-
Cucumber (Cucumis sativus L.), with a total cultivation area of 1.98 million hectares in 2020, is popular worldwide for its crispy texture and special flavor[1,2]. Cucumber fruit is rich in minerals and nutrients, including calcium, protein, iron, and vitamins and thus provides numerous health benefits to the human body[3]. Cucumber fruit also supplies polyols, flavonoids, and polysaccharides with antioxidant properties, which help scavenge free radicals, delay aging, and boost immunity and mental health[4,5]. Cucumber fruit contains Cucurbitacin B and C that inhibit the growth of various tumors and cerebral reperfusion injury and protect the liver from inflammation[6,7]. In addition, the wild cucumber fruit has been used as a herbal medicine in several health-related products[6].
Auxin was first isolated from maize in 1941[8]. Initially, it was proposed as a mobile molecule regulating the phototropic growth of coleoptiles. Since then, auxin received great attention in plant biology research as it is involved in every developmental process from embryogenesis to postharvest ripening[9]. In cucumber, auxin is involved in various agronomic trait development and stress responses[10,11]. Auxin participation is largely dependent on its biosynthesis, transportation and signaling. Auxin biosynthesis pathways mainly include IAOx (indole-3-acetaldoxime), tryptamine (TAM), IAM (indole-3-acetamide), and Trp-IPyA (tryptophan-indole-3-pyruvic acid). Among them, the most discussed auxin biosynthesis pathway is the Trp-IPyA pathway. TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS (TAA1) genes begins the conversion of Trp into IPyA. Following that, the irreversible and rate-limiting reaction catalyzed by YUCCA genes (flavin-containing monooxygenases) ensures the decarboxylation of IPyA into IAA[12−16]. PIN and AUX1/LAX are key auxin transporter genes, instrumental in regulating plant phenotype and auxin homeostasis[17−19]. Auxin signaling genes such as AUX/IAA, TIR1/AFB and ARF are responsible for tightly regulating the different processes, including embryo development, seed abortion and fruit setting[20−22].
The present review summarizes recent progress on the auxin regulation of growth and stress response in the cucumber plant. The future directions to develop high-yielding and climate-resilient cucumber lines are also emphasized.
-
Auxin regulates plant development mainly by its biosynthesis, transport, and signal transduction. It is generally regarded as the growth hormone owing to its involvement in almost every developmental process[23]. In cucumber, auxin regulates the development of different organs (root, hypocotyl, shoot, leaf, tendril, flower, and fruit) (Fig. 1). Below, we summarize how auxin regulates the growth of cucumber.
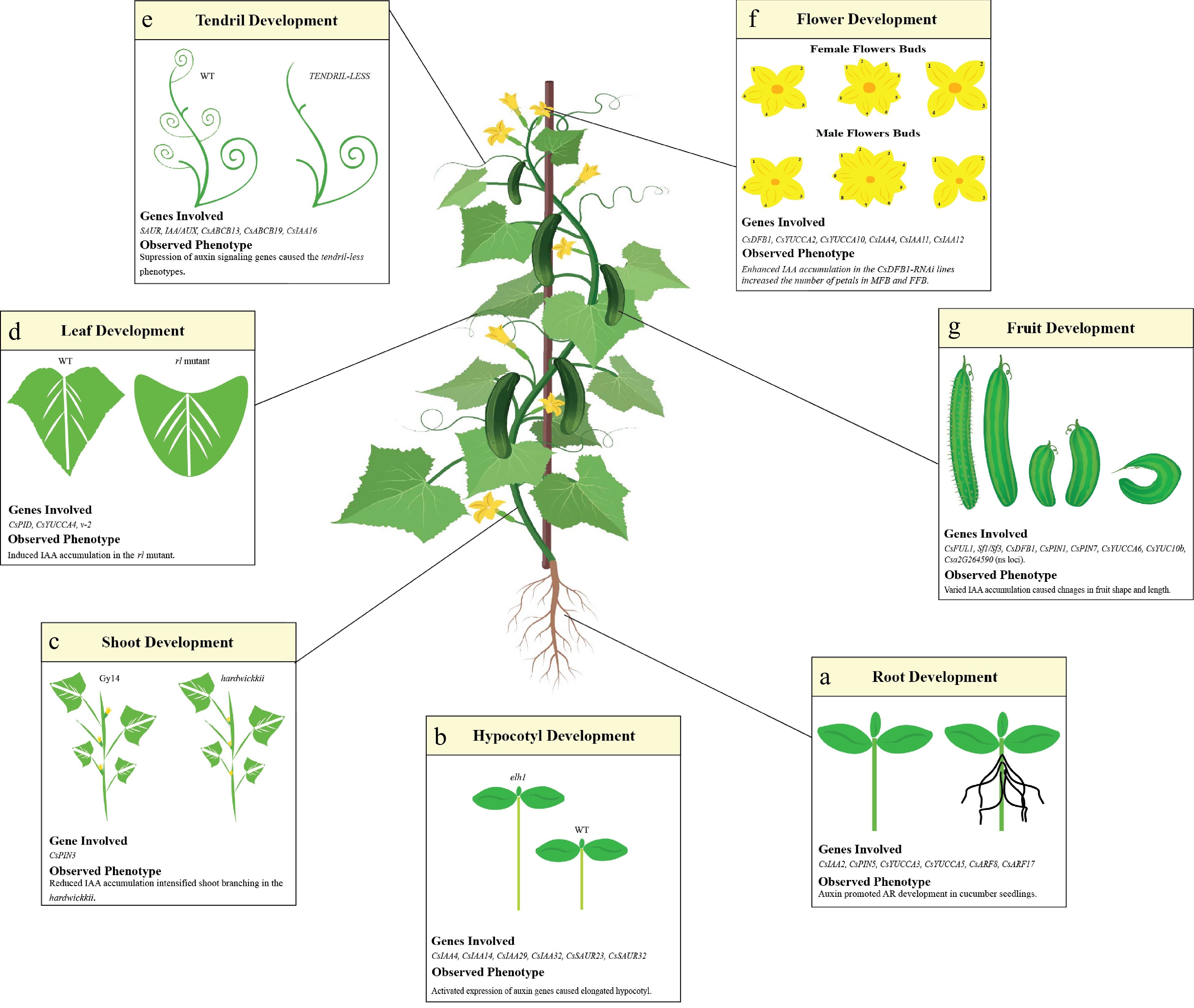
Figure 1.
Auxin mediated cucumber growth and development. Auxin genes involved in the modulation of cucumber developmental stages has been presented in each box. (a) In roots, the auxin signaling genes (CsIAA2, CsARF8, CsARF17), auxin biosynthesis genes (CsYUCCA3, CsYUCCA5) and auxin transporting gene (CsPIN5) are shaping its structure and directional growth. (b) Majority of the auxin signaling genes (CsIAA4, CsIAA14, CsIAA29, CsIAA32, CsSAUR23 and CsSAUR32) are the main drivers of the hypocotyl length. (c) For shoot development, the auxin transporting gene CsPIN3 regulate the IAA accumulation in the wildtype cultivar hardwickii. Reduced IAA accumulation intensified the shoot branching in hardwickii. (d) The upregulation of CsYUCCA4 in v-2 mutant caused the rolled phenotype by inducing the IAA accumulation. (e) Suppression of auxin signaling genes SAUR and IAA/AUX resulted in the tendril-less phenotype in mutant line CG9192. (f) The enhanced expression of auxin biosynthesis genes (CsYUCCA2 and CsYUCCA10) amplified the IAA accumulation, increased the number of petals in male and female flower bud. (g) The higher accumulation of IAA in fruit caused its length to increase. The curved fruit phenotype was caused by the asymmetric distribution of IAA in the concave and convex side of the fruit.
Root development
-
Auxin plays a key role in maintaining the root growth of the cucumber plant. From directional growth to lateral and adventitious root developments, auxin influence is instrumental[24]. Adventitious roots (ARs) and Lateral roots (LRs) arise from the stem and root tissues, respectively, and are pivotal in plant growth and development[25]. A series of research articles have highlighted the auxin role in regulating cucumber ARs and LRs development. Applying gibberellic acid (GA3) to cucumber has significantly induced LR through enhancing auxin biosynthesis genes CsYUCCA3 (Csa3G133910) and CsYUCCA5 (Csa3G619930) and suppressing the auxin inactivation genes CsGH3.3 (Csa3G198490) and CsGH3.5 (Csa3G431430)[26]. Application of methane (CH4) induced AR in cucumber[27]. Amplified expression of auxin signaling genes CsAux22D-like and CsAux22B-like was observed in the CH4 treated cucumber[27]. Exogenously applied glutathione (GSH) promoted AR formation in cucumber by upregulating the expression of CsARF8, CsARF17, CsAux22D-like, and CsAux22B-like[28].
The movement of roots toward water is called hydrotropism[24]. Auxin transporter genes CsPIN5 were reported to regulate cucumber root hydrotropism. The Clinorotation method was employed to analyze the hydrotropic response of cucumber roots. Four hours after hydrosimulation in the microgravity (µG) environment, faster and better root growth was observed on the high humidity side (concave) than on the low humidity side (convex). Elevated expression of CsPIN5 was recorded on the concave compared to that of the convex. The study revealed that under µG conditions, roots become hydrotropically sensitive. This hydrotropic sensitivity of cucumber root is mainly driven by the higher accumulation of the CsPIN5 gene[29]. Another study reported the prominent role of auxin-inducible genes in regulating hydrotropism. The gene CsIAA2 is expressed more dominantly on the concave side than the convex[30].
Hypocotyl and shoot development
-
Hypocotyl length is a key factor in determining the quality of seedlings. Seedlings with moderate hypocotyl length are easy to handle during transplantation and generally grow well. Local auxin biosynthesis in the leaves and cotyledon promote hypocotyl elongation in Arabidopsis[31]. In cucumber, an elongated hypocotyl 1 (elh1) mutant, which showed an elongated hypocotyl because of the longitudinal cells, was identified[32]. CsHY2 (CsGy1G030000) encoding a phytochromobilin (PΦB) synthase was found to be responsible for the elongated hypocotyl phenotype in elh1. Elevated expression of CsHY2 was recorded in elh1 in comparison with WT. RNA-seq analysis of the WT and elh1 revealed the upregulated expression of AUXIN RESPONSIVE GENES (CsIAA4, CsIAA14, CsIAA29, CsIAA32, CsSAUR23, CsSAUR32) in elh1[32].
Shoot branching is an important agronomic trait determining cucumber fruit yield[33]. These branches must be removed manually from the cucumber plant to avoid unnecessary nutrient consumption[33]. Auxin's role in cucumber shoot branching has been investigated in detail[34]. The cucumber line Gy14, in comparison to wild ancestor hardwickii, has relatively small numbers of shoot branches. CsBRC1 (BRANCHED1) exhibited high expression in the auxiliary buds of Gy14 than the hardwickii. CsBRC1 binds to CsPIN3 and suppresses its expression level, thus conferring a low number of shoot branching. The RNAi lines of CsBRC1 generated a high number of branches by increasing the expression level of CsPIN3 and decreasing the IAA accumulation in auxiliary buds[34]. Dramatic inhibition of auxiliary buds was observed in the TIBA (2,3,5-Triiodobenzoic acid) treated hardwickii. The IAA accumulation in the TIBA treated plants was also increased[34]. This revealed the crucial role of the auxin transporter gene in regulating shoot branching.
Leaf development
-
Auxin regulating cucumber leaf development has been reported numerous times[35,36]. Leaf mutation provides the perfect platform in understanding the genetics and genomics of leaf biology. One cucumber round leaf (rl) mutant was identified from an ethyl methanesulfonate-induced mutagenesis population. Genetic screening and MutMap analysis mapped a single recessive gene encoding PINOID kinase protein (Csa1M537400), active in auxin transport, as the candidate gene responsible for rl mutation. The gene displayed relatively lower expression in the rl mutant than in the WT plant. Obvious induction in the endogenous IAA content and the higher expression level of CsYUCCA4 were also recorded in the rl mutant. The auxin transport inhibitor NPA (1-N-naphthylphthalamic acid) -treated plants displayed similar leaf phenotypes to the rl mutant, which further validates the involvement of IAA in leaf development[37]. Auxin was also found to be responsible for regulating the leaf color of cucumber. A single recessive gene v-2 (Csa3G890020) encoding an auxin F-box protein was fine mapped, controlling the virescent leaf that developed yellow cotyledon and upper five true leaves. Auxin signaling and chlorophyll biosynthesis genes were decreased significantly in the v-2 mutant[38]. Overall, these finding elucidates auxin involvement in leaf development and color change.
Tendril development
-
The tendril is a specialized organ arising from the lateral meristem, providing extra support to the main stem and branches. Cucumber has typically branchless tendrils. In response to a touch stimulus, tendrils twine and climb quickly by sensing a thigmotropic signal[33]. Since cucumber cultivation is often carried out in a protected environment, tendrils become a problem by causing disorderly growth and increasing crop management labor[33]. Several tendril-less cucumber mutants have been previously reported[39,40]. Auxin was found to play a key role in regulating the growth and numbers of tendrils in cucumbers. A unique tendril-less mutant was identified from more than 3,000 cucumber lines by Wang et al.[41]. Instead of developing tendrils, the tendril-less mutant line CG9192 forms branches and, in the process, loses its climbing ability. The gene TEN (Csa5G644520) encoding a TCP transcription factor was responsible for the underlying tendril-less phenotype in the CG9192 line[41]. In situ and PCR analysis revealed that TEN was expressed specifically in tendrils. The variation in the protein motif CNNFYFP of TEN impairs the transcriptional activation domain (TAD), hindering the normal tendril development in cucumbers. Transcriptome-wide analysis showed genes downstream of TEN required for sensing and climbing ability[41]. Small auxin up-regulated RNA (SAUR) (Csa7G009080, Csa7G009070, Csa2G258640), and IAA/AUX transcription factors (Csa1G397130, Csa3G143570) were all downregulated in TEN. Also, these genes are preferentially expressed in tendrils. Therefore, it can be assumed that TEN recruits and suppresses the expression of SAUR and IAA/AUX genes in the tendril-less mutant[41]. The CsPID gene controlling the rl mutant phenotype was shown to facilitate the initiation of tendrils in the first 20 nodes[42]. Further, the transcriptomic analysis revealed that auxin transporting CsABCB13, CsABCB19 (Csa3G873270, Csa5G636450), and signaling CsIAA16 (Csa1G397130) genes were significantly downregulated in the rl mutant[41]. Auxin's role in modulating tendril development in cucumbers is influential. The rarity of cucumber tendril-less lines provides grounds for the application of auxin in controlling the growth of tendrils.
Flower development
-
Cucumber flowers are generally small and yellow, with 3-6 petals per blossom. Auxin plays a fundamental role in cucumber flower development. For instance, a cystatin-like protein, CsDFB1 (DEFORMED FLORAL BUD1) was identified as a key gene indirectly regulating local auxin biosynthesis and distribution[43]. The CsDFB1 gene displayed dominant expression in the floral meristem, primordia, and vasculature. RNAi-mediated silencing of CsDFB1 increased the IAA accumulation in the shoot by triggering and suppressing the expression of the CsYUCCA2 and CsPIN1 genes, respectively. Conversely, significantly lower IAA accumulation was observed in the CsDFB1 overexpressed lines. The CsDFB1-RNAi lines had an increase of 20%−80% in floral organ (petals, stamens, and carpels) numbers. CsDFB1 interacted with CsPHB (HD-ZIP III transcription factor PHABULOSA), resulting in the truncated expression of CsYUCCA2 and CsPIN1 in WT plants. Overexpression of CsPHB resulted in a similar phenotype to CsDFB1-RNAi lines[43]. It highlighted the importance of auxin distribution in cucumber flower organogenesis.
Female flowers, on the other hand, determine the fate of cucumber fruit yield. Phytohormone such as ethylene has long been linked with female flower induction[44]. Recently, the participation of auxin in cucumber female flower production has been reported. For instance, blue light-induced female flower by up-regulating auxin biosynthesis and signaling genes in cucumber[45]. A more detailed explanation of the auxin role in female flower induction was presented in two androecious cucumber lines (406an, 406a)[46]. Different concentrations of IAA were used to investigate whether IAA induces femaleness in the 406an and 406a lines. An increased number of female flowers was observed under 50 and 500 mg/L of IAA. However, 500 mg/L IAA suppressed the plant height because IAA over-accumulation caused toxicity. Therefore, 50 mg/L of IAA-treated flowers were used for further experimental work. Post IAA treatment, induction in the endogenous ethylene was also observed. The expression of ethylene biosynthesis genes (CsACS1, CsACS2 and CsACS11) were enhanced whereas sex controlling genes (CsWIP1) were significantly inhibited. The ENHANCER OF SHOOT REGENERATION 2 (ESR2) was also triggered by exogenous auxin. The CsESR2 binds with the promoter region of CsACS2 and activates its expression. Therefore, the application of auxin enhanced femaleness in the cucumber androecious lines via CsESR2-CsACS2 signaling cascade[46].
Male sterility is an important agronomic trait in cucumber hybrid seed production[47]. A normal supply of carbohydrates is important for the development of male gametophytes. In this regard, a sucrose transporter gene, CsSUT1, was functionally characterized in cucumber to understand its role in male sterility[48]. The CsSUT1 silenced plants bare male flowers with abnormal anthers and microsporocytes producing unviable pollens. Compared to WT, the CsSUT1 antisense lines possessed a significantly low starch, sucrose, and hexose level in the male flower buds. Auxin biosynthesis (CsYUCCA10) and signaling (CsIAA4, CsIAA11, CsIAA12) genes were downregulated in the CsSUT1-RNAi lines showing the association of auxin with male sterility[48].
Fruit development
-
Auxins regulate key cucumber traits, including fruit setting, length, shape and trichomes/fruit spines[22]. There are two types of fruit setting, i.e., pollination dependent and parthenocarpic[49]. Auxin has been widely utilized to achieve a successful fruit setting without fertilization[22]. A study by Qian et al.[1] presented the benefits of exogenously applied auxin inducing parthenocarpic fruit in cucumber. Naphthaleneacetic acid (NAA) at 100 mg/L was sprayed on the unpollinated cucumber ovaries. The NAA-treated unpollinated ovaries had successful fruit settings, whereas untreated ovaries were deceased[1]. Similarly, Su et al.[10] used two cucumber inbred lines, namely ZK (weak parthenocarpic) and DDX (strong parthenocarpic). Unfertilized ovaries of ZK failed to grow. On the contrary, unfertilized ovaries of DDX generated normal-sized parthenocarpic fruits. ZK, compared to DDX, was quantified for low endogenous IAA content. Applying 100 mg/L NAA to the unpollinated ovaries of the ZK line produced parthenocarpic fruit[10]. The spraying of sugars (sucrose, glucose, and fructose) on the ZK line caused parthenocarpic fruit induction[50]. The auxin signaling gene CsIAA14 was induced significantly in the sugar-treated ovaries. On the other hand, the auxin degradation gene CsGH3.1 was suppressed post-sugar treatment[50].
Fruit length is a prominent agronomic trait in cucumber breeding. Two alleles (CsFUL1A, CsFUL1C) of the FRUIT (FUL)-like gene were mapped across 150 cucumber lines. The CsFUL1A was enriched specifically in the long-fruited cucumber lines of East Asia (China and Japan). Random distribution of CsFUL1C was observed in the wild and semiwild cucumber lines[51]. Silencing of CsFUL1A resulted in the elongated fruit phenotype. By contrast, overexpressing the CsFUL1A generated shorter fruits. Higher IAA accumulation was observed in CsFUL1A antisense transgenic fruits. Also, an elevated expression level of CsPIN1 and CsPIN7 was observed in the CsFUL1A -RNAi fruits[51]. A natural cucumber mutant with short fruit (sf1) was isolated from the North China-type inbred line CNS2[52]. A total of 15 genes were identified in the 174.3 kb region on chromosome 6. Various auxin signaling genes were downregulated in the sf1 mutant[52]. Genetic analysis showed that the CsKTN1 gene encoding the katanin p60 subunit was responsible for the short fruit3 (sf3) phenotype[53]. Interestingly, no change was noted in the expression of CsKTN1 between WT and sf3. However, suppressed expression of CsYUCCA6 and lowered IAA accumulation were recorded in the cucumber (sf3) mutant[53]. Apart from the cucumber fruit length, its fruit shape is also a key factor affecting the cosmetic appearance and marketability of cucumber fruits. Asymmetric auxin distribution is central to fruit shape. For example, samples were taken from the curved and straight cucumber fruit to measure endogenous auxin levels[54]. Compared to the concave side, high auxin concentration was recorded on the convex side of the curved fruit. No significant difference was recorded in the auxin level on both sides of the straight fruits[54]. This asymmetric distribution in the curved cucumber fruit might be brought about by the impaired local auxin biosynthesis and transport. The application of 0.15 μM NAA inhibited the curvature at 2 d post-anthesis (DPA). Transcriptomic analysis revealed that the CsYUC10b gene level was significantly higher in the convex side in comparison to the concave side. Overexpression of CsYUC10b induced the equal biosynthesis of auxin on both sides and finally produced straight fruits[54]. Overexpression of CsDFB1 suppressed the IAA accumulation by impeding the CsYUCCA2 expression resulting in a curved fruit phenotype, whereas CsDFB1-RNAi lines showed contrasting results for fruit phenotype[43]. Interestingly, CsYUC10b and CsDFB1 had no relationship with the previously identified loci involved in fruit shape[55,56]. Therefore, further genetic work is required to develop markers for fine mapping the QTL and identify major loci regulating cucumber fruit shape.
Fruit trichomes or spines are important traits affecting fruit smoothness, storage, and transportation. Thus far, various fruit spine mutants have been identified, namely tiny branched hair (tbh), microtrichome (mict), and glabrous (csgl1)[57]. Auxin's role in cucumber fruit spine formation has been previously studied. Such as, a numerous spine (ns) locus harboring a Csa2G264590 gene was mapped in the F2 population (numerous fruit spines lines X few fruit spines lines). The Csa2G264590 gene was annotated as the auxin transporter-like 3 and was significantly downregulated in the fruit skin of ns lines. Several signaling genes (Csa7M440550, Csa5M610430, Csa1M397130) were upregulated in the ns lines[58]. More recently, nine loci for cucumber fruit spine density were identified by using genome wide association mapping analysis[59]. Among them, fsdG2.1 showed a closer association with the previously reported ns loci (Csa2G264590). Overexpression of NS generated fruits with lower spine density by increasing the IAA accumulation in fruit peel. On the contrary, CRISPR/Cas9 generated ns-cr lines developed fruit with a high number of spines, and the level of endogenous IAA also plummeted in peel tissue. NPA treatment significantly induced the fruit spine density, confirming the auxin regulation in spine formation[59]. However, Yang et al.[60] discovered that auxin level was unaltered between the two cucumber fruit spine-specific parental lines (S-SB and L-SB). It indicated that other unknown pathways might be regulating fruit spine density, independent of auxin.
-
Some environmental stresses such as drought, waterlogging, salinity, heat, and other biotic stressors inflict serious damage on cucumber growth by disturbing the physiological activities[61−65]. As an essential growth hormone, auxin ensures the proper functioning of plant physiology under stressful conditions. In the subsequent sections, we discuss auxin's role in regulating cucumber stress biology. Summarized information regarding the auxin-mediated stress response in cucumber is presented in Table 1.
Table 1. Auxin participation in regulating cucumber response to multiple stresses.
Stress Auxin activity Functions Reference Heat CsYUC8, CsYUC9 ↑ Enhanced IAA accumulation under high temperature stress. [68] Iron YUC1, PIN1 ↑ Higher expression of PIN1 gene increased cucumber tolerance. [70] Waterlogging IAA accumulation ↑ Boosted AR formation, ethylene accumulation, and expression of CsRBOHB and CsRBOHF3. [71] Cold CsYUCCA2 ↑ Decrease harmful ROS and stress-induced electrolyte leakage. [75] CsARF6 ↑ Activating the expression of cold stress-responsive gene CsDREB3. [76] Salinity AUX/IAA ↓ Reversed the harmful effects of salinity stress. [79] Csa6G104650 ↑ Regulate the silicon-mediated salinity resistance. [81] Drought IAA accumulation ↓ CO2 suppressed the IAA accumulation and boosted GA. [83] Cadmium ABP19a-like ↑ Improve photosynthesis and antioxidant enzyme activities. [85] Powdery mildew CsIAA4, CsIAA6 ↓ Suppressed auxin signaling hinders the pathogenicity of powdery mildew pathogen. [88] Downy mildew IAA accumulation ↑ Positive regulation of the salicylic acid pathway. [91] Nematode Auxin transport ↓ Augmented flavonoid biosynthesis inhibited the formation of the giant cell on the root. [93] Downregulated = ↓, Upregulated = ↑. Abiotic stresses
-
Global climate change events causing the surface and air temperature to rise. Temperature above the optimum level required for normal growth induces heat stress in plants. This causing detrimental and irreversible damage not only to plant growth, but also threatening the world food security by plummeting the overall crop productivity[66]. The phytohormone auxin plays an important role in heat stress-induced thermomorphogenesis, including stem (hypocotyl) elongation and leaf hyponasty[67]. In cucumber, the IAA accumulated in abundance under high temperature stress (38 °C)[68]. The auxin biosynthesis genes CsYUC8 and CsYUC9 also displayed an induced expression pattern under 38 °C[68]. Therefore, it can be suggested that auxin regulates the response of cucumber seedlings to high temperature stress; however, no functional study is available.
Iron (Fe) is an essential micronutrient involved in photosynthesis, respiration, nucleic acid synthesis, protein functions and chlorophyll structure[69]. However, Fe deficit or excess is harmful to plants for normal growth and development and causes a significant yield penalty in terms of quantity and quality. The cucumber plant was grown in Fe deficient soil to understand its response to Fe stress[70]. Increased Gamma-aminobutyric acid (GABA) was observed in the cucumber plants grown in Fe deficient soil. Because of this, exogenous GABA was used over cucumber plants subjected to Fe deficiency. Plant treated with 20 mM GABA suppressed the chlorosis by increasing the expression of iron transporter genes FRO2, IRT1 and HA1. Additionally, GABA application induced the endogenous IAA level by boosting the expression of YUC1 and PIN1 genes. However, the use of NPA, an auxin transport inhibitor, reversed the beneficial effects of GABA[70]. It can be suggested that iron and auxin transporting genes work in concert, thus regulating the response of cucumber to Fe stress.
Excess water blocks oxygen to the root and can cause moderate to severe damage[11]. The development of AR in cucumber is one kind of major phenotype response to waterlogging stress. AR formation largely depends on the local auxin biosynthesis and transportation[71]. For instance, the level of endogenous auxin in the hypocotyl increased at 72 h post waterlogging stress. Applying 10 mg/L of NAA enhanced the AR formation[71]. Further analysis revealed that auxin treatment upregulated the expression of ethylene biosynthesis genes (CsACS1, CsACS2, CsACO5) and reactive oxygen species (ROS) signaling genes such as CsRBOHB and CsRBOHF3 under waterlogging stress. By contrast, NPA treatment substantially hindered AR formation[71]. Exogenous NAA improved the formation of AR; however, elongation was unnoticed. AR elongation has been achieved by sugar treatment, as revealed by the study[72]. The removal of shoots inhibited the AR formation and elongation. Sugar treatment (300 µL) positively modulated the AR formation and elongation by strongly up-regulating the expression of CsPIN1, CsPIN1b, CsPIN8, CsARF5, CsARF6, and CsSAUR29[72]. Interestingly, no significant difference in ethylene biosynthesis genes was noted. However, the previous research did not separate the AR emergence and elongation. Further study focusing on these two different processes might explain the exact role of auxin in each stage.
Cold stress mainly confines plant growth by causing chilling injuries to the tissue. Cucumber is highly sensitive to cold stress, and the plant will stop growth under 15 °C[73]. QTL named qLTT6.2 was identified in the F2 population of 'CG104 (LT-tolerant inbred line) and 'CG37' (LT-sensitive inbred line). Two candidate genes (Csa6G445210, an auxin response factor, and Csa6G445230, an ethylene-responsive transmembrane protein) were fine mapped in the 42-kb interval region of qLTT6.2. Compared to CG37, the dominant expression of Csa6G445210 was recorded in the CG104 line[74]. Though, the study failed to provide any detail about crosstalk of auxin and ethylene in the CG104. A study by Zhang et al.[75] explained the role of endogenous IAA in cucumber plants subjected to cold stress. The 1.0 mM sodium hydrosulfide (H2S) application enhanced cucumber tolerance to cold stress. Post H2S treatment, a sharp increase in the endogenous IAA content was observed. The transcription of auxin biosynthesis gene CsYUCCA2 was also triggered in the H2S treated cucumber plants. Conversely, NPA application significantly compromised the cucumber defense against the cold by decreasing the endogenous IAA and H2S contents[75]. Similarly, H2S treatment to cucumber boosted the expression of CsARF6, an auxin-responsive gene. Overexpression of CsARF6 confers cucumber plant tolerance to cold stress by augmenting the endogenous IAA and H2S levels. Molecular analysis revealed that CsARF6 bind to the promoter region of CsDREB3 (DEHYDRATION-RESPONSIVE ELEMENT-BINDING 3) and transactivate its expression[76].
Salinity/salt stress is the second biggest abiotic factor affecting agricultural productivity worldwide by damaging numerous physiological, biochemical, and molecular processes[77,78]. Cucumber is extremely sensitive to salinity stress. Auxin involvement in enhancing cucumber tolerance to salinity has been recently documented. At the seedling stage, the cucumber cultivar 'Jinyou 1' was subjected to 100 mM NaCl[79]. Transcriptomic analysis revealed numerous differentially expressed genes. Among them auxin signaling genes SAUR (LOC105436055), Aux/IAA (LOC101219209, LOC101217817), and GH3 (LOC101208132) displayed a downregulated trend[79]. Silicon application has been proved to be pivotal in alleviating the detrimental effects of salinity stress[80]. Cucumber seedlings were subjected to 75 mM NaCl stress. Silicon at the rate of 0.3 mM was added to the nutrient solution. Meanwhile, silicon addition to nutrient solution significantly minimized the harmful effects by augmenting the stomatal conductance, net photosynthesis rate, and dry weight of fully expanded cucumber leaves. The auxin-induced protein 5NG4-like (Csa6G104650), a key molecule transporting gene, was upregulated in silicon-NaCl treated seedlings[81]. It can be assumed that auxin signaling genes are key in cucumbers' silicon-mediated salinity tolerance. However, functional studies are missing to elucidate the underlying mechanism.
Similarly, drought stress adversely affects agricultural productivity worldwide and is expected to rise in the coming years[82]. There are only a few auxin-related studies on drought stress in cucumbers. For instance, cucumbers have been subject to drought stress along with CO2 to understand its effects on root biology. Drought stress alone inhibits the root growth and root biomass. CO2 enrichment reversed the adverse effects of drought stress on the cucumber plant by regulating the endogenous phytohormones. IAA accumulation decreased in the drought, and CO2 treated cucumber roots. GA, on the other hand, induced significantly. Auxin may work downstream of GA in regulating cucumber response to drought stress[83]. However, functional studies are lacking that highlight the constitutive role of auxin in cucumbers under drought stress.
Increasing industrialization has increased heavy metal content in air and soil. Heavy metals cause injury to plant cells and cause the malfunction a variety of physiological processes[84]. Studies on auxin regulating the response to heavy metals in plants have been reported; however, not enough literature is available on cucumber. A recent study highlighted the role of auxin in cucumber response to cadmium stress[85]. The application of 3 µM selenium to cadmium stressed cucumber was performed. Selenium application significantly inhibited the detrimental effects of cadmium. Auxin binding protein (ABP19a-like) showed abundance in the selenium-treated seedlings compared to the control[85]. However, further functional studies are required to rewire the auxin involvement to mitigate the stress of the cadmium or other heavy metals.
Biotic stresses
-
Modern research has emphasized the role of auxin homeostasis in plant-pathogen interconnections. Below, we have briefly discussed the role of auxin in regulating cucumber response to various biotic stresses.
Powdery mildew is Cucurbitaceae's most devastating fungal disease, causing serious damage to the yield. The Podosphaera fusca (Fr.) and Sphaerotheca fuliginea are the main fungi causing powdery mildew in cucumber[86]. So far, no functional study has characterized the direct involvement of auxin in mitigating powdery mildew stress in cucumbers. However, RNA-seq studies have highlighted the participation of auxin in regulating cucumber response to powdery mildew. For instance, transcriptomic analysis was applied to two cucumber lines XY09-118 (resistant) and Q10 (susceptible). Two auxin-responsive protein genes, six auxin-induced proteins, and one auxin efflux carrier gene were downregulated in XY09-118 compared to Q10[87]. Similar downregulation of auxin signaling genes was recorded in the powdery mildew resistant line 'BK2'[88]. From the studies, auxin signaling genes could be involved in the negative regulation of plant immunity. The recent work of Navarette et al.[89] also supported this notion. Functional studies will expand our understanding of auxin signaling in cucumber immune response to powdery mildew stress.
Downy mildew caused by a biotrophic fungus Pseudoperonospora cubensis, is another serious fungal disease in cucumbers. Jasmonic acid and salicylic acid are key in regulating the response of plants to biotrophic fungus[90]. However, studies relating to auxin participation in the defense mechanism against P. cubensis are rare. The irregular vasculature patterning (CsIVP) is a transcription factor from basic Helix-Loop-Helix (bHLH) family[91]. Post P. cubensis infection, the mRNA level of irregular vasculature patterning (CsIVP) reduced significantly. The CsIVP interacts with NIM1-INTERACTING1 (CsNIMIN1), a key suppressor of the salicylic acid pathway. Knockdown of CsIVP increased resistance against P. cubensis by denying CsNIMIN1 from suppressing the production of salicylic acid. Interestingly, the IAA accumulation in the leaves of CsIVP-RNAi lines was boosted many fold. By contrast, a low level of IAA accumulation was observed in the WT leaves which also showed increased susceptibility to P. cubensis[91]. There may be synergistic crosstalk between auxin and salicylic acid to regulate the response of the cucumber plant to downy mildew.
The root-knot nematode such as Meloidogyne incognita is a serious parasite, infecting the roots of almost all land plants. In cucumber, Meloidogyne incognita causes massive losses to overall yield by infecting the root and disturbing the hormonal pathways[92]. Despite the large germplasm, very few Meloidogyne incognita cucumber resistant lines have been developed. The Meloidogyne incognita generally make gigantic galls on the roots via parasitism. The cucumber line IL10–1 displayed resistance against Meloidogyne incognita by inhibiting the development of giant cells on the root[93]. Compared to CC3 (susceptible line), the enriched flavonoid biosynthesis pathway 3 d after Meloidogyne incognita inoculation was observed in IL10-1. Curtailed auxin transport in the roots of IL10-1 was also recorded. The induced flavonoid biosynthesis could be involved in the restricted auxin transport, which resulted in the inhibition of giant cells on cucumber root, thus conferring resistance to Meloidogyne incognita[93]. However, the study failed to explain how auxin transport regulates gall formation on cucumber roots.
Conclusions
-
Auxin plays an important role in cucumber growth and stress response, and the application of auxin in cucumber production is very popular in farming operations. For example, auxin control of branch numbers and parthenocarpy in cucumber are key factors significantly reducing labor and increasing the overall production. In the last decade, extensive genetic studies in cucumbers have identified key genes regulating agronomic traits by directly or indirectly modulating auxin. Nonetheless, the auxin-mediated stress response of cucumber added to its versatility. Despite the evidence, future research work is required to answer the following queries:
• Identifying the functional genes and QTLs controlling fruit setting and shape and explain its regulatory mechanisms;
• Auxin induces femaleness in cucumbers by activating ethylene biosynthesis. However, the question remains open whether auxin treatment affects other hormones such as GA when enhancing femaleness. It is important to understand these different signaling cascades;
• A preliminary study indicated auxin's involvement in regulating cucumber response to heat stress. It would be interesting to see how auxin minimizes the detrimental effects of heat stress on cucumber, particularly at the fruit setting stage;
• Auxin is still less understood in response to environmental stresses which requires further exploration.
This work was supported by National Natural Science Foundation of China, Grant/Award Number: 31972422, 32272739.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Sharif R, Su L, Chen X, Qi X. 2022. Involvement of auxin in growth and stress response of cucumber. Vegetable Research 2:13 doi: 10.48130/VR-2022-0013
Involvement of auxin in growth and stress response of cucumber
- Received: 28 July 2022
- Accepted: 07 September 2022
- Published online: 30 September 2022
Abstract: Cucumber (Cucumis sativus L.) is an important vegetable, popular worldwide for its crispy texture and special flavor. Plant hormones such as auxin stand out for its dominating function in morpho- and organogenic processes, formation of organs as well as regulation of tropic responses. These developmental processes are entirely, or partially dependent on auxin biosynthesis, transport, and signal transduction. In cucumber, auxin not only fine-tunes its morphogenesis but also its response to environmental stress. The role of auxin in regulating different organs (root, hypocotyl, shoot, leaf, tendril, flower, and fruit) development in cucumber is reviewed in the present paper. Moreover, the role of auxin in cucumber response to biotic stresses (powdery mildew, downy mildew, and nematode infections) and abiotic stresses (heat, iron, waterlogging, cold, salinity, drought, and heavy metal stresses) is discussed. Finally, we point out the blind spots and future research thoughts to extend our understanding of this myriad molecule in cucumber growth and stress biology.
-
Key words:
- Auxin /
- cucumber /
- growth /
- stress tolerance












