-
The worldwide public healthcare is challenged by various bacterial pathogens that cause infectious diseases leading to a large mumber of deaths[1]. The emergence and re-emergence of infectious diseases pose a formidable threat to public health and welfare. Because of this, researchers and scientists are focused on the discovery and development of new and effective drugs[2], and plants have been a valuable source of natural products with antibacterial properties[3]. Bioactive molecules derived from plants inhibit the growth of pathogenic bacteria[4], thus were developed into most medicines. However, some bacteria are resistant to antibiotics, thus alternative sources of pharmaceutically important secondary metabolites are being explored.
Lichens which are symbiotic organisms composed of fungi and algae and/or cyanobacteria in an intimate biological union[5] has been one of the many alternative sources of biologically active metabolites. To date, 17,000 species worldwide[6] and 789 species in the Philippines[7] have been described and some are being used as traditional medicine despite the emergence of different modern medicines. Lichens have been used in the treatment of diverse diseases like arthritis, alopecia, constipation, kidney diseases, leprosy, pharyngitis, rabies, infection, worm infestation[8] and other gastro-intestinal parasites in humans and livestock in Nordic countries such as Scandinavia[9]. Lichen secondary metabolites exert a wide variety of biological actions including antibiotic, antimycobacterial, antiviral, anti-inflammatory, analgesic, antipyretic, antiproliferative and cytotoxic effects[8].
One of the most common growth forms of lichen is the foliose lichen. Foliose lichens are characterized by flattened leafy thalli, and an upper and lower cortex. It has a highly structured growth form with a distinct lower cortex attached to the substratum usually by thread-like rhizinae[10]. Leptogium is a well-known lichen genus under the family Collemataceae and is distributed throughout the tropical regions of the world[11]. It is characterized by a foliose to subsquamoulose thallus, which can be gelatinous, blue-grey to brown or blackish, adnate to loosely adnate, with isidia or lobule. There was initially no secondary metabolites reported in most of its species including L. phyllocarpum[12] and L. burnetiae[12,13]. However, a recent report noted two unknown chemical constituents in L. azurem and the presence of atranorin and norstictic acid in L. burnetiae, L. burnetiae var. hirsutum, and L. cyanescens[11]. Thus, to determine if this report is true for other species of Leptogium, both species of Leptogium cochleatum and Leptogium moluccanum collected in Mt. Isarog, Camarines Sur were characterized, and the crude extracts were analyzed and tested for antibacterial activity against bacterial pathogens.
-
Standing 2,000 meters above sea level, Mount Isarog is an inactive stratovolcano located in Luzon Island, Philippines. It has the coordinates 13°39'33'' N 123°22'24'' E, specifically located in the province of Camarines Sur. A dry period occurs from the month of January to May and the wet season starts in August but rainfall varies. In the lowlands it has a temperature ranging from 20.8 to 33.6 °C. The temperature decreases by 0.6 °C for every 100 m elavation.
Collection of lichen samples
-
Foliose lichens were collected along the trails in Mount Isarog, Camarines Sur with purposive sampling technique which is a non-probability sampling technique. The lichens were obtained by detaching them from the substrate using a knife and scalpel. The collected samples were manually cleaned of any debris using syringe needles and were initially stored in paper bags. The samples were air-dried for 24 h and stored in a moist, cool, dry place until further use[14]. Voucher specimens were stored at the UST Central Laboratories, University of Santo Tomas in Manila, Philippines.
Characterization and identification of foliose lichen samples
-
The lichen samples were characterized through observation of their morphological features and thalline spot tests K (potassium hydroxide), C (sodium hypochlorite), and IKI (iodine potassium iodide). Razor blades were used to cut the thallus open, followed by thalline spot test. A 10% aqueous solution of potassium hydroxide (KOH) was placed dropwise on the thallus for the K test. The same procedure was carried out for C test and I test, with a drop of 5.25% solution of sodium hypochlorite (NaOCl) and a drop of iodine potassium iodide (IKI) respectively. Resulting color change of the thallus, along with morphological characteristics, was the basis for identification in reference to the previously published dichotomous keys[15−18].
Extraction and thin-layer chromotography of the lichen extracts
-
After air-drying, the samples were weighed, cut into small pieces, and crushed using a mortar and pestle. The samples were transferred to Erlenmeyer flasks and 100 mg of lichen thalli were soaked in 100 ml of the extracting solvents for 24 h, hexane being the first solvent used, followed by dichloromethane (DCM), then methanol. After 24 h, extracts were filtered and placed in pre-weighted vials, and the samples were soaked in the solvent again. The process was carried out every after 24 h until the extracts showed a clear and transparent appearance, and the solvent was evaporated using a rotary evaporator. The final amount of the crude lichen extracts of each species per solvent were then weighed[19].
Thin-layer chromatography (TLC) was used to identify the metabolites or compounds present within the foliose lichen crude extracts. The extracts were spotted on TLC plates, and depending on the extracting solvent used, were run on solvent systems (A) hexane-dichloromethane, (B) dichloromethane, and (C) methanol-dichloromethane. Spots were observed under a UV light at a wavelength of 365 nm[20]. Furthermore, the TLC plates were sprayed with the following spray reagents: potassium ferricyanide-ferric chloride, Dragendorff's reagent, Kedde reagent, Bornträger reagent, magnesium acetate in methanol, Van-Urk-Salkowski test, vanillin-sulfuric acid, and Naphthol-sulfuric acid. The visual appearance was used as the basis in identifying the compounds present[21].
Test bacteria and paper disk diffusion assay
-
The test bacteria used include Enterobacter agglomerans, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus. The bacteria were initially grown in Mueller-Hinton agar (MHA) slant tubes. After incubation, each 24-h old culture was inoculated in a Normal Saline Solution (NSS) and the turbidity was adjusted to that of 0.5 McFarland standard[20].
In the paper disk diffusion assay, the standardized test bacteria were swabbed on MHA plates with an agar depth of 4 mm, and the bacteria were allowed to dry for at least 20 min. Sterile filter paper discs, with a diameter of 6 mm and thickness of 390 µm, were impregnated with 40 µL of lichen crude extracts. The plates were sealed and incubated at 37 °C for 1–2 d with constant observation. After 24 h, the diameter of the growth in the inhibition zone was measured using the Vernier caliper and were classified based on the zone of inhibition (ZOI) standards, < 10 mm as inactive, 10–13 mm as partially active, 14–19 mm as active, > 19 mm as very active[21]. Chloramphenicol and tetracycline were used as positive controls.
Statistical analysis
-
Antibacterial activity was evaluated using the mean diameter of the inhibition zones formed. The data was subjected to one-way analysis of variance (ANOVA) to determine the errors and significance of ZOI.
-
Leptogium is a well-known lichen genus found in the tropics. Morphologically, the thallus of both species was black, but L. cochleatum thallus is approximately 90–150 µm thick, while L. moluccanum is 30–60 µm thick. The thalline of L. cochleatum is wrinkled with flat apothecial disc while L. moluccanum has smooth thalline with convex apothecial disc. The morphology of Leptogium species used in the study is presented in Fig. 1.

Figure 1.
The morpho-anatomical structures of Leptogium cochleatum (left) and Leptogium moluccanum (right).
The lichen Leptogium secondary metabolites
-
To test the presence of secondary metabolites in the lichen samples, the lichen thalli were crushed, extracted with different extracting solvents, and subjected to TLC analysis. The spray reagents used in TLC includes potassium ferricyanide-ferric chloride, Dragendorff’s reagent, Kedde reagent, Bornträger reagent, magnesium acetate in methanol, Van-Urk-Salkowski test, vanillin-sulfuric acid, and α-naphthol-sulfuric acid. The results suggest that the Leptogium species contain secondary metabolites including phenols, tannins, flavonoids, alkaloids, anthrones, triterpenes, and sterols. However, TLC analysis did not detect cardenolides, antraquinones, coumarins, indoles, and sugars in both L. cochleatum and L. moluccanum. It is also worth noting that similar results were obtained using polar solvents DCM and methanol, as opposed to hexane, a non-polar solvent (Table 1).
Table 1. Thin-layer chromatography analysis of L. cochleatum and L. moluccanum in solvent system.
Spray
reagentPositive
resultL. cochleatum L. moluccanum Compounds present Hexane DCM Methanol Hexane DCM Methanol A Blue + + + + + + Phenols, tannins, flavonoids B Brown-orange − + + + + + Alkaloids C Blur to red-violet − − − − − − Cardenolides D Orange, Yellow, Blue − − − − − +(2) Antraquinones (1), anthrones (2), coumarines (3) E Orange-violet − − − − − − Anthraquinones F Blue violet − − − − − − Indoles G Blue violet + + + + − − Triterpenes, sterols H Blue − − − − − − Sugars The spray reagents used include A, potassium ferricyanide-ferric chloride; B, Dragendorff’s reagent; C, Kedde reagent; D, Bornträger reagent; E, magnesium acetate in methanol; F, Van-Urk-Salkowski test; G, vanillin-sulfuric acid; and H, α-Naphthol-sulfuric acid. Antibacterial assay
-
For the antibacterial activity assay of the lichen extracts of L. cochleatum and L. moluccanum, hexane extract of L. moluccanum showed the highest ZOI of 17.14 mm against K. pneumoniae, although the methanol extract of L. cochleatum showed activity. Both hexane and dichloromethane extract of L. cochleatum showed the highest ZOI against E. agglomerans, while the dichloromethane extract of L. cochleatum showed a ZOI of 14.84 mm against E. coli. The mean ZOI of the lichen extracts against each bacterium are shown in Table 2. Overall, four extracts showed activity against three bacterial pathogens. Hexane extract of L. moluccanum against K. pneumoniae, hexane and DCM extracts of L. cochleatum against E. agglomerans, and DCM extract of L. cochleatum against E. coli have very active inhibitory potential against the said organisms. Based on the ZOI standards by Guevarra[21], five samples that showed activity against the pathogenic bacteria. One extract exhibited partial activity against K. pneumoniae, while the other showed higher activity against K. pneumoniae (1), E. coli (1) and E. agglomerans (2) (Fig. 2).
Table 2. Mean of the ZOI (mm) of the Leptogium extracts against the test pathogenic bacteria.
CP TC L. cochleatum L. moluccanum Haxane DCM Methanol Hexane DCM Methanol K. pneumoniae 32.06 24.46 7.96 8.94 10 17.14 7.72 6.89 S. aureus 35.7 30.6 7.68 6.79 8.83 8.18 6.34 6 P. aeruginosa 41.54 28.13 6.33 8.14 8.33 8 9.15 7.56 E. agglomerans 25.32 24.67 15.28 15.86 6.83 7.96 7.46 6 E. coli 25.62 20.4 6.72 14.84 6 8.81 6 6 CP, chloramphenicol; TC, tetracycline. 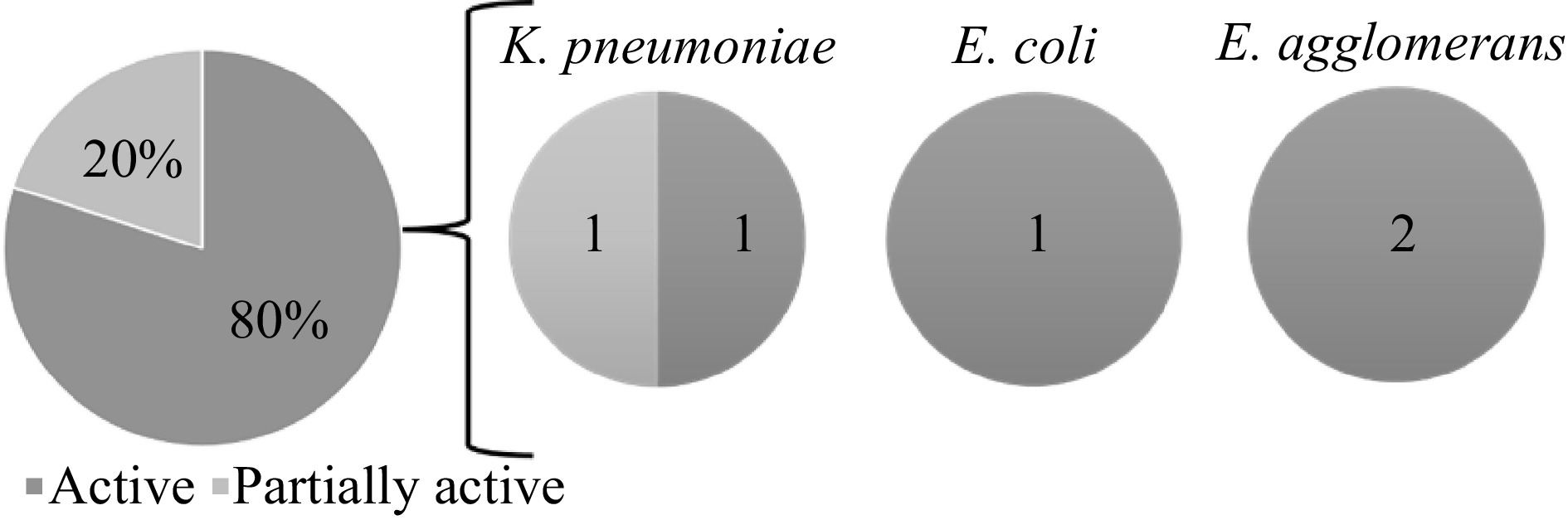
Figure 2.
Antibacterial activity of the lichen crude extracts against the test organisms based on the zone of inhibition (ZOI) standards by Guevara[21].
Statistical analysis using one-way analysis of variance (ANOVA) showed that there is significant difference on the potential of the different lichen extracts and positive controls, tetracycline, and chloramphenicol as antibiotic agents. In the post hoc test, the mean ZOI of hexane extract of L. cochleatum showed no significant difference with the mean ZOI of both the tetracycline and chloramphenicol with p-value of 0.275721 and 0.270317, respectively. This means that the hexane extract of L. cochleatum can also inhibit the growth of E. agglomerans. This suggests that the extracts can inhibit the growth of the bacterial pathogens and the lichen compounds detected in the extracts are responsible for the antibacterial activity of lichens.
-
Lichens are comprised of a fungal and an algal partner which are stable, ecologically obligate, self-supporting composite organisms[22]. Lichens are a diverse group of organisms known to be bio-indicators of pollution and produce numbers of pharmaceutically important secondary metabolites used in traditional medicines for centuries[23]. Thus, lichens and their products hold considerable interest as alternative treatments in various parts of the world[24]. Lichen acids or secondary metabolites are either in crystal forms or liquid that are scattered in different parts of the thallus and are exported outside of the fungal hyphae[25]. They are used in the regulation of primary metabolic pathways and help in the maintenance of balance in the environment, adapting to match the environmental needs. Secondary metabolites exhibit many biological activities such as anti-inflammatory, antifungal, anticancer and antimicrobial[26]. Despite these, some species of Endocarpon and Leptogium showed no presence of secondary metabolites in their thalli[12,13], yet the TLC results of the of L. cochleatum and L. moluccanum indicated that they contain phenols, tannins and flavonoids similar to other Leptogium species[11].
The antibacterial activity indicates the effectivity of the secondary metabolites with antibacterial activity as extracted using hexane and dichloromethane. A study by Kambar et al.[22] have not detected any secondary metabolite from Leptogium burnetiae using methanol as the extracting solvent. However, this study detected phenol, tannins and flavonoids in both Leptogium species, which acts as growth inhibitors (bacteriostasis) on microbial organism[27−29]. Alkaloids, triterpenes and sterols were also detected in L. cochleatum which are a large and structurally diverse group of compounds that have served a scaffold for important antibacterial drugs[23].
-
This study shows the potential of of L. cochleatum and L. moluccanum as sources of biologically active secondary metabolites with hexane and dichloromethane as effective extracting solvents. Further studies on the identification of these secondary metabolites should be performed.
-
The authors are thankful to Mr. Mark Gabriel Galinato, and Dr. Oliver Villaflores for their expertise and guidance in the completion of this study, and to the SRL laboratory technicians for their unwavering support and understanding especially in the use of the different laboratory apparatus needed in the experimentation.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Manlapaz APB, Mariano MI, Reyes ORM, Rodriguez LC, Paguirigan JAG. 2022. Antibacterial activity of Leptogium cochleatum and Leptogium moluccanum. Studies in Fungi 7:19 doi: 10.48130/SIF-2022-0019
Antibacterial activity of Leptogium cochleatum and Leptogium moluccanum
- Received: 31 August 2022
- Accepted: 01 December 2022
- Published online: 23 December 2022
Abstract: Currently there is an increase in the emergence and re-emergence of bacterial infections, and lichens are potential sources of pharmaceutically important secondary metabolites to halt this problem. Lichens are unique plant-like organisms which are relatively less explored in the Philippines. Here, lichen extracts of Leptogium cochleatum and Leptogium moluccanum were tested against Escherichia coli, Enterobacter agglomerans, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus using paper disk diffusion assay. Hexane extract of L. moluccanum led to a 17.14 mm zone of inhibition (ZOI) against K. pneumoniae, while hexane extract of L. cochleatum led to a 15.28 mm ZOI against E. agglomerans. Dichloromethane extracts of L. cochleatum exhibited a ZOI of 15.86 mm against E. agglomerans and 14.84 mm ZOI against E. coli. The bioactivities exhibited by the lichen extracts may be due to the secondary metabolites detected using thin layer chromatography. Indeed, lichen-derived bioactive compounds hold great promise for pharmaceutical applications for the benefit of human life.












