-
The lotus (Nelumbo) is a perennial aquatic herb of the genus Nelumbo in the Nelumbonaceae, and is one of the top ten flowers in China. As one of the earliest angiosperms, the lotus is known as the 'living fossil'. The oldest lotus fossils can be traced back to the late Early Cretaceous about 100.5 to 113.0 million years ago. They lived in the same period as glacial relic plants (such as Metasequoia and Ginkgo)[1,2]. Before the third century (about 100 million years ago), there were 10−12 species of lotus plants. After the ice age, most of the species became extinct due to the cold. There are only two types of Asian lotus (Nelumbo nucifera Gaertn.) and American yellow lotus (Nelumbo lutea Pers.), and their distribution areas have also moved southward as a whole[3]. The cultivation and domestication of lotus has a long history. Lotus has been collected and consumed by humans since the early Stone Age and has been cultivated as food for thousands of years[4]. Lotus has a long history of planting in China, Japan, Vietnam and other countries in the world, especially in China, which is known as the center of the world's lotus cultivation. It has a cultivation history of more than 2,500 years. The cultivation area is China wide with the exception of Tibet and Qinghai province[5,6].
Lotus has high ornamental, economic and cultural value[7]. According to different uses, the lotus is divided into three systems: seed lotus, rhizome lotus and flower lotus[8]. The various species groups selected, and the cultivation techniques of various systems have developed over a large range and of varying degrees in different parts of China. The lotus flower is the theme plant in the waterscape garden layout[9]. It is suitable for growing in relatively stable and calm shallow water, ponds, lakes and marshes. The lotus is light-loving and very intolerant to shade, its petals are rich and colorful, the lotus leaf is green, the plant shape is tall and straight, and it is considered to be ornamental throughout the growth period and even the dead leaf period (Supplemental Fig. S1)[10]. Lotus can also purify sediment and water environment and improve water quality. It is of great significance for the treatment of urban black and smelly river sediment pollution and the purification of urban reclaimed water bodies[11]. Lotus can be used medicinally or as food. In addition to edible lotus root and lotus seeds, lotus seeds, lotus leaves, lotus house, lotus beard and lotus root joints can all be used medicinally and have high medicinal value[12−19]. The lotus also has profound cultural connotations. In Confucian culture, the lotus is often used as a metaphor for gentlemen, and in Buddhist culture, the lotus is regarded as a holy thing[20].
Lotus has a long history of planting in worldwide, but its breeding history is short. In China, the real breeding of lotus began in the 1980's[21], Mainly through traditional manual direct selection and cross/backcross breeding, impressive achievements have been made in the past few years, and a large number of lotus cultivars have arisen. So far, China has nearly a thousand kinds of lotus cultivars. However, in some countries, such as Vietnam and Myanmar, there are few individuals and units engaged in lotus breeding, and its resources are mainly derived from natural differentiation and variation[22]. Therefore, there are still many problems in lotus breeding, such as incomplete understanding of germplasm resources, incomplete breeding information, and relatively backward breeding technology. There are few new molecular-assisted breeding techniques and people engaged in lotus breeding worldwide. This article focuses on the classification and characteristics of wild lotus species and cultivated species resources, phenotypic genetics, molecular marker-assisted breeding, breeding methods and their applications, and molecular breeding. Prospects for the problems and future development trends of lotus breeding, development and utilization are reported in order to provide ideas and inspiration for breeders and scientific researchers.
-
There are only two species of lotus. One is the Asian lotus with red and white flowers. The other is the American lotus with yellow flowers. These two species are separated by the Pacific Ocean, Asian lotus is widely distributed in Asia and Oceania, while American lotus is mainly distributed in America[23,24]. The long history of evolution and domestication of the Asian lotus has led to its widespread distribution and differentiation in rain-rich areas in Asia. Wild lotus is mainly distributed in the three eastern provinces of China, the middle and lower reaches of the Yangtze River, the Pearl River Basin and the Yunnan-Guizhou Plateau. It is also widely grown in South Asian countries such as India, Thailand, Vietnam and Laos. The American yellow lotus is distributed in North America and the northern part of South America, from Canada in the north to Brazil in the south, and its wild communities are found in Brazil, the West Indies and the southeast of Lake Ontario in Canada[24] (Fig. 1). The American yellow lotus is basically wild, and it is rarely cultivated and used as aquatic ornamental plants or aquatic vegetables[23]. Because of its rapid growth and reproduction, it can quickly occupy the growth space in shallow waters. In Connecticut, USA, the lotus was classified as a potential invasive plant, thus prohibiting its cultivation throughout the state[25]. However, the American yellow lotus possesses unique genetic material that regulates yellow flower color, and is an excellent breeding material for breeding yellow lotus cultivars.

Figure 1.
Geographical distribution of Nelumbo. The colored position on the map indicates the planting area of the lotus. The purple area indicates the main planting area of Nelumbo nucifera Gaertn.; the yellow area indicates the main planting area of Nelumbo lutea Pers. Dots on the map indicate the locations where wild species of lotus were collected. Black dots indicate the area where lotus fossils were unearthed, green dots indicate sections where Nelumbo nucifera Gaertn. are found, and orange dots indicate sections where Nelumbo lutea Pers. are found.
China is the origin center of Asian lotus[26]. Most of the wild species of Asian lotus are distributed in the middle and lower reaches of the Heilongjiang River, the middle and lower reaches of the Yangtze River and the lower reaches of the Yellow River, and the lakes and swamps associated with these three river branches in China[27]. Wild lotus is also widely distributed in some countries around China, such as Thailand, Vietnam, Cambodia, India, Pakistan, Southeast Asia and South Asian countries[28−30]. Commonly used lotus varieties for ornamental use are traditional varieties such as Ancient Lotus, Red Qianye, Daseijin, Thousand Petal Lotus and Table Lotus[24], as well as wild type varieties such as Weishan Lake Red Lotus, Baiyangdian Red Lotus, Chaohu Wild Lotus, Honghu Lake Wild Lotus and Dongting Lake Wild Lotus[31]. Traditional lotus varieties have an ancient planting history, most of which have been passed down from ancient times to the present. For example, the ancient lotus was derived from the germination of thousand-year-old lotus seeds under the peat soil excavated by the Beijing Botanical Garden of the Chinese Academy of Sciences in the early 1950's[24]. Red Qianye is an ancient lotus cultivar, and the ancients called the double-petaled flower 'Qianye'. It has appeared in the Jin Dynasty and is one of the first Asian lotuses introduced to the United States. After being crossed with the American yellow lotus, it is very popular in the United States after breeding red, orange and yellow three-color lotus[24]. Thousand-petal lotus is an ancient cultivar that appeared in the Sui and Tang Dynasties. It was discovered in Zhengyi Town, Kunshan County, Jiangsu Province in the 1940's, and became wide spread after being discovered in Yuquan Temple, Dangyang County, Hubei Province in the 1960's[32]. Wild lotus has rough frontal leaves. The flowers are mostly pink or red with single petals, a few are white flowers with single petals, and the fruit is oblong, with enlarged rhizomes (lotus roots) and slender, with high seed setting rate and growth speed. It has the characteristics of fast growth speed and good stress resistance, and its ornamental value is relatively low, but it is an excellent material for breeding new varieties of lotus with excellent stress resistance.
Cultivated lotus
-
Cultivated lotus is artificially selected and cultivated, and there are lotus varieties cultivated in certain areas. So far, there have been about 4,500 lotus cultivars worldwide, of which 2,080 are in official documents (including 142 internationally registered), and 2,412 are currently unpublished[33]. Among them, China has the largest number of cultivar records, followed by Japan and the United States, while a few can be found in Europe and Africa (Table 1); a few cultivars were recorded before 1960, and most of them were cultivated or discovered after 1960[33]. Lotus has high edible, medicinal and ornamental value[34]. According to the data, the American yellow lotus, the Asian lotus and hybrids between the two are all edible[6]. Despite this, there are still many edible lotus cultivars that have not been popularized. In American countries, there are only wild plants for edible lotus, and most of the hybrids of American lotus and Asian lotus are only used for ornamental purposes[23]. These phenomena have greatly affected the promotion of edible lotus and the development of its edible value. In Asia, many countries have had the habit of eating lotus since ancient times, such as China, India and Vietnam[34]. For them, all the vegetative organs of the lotus (roots, stems, leaves, flowers and seeds) can be eaten, such as harvesting rhizomes for cooking and eating, and also used to make candied fruit, soup, fried lotus root slices, and even mixed with sugar for raw food, the unfolded tender leaves and tender lotus root whip are used as vegetables, tender lotus roots are used as accessories, and the lotus seeds can be eaten raw, cooked, marinated or powdered[34]. Lotus also has a high medicinal value. Studies have shown that lotus seeds have strong antioxidant capacity and have certain curative effects for the treatment of inflammation and are anti-viral[35]. In addition, it has been found that NN-B-4 isolated from lotus seeds has a protective effect on the liver. It can be mixed with a cultivar of foods to form a medicated diet, which has an effect on the treatment of insomnia, coughs, and bad breath[36−38]. Ornamental is another important trait of lotus. Among the lotus germplasm resources collected in various countries, the lotus germplasm resources for ornamental use account for the largest proportion, and the ornamental traits of lotus are also a popular direction for lotus breeding in recent years. According to the edible value, medicinal value and ornamental value of the lotus, in order to further develop the economic value of the lotus, people carry out directional breeding for different purposes, and then divide the lotus into three types: seed lotus, rhizome lotus and flower lotus according to the purpose of the lotus[4].
Table 1. The number and geographical distribution of the global lotus cultivars[33].
Origin of cultivar
informationCultivar number Percentage
(%)China 2,888 64.29 Japan 1,519 33.82 American 470 10.46 Australia 47 1.05 Thailand 12 0.22 The other parts* 21 0.71 Global total after weightless 4,492 * Species from Vietnam, India and Russia, and species recorded on the website of the International Water Lily Landscape Horticulture Association but of unknown origin. Seed lotus
-
The seed lotus refers to a lotus cultivar that mainly harvests lotus seeds. It has the characteristics of large flowering, high seed setting rate and full seed. In China, seed lotus has more than 3,000 years of cultivation history. Among the most cultivated seed lotus cultivars, 'Xianglian', 'Ganlian' and 'Jianlian' are the most famous. They are known as the three famous lotuses in China and enjoy a high reputation both at home and abroad[35]. There are not many varieties of seed lotus, and they are mainly distributed in Hunan, Jiangxi and Fujian. Among them, the seed lotus cultivar of 'Xianglian' is the most abundant, with about eight varieties[39]. In 1986, the Wuhan Institute of Vegetable Science listed as 'National Germplasm Wuhan Aquatic Vegetable Resource Nursery' and collected and preserved domestic seed lotus varieties. At present, 33 cultivars of seed lotus have been collected, laying a solid foundation for the selection and breeding of new cultivars of seed lotus[31]. In Vietnam, the seed lotus is the most planted lotus cultivar, which can be seen everywhere from south to north[28]. Although the planting area is large, the lotus seeds produced by the seed lotus varieties it planted are relatively small, coupled with extensive management, so that the overall yield of lotus seeds in Vietnam is low. The Plant Resource Center in Hanoi, Vietnam has collected 42 lotus populations, of which 33 are seed lotus[28]. Because there are no individuals and units engaged in lotus breeding in Vietnam, its resources are mainly derived from natural differentiation and mutation, and there is no information about related varieties from artificial breeding, especially hybrid breeding, so although Vietnam has a large lotus population distribution, there is no development and utilization of it which has caused the lotus resources in Vietnam to be far less than the huge number of cultivars in China and Japan. In addition to the Asian region, non-Asian countries such as the United States, Mexico, and Australia are also engaged in the collection and development of lotus germplasm resources[40]. Auburn University in the United States has been committed to the collection and preservation of lotus germplasm resources (including flower lotus and edible lotus)[33], and its main work is to improve the cultivation and management of various lotus varieties. They believe that the rhizomes of seed lotus are not commercially available. After container planting, the rhizomes of 'Space 36' were larger, and they were considering for other purposes (such as P and N eutrophication phytoremediation, alcohol production). 'Space 36' has a long growth period, large grains, high seed setting rate, high rhizome yield, and excellent agronomic traits. The lotus seed yield is more than three times that of general varieties[41]. Although the seed lotus cultivars are planted in a large area in many countries, the yield and quality of their lotus seeds are not large due to the lack of seed lotus germplasm resources, single cultivars and lack of systematic cultivation and management methods. Degree of development, in addition to the amount of flowering, pollination methods, pollinators, and rainy weather, have a greater impact on the yield and quality of the lotus seeds. These problems have not been solved effectively in production. These areas will be an important direction in the future for the breeding of new cultivars of seed lotus.
Rhizome lotus
-
The main purpose of Asian lotus (Nelumbo nucifera) cultivation is to harvest edible rhizomes. The rhizome lotus is the general name of the lotus resources for the purpose of feeding on the large underground stems. It has high application value in edible, medicinal and processing aspects, and is an important aquatic vegetables. The rhizome lotus will be harvested for 120 days in warm climates and 150−180 days in cold climates. In Thailand, the rhizomes are harvested 8−12 months after the lotus is planted (close to the warm season), and can be harvested at any time after the plants are dormant[34]. In some producing areas (such as Wuhan, China) lotus roots can be planted twice a year[42]. The rhizome lotus are planted in many countries. In Asia, China has the largest planting area (200,000 hm2), followed by Japan (4,490−6,090 hm2), Vietnam (2,000 hm2), and South Korea 291 hm2. In the United States, Asian lotus has been grown in California[6,43]. Mexico and Australia regard Asian lotus as a potentially valuable export vegetable. El Salvador, Guatemala, Honduras and Nicaragua all have rhizome lotus planting[43]. Yamaguchi (1990) believes that lotus root can be cultivated in the southeastern states of the United States[40]. It can be seen that lotus root has been popular in countries all over the world as an important aquatic vegetable.
China is the largest planting country of rhizome lotus and has a long history of planting and consumption. Before the 1980's, the rhizome lotus in China were mostly bred by farmers according to their own purposes, and there was little mutual exchange, so some varieties of resources with local characteristics were formed[31]. For example, there are traditional varieties of early, middle and late maturity in Wuhan. Early maturity includes 'LiuBaozi' and 'Jiayu'; middle maturity includes 'Hunan paozi', 'Zhuweiba', and 'Zhou Ou'; late maturity has 'Damaojie' and 'Xiaomaojie'. The types are also richer, with white skin, yellow skin, less flowering or more flowering, and long and short nodes. In the collection and sorting of rhizome lotus resources, it is found that the lotus resources of various provinces are far more than those recorded, and there are many unique resources. Generally, the resources in the middle and lower reaches of the Yangtze River have high yields, good quality, uniform lotus joints (15−18 cm in length), and are suitable for paddy field cultivation. A large number of wild lotus grows in the lake areas of the Yangtze River Basin. In the north (e.g., Henan, Shanxi, Shandong), there are fewer resources, and the degree of artificial domestication is low. The lotus roots are long and thin (20−25 cm in length) and thin (5 cm in horizontal diameter), low in yield, and deep into the mud. In the south (e.g., Guangdong, Guangxi, Fujian), the lotus root tube is short (12−15 cm in length), thinner, smaller in single branch, and low in yield (1,000 kg/667 m2). Southwestern provinces (e.g., Guizhou, Yunnan, Sichuan), often have some special resources. For example, the rhizome lotus that have been planted for a long time in some places in Yunnan are actually 'thousand petals'. It shows that in addition to the 'Thousand Petal Lotus' in the Yuquan Temple in Dangyang, Hubei, it is also found in Yunnan. The rhizome lotus planted in Simao (Yunnan) is a red flowering double-petaled lotus, with low yield and poor quality. It is a good flower lotus resource. Sichuan and Guizhou lotus roots have a relatively small single branch weight and low output. In addition, the cultivars of rhizome lotus in many areas have no names, and a few areas with a long planting history are named by farmers according to a certain characteristic. Unnamed resource materials are usually named after the place name, plus a certain characteristic, when they are collected[31]. Compared with seed lotus, rhizome lotus has more abundant germplasm resources. Due to the vague classification of lotus plants in the early period and backward breeding methods, the germplasm resources of rhizome lotus have obvious local characteristics. For example, in the middle and lower reaches of the Yangtze River with wild lotus resources, the yield of lotus varieties is the highest; in northern regions such as Henan, Shanxi, and Shandong, not only the yield of rhizome lotus is low, but also the quality is relatively poor; while in the southwest of Yunnan, Sichuan, Guizhou, there is also the phenomenon of using 'thousand-petal lotus' and double-petal red flower lotus, which are mainly used for ornamental purposes, as rhizome lotus cultivation.
Flower lotus
-
The lotus is one of the Buddhist fireworks and is the most common flower in Buddhist temples. When pilgrims go to the temple to worship Buddha and carry out Buddhist services, they usually use lotus to pay tribute to Buddha, and many lotus flowers are also offered on the altar of Buddha[44]. Therefore, in many countries where Buddhism is popular, such as China, Thailand and India, there is a great market demand for cut lotus flowers. Thailand's lotus is mostly wild, with a small number of cultivars. The lotus in Thailand belongs to the tropical type in the ecological type. The rhizomes do not expand and cannot form lotus roots, and basically are not dormant at all year round. There are flowers in all seasons. It is an excellent germplasm resource for cultivating characteristic lotus cultivars with short dormancy period and long flowering period[45]. Japan is also one of the main growing countries of lotus. Fossils of lotus receptacles, pollen and lotus leaves have been found in the Cretaceous strata of the paleo-geological era[46]. At present, the popular cultivars in Japan are the flower lotus introduced to Japan with Buddhism and monks sent to the Tang Dynasty more than 1,400 years ago, as well as their hybrid descendants[46]. Therefore, the lotus planted and bred in Japan is mainly flower lotus. In the first half of the 19th century, there was an upsurge in cultivating lotus flowers in Japan. The lotus photo and painting collection 'Fragrant Fragrance Picture Book' recorded about 60 cultivars of Japanese lotus at that time, which has since declined. The earliest in modern times to comprehensively organize the resources of lotus cultivars is Kitamura Fumio, Version Yuji's book 'Flower Lotus' published in 1972, which records 65 cultivars[33].
As the center of cultivation and origin of lotus, China has had the habit of admiring lotus since ancient times, and the characteristic of lotus being 'out of silt but not stained' is often used to describe a gentleman's noble character. Flower lotus, a cultivated lotus whose main purpose is ornamental, has extremely rich flower types (single, double, duplicated, thousand petals, etc.) and flower colors (white, red, pink, yellow-green, yellow, rainbow colored)[11] (Fig. 2). Among the germplasm resources collected, the number of germplasm resources in flower lotus accounted for nearly half of the total. According to records, flower lotus has at least 2700 years of cultivation history in China, and many excellent flower lotus cultivars have been passed down[47]. In the middle of the 20th century, especially after 1979, the research on flower lotus in China has made rapid progress. According to statistics, in the mid-1990's, there were more than 300 cultivars of lotus in China, of which 80% were new varieties[31,47]. The selection and breeding of ornamental lotus in China started in the Wuhan area. Around 1960, China began to collect lotus resources and cultivate new varieties of ornamental lotus[48]. The cultivation of new cultivars of ornamental lotus started to develop slowly, and the successful cultivation of the new cultivar 'Wei Er' became the pioneer in the cultivation of new varieties of lotus. After 2000, not only Chinese scientific research institutes began to study new lotus cultivars, but related enterprises and individuals also began to engage in the cultivation of new ornamental lotus cultivars. Therefore, the number of new lotus cultivars in China has been increasing year on year. In 2014, under the joint efforts of the Institute of Botany of the Chinese Academy of Sciences in Jiangsu Province, a new cultivar of ornamental lotus 'Qinhuai Danzhuang' was cultivated. This cultivar has a light and elegant flower color. It belongs to a small and medium-sized semi-double multi-color accessions. It has made major breakthroughs in plant type and flower color. In 2015, under the means of artificial hybridization, new cultivars of 'Bumblebee', 'Little Wine Lotus', 'White Fairy' and other miniature lotuses with excellent traits such as American lotus and Asian lotus were selected and bred. The emergence of these cultivars has further enriched the cultivar resources of Chinese small lotus[49]. After decades of breeding, a large number of diversified varieties have been selected in terms of flower color, flower type, flower posture, flower number, and plant type. With the increase of breeding units, the number of flower lotus cultivars has increased rapidly. However, there are no statistics from authoritative departments. It is estimated that there are currently 800−1,000 cultivars[33].

Figure 2.
Typical flower colors and types in lotus. The cultivar names of (a)−(x) can be found in Supplemental Table S1.
-
In the work of plant genetics and breeding, understanding the genetic characteristics of plants, such as plant genetic relationships, chromosome ploidy and trait inheritance, can improve breeding efficiency. In recent years, with the development of breeding technology, researchers from the scientific research institutes involved in lotus genetic breeding have begun to explore some genetic mechanisms of lotus, and have made progress.
The lotus is a common cross-pollination plant. Although a hermaphrodite, the pistil matures a day earlier than the stamen. This difference in sexual maturity time guarantees its natural cross-pollination[9]. The heterozygosity of the genetic basis of the existing lotus resources (rhizome lotus, seed lotus, and flower lotus) has increased the difficulty of studying the genetic rules of certain characteristics of lotus. Research in this area is not yet systematic, and there is still a lot of work to be done. Since 1921, people began to study the number of chromosomes in different lotus varieties, proving that they are all diploid, the number of chromosomes in somatic cells is 16 (n = 8)[50−53]. Huang[53] used the root tip as the material to analyze the karyotype of the American lotus, the number of chromosomes was 16 (n = 8), further verifying that the Asian lotus and the American lotus are closely related. In 1984, Huang[53] formulated 27 hybrid combinations with Asian lotus as the female parent and American yellow lotus as the male parent. The results have shown that all the hybrid combinations were able to set fruit successfully, and the average seed setting rate was as high as 60.9%. There is no significant difference in the rate of seed setting, and the offspring can bear normal fruit. This shows that the karyotypes of the two species of the genus Nelumbo are similar. The chromosomes of the two species of the genus Nelumbo are homologous and can cross each other to be fertile. There is no reproductive isolation, only geographic isolation. Although there are certain differences in morphology, flower color, and flower type, they belong to the same biological species.
Huang[54] studied the inheritance of double petals of lotus, and believed that the double-petal traits of lotus were recessive inheritance. For example, the hybridization of 'single-petal' white flower Jianlian and 'double-petal red flower' Red Qianye. The F1 generation can express a single red flower, indicating that red flowering is dominant to white flowers. F2 generation separates different types such as double safflower, single safflower, single white flower, etc. Studies have shown that the double-petal traits of lotus are mainly the result of mutations in certain chromosomal structures, and have nothing to do with chromosome ploidy. Especially for the highly double-petalled 'duplicated-petal' and 'Thousand-petal', the chromosome structure variation is particularly obvious. Selfing of double-petal varieties can separate varieties with different degrees of petalization. Huang et al.[55] crossed the American yellow lotus and Asian lotus single-petaled cultivars, the F1 generation was still single-petaled, and when crossed with double-petaled varieties, the interspecific hybrids were semi-double or double-petaled. The petal bases of hybrids appear yellow to varying degrees, and other traits such as flower diameter size do not have obvious recessive relationships. This is contrary to the recessive double petals researched by Huang Guozhen, indicating that the lotus flower type and flower color are not Mendelian inheritance.
-
Molecular marker is a genetic marker based on nucleotide sequence variation, which can directly detect the differences between biological individuals at the DNA level and reflect the genetic variation between biological individuals. Compared with other genetic markers, DNA molecular markers eliminate the influence of the environment on species. At the same time, DNA molecular markers have the advantages of genetic stability, large amounts of information, and high reliability[56]. With the rise of genome sequencing technology and related research technologies, more and more molecular markers have been developed, the most commonly used are Restriction Fragment Length Polymorphism (RFLP), Amplified Fragment Length Polymorphism (AFLP), Simple Sequence Repeat (SSR) and Single Nucleotide Polymorphism (SNP). Each molecular labeling technology has different emphasis on the detection of polymorphism level, site specificity, repeatability and technical requirements. There is no ideal molecular labeling technology that can be applied to any situation[57]. At present, these molecular markers have been used in research on lotus classification, genetic diversity analysis, cultivar identification, genetic map construction, and important trait positioning (Supplemental Table S2).
Lotus classification research
-
The ornamental value of lotus has attracted much attention since ancient times. After a long time of artificial cultivation and breeding, abundant lotus cultivars resources have been produced. Scientific and practical classification of lotus cultivars is helpful to the preservation and management of lotus germplasm resources, and lays a theoretical foundation for the further development and utilization of lotus resources. Guo et al.[58−60] used RAPD molecular markers to find that the genetic background of the flower lotus group of the American yellow lotus and the Asian lotus was similar, and did not show obvious specificity at the DNA level, thus supporting the classification of the American yellow lotus as a subspecies of the Asian lotus. However, in recent years, using molecular markers such as ISSR and AFLP to study the genetic relationship between the American yellow lotus and the Asian lotus, results show that there is a large genetic distance between the two. There is a significant difference at the DNA level, that is, the Asian lotus and the American lotus should be separated into two groups[61−64]. Using SSR molecular markers to analyze the genetic relationship of wild lotus resources in Northeast China, Thailand and the Americas, and also support the independence of Chinese lotus and Thai lotus from American yellow lotus[3], Huang et al.[65] used genome resequencing technology to sequence and analyze the entire genes of Chinese lotus, Thai lotus and American lotus, and found that American lotus is completely independent of Chinese lotus and Thai lotus. Therefore, most scholars at this stage support the view that American lotus is an independent species.
Genetic diversity analysis
-
The lotus is rich in resources and has many varieties, showing very rich variation in morphology. For example, the plant type of lotus can be divided into large lotus, medium lotus and small lotus, flower type has few petals, semi-double petals, double petals, duplicated and thousand petals, etc. The flower colors include red, white, pink, yellow, and multiple colors[9]. In order to understand the genetic variation of lotus, determine its inter-specific or intra-species genetic relationship, preserve and use existing germplasm resources, scientific researchers have conducted more research on the genetic diversity of lotus. Li et al.[61] used RAPD and ISSR markers to detect the genetic variation of 70 Chinese lotus, seven Thai lotus, and two American yellow lotus germplasms, and found that these germplasms have high genetic diversity. The percentage of polymorphic sites in RAPD reached 96.40%, and the percentage of polymorphic sites in ISSR reached 91.20%. However, Fu et al.[66] used AELP markers to analyze the genetic diversity of 138 samples that covered the main types of lotus cultivars. The results showed that the genetic diversity of lotus cultivars was low, and the percentage of polymorphic loci was only 28.7%. Yang et al.[3] used 36 pairs of SSR primers to analyze the genetic diversity and population structure of a total of 83 lotus samples from 11 populations from the United States, China, and Thailand. The results show that the level of genetic differentiation among different populations is very high, and the mode of reproduction has a great influence on the level of genetic diversity. Fu et al.[27] used 11 genomic SSR primers, three EST-SSR primers and three chloroplast DNA primers to study the genetic diversity of 69 wild Asian lotus samples from 25 locations in northern China. It was found that the genetic distance between wild Asian lotus samples from Songhua River Basin and Liaohe River Basin in Northeast China is relatively small. Compared with germplasm in other regions, these germplasms may be relatively primitive. Liu et al.[67] performed genome resequencing of 296 lotus accessions from different geographical locations (including wild lotus, flower lotus, rhizome lotus and seed lotus). It is found that the wild lotus in the United States has a distant genetic relationship with the Asian lotus, and the seed lotus and lotus root of the lotus are not from a single source, but have more complex multiple sources. Seed lotus shows high genetic diversity, which may be due to the flow of genes from the male parent to the lotus seeds through artificial hybridization. The genetic diversity of lotus roots is much lower than that of other populations. This research provides information for the analysis of lotus genome diversity. A large number of SNP markers have laid the foundation for molecular assisted breeding of lotus[67].
Judging from the existing research results, if the experimental materials include American lotus and the resources of lotus containing genetic components of American lotus, the overall genetic diversity level is higher[3,61,67]; if all the experimental materials are Asian lotus, the overall genetic diversity level is low[27,66]. In addition, the genetic relationship analysis of flower lotus, rhizome lotus and seed lotus show that the genetic diversity of rhizome lotus is the lowest, while flower lotus has the most abundant genetic diversity, and is closely related to seed lotus. They are clustered into one branch in the branch diagram. This may be related to the different breeding methods adopted by different types of varieties.
Genetic map
-
The genetic linkage map is the genetic map. Its theoretical basis is the recombination and exchange of chromosomes. According to the exchange value detected by genetic recombination, the recombination rate is used to express the relative position of genes or genetic markers on the chromosome. Usually expressed in Limo (cM), 1cM is equivalent to a 1% recombination rate. Genetic mapping is an important basis for research on QTLs analysis and molecular marker-assisted breeding. It is also one of the important tools for research on crop genetic breeding, target gene location and cloning[68,69]. In 2012, the Wuhan Botanical Garden, Chinese Academy of Sciences cooperated with the University of Illinois-Champaign to sequence the genome of the 'Ancient Chinese lotus'. The genome size was about 804 Mb, 26,685 genes were annotated and the genome map of the 'Ancient Chinese Lotus' was drawn for the first time[70]. In the same year, the whole genome sequencing of the sacred lotus was completed. The assembled genome size was about 792 Mb, with 40,348 coding genes[71]. In 2018, Wuhan University further upgraded the two reference genomes using the BioNano optical map and high-density genetic map, and discovered ancient chromosome structural variations[72]. In 2020, the 'Ancient Chinese lotus' was re-sequenced using HI-C sequencing technology and eight chromosomes were mounted, which further improved the genome information of the lotus. At the same time, it revealed the evolutionary status of the lotus in flowering plants and shows that lotus is an ancient angiosperm[73].
Since the lotus genome was sequenced, many lotus researchers began to map the lotus genetics. Yang et al.[74] used the F1 segregation population of the cross between the ancient Chinese lotus and the American lotus, genomic SSR markers and SRAP markers to construct the first genetic linkage map of the ancient Chinese lotus and the American lotus, among which the genetic map of the Asian lotus contains seven linkage groups, 47 markers, and a total length of 365.67 cM; the genetic map of N. chinensis contains 11 linkage groups, 177 markers, and a total length of 524.51 cM. Subsequently, Zhang et al.[75] further used RAD sequencing technology to encrypt this population and constructed a genetic linkage map containing nine linkage groups, 562 RAD markers and 156 SSR markers, with a total length of 543.4 Mb. The drawing of this genetic linkage map not only lays a theoretical foundation for the QTL mapping of important agronomic traits of lotus and the mining of candidate genes, but also has important reference significance for drawing higher-quality genetic maps. In recent years, the continuous development of sequencing technologies, based on genome resequencing and simplified genome sequencing, has promoted the construction of lotus genetic maps. In 2016, the Wuhan Institute of Vegetable Science used the F2 segregation population of the cross of 'Ezilian No. 1' and 'Elian No. 9' to construct a genetic map consisting of eight linkage groups with simplified genome sequencing technology. The map contains 891 Molecular markers, the total length is 556 cM, and the average genetic map distance is 0.74 cM[76]. Li[77] used the F2 segregating population of the hybrid offspring of the temperate lotus 'White Pigeon' and the tropical lotus 'DongHonghua' as the mapping population, and obtained the SNP markers of this population using whole-genome resequencing technology, and constructed a genetic linkage map. The map contains nine genetic linkage groups, 862 bins, 22,728 SNPs, and a total length of 706.31 cM. Although the genetic map of lotus has been constructed, the construction of its genetic map is still in its infancy. The methods used to construct the genetic map mostly use SSR markers. Few genetic maps constructed using SNP markers are used, the sequencing methods also use simplified genome sequencing, and it is difficult to cover the entire genome range of the lotus. The constructed genetic map is insufficient in quantity and quality, the saturation density is not high, and the genetic distance between molecular markers is long, the precise location of the target gene cannot be achieved, and there are certain difficulties in direct use.
Trait positioning
-
On the basis of constructing a genetic map, combined with the abundant phenotypic data of lotus, the researchers mapped many important agronomic traits of lotus (e.g., flower color, flower type, flowering period, lotus seed yield, number of underground stem nodes, plant height). The genetic markers closely linked to the target traits have been obtained, and certain results have been achieved. The location of molecular markers is of great significance to the location of important agronomic traits and molecular marker-assisted selection breeding of lotus.
In 2014, Yang et al.[78] selected 210 lotus germplasms as the research population, and observed their flowering-related phenotype data (flower color, petal number, initial flowering period and duration of flowering period) for two consecutive years, the 11 AFLP markers, 24 SRAP markers and 38 SSR markers were analyzed for the target traits. A total of 13 molecular markers that were significantly related to flower color and 14 molecular markers that were significantly related to the number of petals were detected, they were significantly related to the beginning of the lotus flower. Seven molecular markers significantly related to flowering duration. The discovery of these molecular markers provides an important theoretical reference for the fine positioning of important agronomic traits such as lotus color, petal number, and flowering period. In order to identify genes that regulate the related traits of lotus plant type, Yang[79] used the F1 generation plants obtained by crossing the small plant type cultivar 'Yanyang Gaozhao' and the large plant type cultivar 'Jianxuan-35' as the mapping population, and using SSR and InDel molecular markers constructed a genetic linkage map of lotus, and a total of 14 QTLs related to plant type were detected, which laid a good foundation for the genetic research of lotus plant type. Yan[80] used the F2 generation plants obtained from the cross between flower lotus 'Jinqiu' and seed lotus 'Baihuajianlian' with large differences in petal number as the mapping population, and developed SNP molecular markers and constructed genetic linkage maps using whole-genome resequencing methods. Through QTL mapping, a high-quality QTL locus linked to the number of petals traits was located, 35 candidate genes and three main candidate genes were finally determined.
At this stage, the research on genetic mapping of important genetic traits of lotus is very limited. Therefore, the genetic regulation mechanism of important agronomic traits of lotus is still unclear, which largely hinders the rapid development of the lotus genetic breeding industry. At present, hybrid breeding is still the main breeding method for lotus. This kind of breeding method mainly uses phenotypic characteristics to determine the genetic relationship, and many important agronomic traits of lotus root are often weak in heritability. In addition, many factors such as environment, gene interaction and genotype can also produce inconsistencies. Certainty affects the phenotypic selection of plants and reduces the efficiency of selection. Molecular Marker Assisted Breeding (MMAB) is based on QTL mapping and gene mapping. It starts directly at the DNA level and indirectly selects target genes through genetic markers. The application of molecular biotechnology in actual breeding and selection is fast and accurate. It is not affected by environmental conditions, and has great potential for development in lotus breeding.
-
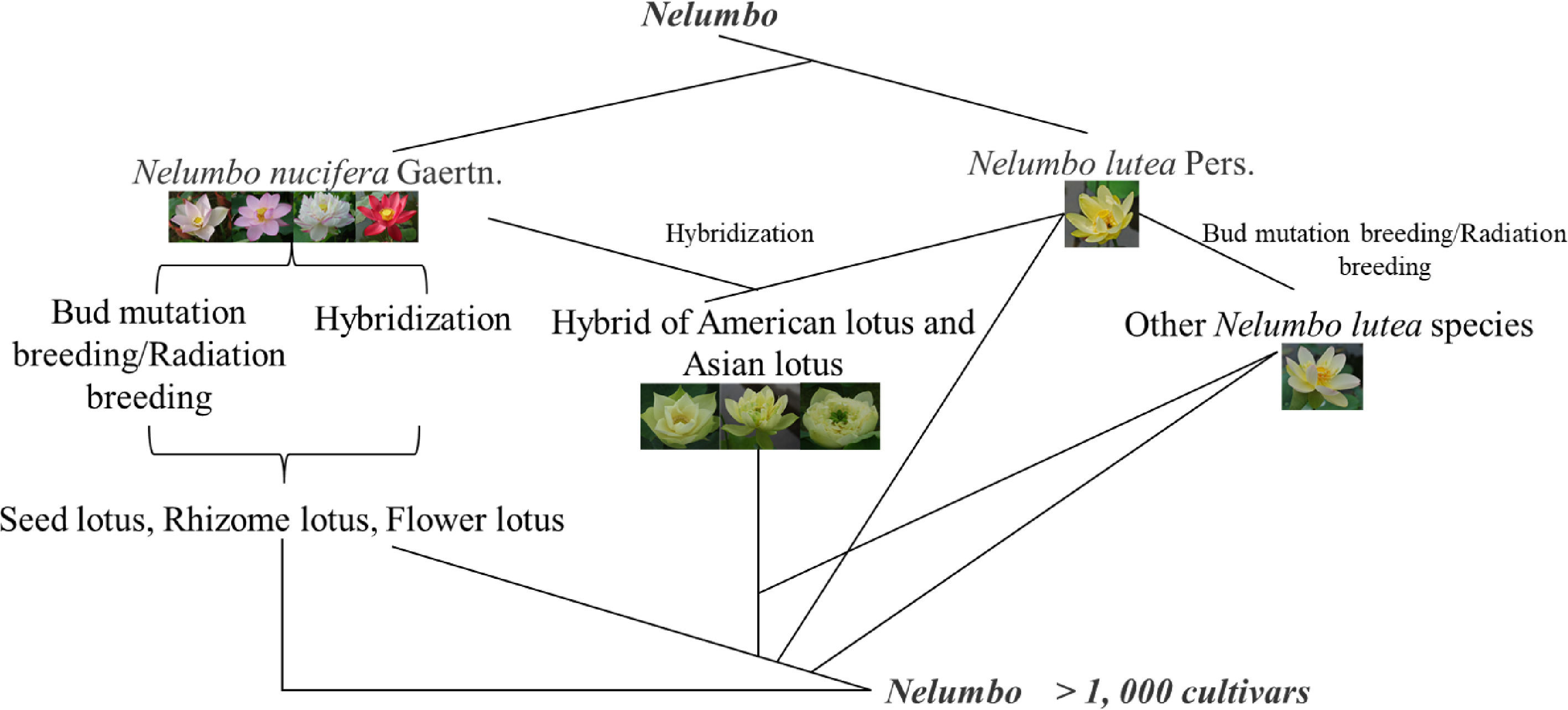
In recent years, the lotus has received more and more attention worldwide, and more and more enthusiasts and scientific researchers have participated in the genetic breeding of lotus. In 2019, Liu et al.[33] conducted a statistical analysis of the world's lotus cultivars resources and found that about 4,500 lotus cultivars have appeared worldwide. Among them, China alone has 2,888 lotus cultivars, more than half the total number of cultivars, followed by Japan, the United States, Australia, Japan, Thailand and other countries. It can be seen that China is the main center of lotus genetic breeding. In China, the collection and genetic breeding of lotus germplasm resources began in the 1960's[26], and its breeding methods are mainly divided into traditional breeding and radiation breeding (Fig. 3).
Traditional breeding
-
Traditional breeding includes selective breeding and artificial cross breeding. The lotus is a cross-pollinated plant with the characteristic that the pistil matures before the stamen, and the genetic background of various types of lotus varieties is highly heterozygous, and a large number of segregations can occur in the F1 generation for selection. Therefore, sexual cross breeding can be used, and then asexually reproduce fixed traits. Based on this characteristic of lotus, natural hybridization has become the most commonly used in lotus breeding, and it is also one of the effective methods for rapid breeding of new lotus cultivars. For example, among the 36 new flower lotus cultivars selected by Wang[21], 24 were selected from 275 seedlings by this method, and the selection rate was 8.7%, accounting for 66.7% of the new cultivars. In addition, eight cultivars were selected from the seedlings of the mixed lotus species, only four lines were selected from the offspring of the 24 combinations that were artificially crossed, accounting for only 11% of the 36 new cultivars. According to statistical analysis, since the 1980s, among the new flower lotus cultivars bred by conventional breeding in China, natural hybrids accounted for 89.5% of the total number, and artificial hybrids accounted for only 10.4%[21]. The method of selecting a mutant bud and developing it into a new cultivar is called bud mutation breeding, which is a kind of natural selection. In nature, the probability of gene mutations in plant cells is very low, only 0.001% to 0.01%, but bud mutations are found in the lotus breeding process and can be bred into new cultivars. The lotus is generally a diploid plant with a chromosome number of 2n = 16. However, triploid lotus varieties have been found in production practice. Therefore, the selection of lotus bud mutation is also one of the important techniques for lotus breeding[81].
Artificial cross-breeding is one of the important methods for the selection and breeding of new lotus cultivars. Combining the purpose of selection and breeding, select suitable parents, integrate the differential genes of both parents to the hybrid individual and the hybrid individual not only possesses the excellent traits of the parent, but also surpasses its parent in terms of flower shape, flower color, and stress resistance, and obtains a new cultivar with excellent traits. Artificial cross breeding can use the mixed pollen of multiple male parents and female parents for artificial pollination, thereby increasing the insemination rate and increasing the probability of breeding new cultivars. The first generation of hybrid individuals with excellent traits can be fixed by asexual reproduction such as lotus root and density division, and further cultivated into new cultivars with stable traits. This breeding method does not require multiple generations of selfing to obtain new varieties with stable traits. Jiang et al.[82] found that there are obvious differences in the seed setting rate of different varieties, and there are also differences in the seed setting rate of cross and reverse crosses. Bao et al.[83] also determined that the seed setting rate of artificial directional crosses was low, and the segregation traits of F1 generation retained some traits of the parents, but most of them tended to the female parents. From this characteristic, some directional breeding direction can be initially explored. Hybrid breeding is the most commonly used and effective method in the breeding of seed lotus, rhizome lotus, and flower lotus. The new cultivars of rhizome lotus and seed lotus are basically derived from hybrid breeding.
Radiation breeding
-
In recent years, people have begun to apply radiation breeding methods to lotus breeding work, and achieved certain results. The most widely used radiation breeding methods include 60Coγ radiation breeding, ion implantation mutation breeding, and space breeding[84]. Among them, 60Coγ-ray is a commonly used radiation source in radiation breeding, and it is also the most commonly used method in lotus radiation breeding. When people use 60Coγ-rays to mutate lotus seeds, it is found that the higher the mutagenesis rate, the lower the germination rate and survival rate. The suitable radiation dose is 30-60 Gy and the degree of radiation variation of ornamental lotus with different flower and color varieties varies. Red varieties are more prone to variability, flower type, flowering period, and number of petals are more difficult to variate[83,85]. At the same time, it is also proved that the degree of variation caused by 60Coγ-ray irradiation of lotus seeds is greater than that of irradiated lotus roots. Deng[86] used ion implantation 'Feng Tuan' lotus seed to cultivate a new cultivar of 'Zi Chan', and Lianhuachi Park used ion implantation technology to cultivate a number of new lotus cultivars. The Bailian Research Institute of Guangchang County first carried out spaceborne mutation breeding in 1994, and successfully selected new cultivars such as 'Space 3' and 'Space 36'. The lotus cultivar of 'Space 36' was widely used in production[87].
-
Molecular breeding is an efficient way to shorten the plant breeding cycle. Since the mid-1990's, researchers have carried out extensive research on lotus tissue culture. He & Liu[88] successfully differentiated immature embryos from callus into complete plantlets. Only the three stages of immature embryo, green embryo leaf and cotyledon can induce callus from the explants of American yellow lotus, while the green embryo can directly induce plants[89]. Mahmad et al.[90]established a lotus in vitro regeneration system using immature lotus embryos as test materials. The establishment of lotus in vitro regeneration system makes it possible to realize lotus transgenic technology, and also lays the foundation for lotus molecular breeding. In 2017, Chen[91] used the pollen tube pathway method to successfully transfer the two genes NnTP1 and NnTP2 into the lotus, and obtained a lotus transgenic plant. With the development of molecular biology research technology, people began to be interested in the molecular mechanism of the formation of lotus traits, and the molecular mechanisms of some important horticultural traits of lotus were revealed (Table 2). These molecular mechanisms, related to production and practical applications, laid the foundation for the molecular breeding of lotus.
Table 2. Genes related to ornamental characteristics that have been cloned in Nelumbo.
Horticultural traits Genes References Flower color NnCHS, NnCHI, NnF3H, NnF3'H, NnF3'5'H, NnDFR, NnANS, NnMYB5, NnTT8 [92−95] Flower type NnTP1, NnTP2, NnSEP1, NnAGL9, NnMADS6, NnCMB1, NnMADS14, NnAG1, NnAG2 [91,96−99] Flowering period NnAP1, NnAP2, NnLFY, NnFT, NnCO, NnSnRK1 [98,100−102] Abiotic stress resistance NnPCS1, NnTPS, NnMT2a, NnANN1, LrZIP, NnsHSP17.5, NnGPX1, NnUBC, NnGPX, Mn-SOD, NnCDPK, NnCNF2, NnNHX1, NnWRKY41, NnγGGS, NnCBF [10,103−107] Root and seed NnGBSS, LrSSS, NnPPO, NnAGPL1, NnAGPLS, NnADAP, NnSSSIII, NnEXPA1, NnCOL5, NnSBE [108−110] miRNA miR408-5p, miR4414a-3p, miR5077, miR156, miR159, miR393, miR319c [111−114] Flower color
-
Flower color is one of the important horticultural traits of lotus, and it is also an important index to evaluate the ornamental value of them. The main color-forming pigments in plants are divided into four major groups; chlorophyll, carotenoids, flavonoids, water-soluble alkaloids and their derivatives[115]. The main components of lotus petal pigments are flavonoids, including flavonoids, flavonols and dihydroflavonols[116], without carotenoids; red lotus type and pink lotus type lotus anthocyanins contain anthocyanins; multicolor lotus type lotus contain trace amounts of anthocyanins, which may be distributed in the tip of the petals[117]. In lotus cultivars with different flower colors, flavonoid biosynthesis is differentially regulated by the expression of flavonoid biosynthesis. Among them, NnCHI, NnF3'H, NnDFR and NnANS are considered to be the key to pigment accumulation and regulate the different flower colors of lotus flowers[92]. Deng et al.[118] analyzed the proteomics and epigenetics of the petals of red lotus and white lotus, and identified four enzymes in the anthocyanin pathway. The expression pattern analysis showed that the anthocyanin synthase gene (ANS) may be a key gene to determine the lotus anthocyanin for biosynthesis and accumulation. The different methylation intensities of ANS gene promoter sequence may cause different flower colors of red lotus and white lotus. In the process of flavonoid biosynthesis pathways, MYB, bHLH and WD40 are the most studied and detailed types of transcription factors. Most of these three types form a complex through the combination of MYB and bHLH, or the three combine to form a complex and then bind to the structural gene promoter to regulate the metabolism of flavonoids[119]. There are a total of 116 R2R3-MYB genes in the lotus genome, 13 of which are related to the synthesis of flavonoids in different tissues of lotus. Two of these genes positively regulate the biosynthesis of anthocyanins and procyanidins in flowers and seeds, respectively[120]. NnMYB5 is a transcriptional activator of anthocyanin synthesis. Overexpression of NnMYB5 in Arabidopsis will result in the accumulation of anthocyanins in immature seeds and pedicels and the up-regulation of AtTT19 expression. In addition, NnMYB5 can also be combined with NnbHLH1 for co-regulation of the accumulation of anthocyanins in lotus petals[94]. Deng et al.[93] transferred NnTT8 into the mutants of Arabidopsis AtTT8 and found that NnTT8 has a function similar to AtTT8 in regulating the biosynthesis of anthocyanins and procyanidins, and can be combined with NnMYB to jointly regulate the synthesis of anthocyanins.
Flower type
-
After thousands of years of domestication and selection, the lotus has formed a rich variety of flower types, including five different flower types: single, semi-double, double, duplicated, and thousand petals. The double-petal, duplicated-petal and thousand-petal lotus cultivars have high ornamental value. They have huge future market potential for the development and application of cut flowers and potted flowers, and they are also important breeding materials. Although a lot of double-petaled lotus cultivars have been bred, most of the current research on double-petaled lotus flowers only focus on the morphological observation and study of the developmental characteristics of their floral organs and tissues[121,122]. The research on the molecular mechanism of the formation of double-petaled lotus is still lacking, which limits the development of genetically engineered breeding of double-petaled lotus, and cannot meet the demand for double-petaled lotus varieties in production. A study by Li et al., found that the flower bud differentiation process of the double-petaled lotus cultivar 'Youyi Mudanlian' is basically the same as that of the single-petal flower, while the double-petaled cultivar 'Hongtailian' lacks the differentiation stage of sex cells because the pistil is completely petalized[122]. In addition, there are temporal and spatial differences in the differentiation of flower buds of different types of lotus flowers; the double-petalization of lotus belongs to the origin of pistil and stamen, while the petal origin of thousand-petal varieties is more complicated and has not been thoroughly investigated[121].
Among the molecular regulation mechanisms of flower development, the ABCDE model is the most classic regulation method[123]. Chen[91] studied the formation mechanism of the lotus flower type and found that the expression of NnTP1 and NnTP2 genes (belonging to the C/D gene in the ABCDE model) in the thousand-petal cultivar showed a sharp decline compared with the single-petal cultivar, there is almost no expression at the full flowering stage. It is speculated that the decrease of NnTP1 and NnTP2 gene expression is one of the main reasons for the petalization of lotus flowers. There are a total of 44 MADS-box genes in the lotus genome (MADS-box genes are a type of transcription factor with homologous conserved regions that can regulate the development of floral organs), which are mainly located in the nucleus. Among them, NnMADS14, which is homologous to Arabidopsis SEPALLATA3 (SEP3), has been shown to be related to the development of floral organs[96]. Liu et al.[97]analyzed the E-type genes in lotus that affect the formation of sepals and isolated three E-type gene family members from Nelumbo nucifera Gaertn. flower buds, namely NnSEP1, NnMADS6 and NnAGL9. All three genes have been confirmed to be located in the nucleus and have typical MADS-box characteristics. NnSEP1 and NnMADS6 are specifically expressed in sepals, while NnAGL9 is highly expressed in petals, indicating that there are different developmental mechanisms for the formation of sepals and petals in lotus. Kashiwamura et al.[99] studied and analyzed the C-type genes in lotus and found that NnAG1 and NnAG2 are specifically expressed in the stamens and carpels of single-flowered varieties, but their expression levels are down-regulated in double-flowered varieties. In situ hybridization experiment analysis further showed that these two C-type homologues are specifically expressed in the inner wheels such as stamens and carpels of single-petal flower cultivars, but the signal is weakened in lotus double-petaled cultivars, indicating that the down-regulation of type C genes is one of the reasons for the double-petaled lotus flower. The double flower type of the lotus is formed by the gradual petalization of the stamens and pistils of the lotus, and is not directly differentiated. Transcriptome sequence analysis of double lotus flower buds, before and after differentiation, revealed that seven MADS-box gene family members (including DEF, PMADS2, CMB1 and SEP genes) were up-regulated and one MYB gene NnMYB44 was down-regulated during flower bud development. It shows that these genes are involved in the regulation of lotus flower development and the regulation of double petal traits of lotus[114].
Flowering period
-
Lotus flowers mainly bloom in summer. The high temperature in summer greatly affects people's interest in viewing lotus flowers, and the blooming period of lotus is relatively short, and there are few lotus flowers in autumn. It is of great significance to study the molecular mechanism of the lotus flowering period to cultivate early-flowering and long-flowering cultivars. Hormones are an important factor in regulating the blooming of lotus flowers. Treatment of 'Wan Lian' with 150 mg/L IAA can increase and advance its flowering period. GA3 treatment (150 mg/L) can prolong the flowering period. 6-BA (100 mg/L) treatment has a significant effect on the leaf area, number of standing leaves, and absolute growth rate of leaf height[124]. Using 0.1 mmol/L spermidine to treat lotus can increase the length and width of the largest stem and petals of lotus, and prolong the life span of a single flower[125]. Shen[126] sequenced the transcriptome of the lotus cultivar 'Qingyu' and screened out genes related to flower opening, and performed real-time fluorescence quantitative analysis on genes with relatively high expression levels. It can be seen that the expression of ethylene, auxin, and cytokinin-related genes all show a gradual increasing trend during the opening of the lotus flower, which plays a regulatory role in the opening of the lotus. The expression of cell wall-related genes is higher in the early stage of lotus flower opening, and the expression of this gene gradually decreases with the senescence of lotus petals. LYF and AP1 play an important role in the differentiation and development of flower organs. Studies have found that NnLYF and NnAP1 of lotus are expressed in all stages of lotus growth and development, and the expression of these two genes reaches the highest level in the bud stage. This shows that NnLYF and NnAP1 play an important role in controlling the characteristics of floral meristems and the formation of floral organs[98,102]. Liu[103] transferred the NnAP2 of the lotus into Arabidopsis and found that the NnAP2 transgenic Arabidopsis showed an early flowering phenotype. Further research found that the early flowering phenomenon of transgenic Arabidopsis is that NnAP2 indirectly promotes the increase of GA content by inhibiting the expression of the GA2-oxidase gene, thereby promoting early flowering of transgenic plants. Yang et al.[101] conducted transcriptome sequence analysis on the two stages of bud development of tropical lotus and temperate lotus, and identified 147 genes related to the flowering period of lotus flowers. These genes are homologous to the genes that control the flowering period pathways of other plants. Among them, the differential expression of COP1, CCA1, LHY, CO-LIKE, VIN3, GAI and FT genes involved in photoperiod, vernalization and gibberellin pathways indicates that they may be the key factors controlling the early flowering of lotus. Cao et al.[100] used VIGS technology to silence the expression of NnSnRK1 in lotus seedlings. NnSnRK1 silent lotus seedlings have strong flowering ability, and the flower-to-leaf ratio increased by 40%. When the seedlings were treated with ABA, the expression level of NnSnRK1 and protein kinase activity were significantly reduced, and ABA treatment can also enhance the phenotype of NnSnRK1 silent seedlings.
Abiotic stress
-
Plants have strong environmental adaptability. They can adapt to different growth environments by enhancing their resistance to environmental stress. The research on the abiotic stress of lotus mainly focuses on flooding, high temperature, salt, low light and heavy metals. NBS-type disease resistance genes (Nucleotide-binding site) are the most important type of disease resistance genes in plants, and their evolutionary model, structural characteristics and functional regulation have always been hotspots in the field of disease resistance gene research. There are a total of 137 NBS-type disease resistance genes in the lotus genome. Analysis shows that these genes in the lotus genome have ancient replication and recombination events like the whole genome sequence. Among them, 52 NBS genes contain the NACHT domain that has only been reported in the NBS genes of animal, fungal and bacterial genomes. It implies that the NACHT gene and the NBS gene may have similar ancient origins and play an important role in the resistance to disease migration of aquatic to terrestrial plants[127]. The plant chelating peptide synthase (Phytochelatin synthase, PCS) NnPCS1 in lotus participated in the stress response to metallic chromium. After chromium stress treatment, the expression of NnPCS1 gene in lotus leaves increased significantly, and NnPCS1 was expressed in Arabidopsis thaliana. Expression of NnPCS1 gene in Arabidopsis can significantly increase the absorption and accumulation of chromium[128]. Li[129] used SMART technology to successfully construct a cDNA library of rhizome lotus and flower lotus top bud, which provided sequence resources for the cloning of lotus-related functional genes. The lotus was subjected to stress treatments with different time gradients such as cold, heat, mechanical damage, high salt, ABA and PEG, and it was found that the expression levels of NnGPXl and NnUBC increased rapidly after various treatments, indicating that the lotus grows and faces stress. The environment plays an important role in regulating growth. Diao et al.[130] cloned the lotus glutathione peroxidase (NnGPX) gene in 2014 and proved that lotus glutathione peroxidase is salt-tolerant. The seed defensin gene, lotus small molecule heat shock protein gene sHSP17.5, polyphenol oxidase gene and CBF2 gene have been cloned, and all have been proved to be related to the stress resistance and stress of lotus[106,107,130−133]. Although lotus is an aquatic plant, it is not resistant to flooding. Jin et al.[105] found that the Trehalose-6-phosphate synthase (TPS) gene family in lotus was subjected to purification selection during the evolution process, and the expression of TPS gene was significantly increased under flooding treatment. It is believed that two of the TPS genes may play an important role in the energy metabolism and stress response of lotus. Through transcriptome analysis, Wang et al.[134] found that lotus has 4,002 differentially expressed genes under waterlogging stress. After GO analysis and KEGG enrichment analysis, it is found that they are mainly involved in redox, non-biological stimulation, cell metabolism and small molecule metabolism processes. Further analysis of the lotus WRKY gene family found that the expression of NnWRKY41 gene increased after flooding, and the tobacco transient expression experiment was used to further verify that NnWRKY41 can improve the spoilage resistance of lotus after flooding[104].
Lotus root and lotus seeds
-
Lotus root and lotus seeds are the main edible parts of lotus. The research on lotus root and lotus seeds has mainly focused on the starch synthesis and shape and size of lotus root and lotus seeds. Starch is the main nutritional component of lotus root. The related enzymes regulating lotus root starch synthesis mainly include ADPG-pyrophosphorylase, starch synthase, starch branching enzyme and starch debranching enzyme[135]. Cheng et al.[136,137] sequenced the transcriptome at different stages of lotus root development, summarized and analyzed 86 genes that may be related to lotus root rhizome enlargement, including 35 hormone-inducible protein genes, four light-inducible protein (MADS-BOX) genes, and 11 rhizome storage protein gene (Patatin), 35 genes related to starch metabolism, and one gene related to rhizome formation. Further research found that 34 genes participated in the starch synthesis process of lotus rhizomes, among which the expression of four genes LrGBSS, LrSBEI, LrSBEII and LrSBEIII increased significantly in the late stage of underground stem swelling. Yang et al.[138] used the transcriptome to analyze the differential genes in the development of underground stems of tropical and temperate lotus, 24 genes were discovered and were related to photoperiod, starch anabolism, and hormone transmission that may be involved in the process of lotus rhizome enlargement. Cao et al.[139] analyzed the morphology, physiology and proteome of the lotus rhizome during the expansion process, and found that light and auxin signals may be transmitted through the secondary messenger Ca2+, and play an important role in the lotus rhizome expansion. Wang et al.[140] used the metabolome-binding protein group and found that there are significant differences in the processes of sugar metabolism, glycolysis, tricarboxylic acid cycle, and amino acid metabolism in the four different stages of lotus seed development. Cao et al.[141] conducted SNP verification on a population of 95 lotus cultivars. Through population typing experiments and SNP mapping, a SNP related to the rhizome phenotype of lotus root was located in the NADAP gene of the AP2 subfamily. It was found that the lotus roots in the SNP-CC group were significantly larger and have higher soluble sugar content. It is speculated that the change of the SNP of the NADAP gene in the roots of lotus roots affects the size and sugar content of the roots of lotus. In addition to its important edible value, lotus seeds are also rich in flavonoids[142,143] and have strong antioxidant capacity[144]. They play an important role in anti-aging and anti-oxidation for people and can be used as an important medicine[145].
-
The lotus is a relic plant left over from the glacial period. Following the glacial period, only two kinds of lotus remained, Asian lotus and American yellow lotus, which have a long history of cultivation[1,2]. On the basis of only two species of Asian lotus and American yellow lotus, through modern scientific research, more than 4,500 cultivars of lotus have been recorded, and it has become the largest economic aquatic crop with the largest cultivation area and the richest accession resources in China. Now it is grown as an aquatic vegetable in various countries worldwide[11,33,40]. Wild lotus is a lotus resources which grows naturally and is a high-quality germplasm resource for lotus hybrid breeding. However, with the development of economy and the expansion of human activities, the habitat of wild lotus is gradually shrinking, which greatly affects its growth. In addition, with the development of lotus breeding, a large number of cultivated lotus cultivars have been developed, and the cultivation area has gradually expanded, and its pollen has entered the habitats of wild lotus through a variety of means, resulting in wild lotus being affected by foreign gene infiltration. Based on this situation, research on the genetic diversity and population structure of wild lotus populations should be strengthened to provide a theoretical basis for wild lotus conservation. The collection and research of resources in Yunnan, China and abroad, (especially tropical lotus in Southeast Asia and South Asia, and American yellow lotus in different regions) should be strengthened. The different ecotypes of lotus preservation methods in different regions should be studied to ensure survival while preserving their genetic integrity. In order to protect wild lotus populations, artificial pollination and sowing of wild lotus should be encouraged, and it should be prohibited to collect or destroy wild lotus populations for any purpose. Ex situ conservation is also an effective way to protect wild lotus. Wild lotus germplasm resources should be collected, and wild lotus seed resource nursery for germplasm preservation should be initiated.
Germplasm resources are important materials for studying the origin, cultivation history and genetic breeding of lotus. Strengthening the collection and identification of domestically famous and high-quality cultivars, and perfecting the existing germplasm resource nursery is a long-term goal for lotus researchers. China has been collecting lotus resources for 40 years. Most of the domestic resources have been collected, but some excellent resources are still scattered in the private sector[31]. In recent years, the large-scale application of new cultivars of rhizome lotus and seed lotus in China's lotus root production areas has led to a significant reduction in the planting area of the original local varieties. If protection measures are not taken in a timely fashion, local cultivars may disappear. In addition, there may be some rare resources in the private sector that need to be excavated and sorted. In particular, the rhizome lotus are widely planted in various parts of China, and there was little exchange in the past, thus forming a batch of resources adapted to different ecological environments. In some remote mountainous areas, especially southwestern China, there may be a lot of precious resources. In the research of resources, there is a phenomenon that different names are used for the same material due to collection by different units, resulting in repeated naming of resources. The same situation exists in seed lotus. Since there is no local name, it is often named as the location in which it is found. There are many breeding units of flower lotus, most of which are named by themselves based on certain traits. The rhizome lotus will further increase local resources, flower lotus will add new cultivars. For example, although there are more than 300 cultivars of flower lotus, they are all evolved from more than 50 original specimens, and some of these are core resources. Therefore, in the future, researchers need to pay attention to the collection and preservation of these rare germplasm resources, and make full use of them to enrich the gene pool of different varieties of lotus.
Hybrid breeding is the main method of lotus breeding, and it is also the most widely used breeding method. Among the cultivars bred by cross-breeding, the flower lotus accounts for the largest proportion. The cultivars of different flower types, flower colors, and plant types have emerged in large numbers. However, the bred cultivars of flower lotus lack uniform identification, and the homogeneity is more serious[146]. Most cultivars of flower lotus are extremely shade-tolerant, and they will have few flowers or even no flowering under low light conditions, and they will bloom later in June to October each year, and the viewing period is short. In addition, most of the flower lotus cultivars have a short flowering period and the petals fall easily making it difficult to enter the cut flower market. Therefore, for flower lotus breeding, it is necessary to use the rich germplasm resources of the lotus to breed different flower types and colors, and to cultivate long-flowering cultivars in the middle and lower reaches of the Yangtze River until late November to early December. Cultivation of low-light-tolerant bowl lotus cultivars will encourage lotus into thousands of households; cultivate fresh cut flower varieties and dried flower varieties, varieties with a long single flower blooming period, and varieties that are not easy to drop or do not drop. For rhizome lotus breeding, high-yield is still the first breeding goal, followed by high-quality, shallow soil suitable for mechanical mining. In terms of quality breeding, brittle lotus roots require high moisture and soluble sugar content; silty lotus roots require high dry matter and starch content. In terms of appearance quality, since traditional local cultivars and wild lotus roots are mostly long cylindrical or long strips, the flavor is good, but the yield is low and the mud is deep. How to maintain the long cylindrical or striped lotus root flavor is one of the breeding goals for the future to get shallow into the mud and increase the yield. High yield is the primary breeding goal of seed lotus breeding, followed by appearance quality and nutritional quality. Most of the seed lotus cultivars are single-petal varieties with high seed setting rate, and there are few double-petaled varieties. Breeding varieties that share ornamental and production value is the direction for seed lotus breeding. In addition, the flower stalk and petiole of lotus have thorns, which greatly affects the harvesting efficiency of lotus seeds and labor costs. It is necessary to breed thornless seed lotus cultivars.
There are two main ways for the reproduction of lotus, namely seed reproduction and lotus root reproduction. In order to improve the reproduction efficiency of lotus, researchers have been studying lotus tissue culture technology since the mid-1990's. So far, a lotus regeneration system has been successfully established, and even foreign genes can be introduced into the lotus genome[95]. Because of the low transformation efficiency and low callus proliferation rate, it has not been widely used, but this is still an important basis for lotus to enter the era of molecular breeding. The lotus has an extremely important position in the evolution of angiosperms, which has attracted the attention of a large number of researchers. At present, two lotus genomes have been sequenced and published[70,71,147]. Based on lotus genome data, a large number of molecular markers have been developed. However, most of these developed molecular markers were used to study the genetic diversity of lotus, and there are few reports on molecular markers related to important horticultural traits of lotus. In general, lotus is still in its infancy in molecular breeding, and many important horticultural traits such as flower type, flower color, stress resistance and other aspects of the mechanisms involved have only had minimial research on the surface, and the deeper research is relatively backward. Researchers of lotus need to make full use of lotus genome information and modern molecular biology technology to further understand the gene functions and regulation methods that control important horticultural traits of lotus. This will be of great significance to the use of molecular breeding methods to select new varieties of lotus with distinctive traits and to improve the breeding efficiency of lotus.
This work is supported by the National Natural Science Foundation of China (Grants No's 31971710 and 32071829), Fundamental Research Funds of the Central Government (Special Project of Lotus Germplasm Resources) (KYZZ2021003).
-
The authors declare that they have no conflict of interest.
- Supplemental Fig. S1 The plant morphology of lotus.
- Supplemental Table S1 The cultivar names of Fig. 2.
- Supplemental Table S2 Comparison of different molecular marking techniques and the application in lotus.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhou P, Jin Q, Qian P, Wang Y, Wang X, et al. 2022. Genetic resources of lotus (Nelumbo) and their improvement. Ornamental Plant Research 2:5 doi: 10.48130/OPR-2022-0005
Genetic resources of lotus (Nelumbo) and their improvement
- Received: 13 October 2021
- Accepted: 19 January 2022
- Published online: 23 March 2022
Abstract: Lotus (Nelumbo) is one of the top ten flowers in China, which has high ornamental, edible and medicinal value. Lotus has a been cultivated for thousands of years. Through discovery and cultivation, more than 4,000 cultivars have been recorded. However, the information related to lotus breeding is quite scattered, and the related genetic rules and trait formation mechanisms are still poorly understood, which has caused a greater impact on lotus genetic breeding. This article systematically introduces lotus germplasm resources, including wild species and cultivated species, summarizes lotus breeding methods and breeding directions, and focuses on the latest progress in the isolation and functional identification of structural and regulatory genes related to important horticultural traits. Prospects for the protection and utilization of lotus resources, breeding and industrialization are reported.