-
China has cultivated the sacred lotus (Nelumbo nucifera), a perennial rhizome aquatic plant in the Nelumbonaceae family, for over 2,000 years on 330,000 hectares[1]. Two species of Nelumbo exist: nucifera and lutea. N. nucifera inhabits Asia and Oceania[2]. N. lutea inhabits North and Northern South America[3,4]. N. nucifera and N. lutea are only geographically separated, not reproductively[5]. Hybrid breeding of N. nucifera and N. lutea may enhance sacred lotus diversity. As a food-drug dual, sacred lotus is popular in East Asia, especially China[2]. According to the 2020 edition of the 'Pharmacopoeia of the People's Republic of China'[6], sacred lotus leaves, flowers, seeds, stamens, receptacles, and internodes are commonly used medicinal materials and have important medicinal value. For example, the lotus leaf may clear heat, relieve summer heat, and send clarity (pure) upward. Lotus has been shown to promote blood circulation and hemostasis, as well as to remove dampness and wind and nourish the heart and kidney. The lotus seed can tonify the spleen and kidney, alleviate diarrhoea, and stop seminal secretions[6]. Lotus plumules can clear the mind and clear the heart, as well as restore appropriate heart-kidney coordination, boost essence, and stop bleeding[7−9].
BIAs with medical potential and healthcare benefits are being studied. BIAs are various plant-specific tyrosine-derived metabolites[10]. Most sacred lotus alkaloids are 1-benzylisoquinoline, aporphine, and bisbenzylisoquinoline. Norcoclaurine, a typical 1-benzylisoquinoline alkaloid, treats heart failure, arrhythmia, bradycardia, myocardial ischemia-reperfusion injury, and cardiac fibrosis in traditional Chinese medicine[11,12]. Norcoclaurine has anti-inflammatory, anti-arrhythmic, and antithrombotic properties and is a β2-adrenergic receptor agonist[13]. Neferine and isoliensinine, the main bisbenzylisoquinoline alkaloids in sacred lotus plumule extract, are pharmacologically significant[7]. Neferine possesses anti-inflammatory, anti-oxidative, anti-hypertensive, anti-arrhythmic, anti-platelet, anti-thrombotic, anti-amnesic, anti-anxiety, and anti-cancer characteristics. Isoliensinine is anti-tumor, cardioprotective, antioxidant, antidepressant, anti-HIV, and anti-Alzheimer's[14,15]. According to recent research, bisbenzylisoquinoline alkaloids may cure new coronavirus pneumonia[16]. Lotus leaves contain high-purity aporphine alkaloid nuciferine (NF). NF is anti-obesity, anti-hyperlipidemia, hypoglycemia, hypouricemic, anti-inflammatory[17], and otherwise therapeutic[18−20].
The metabolic pathways, biosynthesis, and corresponding enzymes involved in the formation of benzylisoquinoline alkaloids derived from the sacred lotus plant have yet to be elucidated, despite their significant pharmacological properties. Currently, the primary focus of research on the biosynthesis of benzylisoquinoline alkaloids (BIAs) lies in opium poppy (Papaver somniferum) and other related species within the Ranunculales order. Extensive investigations have successfully revealed the complete biosynthetic pathways of various alkaloids possessing significant pharmacological properties, including morphine (morphinan), noscapine (phthalideisoquinoline), and sanguinarine (benzophenanthridine)[21]. Although the structure of BIAs in members of the Ranunculales order is characterised by complexity and diversity, it is important to note that all BIAs share a common biosynthetic origin. Specifically, metabolites derived from L-tyrosine, dopamine, and 4-hydroxyphenylacetaldehyde (4-HPAA) undergo a Pictet-Spengler condensation catalysed by norcoclaurine synthase (NCS), resulting in the formation of (S)-norcoclaurine. Subsequently, this compound is transformed into the key intermediate (S)-reticuline through the action of three methyltransferases (6OMT, CNMT, 4'OMT) and one cytochrome P450 monooxygenase (CYP), known as N-methylcoclaurine 3'-hydroxylase (NMCH)[22−26]. The processes outlined above are often known as the upstream universal synthesis pathway. Subsequently, a series of oxidative enzymes facilitate the specific coupling of C-C and C-O bonds, leading to the transformation of (S)-reticuline into protoberberine, which serves as a precursor for the synthesis of benzophenanthridines and phthalideisoquinolines. Additionally, the conversion of (S)-reticuline gives rise to the formation of aporphine and morphinan alkaloids.
In the BIAs biosynthetic pathway of sacred lotus, from L-tyrosine to dopamine and 4-hydroxyphenylacetaldehyde (4-HPAA) to the formation of N-methylcoclaurine and reticuline is common to the synthesis pathway of Ranunculales species such as opium poppy, and the synthesis pathway is clear. However, the synthesis of bisbenzylisoquinolines (liensinine, neferine), the different methylation modifications between bisbenzylisoquinoline alkaloids, and the synthesis of aporphine compounds (nuciferine, etc.) are not clear. Therefore, it is crucial to investigate the pharmacological importance of these particular chemicals by studying the sacred lotus' functional enzymes. Hence, it is essential to conduct a comprehensive investigation on the functional enzymes present in the sacred lotus in order to elucidate the pharmacological potential of these distinct substances. Furthermore, it is worth noting that several benzylisoquinoline alkaloids (BIAs) derived from the sacred lotus have a conformation mostly composed of the R-enantiomer. This is in stark contrast to the prevalent S-enantiomer conformation seen in BIAs derived from opium poppy and plants connected to the Ranunculales order. Hence, the investigation of the atypical stereochemistry of BIAs in the sacred lotus has significance in terms of its molecular and biochemical aspects. Furthermore, the study of the metabolism and biosynthesis of angiosperms, which constitute the fundamental group of flowering plants, has significant implications for the understanding of plant evolution.
-
The sacred lotus contains three forms of BIAs: 1-benzylisoquinoline, aporphine, and bisbenzylisoquinoline alkaloids (Table 1). Their structure, concentration, and physiological functions in sacred lotus have been extensively studied. Their chemical formula, stereo configuration and distribution in sacred lotus organs are shown in Table 1.
Table 1. Benzylisoquinoline alkaloids (BIAs) were identified in several organs of Nelumbo nucifera, together with their respective chemical formulas and stereochemical properties. L, lotus leaf; E, lotus embryo; F, lotus flower; S, lotus seed; R, lotus rhizome.
No. Alkaloid Formula Enantiomer Organ Reference 1-Benzylisoquinoline 1 Norcoclaurine C16H17NO3 (+)-R and (−)-S L, E [27−30] 2 Coclaurine C17H19NO3 (+)-R L, E, F [27,29,31] 3 Norjuziphine C17H19NO3 NS F [32] 4 Isococlaurine C17H19NO3 NS F [33] 5 N-Methylcoclaurine C18H21NO3 (−)-R L, E, F [27,29,31] 6 6-Demethyl-4'-O-methyl-N-methylcoclaurine C18H21NO3 NS E [29] 7 Norarmepavine C18H21NO3 (+)-R F [31] 8 N-Methylisococlaurine C18H21NO3 NS L, E [29,34] 9 Norroefractine C18H21NO3 NS F [33] 10 Juziphine C17H19NO3 NS F [33] 11 Armepavine C19H23NO3 (−)-R and (+)-S L, E, S [29,31,35,36] 12 4'-O-Methyl-N-methylcoclaurine C19H23NO3 NS E [29] 13 Lotusine C19H24NO3+ NS E [29] 14 Isolotusine C19H24NO3+ NS E [29] 15 4'-O-Methylarmepavine C20H25NO3 NS L [37] Aporphine 16 Caaverine C17H17NO2 (−)-R L [35,38] 17 Asimilobine C17H17NO2 (−)-R L, F [31,38,39] 18 Glaziovine C18H19NO3 N/A F [33] 19 O-Nornuciferine C18H19NO2 (−)-R L, F [13,38,40] 20 N-Nornuciferine C18H19NO2 (−)-R L, E, F [13,29,38] 21 Lirinidine C18H19NO2 (−)-R L, F [13] 22 N-Methylasimilobine C18H19NO2 N/A F [32] 23 Roemerine C18H17NO2 (−)-R L, F [38,40−42] 24 Dehydronuciferine C19H19NO2 N/A L, R [13,34,41] 25 Dehydroanonaine C17H13NO2 N/A L [34] 26 Dehydroroemerine C18H15NO2 N/A L [34] 27 Pronuciferine C19H21NO3 (+)-R and (−)-S L, E, F [13,29,35,37] 28 Nuciferine C19H21NO2 (−)-R L, E, F [29,31,38,40] 29 7-Hydroxydehydronuciferine C19H19NO3 N/A L [38] 30 Lysicamine C18H13NO3 N/A L, F [13] 31 Cepharadione B C19H15NO4 N/A L [32] 32 Anonaine C17H15NO2 (−)-R L, F [38,41] 33 Liriodenine C17H9NO3 N/A L [38] Bisbenzylisoquinoline 34 Nelumboferine C36H40N2O6 NS E, S [41,43] 35 Liensinine C37H42N2O6 1R,1'R L, E, F, S [39−41,44] 36 Isoliensinine C37H42N2O6 1R,1'S E [40,44] 37 Dauriciline C36H40N2O6 NS S [5] 38 6-Hydroxynorisoliensinine C36H40N2O6 NS E [29] 39 N-Norisoliensinine C36H40N2O6 NS E [29] 40 Nelumborine C36H40N2O6 NS E [43] 41 Dauricinoline C37H42N2O6 NS S [5] 42 Neferine C38H44N2O6 1R,1'S E, S [40,41,44] 43 Dauricine C38H44N2O6 NS S, R [45] Tribenzylisoquinoline 1 Neoliensinine C63H70N3O10 1R,1'S,1''R E [44] 1-Benzylisoquinoline alkaloids
-
1-Benzylisoquinoline alkaloids are traced in lotus leaves, flowers, embryos, and seeds (Table 1). The 1-benzylisoquinoline alkaloids in sacred lotus mainly include norcoclaurine, coclaurine, norjuziphine, isococlaurine, N-methylcoclaurine, 6-demethyl-4'-O-methyl-N-methylcoclaurine, norarmepavine, N-methylisococlaurine, norroefractine, juziphine, armepavine, 4'-O-methyl-N-methylcoclaurine, lotusine, isolotusine, 4'-O-methylarmepavine.
The pharmacological effects of these 1-benzylisoquinoline alkaloids are diverse. Norcoclaurine's pharmacological action is one of the most extensively researched. It possesses anti-oxidant, anti-HIV, and anti-Alzheimer's disease pharmacological actions[46], as well as cardiovascular pharmacological activities such as treating heart failure, lowering myocardial ischemia injury, and reducing pathological cardiac fibrosis and dysfunction[27,31,46]. Other 1-benzylisoquinoline alkaloids' pharmacological properties are also noteworthy. Armepavine, for example, inhibits melanin formation and regulates the immunological system[36]. Furthermore, it has been shown that this therapeutic approach may be used for the treatment of autoimmune disorders, including systemic lupus erythematosus and crescentic glomerulonephritis[47]. Lotusine contains anti-wrinkle, neuroprotective, and liver-protective properties[48, 49].
Aporphines
-
The aporphine and pre-aporphine compounds found in sacred lotus are caaverine, asimilobine, glaziovine, O-nornuciferine, N-nornuciferine, lirinidine, N-methylasimilobine, roemerine, dehydronuciferine, dehydroanonaine, dehydroroemerine, pronuciferine, nuciferine, 7-hydroxydehydronuciferine, lysicamine, cepharadione B, anonaine, liriodenine. Among them, the pharmacological effect of NF is the most concerning which has anti-obesity, anti-hyperlipidemia, anti-diabetes, anti-arteriosclerosis, anti-tumor and other effects[50−52].
Among them, aporphine and pre-aporphine chemicals found in sacred lotus, such as lirinidine, asimilobine, N-methylasimilobine, and pronuciferine, O-nornuciferine, have anti-Alzheimer's disease properties[53, 54]; Lirinidine in lotus petals has an anti-cervical cancer effect[33].
Bisbenzylisoquinolines
-
Bisbenzylisoquinoline alkaloids are mainly accumulated in the seed embryo of sacred lotus. The main bisbenzylisoquinoline compounds are included nelumboferine, liensinine, isoliensinine, dauriciline, 6-hydroxynorisoliensinine, N-norisoliensinine, nelumborine, dauricinoline, neferine, dauricine. There are several investigations being conducted on bisbenzylisoquinoline alkaloids at the moment. The most noteworthy is that bisbenzylisoquinoline alkaloids have the potential to be exploited as therapeutic agents for new coronavirus pneumonia. Neferine, in particular, can prevent SARS-CoV-2 infection by inhibiting Ca2+-dependent membrane fusion[16]. Furthermore, neferine possesses anti-tumor, anti-inflammatory[25], anti-hypertension, anti-diabetes, anti-arrhythmia, anti-platelet, anti-thrombosis, neuroprotective, anti-amnesia, anti-anxiety, and other properties[55−58]. Neferine anti-tumor research has been on the rise in recent years. Isoliensinine and liensinine have notable pharmacological actions. Isoliensinine provides several health benefits, including anti-tumor, heart protection, anti-oxidation, anti-depression, anti-HIV, and anti-Alzheimer's disease[14, 15].
-
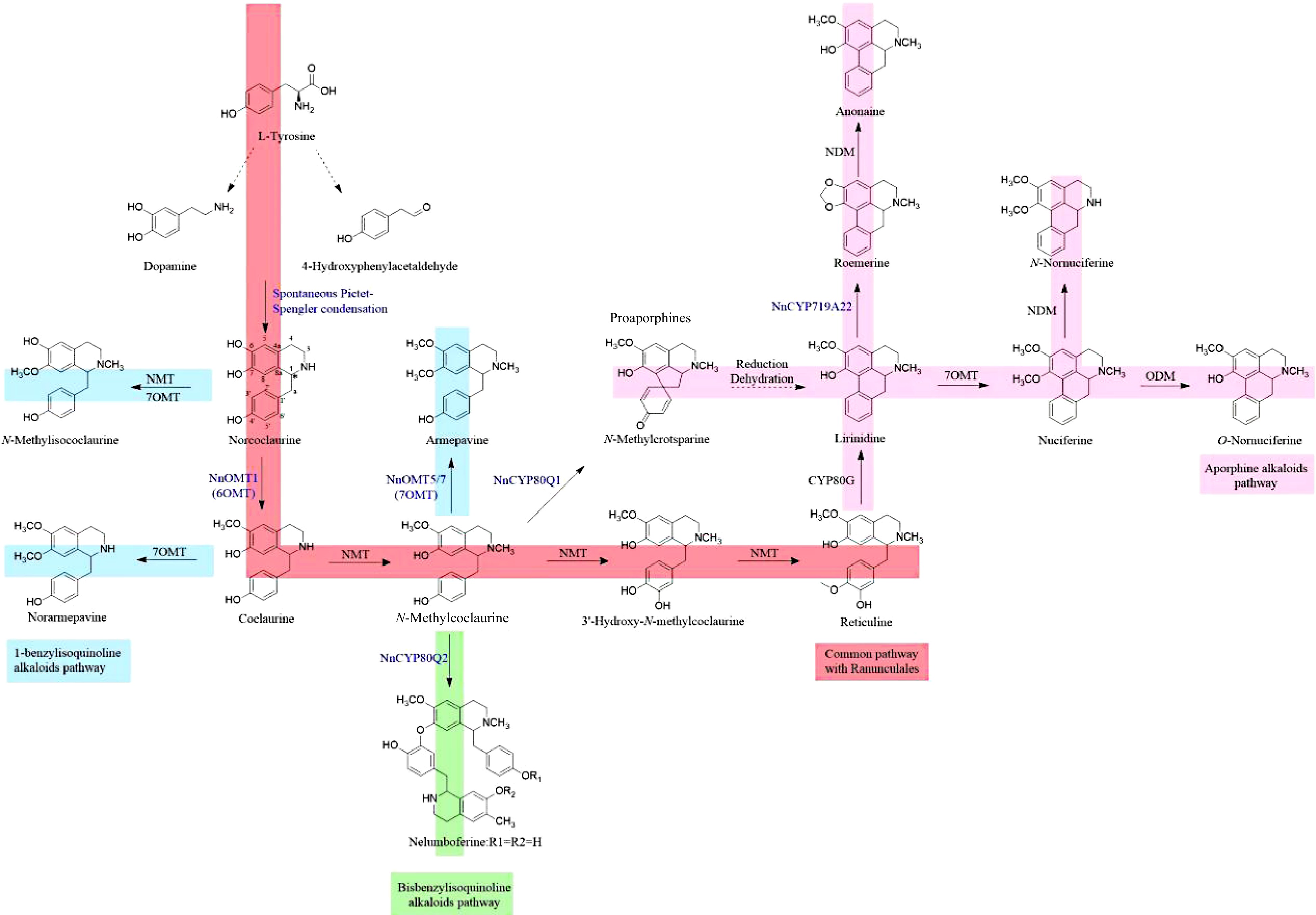
Because of the monophyletic evolution of BIAs biosynthesis in angiosperms, the selection of genes related to BIAs biosynthesis in sacred lotus can be guided by the opium poppy BIAs metabolic pathway. Hence, it is anticipated that the biosynthetic route of benzylisoquinoline alkaloids (BIAs) in the sacred lotus involves the condensation of dopamine and 4-hydroxyphenylacetaldehyde (4-HPAA) catalysed by NCS, followed by the enzymatic conversion of (R,S)-norcoclaurine into various substituted 1-benzylisoquinoline, protoaporphine, aporphine, and bisbenzylisoquinoline alkaloids. This conversion is facilitated by specific enzymes such as O-methyltransferase (OMT), N-methyltransferase (NMT), cytochrome P450 oxidoreductases (CYPs), and others, which belong to a restricted enzyme family[59]. In contrast to the preponderance of S-conformational BIAs in Ranunculales, the majority of BIAs observed in sacred lotus are R-conformational. As a result of the presence of this anomalous stereochemistry in sacred lotus, it is possible that the biosynthesis of sacred lotus will contain unique pathways or homologous enzymes. The most recent study conducted by Menéndez-Perdomo and J. Facchini has provided further validation that dopamine and 4-HPAA, both derived from L-tyrosine, serve as the precursors for the synthesis of (R,S)-norcoclaurine in the sacred lotus plant. Conversely, it was observed that in other plant species, the production of (R)-norcoclaurine by-products was predominantly favoured due to the presence of R-enantiospecific methyltransferase and CYPs. The presence of these enzymes has been shown to have a role in the synthesis of diverse 1-benzylisoquinolines inside the sacred lotus plant. The study also shown that the enzymes accountable for the production of R-enantiomers of pre-aporphine (NnCYP80Q1) and bisbenzylisoquinoline (NnCYP80Q2), as well as the incorporation of methylenedioxy bridges on the aporphine substrate (NnCYP719A22), exhibit identical characteristics[59].
Nevertheless, there are still unresolved matters pertaining to the examination of the biosynthetic pathway of the sacred lotus' benzylisoquinoline alkaloids (BIAs). Firstly, it is observed that BIAs mostly occur in R-enantiomers, whereas S-enantiomers are more prevalent in the order Ranunculales. The enantioselective synthesis of (S)-norcoclaurine has been facilitated by NCS catalysis. However, it has been shown that both (R)-norcoclaurine and (S)-norcoclaurine are present in sacred lotus, suggesting the presence of diastereoselective enzymes or two distinct NCS orthologs that selectively favour either the R or S enantiomer[59]. Secondly, it was observed that no benzylisoquinoline alkaloids (BIAs) bearing a 3'-hydroxyl group were detected in the benzyl moiety. This absence may be attributed to the absence of the NMCH enzyme. This suggests that N-methylcoclaurine serves as a pivotal intermediary in the production of proaporphine, aporphine, and bisbenzylisoquinoline alkaloids in the sacred lotus[59]. Thirdly, in contrast to the direct conversion of (S)-reticuline bases to aporphine alkaloids through C-C and C-O coupling in plants of the order Ranunculales, the appearance of proaporphine in the sacred lotus plant indicated that 1-benzylisoquinoline substrates were indirectly transformed into apophine in the absence of ortho- or para- substituents in the phenyl moiety[59]. Fourthly, it was shown that bisbenzylisoquinolines exist as head-to-tail dimers in the sacred lotus, but only tail-to-tail couplings were detected in plants belonging to the Ranunculales order. The occurrence of aporphine and bisbenzylisoquinoline alkaloids in Ranunculales plants is attributed to the intramolecular C-C and intermolecular C-O coupling of BIAs. These coupling reactions are facilitated by enzymes belonging to the CYP80 family. The major aporphine alkaloid found in sacred lotus has an isoquinoline component that is characterised by the presence of a methylenedioxy bridge. The production of protoberberine in Ranunculales involves the participation of many enzymes belonging to the CYP719A subfamily, which catalyse the formation of methylenedioxy bridges. Notably, this biosynthetic pathway is absent in the sacred lotus plant[59].
Possible biosynthetic pathways
-
According to the study of BIAs biosynthesis, the possible BIAs biosynthesis pathway of sacred lotus can be obtained, which is mainly divided into three parts (Fig. 1). Firstly, the common biosynthetic pathway of sacred lotus and Ranunculales species is the Pictet-Spengler condensation of two L-tyrosine derivatives dopamine and 4-hydroxyphenylacetaldehyde (4-HPAA) to produce (R,S) -norcoclaurine. It was catalyzed by NnOMT1 (6-O-methyltransferase) to generate coclaurine, which was then catalyzed by N-methyltransferase to generate N-methylcoclaurine. It also served as a central branch point for the biosynthesis of various BIAs. N-methylcoclaurine was catalyzed by cytochrome P450 monooxygenase 80B to generate 3'-hydroxy-N-methylcoclaurine, and it was catalyzed by 4'-O-methyltransferase to generate reticuline. Secondly, the synthesis pathway of bisbenzylisoquinoline alkaloids in sacred lotus heart and aporphine alkaloids in sacred lotus leaves: N-methylcoclaurine was catalyzed by NnCYP80Q1 in sacred lotus to nelumboferine (bisbenzylisoquinoline alkaloids). N-methylcoclaurine is catalyzed by NnCYP80Q2 to N-methylcrotsparine (proaporphine), which may generate lirinidine through reduction, dehydration and aromatic ring rearrangement, and generate anonaine under the action of NnCYP719A22. In addition, it is speculated that the aporphine alkaloids in sacred lotus may also come from reticuline and generate various aporphine alkaloids under the catalysis of CYP80G, 7OMT, ODM, NDM and other enzymes. Thirdly, the synthesis of 1-benzylisoquinoline alkaloids, N-methylcoclaurine was catalyzed by NnOMT5 / 7 (7OMT) to armepavine.
-
The enzymatic pathway leading to the surprising diversity of benzylisoquinoline derivatives has been shown to originate from a common route, in which the first step is the NCS-catalyzed PictetSpengler condensation of dopamine with (4-HPAA) to produce (S)-norcoclaurine[60, 61]. However, (R)- and (S)-norcoclaurine were both detected in sacred lotus. NCS selectively catalyzed the formation of (S)-norcoclaurine, which indicated that there may be diastereoselective enzymes or two different R- and S-enantiomerically selective NCS orthologs[59]. Recently, the study of Menéndez-Perdomo & Facchini proposed a new possibility that the formation of (R)- and (S)-desmethylhengzhouaconitine in lotus is a spontaneous, non-enzymatic Pictet-Spengler condensation reaction of dopamine and 4-HPAA[59]. It can be seen that the study of NCS in lotus is of great significance to the interpretation of lotus-specific R configuration, and the study of NCS needs to be further promoted.
Methyltransferases
-
Methylation, a frequent biological change in plants, plays an important role in the structural and functional diversity of BIAs. By adding methyl groups, BIAs' chemical characteristics, including as steric effects, overall hydrophobicity, and electronic properties, can be altered, resulting in a shift in biological activity. Methylation processes known as methyltransferases employed S-adenosyl-L-methionine as a methyl donor[62]. The widespread terminal alteration on BIAs of sacred lotus by methyltransferases, including O-methylation and N-methylation, is also a source of its variety.
OMT
-
So far, OMTs in sacred lotus have largely been studied in terms of gene expression, with little functional characterisation of the encoded proteins[63−65]. Despite the fact that BIAs were largely active metabolites in N. nucifera, only three OMTs engaged in the 1-BIA upstream biosynthetic pathway in N. nucifera were discovered in vitro[66]. Two OMTs implicated in BIA metabolism in sacred lotus, which catalysed the 6-O and 7-O-methylation of the 1-benzylisoquinoline backbone, have been functionally characterised. In sacred lotus, the 1-benzylisoquinoline backbone was mostly O-methylated at the C6, C7, and/or C4' locations, yielding a range of 1-benzylisoquinoline alkaloid compounds[66]. Our lab discovered a new and regiospecific O-methyltransferase (NnOMT6) that methylated monobenzylisoquinoline 6-O/7-O, aporphine skeleton 6-O, phenylpropanoid 3-O, and protoberberine 2-O[67]. Monobenzylisoquinoline was converted into aporphine and bisbenzylisoquinoline alkaloids in sacred lotus. However, no reports of OMTs catalysing the aporphine and bisbenzylisoquinoline backbones in sacred lotus have been found.
NMT
-
It is unknown how BIAs are N-methylated in sacred lotus. According to chemical structural suggestions, the N position occurred in once or twice methylation to form tertiary amine or quaternary amine (e.g., N-methylcoclaurine and lotusine). Based on transcriptome analysis, two N-methyltransferases, NnCNMT1 and NnCNMT2, were identified from sacred lotus[68]. However, the role of the N-methyltransferase involved in the production of BIAs in sacred lotus has not yet been determined. It is critical to identify the NMT in the biosynthesis of BIAs.
Cytochrome P450 monooxygenases
-
Cytochrome P450 monooxygenases (CYPs) include a heterogeneous collection of heme proteins that facilitate a multitude of reactions within plant-specific metabolic pathways. NADPH-cytochrome P450 reductase, an enzyme responsible for transferring a pair of electrons from NADPH, facilitates the activation of these enzymes[69]. The formation of sacred lotus benzylisoquinoline alkaloids (BIA) is believed to be influenced by two primary cytochrome P450 (CYP) families, namely CYP80 (subfamilies A and G) and CYP719A[69, 70].
Menéndez-Perdomo & Facchini's most recent study characterised the functions of NnCYP80Q1, NnCYP80Q2, and NnCYP719A22, which were responsible for the formation of pre-aporphine R-enantiomers, dibenzylisoquinoline R-enantiomers, and the formation of methylenedioxy bridges on the aporphine substrate[59]. Based on predictions, the catalytic mechanism of cytochrome P450 enzymes (CYPs) involves several key reactions. Firstly, an intramolecular C-C phenol coupling occurs between the C8 and C1' positions of 1-benzylisoquinoline substrates, resulting in the formation of the corresponding pro-aporphine compound. Additionally, an intermolecular head-to-tail C-O phenol coupling reaction takes place between the C7-hydroxyl and C3' positions of two 1-benzylisoquinoline substrates, leading to the production of the corresponding bisbenzylisoquinoline compound. Furthermore, the oxidative cyclisation of the ortho-hydroxyl group of the isoquinoline moiety in the aporphine substrate, along with the methoxy-substituted aromatic ring, results in the formation of a methylenedioxy bridge[59].
-
The extraordinary therapeutic potential of BIAs is one of the reasons why they have garnered so much interest. In contrast to the S-conformation seen in Ranunculaceae, the sacred lotus, which belongs to an ancient group of aquatic basal plants, has an exceptionally high number of BIAs that have an R-conformation. The investigation of the in vitro synthesis and the pharmacological efficacy of BIAs will be helped along by the discovery of important genes and functional enzymes connected to the BIAs biosynthesis.
-
The authors confirm contribution to the paper as follows: conceptualization and supervision: Chen S; draft manuscript and figure preparation: Chen Z; manuscript review and editing: Zhao H. All authors reviewed and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Chen Z, Zhao H, Chen S. 2023. Progress on synthesis of benzylisoquinoline alkaloids in sacred lotus (Nelumbo nucifera). Medicinal Plant Biology 2:20 doi: 10.48130/MPB-2023-0020
Progress on synthesis of benzylisoquinoline alkaloids in sacred lotus (Nelumbo nucifera)
- Received: 16 August 2023
- Accepted: 03 November 2023
- Published online: 18 December 2023
Abstract: Sacred lotus (Nelumbo nucifera) is a 2,000-year-old perennial rhizome aquatic crop that is primarily employed as a food and drug dual-use crop in East Asia. One of the key bioactive components of sacred lotus is benzylisoquinoline alkaloids (BIAs). Existing research has demonstrated that they have therapeutic and preventive benefits on obesity, diabetes, cancer, and cardiovascular disease. Despite their broad pharmacological relevance, the metabolism of BIA in sacred lotus has received little attention. We reviewed the biosynthetic process of the BIA in sacred lotus in this research. We concluded that a thorough functional characterization of BIAs biosynthesis enzymes provides a wide range of significant therapeutic applications for sacred lotus.
-
Key words:
- Benzylisoquinoline Alkaloids /
- Nelumbo nucifera /
- Sacred lotus /
- Biosynthesis