-
Carnation (Dianthus caryophyllus L.), a perennial herbaceous plant with a long flowering period and rich flower color, is one of the most important commercial ornamental flower crops in the world[1]. Compared with cut carnations, potted carnations have been gradually promoted by businesses in recent years due to their advantages such as transport resistance, simple cultivation facilities, and perennials, which has seen them rapidly become a high-taste Mother's Day gift and a type of home potted plant[2,3].
Ethylene regulates multiple aspects of plant growth and development[4], including flower opening, petal senescence, and abscission, in a wide range of flower species[5,6]. When ethylene is perceived by specific receptors, an ethylene signal is sent through a sequence of biochemical events that regulate the expression of ethylene-responsive genes[7−11], leading to ethylene synthesis and ultimately flower senescence[12,13]. It has been established that there are two rate-limiting steps in the ethylene biosynthesis pathway: the conversion of S-adenosyl methionine to 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC synthase (ACS), and the subsequent conversion of ACC to ethylene by ACC oxidase (ACO)[14]. Carnation flowers are studied as model systems of ethylene-induced flower senescence.
Inhibition of ethylene biosynthesis and signal transduction effectively delays senescence in carnations[15−19]. The accumulation of ACS and ACO gene expression can contribute to the release of ethylene from flowers, which can induce flowering or accelerate flower senescence[20,21].
Treatment of carnation flowers with exogenous ethylene and ethylene analogs such as vinblastine increased ACC synthase (ACS) activity in petals, which in turn increased ACC content and ethylene production. It has been shown that the expression level of ethylene biosynthesis genes was negatively correlated with the vase life of carnation, with lower ethylene production and the expression of Dc-ACS and Dc-ACO in varieties with late petal senescence, although treatment with low concentrations of exogenous ethylene up-regulated the expression of Dc-ACS and Dc-ACO in short vase life varieties, and not in long bottling life varieties[22].
Carnation lines that vary in ethylene biosynthesis and sensitivity are of great value in the study of ethylene-related processes[23]. The flowers of some carnation cultivars have a long-life span, which is associated with low ethylene production and low ethylene sensitivity.
The differentiation of cut flower senescence types according to their response to ethylene is a generalization and extension of the extensive research on respiratory metabolism over the years. In 1922, British scientists Kidd and West proposed the concept of respiratory climacteric[24]. In 1969, Iwata added at the end-rising type[25], and the types of respiratory climacteric were summarized into three types: (1) climacteric type, (2) non-climacteric type, and (3) increasing later stage. Due to the complex genetic background of potted carnations, there are differences in the types of endogenous ethylene production among different varieties. Therefore, it is important to determine the ethylene sensitivity and endogenous ethylene production of potted carnation varieties.
The brief vase life of cut flowers is mainly attributable to the early failure of tissue water relations, which is related to a decrease in water absorption due to vascular occlusion and the rapid loss of water from cut flowers under unfavorable postharvest conditions[26−28]. Water stress promotes senescence in cut flowers. The vase life of cut carnations was shortened by 1.5 d when they were left without water for 24 h[29].
The floral fragrance of carnation is mainly composed of benzene/phenylpropane compounds, terpenoids, and fatty acid derivatives, of which benzene/phenylpropane compounds are the main components[30]. It was found that in many species, ethylene has a regulatory role in the release and synthesis of volatiles in their flowers. Ethylene regulates the release of methyl benzoate, a characteristic aroma of Petunia hybrida, and down-regulates the expression of BSMT, BPBT, and PAL, genes related to the synthesis of methyl benzoate[31,32].
In this study, we investigated ethylene sensitivity, ethylene metabolism types, and expression of ethylene synthesis-related genes in 17 varieties of potted carnations, and also analyzed the effects of ethylene on water relations of cut flowers and major volatile substances of petals to provide some scientific basis for selecting carnation potted flower varieties with long flowering period and high ornamental quality.
-
Seventeen potted carnation varieties were used for the experiment, twelve of which were from Chengdu Jinpin Flower Seedling Co. (Chengdu, China), five varieties were from Kunming Haisheng Seedling Co. (Kunming, China). All varieties were planted in the Smart Greenhouse at the Flower Base of Huazhong Agricultural University (China). Potted carnation were grown under standard greenhouse conditions (20 °C day/night, 50% RH). Plants were watered as needed and fertilized once a week with a 20N : 20P : 20K fertilizer (Rongfeng Gardening Co., Guangzhou, China).
Potted carnation flowers were collected from 8:00−9:00 am, when they were in full bloom (the outermost petal is at an angle of 90° to the flower stem) and free of pests and diseases. After harvesting, the flowers were immediately placed in a container with tap water, placed in a foam box with ice at the bottom, and transported back to the laboratory within 15 min. The stems were trimmed to 3−4 cm in length in the water, leaving 2 leaves per stem, and the flowers were then placed in a 10 mL centrifuge tube with distilled water and rehydrated for 1 h before the experiment. The outermost 6−8 petals of the flowers were selected and immediately frozen in liquid nitrogen and stored at −80 °C for RNA extraction and analysis of volatile substances.
Ethylene treatment
-
For the ethylene treatment, centrifuge tubes containing potted carnations were placed in a 300 mm diameter glass desiccator with 10 mL of NaOH solution at a concentration of 1% under a perforated porcelain plate to prevent the accumulation of carbon dioxide released by respiration during the treatment. A thin and uniform layer of petroleum jelly was applied to the body of the apparatus and the grinding mouth of the lid to isolate it from the outside air. A 1 mL syringe was used to slowly inject 0.1 mL of ethylene gas into the desiccator at a concentration of 10 μL·L−1 and treated for a certain time. Untreated flowers were incubated in the same environment in a chamber with normal air. The ethylene treatment was terminated by removing the flowers from the glass desiccator.
Evaluation of vase life and water balance
-
Vase life is the time from ethylene treatment (the morning after harvest) to the appearance of senescence symptoms such as severe wilting and drying and crinkling of petals under the same environmental conditions. Water balance is calculated by deducting daily transpiration from daily water uptake.
Determination of ethylene release
-
Weighing and recording of potted carnation flowering branches, and then the flowers to be tested were placed in a sealed 50 mL bottle containing 10 mL of distilled water and sealed for 6 h. The endogenous ethylene release was measured by GC-2010 gas chromatograph. 1 mL of gas was extracted from the sealed vial and injected into the gas chromatograph using a GDX-502 column with an inlet temperature of 130 °C, a pressure of 12 psi, a spacer purge flow rate of 3 mL·min−1, a column chamber temperature of 90 °C, an equilibration time of 1 min, a shunt ratio of 1:1, helium (99.999%) as the carrier gas, a holding time of 3 min, and a detector temperature of 220 °C. Detector flow rate: tail blow gas flow rate (N2) 5 mL·min−1, reference flow rate 10 mL·min−1. Then the amount of ethylene released (ng·g−1·h−1) was calculated based on the volume of the sealed vial, sample weight, and ethylene treatment time.
Extraction of total RNA from flower petals
-
The ultra-low temperature frozen samples were quickly transferred to a mortar and pestle pre-cooled with liquid nitrogen, and the tissue was well ground into powder form using a mortar and pestle. The ground samples were transferred to a centrifuge tube, 1 mL of TransZol UP per 50−100 mg of tissue was added, then homogenized with a homogenizer, and left to stand at room temperature for 5 min. After standing, 200 μL of chloroform was added and then shaken vigorously for 30 s, then incubated at room temperature for 3 min, and centrifuged at 10000 r·min−1, 4 °C for 15 min, at which time the sample was divided into three layers, and the RNA was in the uppermost aqueous phase. Five hundred microliters of aqueous phase was transferred into a new centrifuge tube, 500 μL of isopropanol was added, then mixed upside down, incubated at room temperature for 10 min, centrifuged at 10,000 r·min−1, 4 °C for 10 min, the supernatant was then removed leaving a gelatinous precipitate on the side and bottom of the tube. One mililiter of 75% ethanol (prepared with DEPC water) was added and then vortexed vigorously, and centrifuged at 7,500 r·min−1, 4 °C for 5 min. The supernatant was discarded and the precipitate dried at room temperature (it takes about 5 min). Finally, 30 μL of RNA lysis solution was added, incubated at 55−60 °C for 10 min, and then stored at −80 °C for backup.
cDNA synthesis
-
Reverse transcription was performed using the QRT SuperMix for qPCR Reverse Transcription Kit (Vazyme, China). According to the manufacturer's instructions, 1 μg of total RNA and 4 μL of 4× gDNA wiper Mix were added to the PCR tube, supplemented with ribonuclease-free water to 16 μL, mixed by gently pipetting using a pipette, and incubated for 2 min at 42 °C in a PCR instrument. After the reaction was completed, 4 μL of 5× HiScriptII QRT SuperMix II was added to the above reaction product, and the mixture was incubated in the PCR instrument at 50 °C for 15 min and 85 °C for 5 s. After finishing the procedure, it was placed in a −20 °C refrigerator for storage.
Quantitative real-time PCR (qRT-PCR)
-
Quantitative real-time PCR reactions were performed on LightCycler® 480 II Real-time PCR Instrument (Roche, Switzerland). The reaction mix was prepared according to the instructions of the Hieff UNICON® Universal Blue qPCR SYBR Green Master Mix kit (Vazyme, China). The reaction mix consisted of 4 μL of cDNA (cDNA stock solution diluted 10 times), 10 μL of Master Mix, 0.4 μL of forward primer, 0.4 μL of reverse primer, and 5.2 μL of sterile ultrapure water, with a final volume of 20 μL. The reaction solution was added to a 384 well optical plate (Roche, Switzerland). The qRT-PCR reaction procedure was: 50 °C for 2 min, 95 °C for 2 min, then 95 °C for 10 s, 60 °C for 20 s, and 72 °C for 20 s for 40 cycles. The final Ct value was the average of three independent biological replicates, and the 2−ΔΔCᴛ method was applied for data analysis[33,34]. The gene-specific primers used for qPCR of the target genes are shown in Table 1, and the DcUbq3-7 gene was chosen as the internal reference[35].
Table 1. Primers used for quantitative real-time PCR.
Gene Primer DcACS1 Forward (5‘-3’) CAGTGTGACGCCATTGAAAC Reverse (5‘-3’) TTAAGTTCAATGTTTTGTTGAGAAGA DcACO1 Forward (5‘-3’) TGCAATGCTTCTCATCAACC Reverse (5‘-3’) CAATCCATAGGACATGGAACA DcUbq3-7 Forward (5‘-3’) GTTGTTGGTTTCAGGGCTGGTTTG Reverse (5‘-3’) CTACGGTAATTGAGAATTCACACCGAAATG Extraction of volatile substances
-
Add 0.2 μL of benzyl acetate (internal standard) to 20 mL of ethyl acetate and mix thoroughly to prepare ethyl acetate extract, store in a 4 °C refrigerator for spares. Take appropriate amounts of petals in a mortar and grind them into powder under liquid nitrogen freezing. Weigh about 0.5 g of powder in a 2 mL centrifuge tube, add 500 μL ultrapure water to dissolve, vortex, and mix well, then add 600 μL ethyl acetate extraction solution and vortex and mix well, and rotate at low speed overnight at 4 °C in the refrigerator to improve the extraction rate. After centrifugation at 10,000 r/min for 15 min, the supernatant was aspirated with a 1 mL sterile syringe, filtered through a membrane filter (pore size 0.22 μm), and placed in a 2 mL injection vial with a glass inner cannula, stored in a refrigerator at 4 °C, and then detected by GC-MS.
GC-MS detection method
-
The chromatographic column was DB-5MS (30 m × 0.25 mm × 0.25 μm, Themo Scientific, Bellefonte, PA, USA), and the carrier gas was high-purity helium (99.999%) in non-split mode with a flow rate of 1 mL·min−1. The temperatures of the ion source and the injection port were 280 °C and 230 °C, and the temperature of the transmission line was 260 °C. The ramp-up procedure was 40 °C for 3 min, followed by ramp-up to 120 °C for 2 min at 3 °C·min−1 and to 240 °C for 25 min at 5 °C·min−1. El (electron bombardment) ion source with an electron bombardment energy of 70 eV and a mass scan range of 40−450 amu.
The retention indices (RI) of the n-alkane standards (C8-C30) were calculated using a computer search and the National Institute of Standards and Technology (NIST) library, and the relative content of each component in the samples was calculated by the peak area normalization method.
Data analysis
-
Data recording and analysis were performed in Excel 2019 and data ANOVA (P < 0.05) were performed using SPSS software. Graphs were prepared using GraphPad Prism7 software. Experimental materials were collected mainly focusing on the period from June to August 2021 and from March to May 2022, and flower lifespan recording was performed during this period.
-
Among the 17 potted carnation varieties, the single flowering duration and vase life of potted carnations varied considerably in this study (Fig. 1; Table 2). 'Purple Wings' had the longest single flowering period at 24 d, followed by 'Red', 'Cherry', and 'Oscar'. 'Grace Bay' and 'Grane Beach' had the shortest single flowering period, both at 10 d, with a variation of 234% among the 17 varieties (Fig. 1; Table 2). Comparing the vase life, it was found that 'Grane Beach' and 'Grace Bay' had the shortest vase life of 6.2 d and 6.9 d, respectively, 'Cherry' and 'Salmon' had the longest vase life, both at 14 d, with a significant difference of 229% (Fig. 1; Table 2). Based on the results of the differences in vase life of 17 potted carnation varieties, they can be divided into three variety groups: short vase life variety group (< 9 d), medium vase life group (9−12 d), and long vase life variety group (> 12 d).
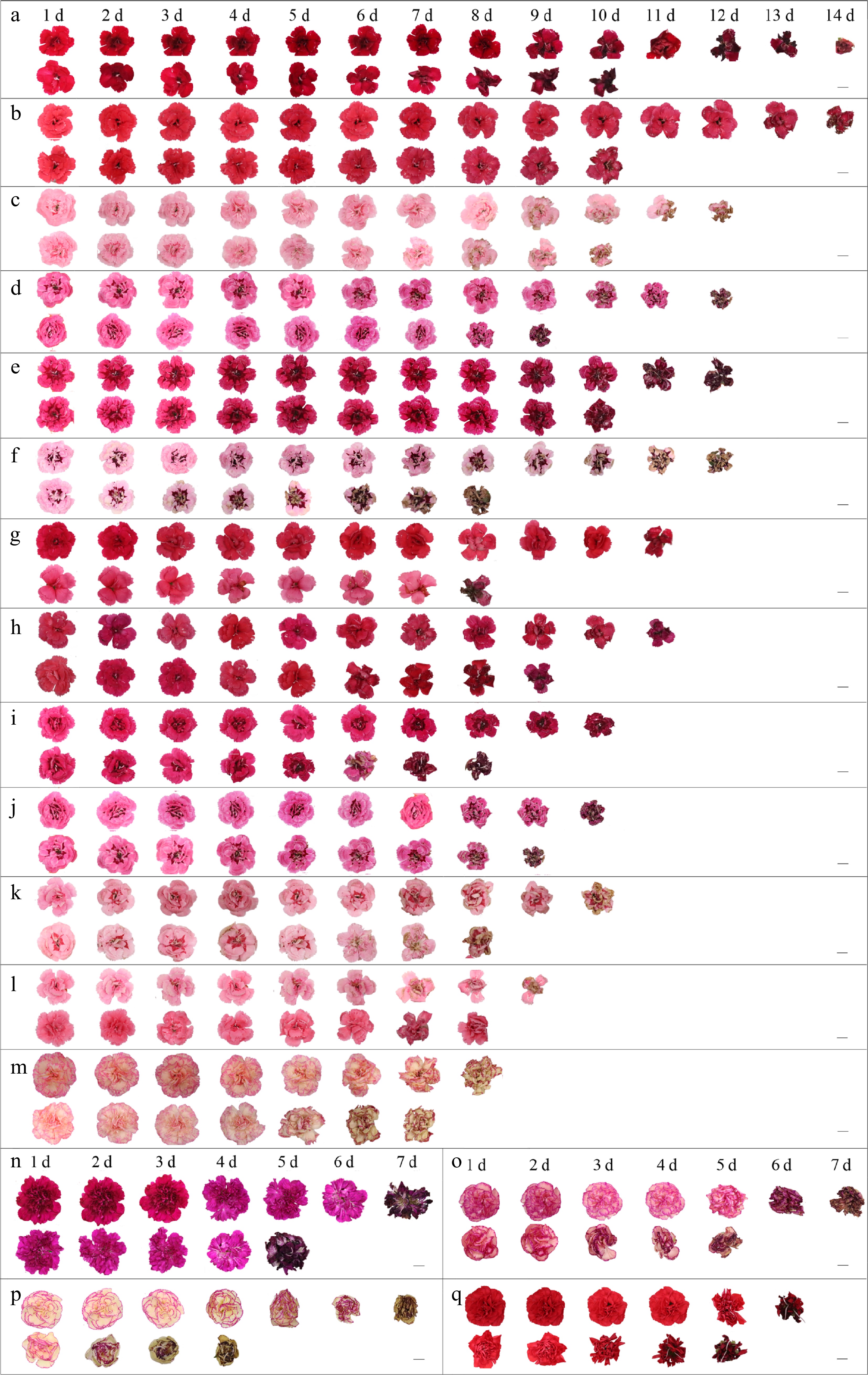
Figure 1.
Representative petal views of 17 potted carnation varieties during bottling. For each variety, the first row is a view of the petals in normal air and the second row is a view of the petals after treatment with 10 μL·L−1 exogenous ethylene for 4 h. (a) 'Cherry'. (b) 'Salmon'. (c) 'Pink Star'. (d) 'Oscar'. (e) 'Purple Wings'. (f) 'Purple Star'. (g) ‘Red’. (h) ‘Magenta’. (i) 'Violet and Pink'. (j) 'Pink and Purple'. (k) 'Red Star'. (l) 'Pink'. (m) 'Bondi Beach'. (n) 'Blinky Beach'. (o) 'Shark Bay'. (p) 'Grace Bay'. (q) 'Grane Beach'. Scale bar = 1 cm.
Table 2. Variation in vase life and flower longevity of 17 potted carnation cultivars.
Cultivar Vase life (d) In planta flower longevity (d)3 Air1 Ethylene2 'Cherry' 14.20 ± 2.44 a 9.20 ± 3.55** ab 21.92 ± 3.47 ab 'Salmon' 13.92 ± 2.66 a 10.00 ± 2.44** a 19.70 ± 3.30 abc 'Pink Star' 12.83 ± 3.04 ab 10.67 ± 2.74* a 19.09 ± 3.85 abc 'Oscar' 12.78 ± 2.95 ab 8.89 ± 2.97** ab 21.60 ± 3.80 ab 'Purple Wings' 12.55 ± 2.91 ab 10.64 ± 3.04* a 23.78 ± 3.70 a 'Purple Star' 12.13 ± 2.10 abc 8.40 ± 3.06** ab 20.77 ± 4.04 ab 'Red' 11.60 ± 2.41 abc 9.33 ± 1.52* ab 22.00 ± 3.36 ab 'Magenta' 11.17 ± 2.59 abc 9.50 ± 1.08 ab 15.20 ± 3.39 cde 'Violet and Pink' 10.79 ± 3.06 bc 8.93 ± 2.95** ab 17.58 ± 3.53 bcd 'Pink and Purple' 10.31 ± 1.38 bcd 9.46 ± 3.59 ab 18.73 ± 4.23 abc 'Red Star' 10.00 ± 1.14 bcd 8.25 ± 1.58 ab 18.17 ± 3.76 bcd 'Pink' 9.17 ± 3.61 cde 8.00 ± 2.04 ab 14.15 ± 3.82 def 'Bondi Beach' 8.33 ± 1.21 def 7.50 ± 1.76 bc 11.71 ± 2.36 ef 'Blinky Beach' 7.33 ± 0.57 ef 5.67 ± 0.57 cd 12.00 ± 0.84 ef 'Shark Bay' 7.11 ± 0.93 ef 4.78 ± 0.97 d 11.25 ± 3.45 ef 'Grace Bay' 6.86 ± 0.69 f 4.14 ± 0.38 d 10.18 ± 3.02 f 'Grane Beach' 6.20 ± 0.84 f 5.60 ± 0.55 cd 10.30 ± 2.40 f (1) Air: vase life of cut flowers was incubated in chambers with normal air at 25 °C, 50% RH, and natural light. (2) Ethylene: vase life of cut flowers was incubated in chambers with 10 μL·L-1 ethylene for 4 h at 25 °C, 50% RH, and natural light. (3) In planta flower longevity: Number of days from full bloom (outermost petals at a 90° angle to the flower stem) to complete wilting and crumpling of complete potted carnations (not cut flowers) grown in a greenhouse at 20 °C, 50% RH, and natural light. Data represent the mean ± SE of three replications (three subsamples per replication). Different lowercase letters indicate significant differences between cultivars using the one-way ANOVA test in SPSS software at P < 0.05. (*, **) represent a significant difference between air and ethylene treatment, as determined by Student's t-test at P < 0.05 and P < 0.01. Ethylene treatment reduced the vase life of 17 potted carnation varieties, and different varieties showed significant differences in their response to ethylene (Fig. 1; Table 2). Among them, the vase life of 'Grace Bay' and 'Cherry' was shortened by 39.7% and 36.8%, respectively, compared with the untreated flowers, indicating that they were highly sensitive to ethylene (Fig. 2). In contrast, three of the varieties, 'Pink and Purple', 'Bondi Beach', and 'Grane Beach', had less than 10% shorter vase life and were very insensitive to ethylene. The rest of the varieties showed a 12.7%−32.7% reduction in vase life (Fig. 2).
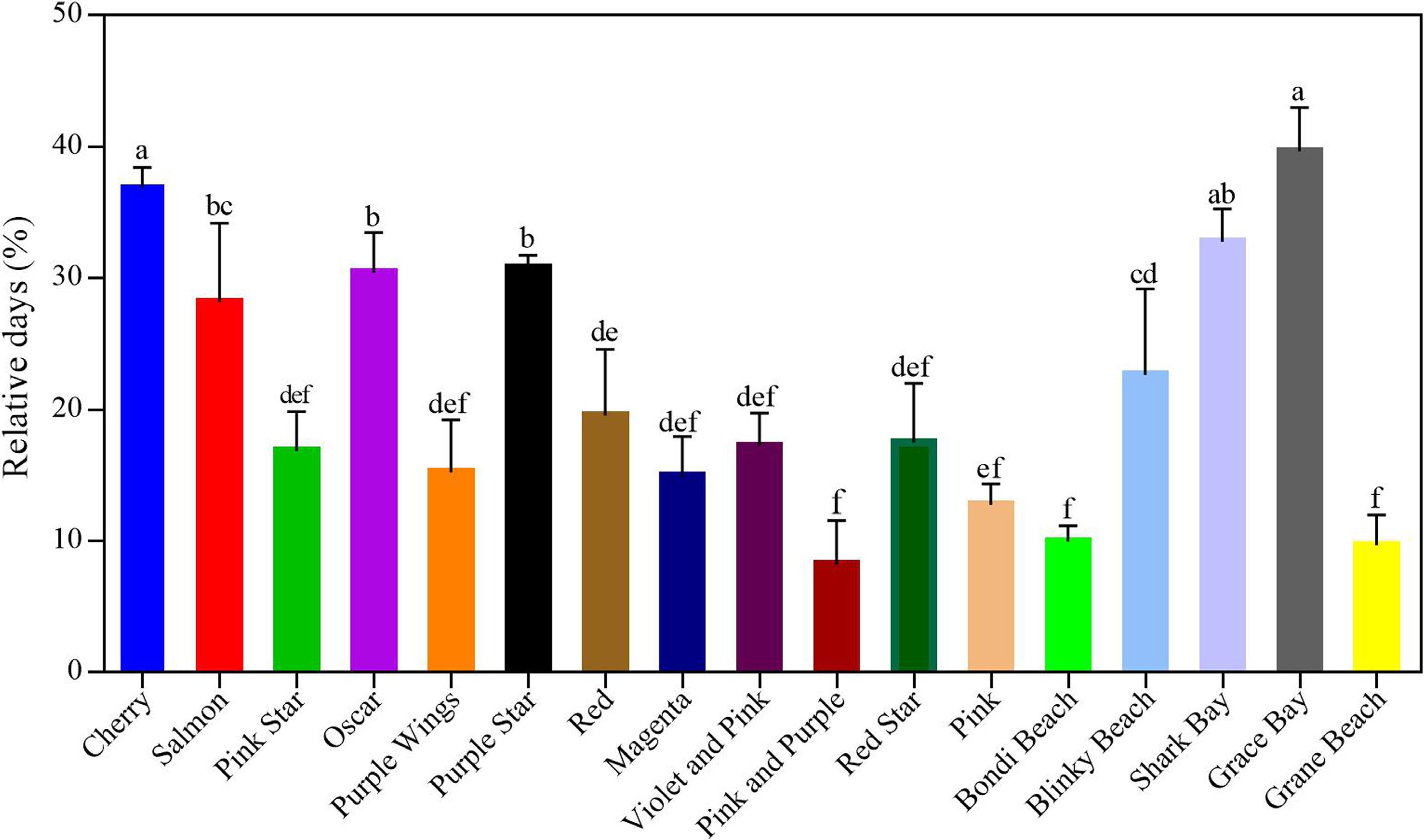
Figure 2.
Relative days of reduced vase life of 17 potted carnation varieties after 4 h of 10 μL·L−1 ethylene treatment. The error bars are represented by the standard deviations of three replicates. Different lowercase letters indicate significant differences between cultivars using one-way ANOVA test in SPSS software at P < 0.05.
Changes in the water relations of flowers after ethylene treatment
-
The number of days to maintain positive water balance was reduced in different varieties after ethylene treatment in different magnitudes, the range of variation was 0−2 d (Fig. 3a). After ethylene treatment, the number of days of positive water balance was reduced by 60% and 50% for the more ethylene-sensitive varieties 'Cherry' and 'Grace Bay', respectively, while the three varieties less sensitive to ethylene 'Pink and Purple', 'Bondi Beach', and 'Grane Beach' had 4.3%, 18.5%, and 18.2% lower positive water balance days, respectively (Fig. 3a).
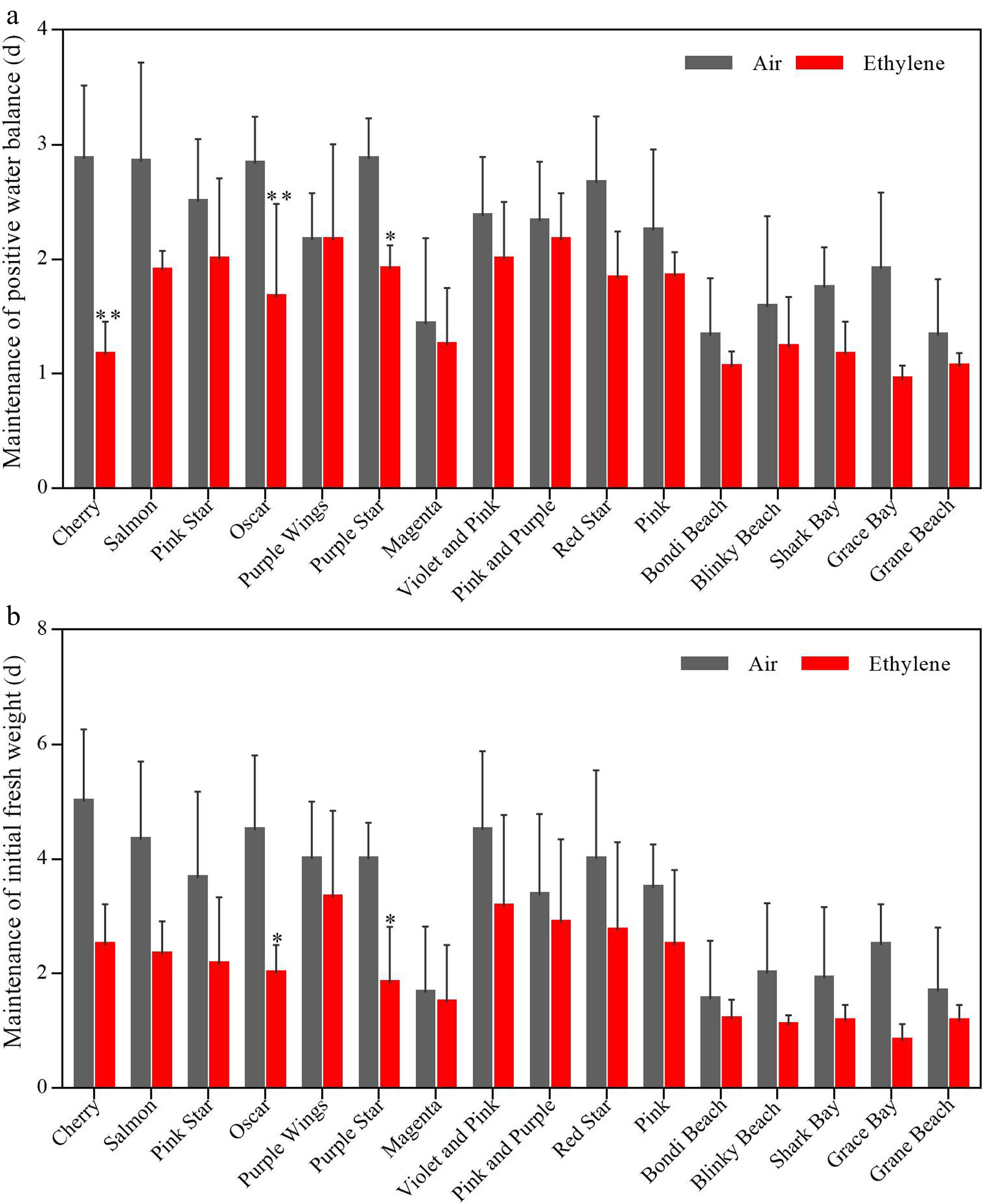
Figure 3.
(a) Number of days in which flowers retained their positive water balance and (b) initial fresh weight for 16 potted carnation cultivars after ethylene treatment. Air: cut flowers were incubated in chambers with normal air at 25 °C, 50% RH, and natural light. Ethylene: cut flowers were incubated in chambers with 10 μL·L−1 ethylene for 4 h at 25 °C, 50% RH, and natural light. The error bars are represented by standard deviations of three replicates (three subsamples per replication). (*, **) represent a significant difference between air and ethylene treatment, as determined by Student’s t-test at P < 0.05 and P < 0.01.
The number of days to maintain the initial fresh weight of flowers varied greatly from 1 to 5 d among varieties, and the response pattern to ethylene was similar to that of positive water balance (Fig. 3b). After ethylene treatment, the number of days to maintain the initial fresh weight of the more ethylene-sensitive varieties 'Cherry' and 'Grace Bay' decreased by 50% and 66.8%, respectively, while the less ethylene-sensitive varieties 'Pink and Purple', 'Bondi Beach', and 'Grane Beach' decreased by 14.3%, 23%, and 30.7%, respectively (Fig. 3b).
These results suggest that there is a positive correlation between the water relations of potted carnation flowers and the sensitivity of flowers to ethylene.
Changes in endogenous ethylene release from flowers after ethylene treatment
-
Ethylene treatment increased ethylene release from different potted carnation varieties. The pattern of endogenous ethylene production of all varieties could be generally classified into two categories (Fig. 4).
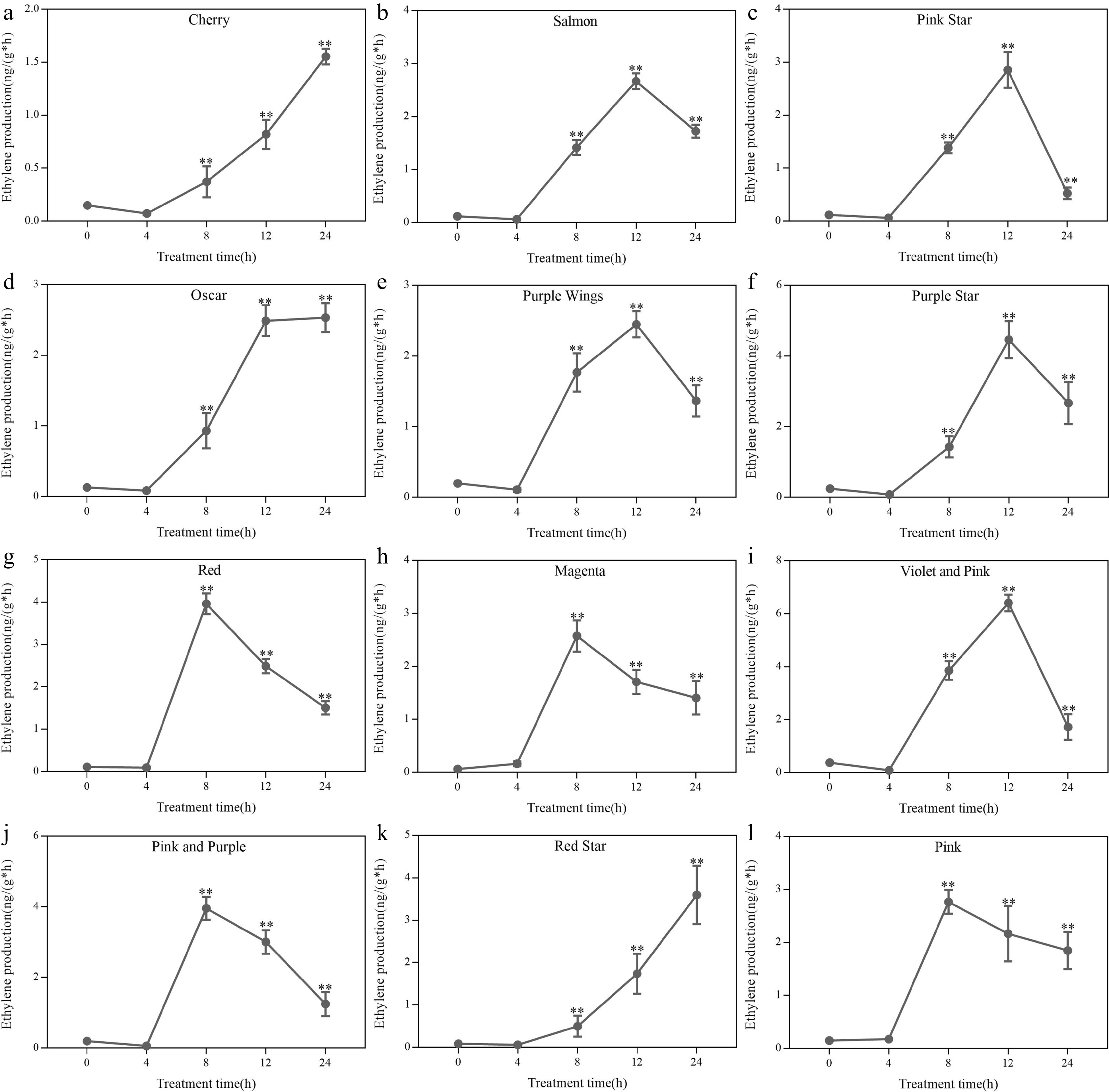
Figure 4.
Effect of ethylene treatment on petal ethylene production in different cultivars of potted carnation. (a) 'Cherry'. (b) 'Salmon'. (c) 'Pink Star'. (d) 'Oscar'. (e) 'Purple Wings'. (f) 'Purple Star'. (g) 'Red. (h) 'Magenta'. (i) 'Violet and Pink'. (j) 'Pink and Purple'. (k) 'Red Star'. (l) 'Pink'. The X-axis indicates cut flowers were incubated in chambers with normal air (0 h) or with 10 μL·L−1 ethylene for 4, 8, 12, and 24 h at 25 °C, 50% RH, and natural light. The Y-axis indicates the endogenous ethylene release from the petals. The error bars are represented by the standard deviations of three replicates. (*, **) represent a significant difference between different treatments, as determined by Student's t-test at P < 0.05 and P < 0.01.
Nine of the varieties showed obvious peaks, consistent with the changing pattern of ethylene climacteric cut flowers, while the remaining varieties showed a continuous upward trend of ethylene release without obvious peak, being the increasing later stage, including 'Cherry', 'Oscar', and 'Red Star' (Fig. 4a, d & k). There was a difference in the time of peak appearance among the climacteric type varieties. The four varieties with longer vase life ('Salmon', 'Pink Star', 'Purple Wings', and 'Purple Star') peaked at 12 h (Fig. 4b, c, e & f), while the short-lived variety 'Pink' peaked at 8 h (Fig. 4l). It is assumed that the time of peak ethylene production correlates with the vase life.
Exogenous ethylene induced carnations to self-catalyze ethylene and endogenous ethylene synthesis was increased. After ethylene treatment, the ethylene release of the longer-lived varieties 'Cherry', 'Salmon', and 'Oscar' was 1.50, 2.57, and 2.50 ng·g−1·h−1, respectively (Fig. 4a, b, & d). In addition, the ethylene release of the shorter-lived varieties 'Violet and Pink' and 'Pink' were 6.86 and 6.4 ng·g−1·h−1 respectively, which were 2.5-4.5 times higher than those of the short-lived varieties (Fig. 4i & l). These results suggest that the long vase life of potted carnation flowers may be caused by low ethylene production.
Changes in the expression levels of ethylene biosynthesis genes after ethylene treatment
-
The transcript levels of DcACS1 and DcACO1 were generally increased in all potted carnation varieties after ethylene treatment.
Four of the five varieties (< 8 d) with shorter vase life ('Grace Bay', 'Shark Bay', 'Bondi Beach', and 'Grane Beach') had the highest transcript levels of DcACS1 after 12 h of ethylene treatment, and five of the six varieties with longer vase life (> 10 d) ('Oscar', 'Purple Star', 'Cherry', 'Violet and Pink', and 'Pink and Purple') had the highest transcript levels of DcACS1 after 24 h of ethylene treatment (Fig. 5a). Based on the above results, it is hypothesized that the use of shorter exogenous ethylene treatment can up-regulate the transcript level of DcACS1 in shorter vase life varieties (Fig. 5a). The increase in DcACS1 transcript levels varied among varieties.
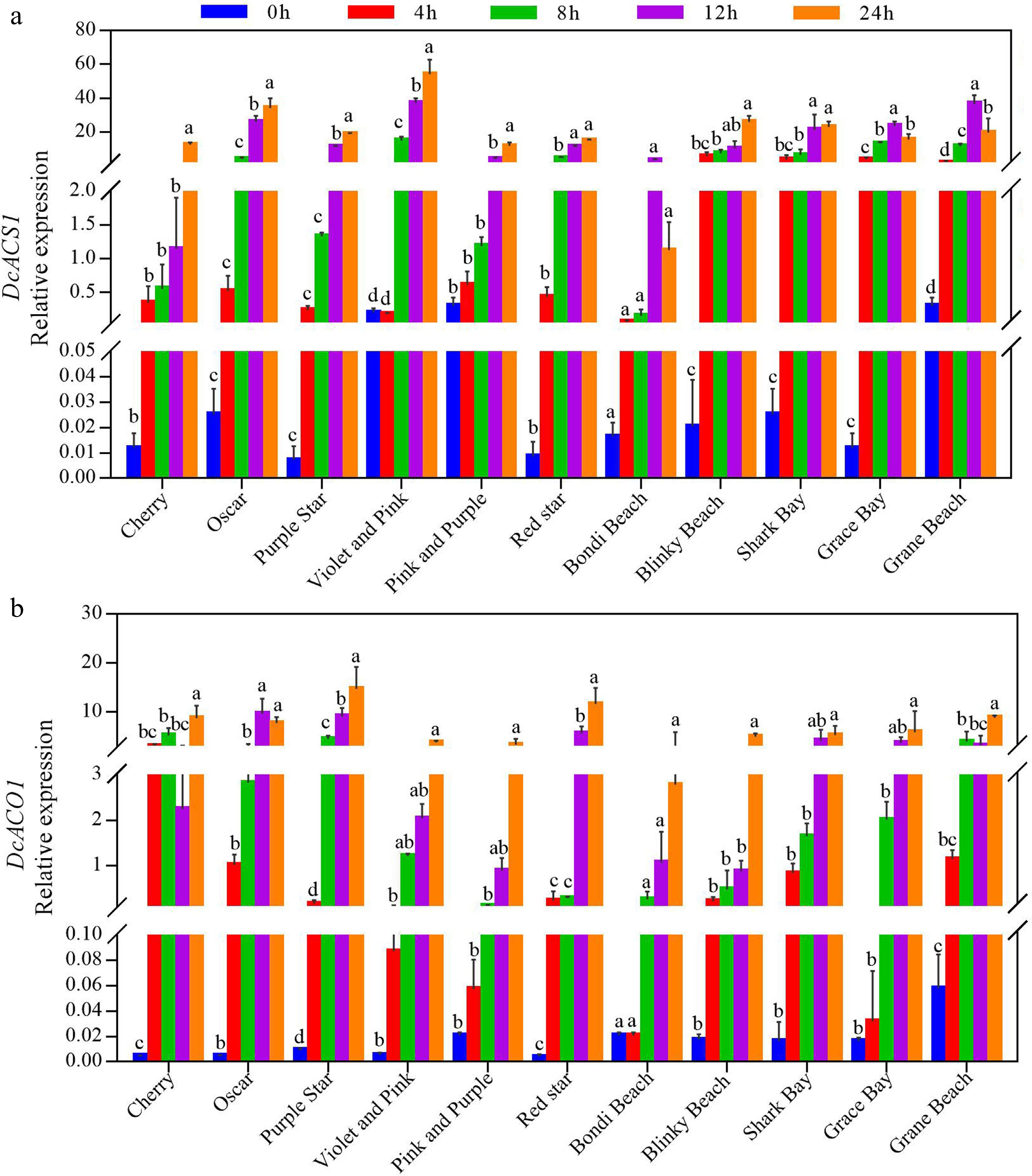
Figure 5.
Effect of ethylene treatment on the relative expression of (a) DcACS1 and (b) DcACO1 in petals of different cultivars of potted carnation. The relative expression level was calculated using the 2−ΔΔCᴛ method. Cut flowers were incubated in chambers with normal air (0 h) or with 10 μL·L−1 ethylene for 4, 8, 12, and 24 h at 25 °C, 50% RH, and natural light. The error bars are represented by the standard deviations of three replicates. Different lowercase letters indicate significant differences between treatments, using the one-way ANOVA test in SPSS software at P < 0.05.
After ethylene treatment, the DcACS1 transcript levels of highly sensitive varieties ('Cherry' and 'Grace Bay') increased 1049 times and 1965 times, respectively, compared with untreated varieties (Fig. 5a). The DcACS1 transcript levels of less sensitive varieties ('Pink and Purple', 'Bondi Beach', and 'Grane Beach') increased only 38 times, 48 times, and 121 times (Fig. 5a).
The expression pattern of DcACO1 in different potted carnation varieties was similar to that of DcACS1 (Fig. 5b). After ethylene treatment, the DcACO1 transcript levels of highly ethylene-sensitive varieties ('Cherry' and 'Grace Bay') increased 1936 times and 579 times compared with the control, while the less ethylene-sensitive varieties ('Pink and Purple', 'Bondi Beach', and 'Grane Beach') were only 108 times, 134 times, and 153 times higher than the control (Fig. 5b).
The above results indicate that the transcript levels of DcACS1 and DcACO1 of highly sensitive varieties increased to a greater extent after ethylene treatment.
Differences in the content of volatile substances of different species and changes in major volatile substances after ethylene treatment
-
Comparing the differences in volatile substance content of different potted carnation varieties, it was found that the relative content of alkanes was significantly highest compared to other compounds (Fig. 6).
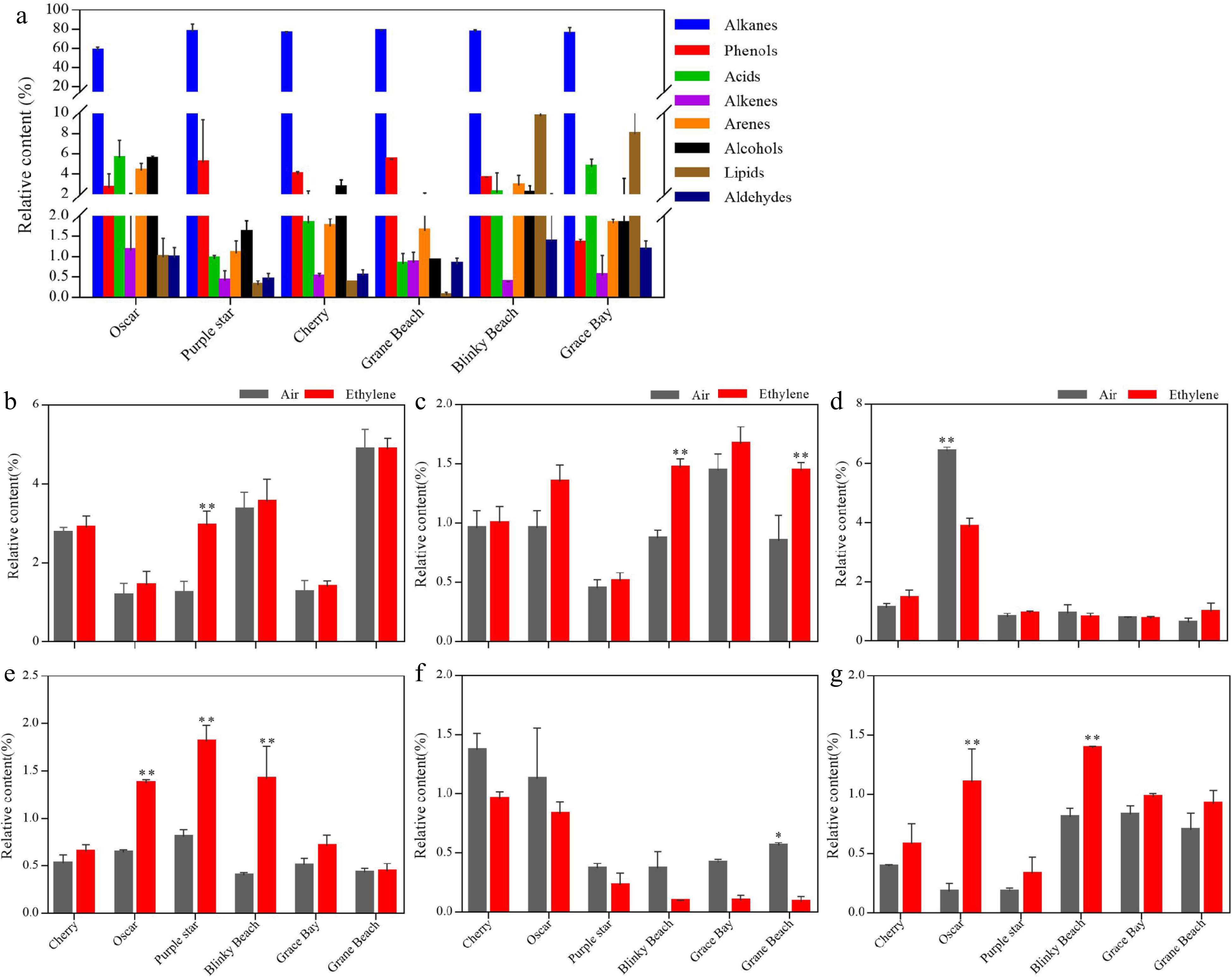
Figure 6.
Differences in the content of volatile substances of different species and changes in major volatile substances after ethylene treatment. (a) Relative content of volatile component in petals of different cultivars of potted carnation. Flowers were incubated in chambers with normal air at 20 °C. Effect of ethylene treatment on relative content of volatile compounds in petals of different cultivars of potted carnation. Air: cut flowers were incubated in chambers with normal air at 25 °C, 50% RH, and natural light. Ethylene: cut flowers were incubated in chambers with 10 μL·L−1 ethylene for 4 h at 25 °C, 50% RH, and natural light. (b) 2,4-Di-tert-butylphenol. (c) 2,4-Dimethylbenzyl chloride. (d) Benzoic acid, 2-amino-4-methyl. (e) Phenol, 2-methoxy-3-(2-propenyl). (f) Behenic alcohol. (h) Benzaldehyde, 2,5-dimethyl. Flowers were incubated in chambers with normal air (Air) or with 10 μL·L−1 ethylene (Ethylene) for 4 h at 20 °C. The error bars are represented by the standard deviations of three replicates. (*, **) represent a significant difference between air and ethylene treatment, as determined by Student’s t-test at P < 0.05 and P < 0.01.
Among the different varieties, 'Grane Beach' had the highest relative content of alkanes at 78.2% and 'Oscar' had the lowest relative content at 57.5%. In addition to alkanes, 'Purple Star', 'Cherry', and 'Grane Beach' had the highest phenolic compound content with 5.1%, 5.0%, and 5.4%, respectively, and 'Blinky Beach', and 'Grace Bay' had the highest content of lipids with 9.7% and 8.0%, respectively. Aldehydes and alkenes were extremely low in all varieties, none exceeding 1.4% (Fig. 6a).
The relative content of 2,4-Di-tert-butylphenol in 'Purple Star' increased 1.4 times from 1.25% to 2.95% after ethylene treatment, while the remaining varieties did not change significantly (Fig. 6b). The relative content of 2,4-Dimethylbenzyl chloride in both 'Grane Beach' and 'Blinky Beach' increased about 0.7 times, while the rest of the varieties increased only slightly (Fig. 6c). The relative content of benzoic acid, 2-amino-4-methyl in 'Oscar' was significantly higher than that of the other varieties, and ethylene treatment led to a decrease from 6.40% to 3.86%, while the relative content of the other varieties was almost unchanged (Fig. 6d).
In a previous study, eugenol (Phenol, 2-methoxy-3-(2-propenyl)) was the main volatile substance of carnation[30]. In this study, ethylene significantly increased the relative content of eugenol in the petals of 'Oscar', 'Purple Star', and 'Blinky Beach' by 1.1, 1.2, and 2.6 times, respectively, compared with the control (Fig. 6e). Meanwhile, ethylene treatment reduced the relative content of behenic alcohol in the petals of the test varieties, with a significant decrease of 0.47% in the relative content of behenic alcohol in 'Grane Beach' (Fig. 6f). In contrast to behenic alcohol, ethylene treatment increased the relative content of benzaldehyde, 2,5-dimethyl in the test varieties, with 'Grane Beach' and 'Blinky Beach' significantly increasing the relative content of benzaldehyde, 2,5-dimethyl by 5.1 and 0.7 times (Fig. 6g).
-
Flower longevity is usually considered as one of the important indicators in the evaluation of the ornamental quality of flowers, and longer-lived varieties have higher ornamental value. Genetic factors are the main determinants of flower longevity and interact with environmental conditions to determine flower longevity[36]. In this study, we considered the vase life under standard postharvest environmental conditions as the genetically determined flower inherent longevity of potted carnations. Although under the same environmental conditions, there was a 234% difference between the shortest and longest floret longevity among 17 potted carnation varieties, while there was a 229% difference between the shortest and longest vase life, indicating a large genetic difference among the varieties selected for this study. This result is consistent with previous studies in which carnation varieties 'Miracle Red' and 'Miracle Symphony' had the longest vase life of 22.4 and 24.3 d, respectively, while another variety 'White Sim' had a bottle life of 7.4 d. These results indicate that there is a large difference in the floret longevity and vase life of single flowers between varieties[37].
All 17 potted carnation varieties showed varying degrees of reduction in vase life after 4 h of exogenous ethylene treatment. Ethylene significantly reduced the vase life of two of the varieties ('Cherry' and 'Grace Bay'), both of which were highly sensitive to ethylene, in addition to ethylene slightly reducing the vase life of 12 varieties, while the remaining three varieties ('Pink and Purple', 'Bondi Beach' and 'Grane Beach') showed almost no change in vase life, indicating their low sensitivity to ethylene. Although 'Purple Wings', 'Pink and Purple' and 'Pink' varieties had a significantly longer vase life than varieties 'Shark Bay' and 'Grace Bay', they were less sensitive to exogenous ethylene than 'Shark Bay' and 'Grace Bay', indicating that there was no significant correlation between vase life of potted carnations and sensitivity to ethylene.
Water channel proteins are thought to play an important role in regulating water transport in plants, increasing cell membrane water permeability, and facilitating water transport. It has been hypothesized that ethylene may inhibit petal water channel protein activity at the transcriptional level, blocking water transport, and thus significantly inhibiting their petal cell elongation, reducing petal water content, and accelerating the senescence process[38]. In this study, the number of days that flower of different potted carnation varieties maintained positive water balance and initial fresh weight of flowering branches decreased to different degrees after ethylene treatment, and the decrease was greater in the varieties that were more sensitive to ethylene, indicating a correlation between the water relationship of potted carnation flowers and ethylene sensitivity.
After ethylene treatment, three potted carnation varieties ('Oscar', 'Red Star' and 'Cherry') were ethylene increasing later stage, and the rest was ethylene climacteric type, with the peak of a leap at 8 or 12 h of ethylene treatment. The ethylene release of shorter vase life varieties ('Violet Pink' and 'Pink') was higher than that of the longer vase life varieties ('Salmon', 'Oscar', and 'Cherry') by a factor of 2.5−4.5, that is consistent with the results of previous studies. In this study, compared to the short-lived variety 'White Sim', the long-lived carnation varieties 'Sandrosa', 'Miracle Rouge', and 'Miracle Symphony' had low ethylene release, and these results suggest that the longer vase life of potted carnations is caused by lower ethylene release[39].
Ethylene-induced flower senescence and abscission are associated with transcriptional regulation of ethylene biosynthesis genes, and increased expression of these genes is positively correlated with ethylene biosynthesis[40]. In this study, the transcript levels of DcACS1 and DcACO1 in the petals of the highly ethylene-sensitive variety 'Cherry' increased 1,049 times and 1,936 times, respectively, compared with the control after ethylene treatment. In contrast, the transcript levels of DcACS1 and DcACO1 in the petals of the less ethylene-sensitive variety 'Bondi Beach' were only increased 45 times and 290 times, respectively. This result indicates that the transcript levels of DcACS1 and DcACO1 are closely related to the degree of ethylene sensitivity and insensitivity, and the transcript levels of DcACS1 and DcACO1 in varieties highly sensitive to ethylene increased more after ethylene treatment. Variation in ethylene sensitivity may be related to the ability to perceive or respond to ethylene, which is regulated through changes in ethylene signaling during flower development[12,41]. It has been proposed that CTRs immediately downstream of ethylene receptors play a negative regulatory role in ethylene signaling and are negatively associated with ACS1 and ACO1 transcripts through the protein kinase module mediating mitogen activation (MAPK)[42,43].
The synthesis and release of plant volatiles are susceptible to regulation by various environmental factors as well as phytohormones. In this study, the main volatile components closely related to the floral fragrance were tentatively identified based on the sensory appearance that carnation aroma is the strongest during the blooming period. The volatilization of 2,4-di-tert-butylphenol, 1-(chloromethyl)-2,4-xylene, eugenol, and benzaldehyde, 2,5-dimethyl in petals increased after ethylene treatment, with 'Oscar', 'Purple Star', and 'Blinky Beach' showed a significant increase in the relative content of eugenol by 1.1, 1.2, and 2.6 times. In addition, ethylene treatment reduced the volatility of behenic alcohol, among which the relative content of behenic alcohol in 'Grane Beach' was significantly decreased by 0.47%. These results indicate that the effect of ethylene on volatile substances in potted carnations was not significant, that is consistent with previous findings that exogenous ethylene treatment does not significantly regulate the synthesis and release of floral substances in carnations[44].
-
It has generally been thought that cut carnations are highly susceptible to postharvest ethylene damage. Our results show that most potted carnations are not extremely ethylene-sensitive, and that the factors determining flower longevity of potted carnations are mainly water stress and endogenous ethylene release, while exogenous ethylene does not have a significant effect on potted carnation volatile substances. Secondly, there are large differences between the single flowering and bottle life of different potted carnation varieties, so insight into potted carnations will help us to improve measures to extend flower life and cultivate new potted carnation varieties that are not ethylene-sensitive.
This work was supported by the Fundamental Research Funds for the Central Universities (Program No. 2662019PY049), the Thousand Youth Talents Plan Project and the Start-up Funding from Huazhong Agricultural University to FZ.
-
Fan Zhang is the Editorial Board member of JournalOrnamental Plant Research. He is blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and his research groups.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang M, Ni C, Wang R, Zhong L, Cheng Y, et al. 2023. Variation in longevity of cut and in planta flowers of potted carnation varieties affected by their relationship with ethylene and water. Ornamental Plant Research 3:2 doi: 10.48130/OPR-2023-0002
Variation in longevity of cut and in planta flowers of potted carnation varieties affected by their relationship with ethylene and water
- Received: 17 November 2022
- Accepted: 04 January 2023
- Published online: 17 January 2023
Abstract: Carnation (Dianthus caryophyllus L.) is a typical ethylene-sensitive cut flower, but a few differences in ethylene sensitivity have been reported for different potted carnation species. In this study, we investigated the relationship between vase life, ethylene sensitivity, ethylene biosynthesis gene expression, and flower volatile substance content of 17 different potted carnation varieties. It was found that under the same post-harvest environmental conditions, the vase life of different varieties ranged from 6.2−14.2 d. Among the 17 varieties, 'Cherry' and 'Grace Bay' were highly sensitive to ethylene, and qRT-PCR analysis showed that their ethylene biosynthesis genes DcACS1 and DcACO1 expression increased the most, while 'Pink and Purple', 'Bondi Beach', and 'Grane Beach' showed the opposite pattern. In addition, the lower ethylene release was important in leading to longer vase life of potted carnations, and that ethylene release from shorter vase life varieties was 2.5−4.5 times greater than that of longer vase life varieties. Varieties that are more sensitive to ethylene are more likely to have a shorter vase life due to early disruption of water relations, which is mainly the result of reduced stem hydraulic conductivity and transpiration water loss. Analysis of volatile substances showed that ethylene had no significant effect on the release of volatile substances from potted carnations. Therefore, a better understanding of petal senescence in potted carnations will help us to improve measures to extend flower longevity according to the ethylene sensitivity of different varieties.
-
Key words:
- Dianthus caryophyllus L. /
- Carnation /
- Petal senescence /
- Ethylene /
- Flower longevity /
- Vase life /
- Water balance /
- Ethylene biosynthesis gene /
- Volatile substance /
- Postharvest












