-
As an invasive sap-sucking hemipteran initially found on crapemyrtle (Lagerstroemia sp.) in Richardson (Texas, USA), crapemyrtle bark scale (CMBS; Acanthococcus lagerstroemiae) has spread across 16 US states[1−5]. Sooty mold accumulation resulting from feeding and honeydew secretion of CMBS leads to reductions in growth and blooming of host plants[6] and even branch die-back[7], which negatively impacts the landscape use of crapemyrtles in the US. Besides on its primary host, increasing observations of CMBS infestation were reported on other economically important plants[8−11] and native species[7,9,12], indicating that the CMBS is a polyphagous invasive insect and poses a great risk to the ornamental plant and landscape industry[13−15] and ecosystems[16,17] in the US.
The effectiveness of bark-sprayed insecticides to control CMBS is limited due to: (1) the ability of CMBS to shelter under plant crevices and suck phloem-sap of hosts; (2) the protective wax coverings secreted by adult females and late-instar males of CMBS; and (3) its high fecundity[2,18,19]. Neonicotinoids systemically applied through soil drench are effective in suppressing CMBS[6]. However, crapemyrtle is an important pollen source for native and non-native bees in the US[20−22] from late spring to early fall[23,24], especially when other resources are scarce. The negative impact of neonicotinoid residuals in crapemyrtle pollens on pollinators raises great concern[19,25,26]. Hence, environmentally friendly and effective non-chemical alternatives for CMBS management, including plant resistance breeding and biocontrol agents, are needed[9]. To date, cactus lady beetle (Chilocorus cacti) is the only biocontrol agent confirmed in laboratory conditions as a predator of CMBS[7,27]. Given the relatively broad host range of CMBS and limited pest management strategies, it is imperative to evaluate other potential biocontrol agents against this invasive pest in the US.
Chrysoperla rufilabris is a common green lacewing in many horticultural and agricultural cropping systems throughout much of the US[28−30]. The larvae of C. rufilabris are generalist predators of various soft-bodied arthropods with a relatively high prey searching and consumption capacity[30−33]. In practice, the C. rufilabris larvae have been applied to control Aphididae[34,35] and Heliothis spp.[36] in important crops[32,37,38]. Even though the foraging efficiency of Chrysoperla carnea or lady beetles upon prey was determined by various cues[39−45], little information is available regarding the predation behavior of C. rufilabris on CMBS. This study hypothesized that C. rufilbaris located CMBS through certain odors emitted by CMBS. Furthermore, these odors could be exploited to attract and retain C. rufilabris on the plants as a preventive measure to suppress CMBS infestation[46,47]. To validate whether C. rufilabris can be integrated into sustainable CMBS management programs, this study: (1) investigated predation activities of the green lacewings upon CMBS in landscape and laboratory conditions; (2) evaluated in-vitro predation potential of the green lacewing by different developmental stages; and (3) tested its foraging performance under dark conditions.
-
Two batches of Chrysoperla rufilabris larvae and eggs were purchased from ARBICO OrganicsTM (Oro Valley, AZ, USA) in June and October 2019. Upon arrival, the individual lacewing larvae and eggs were each placed in a VWR® disposable Petri dish (60 mm in diameter) and maintained inside a CONVIRON®-BDR 16 growth chamber (Controlled Environments Ltd., Winnipeg, Manitoba, Canada) at 25 ± 1 °C, 60 ± 5% RH, and a 16 h L:8 h D photoperiod. The lacewings were provided with eggs and nymphs of CMBS, 20 μL-30 μL droplets of artificial diet[48], and water separately placed on fresh crapemyrtle leaves in the Petri dish (Supplemental Fig. S1).
Nymphs, adults, and eggs of CMBS were collected from naturally CMBS-infested crapemyrtle plants on the Texas A&M University campus in College Station, Texas (USA).
Investigation of predation activities upon CMBS in landscape and laboratory conditions
-
The CMBS-infected branches were collected at different Texas A&M University campus locations from April to November 2019 for preliminary landscape research to investigate if C. rufilabris are present in plants under CMBS infestation. Nymphs, adults, and eggs of CMBS were randomly distributed in a Petri dish (60 mm in diameter), then one larval C. rufilabris was introduced into the same Petri dish to test the predation response of larval C. rufilabris to CMBS. The predation behavior was documented under a Stemi 2000 stereomicroscope (Carl Zeiss AG, Oberkochen, Germany).
Predation potential in vitro
-
Two independent experiments were conducted in June and October 2019, respectively, to evaluate the predation potential of C. rufilabris on CMBS. Feeding duration, which refers to the time taken by a larval C. rufilabris to consume the first CMBS egg completely, was recorded under the stereomicroscope from the time when the first egg was captured to the time when the egg was utterly consumed. Number of consumed CMBS, which refers to the number of CMBS eggs in a Petri dish (60 mm in diameter) consumed by an individual larval C. rufilabris during a 24-h observation period, was counted with the help of ImageJ (National Institutes of Health, Bethesda, MD, USA). A Petri dish containing approximately 300 fresh CMBS eggs without C. rufilabris feeding served as the reference. The Petri dish images before and after feeding were used to accurately determine the number of eggs consumed by C. rufilabris in 24 h.
In June, regardless of its developmental stages, 13 larval C. rufilabris (starved for 24 h beforehand) were individually placed into separate Petri dishes to test the feeding duration and predation potential. Each Petri dish contained approximately 300 fresh CMBS eggs. The June experiment was repeated six times using the same 13 C. rufilabris.
In October, 20 larval C. rufilabris within the same stage (starved for 4 h beforehand) were utilized to investigate the effect of C. rufilabris developmental stages (1st instar, 2nd instar, and 3rd instar; Supplemental Fig. S2) on the feeding duration and predation potential. The October experiment was repeated three times using 20 new larval C. rufilabris within the same stage.
Foraging performance test in the dark
-
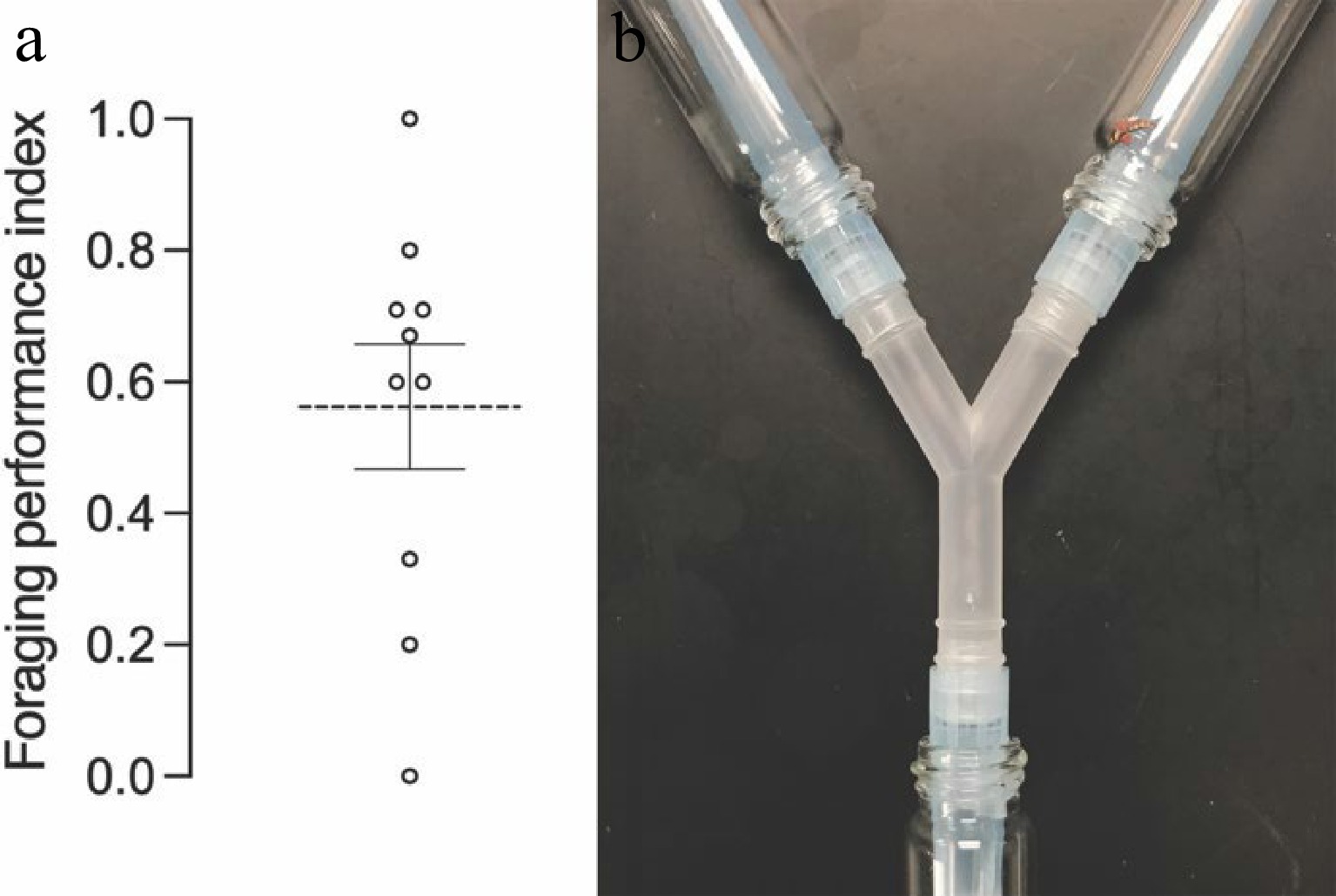
To better understand the cues primarily impacting the foraging efficiency of C. rufilabris, which provides basic information about the CMBS biocontrol strategies, a primary foraging performance test was conducted using a Y-maze in the dark (Fig. 1). The Y-maze consisted of three glass vials, namely loading vial, baited vial, and control vial being joined by a Bel-Art Y-tubing connector (SP Scienceware, Wayne, NJ, USA). Before being fixed with the connector using 1 mL pipette tips and Parafilm®, 10 living gravid females without ovisacs, 10 crawlers, and 20 eggs of CMBS were placed in the baited vial, one larval C. rufilabris was introduced into the loading vial, and the control vial was vacant.
Figure 1.
Y-maze assay. (a) Each Y-tube setup was assembled by a Y-tubing connector and contained a loading vial, a baited vial, and a control vial. (b) Before being fixed to the Y-tubing connector, CMBS females and crawlers were placed into the baited vial. (c) Three 1-mL pipette tips wrapped with Parafilm were used to tightly fix the connector to the three vials. Narrow ends of the two pipette tips were cut to connect the baited vial and the control vial, which could deter the lacewing crawling back once it had made its decision[49]. The wide end of the other pipette tip was cut to connect the loading vial where a larval lacewing was introduced. (d) Twelve Y-mazes were placed horizontally in a box and tested per time and replicated 10 times at 25 ± 1 °C, 60 ± 5% RH in the dark.
Twelve larval C. rufilabris were individually placed into each Y-maze setup for the foraging performance test at 25 ± 1 °C, 60 ± 5% RH and a 24-h dark photoperiod. After 24 h, the number of C. rufilabris that entered the baited vials (B) and the control vials (C) was counted, respectively. This experiment was repeated 10 times using new larval C. rufilabris. Foraging performance index (FPI) of C. rufilabris targeting CMBS in the dark was calculated as:
FPI = (the number of lacewings choosing B – the number of lacewings choosing C) / Total number of lacewings that made a choice
Positive response ratio (PRR) of C. rufilabris foraging performance was calculated as:
PRR = the number of lacewings choosing B / Total number of lacewings that made a choice
Data analysis
-
For the predation potential experiment in June, datasets of the 13 C. rufilabris biological replicates of the feeding duration and the number of consumed CMBS were averaged (mean ± SE) and compared among the six technical replicates. For the October experiment, datasets of 20 C. rufilabris biological replicates with three technical replicates of the feeding duration and the number of consumed CMBS for each larval stage were analyzed by using one-way analysis of variance (ANOVA) with the JMP® 16 (SAS Institute, Cary, NC, USA). Then, the analysis results regarding the feeding duration and the number of consumed CMBS were separated by C. rufilabris developmental stage by using Tukey's honestly significant difference (HSD; α = 0.05) to test if different stages of development impact the in-vitro predation potential of larval C. rufilabris upon CMBS eggs.
-
By examining the samples collected at different campus locations, we observed larval green lacewings feeding on CMBS gravid females [Fig. 2a & b, (30°36'39" N, 96°20'58" W); (30°36'55" N, 96°20'24" W)]. Lacewings’ eggs were deposited on twigs of CMBS-infested crapemyrtles [Fig. 2c & d, (30°37'03'' N, 96°20'08'' W); (30°36'30'' N, 96°21'02'' W)]. These observations allowed us to evaluate the potential of C. rufilabris as a biocontrol agent for sustainable management practices against CMBS. Indeed, in laboratory conditions (Fig. 3), the larval green lacewings not only voraciously consumed CMBS gravid females and eggs but were also able to grab and devour tiny crawling nymphal CMBS. The observations in both landscape and lab conditions confirmed C. rufilabris as the natural predator on CMBS.

Figure 2.
Observations of Chrysoperla rufilabris were reported at different locations on Texas A&M campus (USA). Larval C. rufilabris were observed preying on CMBS gravid females during the landscape investigations on April 9th (a) (30°36'39" N, 96°20'58" W) and June 28th (b) (30°36'55" N, 96°20'24" W). Lacewing eggs were found in CMBS-infested crapemyrtles on Oct 18th (c) (30°37'03" N, 96°20'08" W) and Nov 15th, 2019 (d) (30°36'30" N, 96°21'02" W).

Figure 3.
Larval Chrysoperla rufilabris individuals preying on CMBS under laboratory conditions. Larva of C. rufilabris targeted a female adult of CMBS (a) and voraciously seized and consumed body fluids of the CMBS using its large, sucking jaws (b) after placing them in the same Petri dish. A green lacewing larva easily grabbed a CMBS egg (c) and consumed the egg in about 1 min (d) after placing them in the same Petri dish. Larva seizing a crawling nymphal CMBS (e) and consumed it quickly (f) under the same experimental conditions.
Evaluation of the predation potential upon CMBS eggs
-
In the June test, the results showed that the feeding duration ranged from 53.2 ± 2.5 s to 73.2 ± 2.7 s (mean ± SE) and the number of CMBS eggs consumed ranged from 154.1 ± 2.7 to 195.5 ± 2.5 (mean ± SE). The predation potential (or predation capacity) of C. rufilabris upon CMBS eggs was similar to that reported for 4th instar aphids of Aphis gossypii and Myzus persicae[35].
In the October test, the developmental stages significantly affected the feeding duration (F 2, 177 = 101.1332, p < 0.0001) and the number of CMBS eggs consumed in 24 h (F 2, 177 = 252.6378, p < 0.0001) (Table 1). As C. rufilabris aging, the feeding duration dropped from 141.4
$ \pm $ $ \pm $ Table 1. Feeding duration and numbers of CMBS eggs consumed by Chrysoperla rufilabris at different developmental stages.
Predator developmental stage Feeding duration
(sec)zNumber of
consumed CMBSz1st instar 141.4 ± 4.8a 11.8 ± 1.3c 2nd instar 77.5 ± 4.7b 151.5 ± 6.6b 3rd instar 60.3 ± 3.0c 176.4 ± 6.9a Statistical analysis F 2,177 = 101.1332,
p < 0.0001F 2,177 = 252.6378,
p < 0.0001z Means ± SE (N = 3, representing a total of 60 tested for each developmental stage), in the same column, followed by different letters are significantly different as determined by Tukey's HSD test (α = 0.05). As the lady beetles and green lacewings share the same food resource- CMBS, their predator-predator-CMBS interactions may enhance the pest suppression of CMBS due to predator facilitation[51] or reduce the pest suppression due to predator interference and intraguild predation[52,53]. Therefore, to better implement C. rufilabris as the biocontrol agent of CMBS, further identification of the relative contribution of the lady beetles and green lacewings to CMBS suppression is needed.
Foraging performance test in Y-mazes
-
In the 24-h Y-maze assay (Fig. 4a), the FPI of C. rufilabris upon CMBS was 0.56 ± 0.09 (mean ± SE). Among the green lacewings that made a choice, 78.14 ± 4.74% (PRR) larval C. rufilabris successfully targeted CMBS in the baited vial in the dark (Fig. 4b). The results indicated that some cues primarily associated with olfactory response were likely involved in the foraging performance. Testing olfactory response to volatiles secreted by prey would help ascertain the attractants or repellents related to lacewing-CMBS interaction, which better guides the integrated pest management of CMBS by using C. rufilabris.
-
Our study validated C. rufilabris as a natural predator of CMBS and investigated C. rufilabris predation potential as a biocontrol agent under laboratory conditions. The results regarding its predation potential upon CMBS eggs suggest using 2nd and 3rd instar C. rufilabris could be more efficient in suppressing the CMBS population. The foraging performance of larval C. rufilabris upon CMBS in the dark indicated that olfactory response was likely involved in CMBS predation. Future investigation focusing on the olfactory response of C. rufilabris to CMBS would benefit the development of the integrated pest management of CMBS.
This work is supported by TAMU T3 246495-2019, Crop Protection and Pest Management project ‘Integrated pest management strategies for crape myrtle bark scale, a new exotic pest’ (No. 2014-70006-22632) and Specialty Crop Research Initiative project 'Systematic Strategies to Manage Crapemyrtle Bark Scale, An Emerging Exotic Pest' (grant no. 2017-51181-26831) from the USDA National Institute of Food and Agriculture. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture.
-
The authors declare that they have no conflict of interest.
- Supplemental Fig. S1 Rearing Chrysoperla rufilabris. Each individual of larval C. rufilabris was placed in each Petri dish and fed with eggs and nymphs of CMBS and 20μL-30μL droplets of artificial diet (Prosser and Douglas 1992) (A) . After three larval stages, the third instars secreted silken cocoons to cover themselves to developed as pupae (B) . Winged C .rufilabris adults emerged from the cocoons (C) and were reared using 20 μL-30 μL droplets of artificial diet and water (D) . Several C. rufilabris adults were transferred into the same Petri dish supplemented with the artificial food for reproduction (E) . Green eggs were laid on the lid of the Petri dish nearby the food (F) . The 1st instar C. rufilabris hatched from the eggs (G) and provided with the artificial foods (H).
- Supplemental Fig. S2 Life cycle of Chrysoperla rufilabris. After hatching from eggs (A) in 5-8 d, the C. rufilabris instars developed through 1st (B-1), 2nd (B-2), and 3rd (B-3) larval stages in 14-21 d. Then, the third instars secreted silken cocoons to cover themselves and developed as pupae. Winged C. rufilabris adults emerged from the cocoons in 10-15 d.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wu B, Xie R, Gu M, Qin H. 2022. Green lacewing Chrysoperla rufilabris (Neuroptera: Chrysopidae) is a potential biological agent for crapemyrtle bark scale (Hemiptera: Eriococcidae) pest management. Technology in Horticulture 2:3 doi: 10.48130/TIH-2022-0003
Green lacewing Chrysoperla rufilabris (Neuroptera: Chrysopidae) is a potential biological agent for crapemyrtle bark scale (Hemiptera: Eriococcidae) pest management
- Received: 30 March 2022
- Accepted: 30 May 2022
- Published online: 30 June 2022
Abstract: Crapemyrtle bark scale (CMBS; Acanthococcus lagerstroemiae), an invasive sap-sucking hemipteran, has spread across 16 US states. Infestation of CMBS negatively impacts the flowering and reduces the aesthetic quality of crapemyrtles. The widespread use of soil-applied neonicotinoid insecticides to suppress the CMBS infestation may be hazardous to pollinators and other beneficial insects. Natural enemies of CMBS are important agents for developing integrated environmentally friendly management strategies. This study evaluated the performance of larval green lacewing (Chrysoperla rufilabris) as a biocontrol agent of CMBS. Predatory behavior of the larval C. rufilabris upon CMBS was documented under a stereomicroscope using infested crapemyrtle samples collected from different locations in College Station (Texas, USA). Predation potential of C. rufilabris upon CMBS eggs and foraging performance using Y-maze assay were investigated under laboratory conditions. Results confirmed that larval C. rufilabris preyed on CMBS nymphs, eggs, and adult females. The evaluation of predation potential results showed that 3rd instar C. rufilabris consumed significantly more CMBS eggs (176.4 ± 6.9) than 2nd (151.5 ± 6.6) or 1st instar (11.8 ± 1.3) in 24 hours. Results from the Y-maze assay indicated that larval C. rufilabris could target CMBS in the dark, indicating that some cues associated with olfactory response were likely involved when preying on CMBS. This study is the first report that validated C. rufilabris as a natural predator of CMBS and its potential as a biological agent to control CMBS. Future investigation about the olfactory response of larval C. rufilabris to CMBS would benefit the development of environmentally friendly strategies to manage CMBS.
-
Key words:
- Biocontrol /
- Predation potential /
- Foraging performance /
- Y-maze assay /
- Integrated pest management