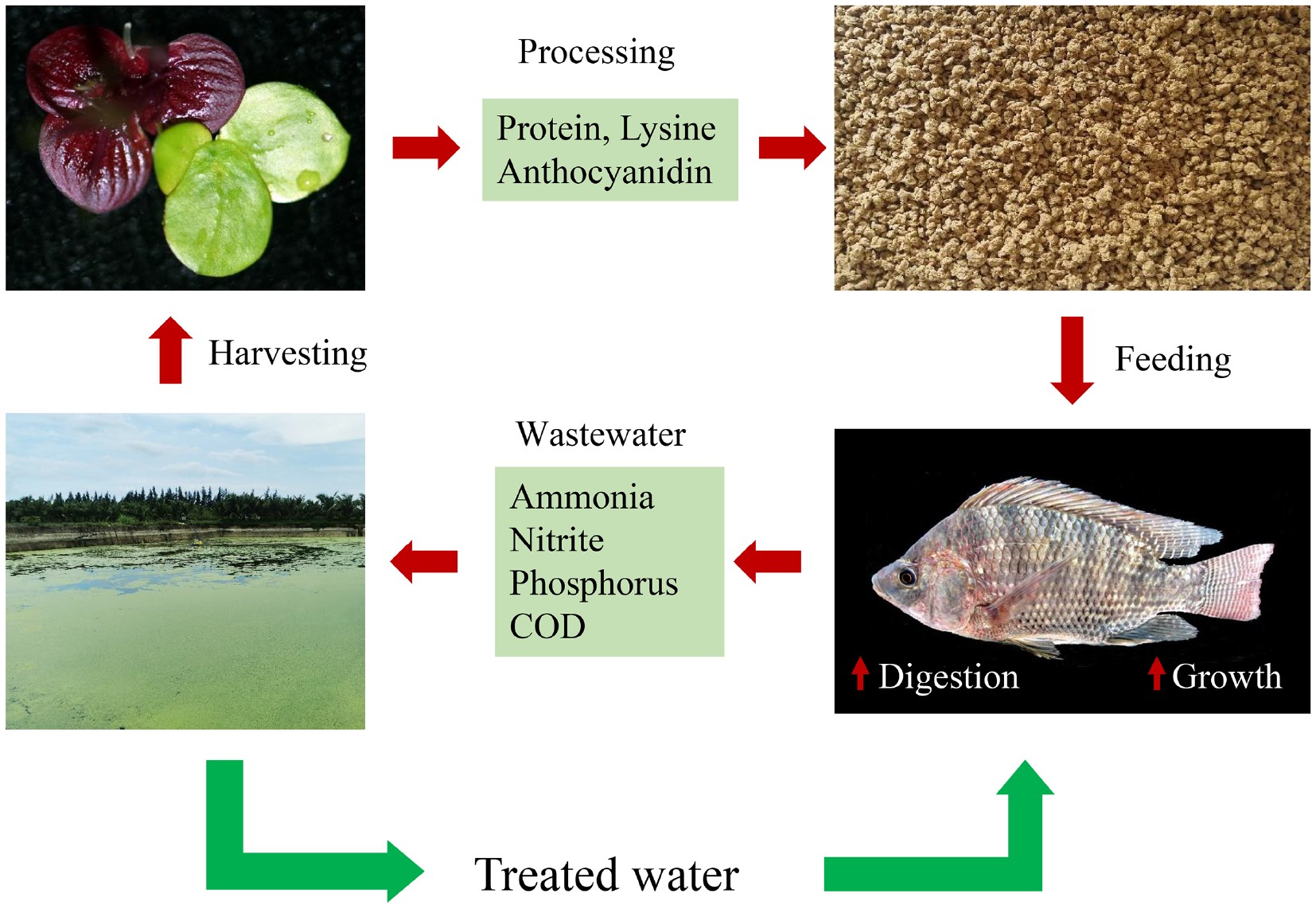
-
The small aquatic plants in the duckweed family (Lemnaceae) are comprised of 37 species[1,2]. They are wild plants and distributed in different areas worldwide. Some species are distributed more widely and globally, while other species are local to a small area[3]. Most duckweed species grow fast and are rich in protein and essential amino acids, and are alternative plant protein resources for human food and animal feeds[4,5]. For example, Wolffia arrhiza has been consumed as a vegetable by the Burmese, Laotians and Thai for many generations[5]. The local name of the plant Khai-nam means eggs of the water, indicating its high nutritional value recognized by the local people[6]. Many duckweed vegetable samples collected in Thai markets have been identified as Wolffia globosa[7], suggesting that at least two duckweed species have been consumed as vegetables by humans for a long time. Since only two Wolffia species are present in southeast Asia, Wolffia species elsewhere may also be a potential for human consumption[7].
Duckweed species, other than those for human food, have been tested for animal feed, including Lemna minor[8,9], L. perpusilla[10], L. gibba[11], and Spirodela polyrhiza[9,12]. These species can accumulate protein with 5 to 10 times higher yields than conventional land-grown crops, such as soybeans, maize, and oats[13], and are easier to harvest than other fast-growing aquatic plants and microalgae[14]. Besides the high yields, duckweed protein is balanced in amino acid composition and can be accepted as 100% duckweed diet for some domestic animals and fish[15]. Therefore, duckweed protein is expected to be one of the novel protein sources as a replacement for animal-derived proteins[4]. However, most previous research performed has used wild-type duckweeds[10,12], which are variable in yield and quality, and there is a gap between research and commercialization. In this research, we used a domesticated variety of duckweed, S. polyrhiza DW2602, as a diet replacement to test its effects on the growth performance and digestive enzymes of tilapia (Oreochromis niloticus) fingerlings. DW2602 is a variety selected from a few hundred wild duckweed strains collected in China, and has been grown for many years. It is of high yield and high protein and relatively resistant to ultraviolet radiation, which makes it possible to grow well in open ponds without sun-shield. The yield of this variety is approximately 30 tons (DW)·ha−1·y−1 with a protein content of 44.5% dry weight.
-
Duckweed strains were taken from the collection of duckweed ecotypes/clones of the Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences (China). A total of 12 strains covering three genera and four species were selected, which were collected in four distinct temperature zones of China, including Heilongjiang (HL), Yunnan (YN), Hubei (HB), and Hainan (DW) Provinces (Table 1), in which DW2602 was a variety local to Hainan Province (China) and has been domesticated for many years. The plants were pre-cultivated for 4 weeks under axenic conditions at 25 ± 1 °C in 150 mL Erlenmeyer flasks containing 50 mL modified Schenk-Hildebrandt medium[16]. The composition of the medium include: KNO3 12.4 mM, (NH4)H2PO4 1.3 mM, CaCl2·2H2O 0.68 mM, MgSO4·7H2O 0.81 mM, MnSO4·H2O 30 μM, H3BO3 40 μM, ZnSO4·7H2O 1.74 μM, KI 3.0 μM, CuSO4·5H2O 0.4 μM, Na2MoO4·2H2O 0.21 μM, CoCl2·6H2O 0.21 μM, FeNaEDTA 27.0 μM, and Na2EDTA·2H2O 2.74 μM. The pH of the medium was adjusted to 5.5. The plants were transferred to fresh medium once a week to prevent nutrient deficiency so that the plants were conditioned to ensure reproducible results. Illuminance was supplied with white-light fluorescence tubes at 100 μmol m−2 s−1 (photosynthetically active radiation).
Table 1. Duckweed strains and/or varieties used in this research.
Strain/varietiy Species Place of collection HL1001 Spirodela polyrhiza Heilongjiang HL1403 Landoltia punctata Heilongjiang HL2002 Lemna perpusilla Heilongjiang HB0203 Spirodela polyrhiza Hubei HB0602 Landoltia punctata Hubei HB0401 Lemna aequinoctialis Hubei YN2701 Spirodela polyrhiza Yunnan YN2702 Landoltia punctata Yunnan YN2704 Lemna aequinoctialis Yunnan DW2602 Spirodela polyrhiza Hainan DW2701 Landoltia punctata Hainan DW1301 Lemna aequinoctialis Hainan To measure the nutritional value of duckweed strains, the pre-cultured plants were grown in plastic trays (area 30 cm × 20 cm) filled with 3 L Schenk-Hildebrandt medium[16]. The trays were covered with transparent plastic wrap film with a few holes punched for ventilation. The illumination and temperature were the same as described for pre-cultivation. The plants were grown for 14 d, during which the plants were transferred into new trays to avoid a stress response if the fronds were going to cover the full surface of the medium. The biomass was then harvested, lyophilized, and ground to fine powder for further analysis.
Analysis of nutritional values
-
The protein content was measured via the Kjeldahl procedure using the N factor 6.25. The proteinogenic amino acid (AA) contents were determined by ion exchange chromatography according to their chemical properties. The majority of the proteinogenic AA was measured after the samples were hydrolyzed with phenolic hydrochloric acid. To measure the sulfur-containing AA, methionine and cysteine, an oxidation was performed before acid hydrolysis.
Total lipid was extracted using a modified single-extraction method[17] with chloroform/isopropanol/methanol/water in the ratio 30/25/41.5/3.5 (v/v/v/v) and a 24-h extraction time.
The starch content was determined by measuring the total glucose content (starch content = glucose content × 0.909) as previously described[18].
Large scale culture of duckweed DW2602
-
The duckweed variety DW2602 with high nutritional value was grown in a full-scale fish pond (60 m × 100 m). Five hundred grams of a compound fertilizer with N:P:K of 15:15:15 was dissolved in water and sprayed on the plants once a week. One third of the biomass was harvested every one to three days depending on the weather and the growth rate of the plants. The harvested biomass was cleaned with tap water, sun-dried for two days, and further dried in an electric dryer at 60 °C for one day. The biomass was crushed with a cell breaker (JP05D-1300, Supor, Zhejiang, China) and sieved to 60 mesh·inch−1. The powder was packed in sealed plastic bags, and stored in a drying cupboard.
Preparation of feed particles containing duckweed
-
A widely used commercial tilapia feed Tongwei-156 (Tongwei, Chengdu, China) was used as a control diet. For formulation of test diets, Tongwei-156 was crushed into a powder with a breaker JP05D-1300, and duckweed was added at 0%, 10%, 15%, 25%, and 30% levels, and 0.5% carboxymethelcellulose was added as a binder. All diets were prepared in particle form of 2 mm in diameter using a particle maker (MGD-120, Shangdong, China). The particles were dried at 60 °C in a drying machine and stored under dry conditions prior to feeding.
Tilapia culture and measurements of growth performance
-
Tilapia variety GIFT (Genetic Improvement of Farmed Tilapia, O. niloticus) fingerlings were obtained from a local fish dealer, and were acclimatized to the laboratory conditions for two days feeding with the control diet (Tongwei-156). The fingerlings with size of 2.0 ± 0.15 g were randomly distributed at the rate of 15 fish per tank with three replicates for each treatment. Some fingerlings were kept in the backup tanks for replacement of any dead fish in the treatments during acclimation. Each tank was supplied with 60 L unchlorinated water and was aerated continuously. All the fish were fed twice a day (9:00 and 15:00) at a fixed daily feeding rate of 3% body weight for 60 d. The fish were weighed every 10 days, and the feeding quantity was increased accordingly. One third of the water was replaced with fresh water every other day.
At the end of the experiment, all the fish were weighed, and their body length was measured. The growth performance was measured as follows:
Weight gain rate (WGR, %) = (Wf – Wi)/Wi × 100
specific growth rates (SGR, %) = (lnWf − lnWi)/d × 100
Condition factor (CF, g/cm3) = Wf/L3 × 100
Viscerosomatic index (VSI, VBR, %) = Wv/Wt × 100
Hepatosomatic index (HSI, LBR, %) = Wh/Wt × 100
where Wf and Wi are final and initial mean body weight, respectively; d is the days of culture; L is final mean body length; Wv and Wh are final mean viscera and liver weight, respectively, and Wt is the mean body weight.
Assay of digestive enzyme activities
-
Three individual fish from each treatment were euthanized at the end of the experiment using anesthetics MS222 (2 g/l)[19]. The entire stomach, liver, and intestine were immediately collected, and washed with 4 oC distilled water. The surface water on the samples was removed with Whatman paper. The samples were weighed and individually homogenized in nine volumes of ice-cold extraction buffer (20 mM Tris/10 mM phosphate, pH 7.0) with a motor-driven homogenizer. The homogenates were centrifuged at 10,000 × g for 10 min at 4 °C, and the supernatants were used immediately for enzyme assay, or stored at 4 °C for a maximum of 24 h.
Proteolytic activity in the stomach and intestine was measured using a pepsin assay kit (A080-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China), following the recommended protocol by the manufacturer, which is based on the reduction of phenol reagents to blue chemicals by tyrosine. The reaction mixture was centrifuged at 10,000 × g for 5 min. The optical density of the supernatant was determined at 660 nm in a spectrophotometer. The proteolytic activity in the liver was measured using a Trypsin Assay Kit (A080-2, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The optical density was determined at 253 nm. The lipase activity was measured using a Lipase Assay Kit (A054-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and the optical density was determined at 420 nm. The amylase activity was measured using an Amylase Assay Kit (C016-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All the measurements were performed following the manufacturer’s protocol.
Statistical analysis
-
Statistical analysis of data was performed by analysis of variance (ANOVA) using SPSS Statistics 17 (Microsoft, USA) followed by Duncan’s multiple range test[20].
-
Nutritional value of 12 strains collected in four distinct climate zones of China (Heilongjiang, Hubei, Yunnan, and Hainan Provinces) were evaluated after the biomass was dried (Table 2). Crude protein content varied from 32.56% to 44.52% (dry weight, hereafter) with an average of 37.91%; The highest protein content (44.52%) was recorded in DW2602, which was a strain collected in Hainan Province. Crude fat content varied from 4.25% to 8.12% and averaged at 5.93%; whereas starch content varied from 2.37% to 6.81% and averaged at 5.30%, suggesting that these strains were poor in starch. However, some duckweed strains were reported to be rich in starch[21], which was most probably induced by stress[18,22−24]. In our research, all strains were grown in nutrient medium and favorable conditions, therefore, they accumulated fewer starch. However, favorable conditions promote the accumulation of protein, and thus are suitable for use in animal feed preparation. All duckweed strains had high water content, with dry matter varying from 4.01% to 7.15% (Table 2), therefore, the biomass has to be dried before use.
Table 2. Nutrient and amino acid contents of duckweed strains (% dry weight).
HL1001 HL1403 HL2002 HB0203 HB0401 HB0602 DW1301 DW2602 DW2701 YN2701 YN2702 YN2704 Average Dry matter 4.01 5.51 6.08 5.39 5.58 7.15 4.79 5.57 5.61 4.82 5.59 5.38 5.46 Protein 38.71 41.18 35.70 38.33 40.70 32.56 39.62 44.52 35.04 33.66 39.52 35.37 37.91 Fat 4.85 5.04 7.75 4.25 8.12 6.33 7.65 4.69 6.35 5.13 5.68 5.35 5.93 Starch 3.56 6.38 2.37 4.39 6.76 6.81 6.34 3.25 6.18 4.37 6.76 6.43 5.30 Arg(R) 2.04 2.42 2.23 2.02 2.30 1.91 2.24 2.35 2.06 1.77 2.32 2.00 2.14 His(H) 0.79 0.81 0.89 0.78 0.82 0.64 0.80 0.91 0.69 0.69 0.77 0.71 0.78 Ile(I) 1.55 1.68 1.70 1.53 1.78 1.33 1.73 1.78 1.43 1.35 1.61 1.54 1.58 Leu(L) 3.42 3.68 3.84 3.38 3.89 2.91 3.79 3.93 3.13 2.97 3.53 3.38 3.49 Lys(K) 2.26 2.41 2.49 2.24 2.53 1.91 2.46 2.60 2.05 1.97 2.31 2.20 2.29 Met(M) 0.33 0.59 0.38 0.33 0.35 0.47 0.34 0.38 0.51 0.29 0.57 0.30 0.40 Phe(F) 2.27 2.29 2.54 2.25 2.41 1.81 2.34 2.61 1.94 1.97 2.19 2.09 2.23 Thr(T) 1.56 1.64 1.66 1.54 1.66 1.30 1.62 1.79 1.40 1.36 1.58 1.44 1.55 Val(V) 2.42 2.58 2.74 2.40 2.81 2.04 2.74 2.78 2.19 2.10 2.47 2.44 2.48 Tyr(Y) 1.00 1.25 0.95 0.99 1.22 0.99 1.18 1.15 1.07 0.87 1.20 1.06 1.08 Pro(P) 1.73 1.82 1.91 1.71 1.89 1.44 1.84 1.99 1.55 1.50 1.74 1.65 1.73 Gly(G) 2.00 2.23 2.15 1.98 2.20 1.76 2.14 2.30 1.89 1.74 2.14 1.91 2.04 Ala(A) 2.20 2.35 2.31 2.18 2.50 1.86 2.44 2.53 2.00 1.91 2.26 2.18 2.23 Ser(S) 1.85 1.92 1.90 1.83 1.82 1.52 1.77 2.13 1.64 1.61 1.85 1.58 1.79 Glu(E) 4.30 4.29 4.56 4.26 4.41 3.39 4.29 4.95 3.65 3.74 4.12 3.83 4.15 The amino acid profiles in the tested duckweed were more balanced than most cereal grains (Table 2), and fit with the recommended amino acid intake for humans by WHO[25]. The 10 indispensable amino acids for fish were high in content, especially the lysine, arginine, and methionine, which are insufficient and are often limiting factors to fish growth in many diets, especially in cereal grains[26,27]. Lysine content in the duckweed strains were between 1.91% to 2.6%, in which DW2602 had the highest and were six to eight times the content of genetically modified rice[26] and maize[27,28].
The majority of the fish show a reduction in feed intake when the feed has an imbalanced essential amino acid composition[29]. The circulating level of some excessive amino acid (e.g. leucine) may induce satiety and thus could impact food intake[30]. On the other side, the most limiting amino acid is best utilized in protein synthesis, while others get wasted, leading to a greater ammonia-genesis and ureagenesis[31]. The balanced amino acid profiles in duckweeds make them a promising fish diet. Strain DW2602 was selected for further test in feeding practices by its highest protein and lysine content (Table 2).
Effects of duckweed on growth performance
-
Fish diets with 0 to 30% of duckweed DW2602 were prepared to feed tilapia fingerlings in net cages. During the two-day acclimation period, one or two fingerlings died in a few tanks, probably due to injury and/or stress during transportation. The number of losses was compensated using fingerlings of the same size from the backup tanks. After acclimation, none of the fingerlings died. Thus, during the experimental period, the survival rates of all treatment tanks was 100%.
Addition of duckweed powder in the fish diets improved the growth performance of the tilapia fingerlings. All the fish fed on diets containing duckweed (10%−30% inclusion) grew faster than those fed on the control diet (Fig. 1a). The growth performance reached the peak when the duckweed inclusion rate was 20%, by which the final weight of the fish increased from 2.00 ± 0.15 g to 37.24 ± 1.21 g in 60 d, which was 18.1% larger than those fed on the control diet (Fig. 1a). The weight gain (Fig. 1b) and the specific growth rate (Fig. 1c) were also the highest when the duckweed inclusion rate was 20% (P < 0.05), which were 1762% and 4.87 %·d−1, respectively, and surpassed the control diet by 19.4% and 5.9% for the weight gain and specific growth, respectively. The duckweed inclusion rate of 25% had the second largest weight gain (1739.5%) and specific growth rate (4.85 %·d−1), which were not significantly different from that of 20% inclusion. When the duckweed inclusion rate reached 30%, the weight gain and specific growth rate decreased significantly compared to that of 20% inclusion, possibly due to the presence of anti-nutrition compounds in the duckweed, such as tannin and phytic acid[12].
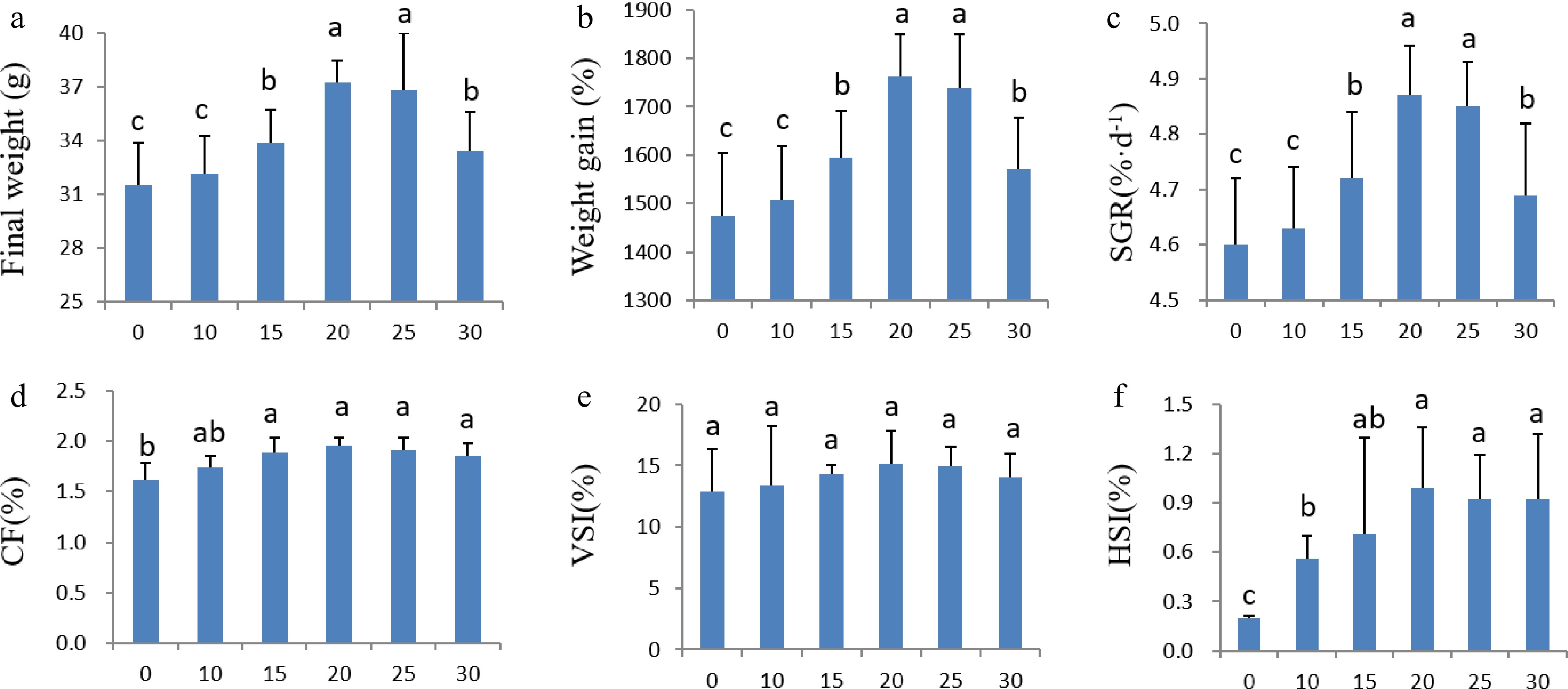
Figure 1.
Effects of dietary duckweed variety DW2602 on growth performance of tilapia. (a) Final weight. (b) Weight gain rate. (c) Specific growth rate (SGR). (d) Condition factor. (e) Viscerosomatic index (VSI). (f) Hepatosomatic index (HIS). The significance of differences was tested by one-way ANOVA and Duncan’s multiple range test using IBM SPSS Statistics version 24. Different letters above columns indicate significant differences at 5% level of significance.
All diets containing duckweed improved the condition factor (CF), and 20% duckweed inclusion showed the highest CF (1.96%, Fig. 1d), which was 21.0% higher than the control diet. Viscerosomatic index (VSI) of fish feeding on different diets was not significantly different, although the VSI of 20% duckweed inclusion appear higher (Fig. 1e). However, the hepatosomatic index (HIS) between treatments was significantly different and duckweed increased the HIS of all treatments with the highest HIS (0.99%) at 20% duckweed inclusion (Fig. 1f), which ws almost four times that of the control (0.2%).
Altogether, addition of duckweed in the fish diet significantly improved the growth performance of tilapia fingerlings, and 20% inclusion showed the best feeding effect and increased the final weight, weight gain, SGR, CF, and HIS.
Effect of duckweed on digestive enzyme activities
-
When fish were fed on the control diet, the proteolytic activity was the highest in the gastric organ, followed by that in the hepatopancreas, enteric organ contained the lowest proteolytic activity (Fig. 2A). Inclusion of 10% duckweed in the diet significantly increased protease activity in the enteric organ, while the changes in gastric and hepatopancreas were not significant. However, when the inclusion rate of duckweed increased to 20%, the proteolytic activities in all digestive organs reached the highest levels, and was increased by 35.9%, 127.9%, and 27.7% in the gastric, enteric, and hepatopancreas, respectively (P < 0.05). When the inclusion rate of duckweed increased to 30%, the proteolytic activity in the gastric and hepatopancreas declined compared to the highest level, but the activity remained high in the enteric organ (Fig. 2a).
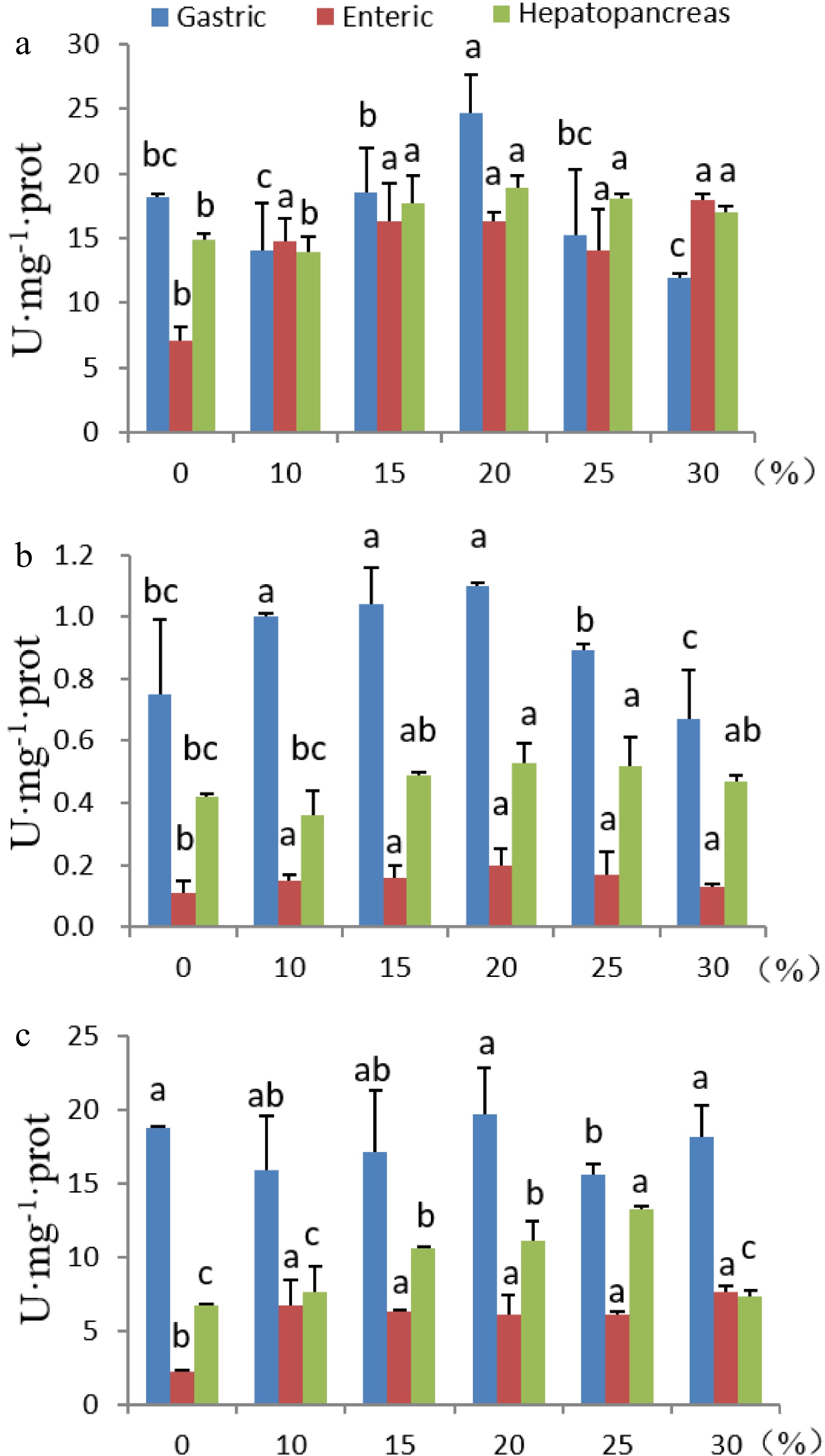
Figure 2.
Effects of dietary duckweed variety DW2602 on digestive enzyme activities. (a) proteolytic activity. (b) Amylase activity. (c) Lipase activity. The significance of differences was tested by one-way ANOVA and Duncan’s multiple range test using IBM SPSS Statistics version 24. Different letters above columns indicate significant differences at 5% level of significance.
Amylase activity in the gastric organ was the highest compared to that in the enteric and hepatopancreas organs no matter how much duckweed was added to the diet. Addition of 10% duckweed significantly increased amylase activity in the gastric and enteric organs, and a further increase of duckweed inclusion to 20% resulted in the highest amylase activity in the three digestive organs (P < 0.05), which was increased by 46.7%, 81.8%, and 26.2% in the gastric, enteric, and hepatopancreas, respectively, compared to the control (Fig. 2b, P < 0.05).
Similar to amylase activity, lipase activity in the gastric organ was the highest among the three digestive organs regardless of the amount of duckweed added in the diets. Addition of duckweed in the diets did not change the lipase activity in the gastric organ, however, significantly increased the lipase activity in the enteric and hepatopancreas (Fig. 2c). Enteric lipase activity was the highest when the duckweed inclusion rate was 30%, and hepatopancreas lipase activity was the highest when the duckweed inclusion rate was 25% (P < 0.05). The percent increase was 238.2% and 97.3% for enteric and hepatopancreas, respectively. When the duckweed inclusion rate was 20%, the lipase activities was respectively 5.0%, 169.8%, and 64.6% higher compared to the controls in the gastric, the enteric, and the hepatopancreas.
Taken together, these results suggested that addition of duckweed in the fish diets promotes the secretion of digestive enzymes, especially when the inclusion rate was 20%.
Apparent digestibility is one of the important indexes to evaluate the nutritional value of a feed formula. After fish ingestion, feed components act as substrates of digestive enzymes, affecting the secretion and activity of digestive enzymes[19,32−34]. The digestive enzymes of tilapia are mainly present in stomach, intestine, and hepatopancreas, and the stomach contained the highest digestive enzyme activity[35]. The important digestive enzymes in tilapia include amylase, protease and lipase[19,36,37]. Amylases hydrolyze glycogen, starch and related polysaccharide glycoside bonds[38,39]. Lipases hydrolyze lipids and its activity increases with the increase of fat content in the diet[40,41]. Under the action of lipase, lipids are decomposed into a mixture of glycerides and free fatty acids and absorbed by fish. In this study, the crude fat content of the feed supplemented with 20% DW2602 dry powder was 26.5% higher than that of the control feed (Table 3). This may be the reason why the lipase activities in the hepatopancreas and intestine of tilapia fingerlings were increased significantly (Fig. 2c), in which the lipase activities in the hepatopancreas and intestine of tilapia were 64.46% and 238.4% higher than those in the control, respectively (Fig. 2c).
Table 3. Stability and nutritional value of control and the diet containing 20% S. polyrhiza DW2602.
Diet Water (%) Dissolution (%) Ash (%) Fat (%) Fiber (%) Protein (%) Lysine (%) Sulfur-AA (%) Control 8.2 10.0 10.9 8.3 4.2 31.2 2.0 0.9 20% duckweed 6.9 8.5 11.3 10.5 4.9 28.9 2.3 1.1 Standard ≤ 12.5 ≤ 10.5 ≤ 14 ≤ 6 ≤ 6 ≤ 28 ≤ 1.6 ≤ 0.8 Flavonoids are mainly stored in stomach, intestine and hepatopancreas after being absorbed by fish. Flavonoids are antioxidants and can protect organs from biotic and abiotic stresses and improve their biological functions[42]. For example, feeding tilapia with anthocyanin-containing diets resulted in increases in the innate immune parameters, gene expression responses, and the survival rate of the fish subjected to ammonia stress[43]. Duckweed are rich in flavonoids[6,24], and may be one of the reasons why inclusion of duckweed in the fish diets increased the digestive enzyme activity and improved the growth performance of tilapia in this research.
Nutritional value and safety of the feed containing duckweed DW2602
-
The amino acid profiles in duckweed are similar to the best composition of amino acids in the diet recommended by FAO, which promotes feed intake and efficient utilization by animals[44]. Duckweed contains high contents of lysine and sulfur-containing amino acids that are essential to animals[45]. In this research, the nutritional indexes of the control diet and the diet containing 20% DW2602 dry powder (duckweed diet, hereafter) met the national standards of China (Table 1). The contents of lysine, sulfur-containing amino acids, and crude fat in the duckweed diet were 2.3%, 1.1% and 10.5%, respectively, which were 12.7%, 17.0% and 26.5% higher than those in the control (Table 3). However, crude protein was 7.4% lower than that of the control diet, although still higher than the national standard (Table 3). Since the protein content of the biomass of DW2602 was higher than that in the control diet, 20% substitution of the control diet by DW2602 dry powder should theoretically increase the protein content. The opposite phenomenon may be a result of protein degradation by high temperature in the feed re-processing, which leads to partial protein loss in previous studies[46]. The content of lysine and sulfur-containing amino acids in the feed supplemented with 20% DW2602 were slightly higher than those in the control group, which is consistent with the amino acid profile of DW2602 itself.
The duckweed diet appeared to be the same color as freshly prepared feed after storage for more than one month, with uniform particle size, no mildew, no deterioration, and no insect damage. Salmonella and mold are the main microbial hazards in feed. Microbiological tests showed that the duckweed diet was negative for Salmonella, and contained only 240 CFU/g of mold, much lower than the national standard (< 30,000 CFU/g). Therefore, the the duckweed diet met the national standards in terms of microbial indicators.
Arsenic, cadmium, lead, and mercury are the four major heavy metal elements that affect animal and human health[47]. Trace amount of intake by animals and humans may cause obvious harm, and thus they are called 'toxic precious metals'. Some duckweed strains were reported to accumulate heavy metals[48−51]. To evaluate the safety of the duckweed diet, the content of arsenic, cadmium, lead, and mercury were measured, and were confirmed to be far below the national standards of China, similar to that of the control feed (Table 4).
Table 4. Content of heavy metals in the control and the duckweed diets (mg/kg).
Diet Arsenic Cadmium Mercury Lead Tongwei-156 0.20 0.05 0.01 0.72 20% duckweed 0.18 0.11 0.01 0.74 National standards ≤ 3 ≤ 0.5 ≤ 0.5 ≤ 5 Lignin is generally the second richest component of the plant cell wall after cellulose. However, lignin content is very low in the duckweed family[52], since the metabolic pathway related to lignin biosynthesis is inactivated in duckweed[53]. At the same time, phenylalanine, the precursor of lignin biosynthesis, flows to the flavonoid biosynthesis pathway[53], resulting in the richness of flavone and flavonoids in the duckweed plants[24]. Therefore, duckweed are easy to be digested by animals, and the flavonoids in duckweed can promote the growth of fish, especially isoflavone, which have antioxidant functions and promote secretion of growth hormones[42].
There have been several reports on the application of duckweed in fish diet. L. minor was used to replace the same amount of rapeseed meal (0%−14%) in fish diet to study the effect of duckweed on the growth performance and digestive capacity of koi carp Cyprinus carpio[54]. The specific growth rate of koi carp in the 14% replacement group was significantly higher than that of the control[54]. At the same time, the expression of TYR gene, a rate limiting enzyme related to melanin synthesis, was significantly up-regulated in the skin of koi[55]. However, the amount of duckweed used in both reports was low. Egyptian researchers used 5%−100% duckweed powder instead of fish meal to prepare pellet feed for tilapia. The results showed that when the replacement rate was 20%, the weight gain, specific growth rate, feed conversion rate, protein efficiency ratio and other parameters of tilapia had no difference with the control, indicating that duckweed could replace 20% fish meal[11]. Ten percent, 20%, 30% and 40% fermented and unfermented dried powder of giant duckweed was used to feed rohu (Labeo rohita Ham.) fingerlings. In the unfermented feed combination, 10% was the best; in the fermented feed combination, 30% was the best[12], indicating that fermentation could make inclusion rate of duckweed higher.
In this research, we tested 10%−30% replacement of the conventional feed of tilapia, and found that the growth performance of tilapia increased initially and then decreased with the increase of the subsititution level, and the growth status was the best when the replacement level reached 20%. This result is similar to previous research[56], in which dry powder of duckweed Wolffia arrhiza was added to the diet of goldfish (Carassius auratu) in different ratios (0%−40%), and 20% of subsititution obtained the best protein efficiency and minimal food conversion ratio, and 30% subsititution resulted in the maximal weight gain and specific growth rate[56]. When the subsititution was more than 30%, the protein efficiency of feed decreased and the food conversion ratio increased[56]. Another study also pointed that tilapia made more efficient use of duckweed when the feeding amount did not exceed 4% of the fish body weight[10].
Statement of animal rights
-
Animal studies were approved by the ethics committee of Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural sciences and were therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
This research was funded by Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences under grant number 1630052020022, the International Science and Technology Cooperation Program of China under Grant number 2010DFA62040.
-
The authors declare that they have no conflict of interest.
-
Received 31 July 2022; Accepted 8 November 2022; Published online 30 November 2022
-
A domesticated duckweed variety DW2602 rich in protein and essential amino acids was obtained.
This variety was grown in full-scale fish pond wastewater and accumulated biomass.
20% substitution of conventional feed by duckweed promoted the growth of tilapia fingerlings.
The digestive enzyme activities were activated by duckweed.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press on behalf of Hainan University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Han B, Chen T, Yu B, Ren Y, Long Y, et al. 2022. Nutritional value of domesticated duckweed variety DW2602 and its feeding effects on the growth performance and digestive activities of tilapia fingerlings. Tropical Plants 1:11 doi: 10.48130/TP-2022-0011
Nutritional value of domesticated duckweed variety DW2602 and its feeding effects on the growth performance and digestive activities of tilapia fingerlings
- Received: 31 July 2022
- Accepted: 08 November 2022
- Published online: 30 November 2022
Abstract: Duckweed are a family of fast-growing aquatic plants, in which wild plants of many species have been tested in animal feed. We domesticated a duckweed variety, Spirodela polyrhiza DW2602, which had 44.5% protein and 2.6% lysine and was grown on a large scale. The effects of DW2602 on the growth performance and the digestive enzyme activities of tilapia variety GIFT (Genetic Improvement of Farmed Tilapia, Oreochromis niloticus) fingerlings were evaluated. The dry powder of DW2602 was added to conventional tilapia feed at six inclusion levels of 0%, 10%, 15%, 20%, 25%, and 30%. The diets were fed to tilapia fingerlings with an initial weight of 2.0 ± 0.15 g for 60 d. Results showed that DW2602 improved the growth performance and digestive enzyme activities significantly. The feeding effect was the best when the inclusion rate was 20%, in which the body weight increased to 37.24 ± 1.21 g in 60 d, 18.15% higher than the control (P < 0.05). The weight gain rate, the specific growth rate, the condition factor, and the hepatosomatic index of the fish were all significantly higher than the control (P < 0.05), and the amylase, protease, and lipase activities in the digestive system were all promoted by DW2602 (P < 0.05). Therefore, the duckweed variety DW2602 has great application prospect in tilapia culture.
-
Key words:
- Spirodela polyrhiza /
- Feed /
- Fish diet /
- Aquaculture /
- Tilapia /
- Protease /
- Lipase.