[41−43]. In this study, heat stress led to serious damages in creeping bentgrass, but chitosan application induced an improvement in heat tolerance as indicated by the reduction in ROS accumulation, better maintenance in antioxidant defense system, as well as the higher gene expression compared with the untreated plants under heat stress as discussed below.
The imbalance between the production and scavenging of ROS in heat-stressed plant resulted in oxidative stress, and influenced normal physiological activities in plants[44, 45]. Jahan et al.[46] observed a significantly increased ROS (
${\text{O}^-_2} $ ${\text{O}^-_2} $ ROS scavenging system plays a key role in mitigating the adverse effects of oxidative stress and conferring heat tolerance in turfgrass species[52, 53]. Various non-enzymatic antioxidants and antioxidant enzymes including SOD, CAT, POD, and ascorbate-glutathione cycle components (ascorbic acid, glutathione, APX, GR, DHAR, and MDHAR) functions in detoxifying the overproduction of ROS[9]. Ascorbate-glutathione cycle is also referred to as the Asada–Halliwell pathway, which is a crucial antioxidant defense pathway to detoxify H2O2 and remain redox homeostasis in plant cells[54]. In lettuce (Lactuca sativa) seedlings, exogenous spermidine positively alleviated oxidative damage via enhancing ascorbate-glutathione cycle under heat stress[55]. Antioxidant enzymes involved in ascorbate-glutathione cycle showed a significant activation due to melatonin application, mitigating heat-induced damage in wheat seedlings[56]. In the present study, heat stress induced a significant increase in multiple antioxidant enzyme activities indicating that antioxidant defense system was triggered in response to heat stress to prevent plants from severe injury. Chitosan application improved heat tolerance mainly associated with positive changes in POD activities and ascorbate-glutathione cycle, as confirmed by significantly increased ascorbic acid content, POD, APX, GR, and DHAR activities compared with untreated plant under heat stress (Figs 3, 4 & 5). Huang et al.[33] previously found that chitosan-pretreated plants had higher heat tolerance through improving activities of four antioxidant enzymes (SOD, CAT, POD, and APX) at 15 d of heat stress. But in our study, no significant difference in activities of SOD and CAT was found between plants with or without chitosan at 42 d of long-term heat stress. Generally, activities of SOD and CAT increased and then declined under heat stress[57]. The different change in activities of SOD and CAT might indicate that both enzymes played an important role in the early duration of treatments. Moreover, heat stress also induced the up-regulation in transcriptional level of antioxidant enzyme genes in order to protect cellular components against oxidative stress[58]. Li et al.[19] revealed that exogenous application of γ-aminobutyric acid up-regulated the transcript of genes encoding antioxidant enzymes (SOD, CAT, POD, APX, MDHAR, DHAR, and GR) under water deficit stress. In this study, exogenous chitosan significantly up-regulated several relative gene expressions compared with untreated plants under heat stress, including SOD (AsCu/ZnSOD), CAT (AsCATB), POD (AsPerox4), APX (AsAPX2, AsAPX3, AsAPX4, AsAPX6, and AsAPX8), GR (AsGR2), and DHAR genes (AsDHAR) (Figs 6, 7, 8&9). Results showed that exogenous chitosan induced a significant increase in POD, APX, GR, and DHAR at both enzymatic and gene expression levels under heat stress. However, antioxidant enzyme activities were regulated by multiple factors, which might lead to variations in the transcript levels of antioxidant enzymes inconsistent with antioxidant enzyme activities[59]. Therefore, in the current study, foliar application of chitosan up-regulated transcript levels of AsCu/ZnSOD and AsCATB, but did not enhance activities of SOD and CAT. Our above results indicated that chitosan-induced promotion in heat tolerance resulted from alterations in antioxidant defense system including non-enzymatic antioxidants, antioxidant enzymes, as well as relative gene expression for ROS scavenging in creeping bentgrass.
In conclusion, exogenous chitosan significantly enhanced heat tolerance in creeping bentgrass. Chitosan-induced heat tolerance could be attributed to the less oxidative damage and better maintenance of antioxidant defense system detoxifying ROS. Foliar application of chitosan suppressed overproduction of ROS (H2O2 and O2−), improved ascorbic acid content and antioxidant enzymes activities (POD, APX, GR, and DHAR), as well as up-regulated transcript levels of AsCu/ZnSOD, AsCATB, AsPerox4, AsAPX2, AsAPX4, AsAPX6, AsAPX8, AsGR2, and AsDHAR compared with untreated control. In the future, further investigating into the molecular mechanisms of chitosan in improving heat tolerance is necessary.
This study was supported by the Fundamental Research Funds for the Central Universities (XUEKEN2022020) and the project of the Innovation and Promotion of Forestry Science and Technology of Jiangsu Province (LYKJ[2021]29).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Li Q, Bian Y, Li R, Yang Z, Liu N, et al. 2023. Chitosan-enhanced heat tolerance associated with alterations in antioxidant defense system and gene expression in creeping bentgrass. Grass Research 3:7 doi: 10.48130/GR-2023-0007
Chitosan-enhanced heat tolerance associated with alterations in antioxidant defense system and gene expression in creeping bentgrass
- Received: 12 March 2023
- Accepted: 19 April 2023
- Published online: 10 May 2023
Abstract: As an effective plant growth regulator, chitosan plays a positive role in enhancing heat tolerance in perennial turfgrass. The objective of this study was to elucidate whether chitosan-promoted thermotolerance was associated with the antioxidant defense system under long-term heat stress in creeping bentgrass (Agrostis stolonifera). Plants were treated with or without 100 mg∙L−1 chitosan under either heat stress (38/28 °C, day/night) or non-stressed condition (25/20 °C, day/night) for 42 d in growth chambers. Foliar application of chitosan significantly enhanced heat tolerance as reflected by the increased turf quality through inhibiting over-accumulation of reactive oxygen species, and increasing ascorbic acid content and antioxidant enzymes activities (peroxidase, POD; ascorbate peroxidase, APX; glutathione reductase, GR; dehydroascorbate reductase, DHAR). Chitosan-treated plants also had higher transcript levels of AsCu/ZnSOD, AsCATB, AsPerox4, AsAPX2, AsAPX3, AsAPX4, AsAPX6, AsAPX8, AsGR2, and AsDHAR genes in comparison to the untreated plants. The results suggested that chitosan-promotion in heat tolerance could be associated with non-enzymatic antioxidants, antioxidant enzymes, as well as relative gene expression.
-
Key words:
- Chitosan /
- Heat tolerance /
- Antioxidant defense system /
- Creeping bentgrass












