-
The atmospheric carbon dioxide (CO2) concentration has increased dramatically, global temperature has increased gradually, and relative humidity has decreased gradually; these changes have resulted in an increase in vapor pressure deficit (VPD) in recent decades[1,2]. VPD affects the water transport from soil to leaves by affecting the water potential gradient of protected vegetables. In the daytime, the optimum VPD for most vegetables is 0.50–1.50 kPa[3,4]. Vegetables experience stress when the VPD exceeds 1.50 kPa. Under high VPD, atmospheric transpiration increases in greenhouses, the water potential gradient and ineffective transpiration of vegetables increase, soil water loss increases, and the water stress experienced by vegetables intensifies[5]. Additionally, the exposure of protected vegetables to long-term high VPD environments affects the absorption of nutrients, induces large-scale plant mortality[6−8], and leads to substantial reductions in fruit yield and quality[9−11]. The temporal stability of certain VPD conditions and diurnal variation in a greenhouse and artificial climate chamber were studied by Zhang et al.[5] and Yu et al.[12], respectively. Protected cultivation via greenhouse and fog generation systems reduces VPD, increases photosynthetic activity, and promotes the water transport of protected vegetables[13,14], which increases crop yield and quality[14,15].
The aim of this review is to clarify the effects of VPD on the water regulation, anatomical structure, stomatal morphology, photosynthetic physiology, nutrient accumulation, yield, and quality of protected vegetables. We discuss the effects of changes in VPD on water transport dynamics, the anatomical structure of plants, stomatal morphology, photosynthetic rate (Pn), and nutrient accumulation of protected vegetables. Over long periods, VPD plays an important role in regulating the yield, quality, and water use efficiency of protected vegetables. Although VPD affects the physiology and productivity of protected vegetables, increases in VPD are usually accompanied by changes in other environmental conditions (including reductions in soil moisture, increases in the atmospheric CO2 concentration, increases in light, and reductions in precipitation). Therefore, the effects of VPD on vegetables are affected by other environmental parameters. The studies discussed in this review were carried out in greenhouses in a controlled environment. VPD can affect plant physiology and productivity independently of other environmental factors. This review provides information that can be used to evaluate the effect of VPD on the physiology and productivity of protected vegetables.
-
Water is transported along the water potential gradient in the soil-plant-atmosphere continuum (SPAC) (Fig. 1)[4,16]. Water flows in each system to form a unified whole. The water potential is used to quantitatively study energy changes in each system. Water absorption, transport, and transpiration need to overcome various sources of resistance (e.g., the soil capillary force, water gravity, and protoplast and apoplast transport resistance)[17]. Normal water metabolism is inhibited if water transport dynamics are insufficient. Root pressure and transpiration are usually the main drivers of water transport[18]. VPD represents the atmospheric evaporation capacity, which is directly related to the potential energy distribution and water flow driving force in SPAC systems[19−21]. The water potential gradient at the leaf–air boundary reaches 48–170 MPa whether tomato plants are under adequate irrigation or deficit irrigation, which is greater than the water potential gradient at the soil–stem boundary (0.08–0.35 MPa) and the water potential gradient at the stem–leaf boundary (0.08–0.21 MPa). The water potential gradient between leaf and air is more than 100 times that between soil and leaf under both adequate irrigation and deficit irrigation, which means that transpiration is the main driver of water transport in vegetable crops[4,12]. The water potential gradient at the leaf–air boundary is key for regulating water transport in SPAC systems, and the water potential gradient at the leaf–air boundary is three times higher under high VPD (2.22 kPa) than under low VPD (0.95 kPa)[12], which indicates that reducing VPD can reduce the atmospheric evaporation demand, ineffective transpiration, and the water potential gradient at the leaf–air boundary, thereby increasing the water potential and water status of plants.
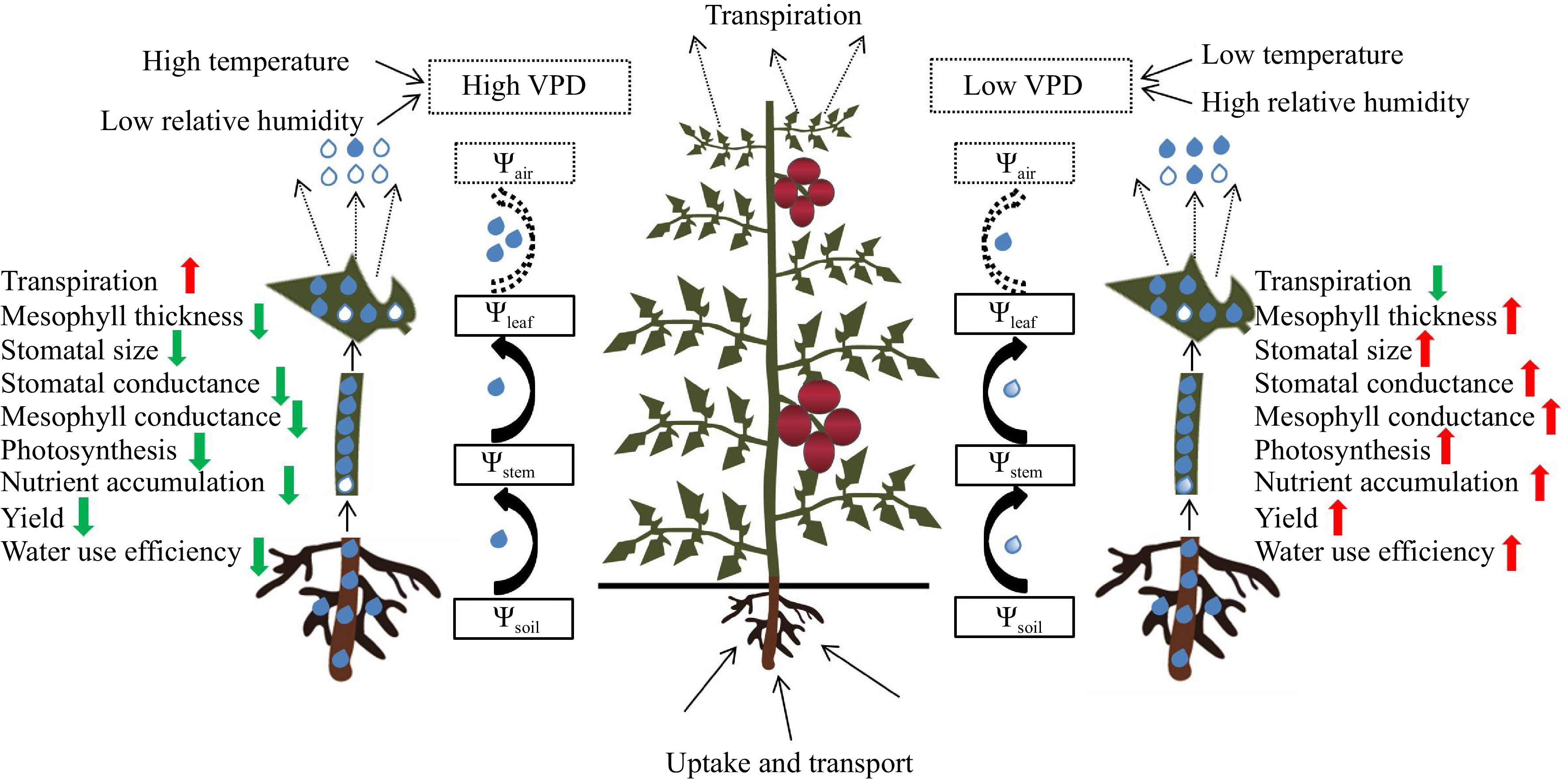
Figure 1.
Effects of the vapor pressure deficit (VPD) on the regulation of water transport along the soil-plant-atmosphere continuum (SPAC) for vegetable crops, as well as its effects on leaf transpiration and plant behavior (adapted from Amitrano et al.[[13]]). The solid black arrows represent the liquid water transport between the soil, stems, and leaves, and the black dotted arrows represent the gaseous water transport between the leaves and the air. Ψair, Ψleaf, Ψstem, and Ψsoil, represent the water potential in air, leaf, stem, and soil, respectively.
No significant difference in leaf water potential before dawn, both under low VPD (1.20 kPa) and high VPD (ranging from 2–5 kPa), was observed under adequate irrigation[22]. Transpiration in vegetables is weak before dawn, the force driving water potential is approximately zero, and the water potential between the substrate and the leaves is approximately balanced. Therefore, the leaf water potential before dawn reflects the soil water status[12]. The leaf water potential decreases with increases in light radiation and the transpiration rate in the daytime under low VPD (1.20 kPa) and high VPD (ranging from 2–5 kPa) under adequate irrigation, and the leaf water potential is lowest around noon; the leaf water potential gradually increases thereafter[5]. This shows that the water potential of tomato gradually decreases as the magnitude of VPD and temporal stability of VPD increase, especially around noon when VPD is high (approximately 5 kPa). Reducing VPD mitigates declines in leaf water potential, and the daily pattern of variation in the leaf water potential is relatively stable; this is particularly obvious around noon[5].
The hydraulic conductance of vegetable crops varies with the water potential and transpiration rate under different VPD treatments, but this change is usually related to the drought tolerance of vegetable crops[23]. The hydraulic conductance of the leaves and individual plants is significantly higher at different growth stages under low VPD (1.20 kPa) than in natural environments (ranging from 2–5 kPa); the hydraulic conductance also first increases and then decreases with growth. This shows that reducing VPD at different growth stages can alleviate hydraulic constraints caused by atmospheric drought[5,22]. The turgor potential of the leaves and the hydraulic conductance of plants are significantly higher under low VPD (1.20 kPa) under both adequate irrigation and deficit irrigation than in natural environments (ranging from 2–5 kPa), but the increase in the plant osmotic potential was not significant. This indicates that the reduction in VPD mainly increases the hydraulic conductance of plants by increasing the leaf turgor potential[4]. Aquaporins play an important role in mediating water transport in cells, and the cytoplasmic calcium concentration regulates the opening and closing of aquaporins and calcium ion (Ca2+) channels[16]. When the cytoplasmic calcium concentration is low, aquaporin and Ca2+ channels open, allowing water and Ca2+ to enter the cell[16]. When the concentration of cytoplasmic calcium is high, aquaporin and Ca2+ channels are closed to prevent the excessive accumulation of cytoplasmic calcium[24]. Tonoplast intrinsic proteins (TIPs) and plasma membrane intrinsic proteins (PIPs) are common aquaporins. The expression of SlTIPs and SlPIPs in tomato leaves is up-regulated under high VPD (2.22 kPa) to compensate for the decrease in the leaf water deficit induced by high VPD[12].
-
Leaves undergo pronounced structural changes during long-term drought adaptation, as they are important organs for sensing changes in the VPD (Fig. 2). In general, increases in the thickness of the leaves and spongy tissues are conducive to reductions in transpiration, increases in the water storage capacity, and increases in crop drought tolerance[25]. Therefore, vegetables growing under high VPD (2.22 kPa) for long periods under adequate irrigation have thicker leaves and spongy tissues compared with those grown under low VPD (0.95 kPa) to reduce transpiration water consumption and increase water storage capacity[12]. However, this varies among crops. The leaf thickness and spongy tissue thickness of cucumber and melon are lower under high VPD (ranging from 2–6 kPa) under adequate irrigation than under low VPD (1.50 kPa), which might be related to variation in the suitable VPD ranges among crops[2]. Palisade tissue is the main site of photosynthesis, and higher palisade tissue thickness is conducive to increases in photosynthesis. Higher palisade tissue thickness and palisade tissue thickness/spongy tissue thickness have been observed under low VPD, and the high thicknesses of these tissues provide sufficient sites for photosynthetic carbon assimilation[2,12,26]. Generally, a high density of veins facilitates the transport of water to all parts of the leaves[27]. High atmospheric evaporation demand under high VPD (2.22 kPa) compared with under low VPD (0.95 kPa) causes water to evaporate into the atmosphere quickly, which reduces water transport in the leaf veins, leaf vein density, and the leaf relative water content[12]. The mesophyll structure determines the diffusion pathway of CO2 in mesophyll tissue. According to the one-dimensional diffusion model of CO2 in mesophyll tissue, the mesophyll conductance (Gm) can be divided into two parts: gas phase conductance and liquid phase conductance[28]. The fraction of mesophyll tissue occupied by intercellular air spaces (fias) and the mesophyll thickness (Tmes) determine the path length of CO2 diffusion from the stomatal cavity to the outer surface of the cell wall[29,30]. Therefore, the diffusion conductance of CO2 in the gas phase is largely affected by fias and Tmes. The response of Gm to environmental changes is mainly regulated by liquid phase conductance. The surface of mesophyll exposed to intercellular air spaces (Sm/S) is the main structure affecting the diffusion of CO2 in the liquid phase. Decreases in VPD (1.48 kPa) increase Sm/S and CO2 diffusion conductance in the liquid phase compared with high VPD (2.55 kPa) under adequate irrigation, which increases the Gm and photosynthesis of protected vegetables. The diffusion of CO2 from outside the cell wall to inside the chloroplast is determined by the structural characteristics at the organelle level[28,31]. Cells are closely arranged under high VPD (2.55 kPa), which results in a reduction in the effective contact area between CO2 and the chloroplast and the amount of CO2 entering the chloroplast. Additionally, the distance between the chloroplast and the cell membrane increases under high VPD (2.55 kPa), which lengthens the diffusion path of CO2 in the cytoplasm, increases the resistance of CO2 transport into the chloroplast, and results in a reduction in Gm and Pn[31].
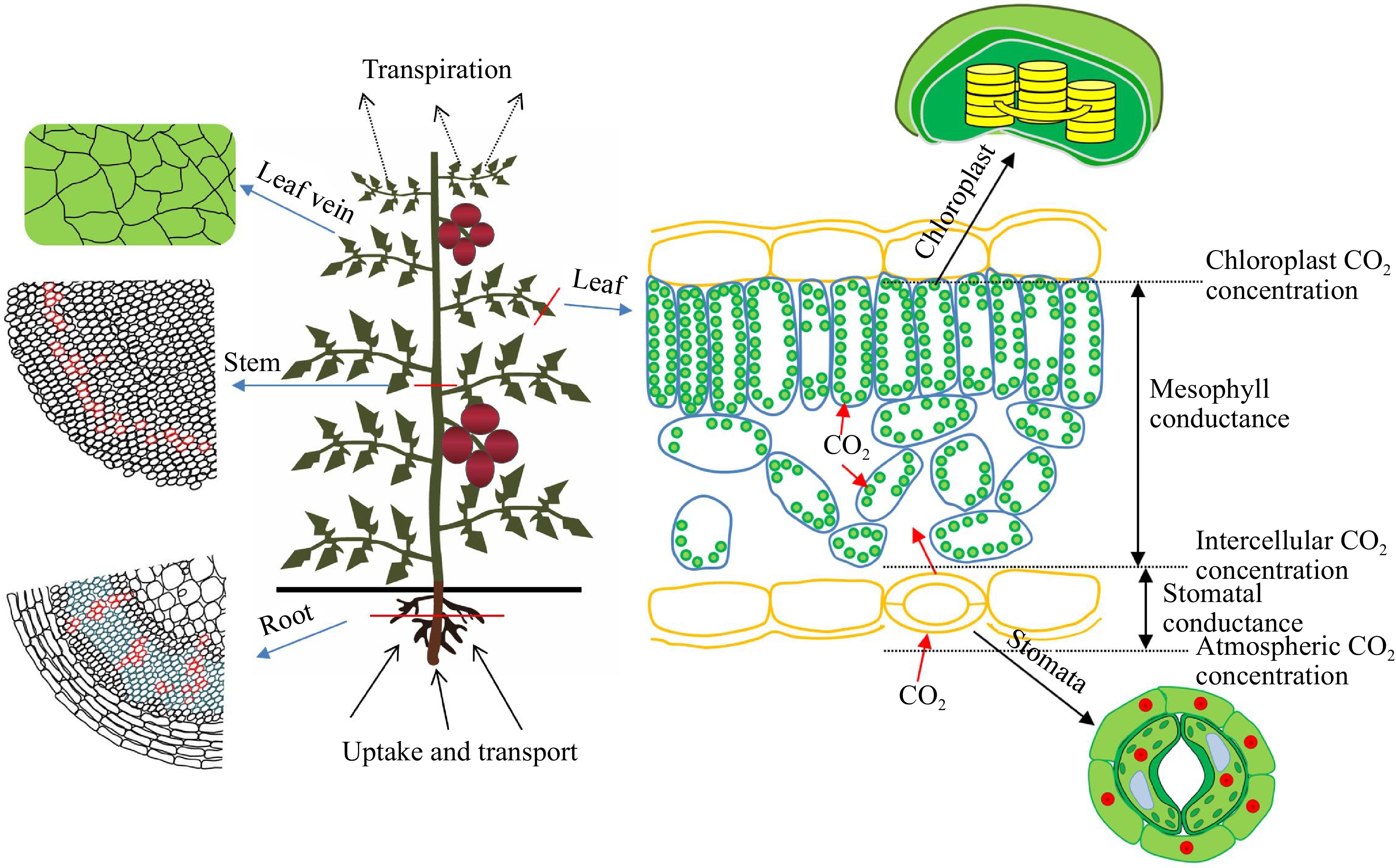
Figure 2.
Schematic model for moderating effects of low VPD on water transport by increasing xylem vessel area and leaf vein density and on photosynthetic limitation by decreasing stomatal and mesophyll CO2 diffusion resistance. There are three main processes. (I) Reducing VPD increases the leaf vein density and the cross-sectional area of xylem vessels in roots and stems via the long-term optimization of plant structure. (II) Reducing VPD reduces stomatal resistance and maintains stomatal openness by reducing excessive transpiration and moderating plant water stress. (III) Reducing VPD reduces mesophyll resistance by reducing the average distance from the cell membrane to the outer membrane of the chloroplast and increasing the number of chloroplasts in a single mesophyll cell.
Although the water transport resistance is highest in the leaves, the stems and roots also play an important role in water transport resistance, which accounts for approximately 40% of the total resistance[23]. Excessive negative air water potential interrupts long-distance water transport through xylem cavitation and xylem embolism under high VPD[32,33]. In general, reductions in the cross-sectional area of xylem vessels can effectively reduce the risk of xylem cavitation and xylem embolism[32,34,35]. Therefore, the xylem vessel cross-sectional area decreased in the stems and roots under high VPD (2.22 kPa) under adequate irrigation. Low VPD (0.95 kPa) increases the cross-sectional area of xylem vessels in the roots and stems, reduces the risk of xylem cavitation and embolism, and increases water transport[12,36,37].
-
Stomata are small pores formed by two guard cells on the epidermis of leaves that control leaf temperature, water transpiration, and photosynthesis[38,39]. The stomata open when the guard cells absorb water, and stomatal movement is mainly regulated by plant water status and the surrounding environment[40]. Rapid increases in VPD lead to decreases in stomatal size and stomatal conductance (Gs). In seed plants, Gs temporarily increases within 2–25 min of increases in VPD; that is, the stoma will 'suddenly open' before closing[41,42]. This transient response is derived from the decrease in the stomatal pressure of epidermal cells under high VPD, and the steady-state response is derived from the increase in the water potential in guard cells caused by ion efflux, the increase in water loss in guard cells, and the reduction or even closure of the stomata[43]. There is no consensus on the exact mechanism by which increases in VPD induce stomatal closure; physiological and metabolic changes might be induced by the perception of changes in VPD by cells in leaves, and hormone signals such as abscisic acid (ABA) might also play an important role[43−45]. The transpiration rate is the product of Gs and VPD under the same boundary layer conductance of the leaves, and the magnitude of the increase in VPD is far greater than the magnitude of the decrease in Gs, which results in an increase in the transpiration rate under high VPD[46]. A higher transpiration rate results in lower turgor of guard cells and higher water loss. Evaluation of the response of transpiration and Gs to VPD has revealed that stomatal closure is the result of increased transpiration through stomata after signals associated with VPD changes are perceived by guard cells. This result confirms that increases in transpiration are proportional to increases in VPD, and increases in leaf transpiration contribute to decreases in leaf temperature[47].
The sensitivity of the stomata to environmental change is enhanced under high VPD, which alleviates the effect of drought stress on vegetable growth[40,48,49]. In angiosperms, the response of the stomata to changes in leaf turgor is mediated by ABA, which stems from the slight decrease in leaf turgor and triggers the rapid synthesis of ABA in the leaves; this is followed by stomatal closure within 10 - 20 min[45,50]. This turgor-mediated ABA synthesis indicates that ABA plays an important role as a metabolic signal in the hydraulic conduction of leaf guard cells[43,51]. The ABA signal pathway comprises ABA receptors, type 2C protein phosphatase co-receptors (PP2Cs), and SnRK2 protein kinases (including OST1). ABA combines with receptors to form receptor-ABA-PP2C complexes, which lead to the inactivation of PP2Cs to activate SnRK2 protein kinases[52,53]. Additionally, OST1 activates the downstream chronic anion channel SLAC1, which triggers stomatal closure[39]. Some studies have shown that ABA is not necessary for the stomatal VPD response because the response of an ABA-deficient and ABA-insensitive Arabidopsis thaliana mutant to VPD is similar to that of wild-type plants[54]. In another study, the final Gs value (expressed as a percentage of the initial value) after increasing VPD was 32%, 55%, and 53% in aba2-13, ost1-4, and wild-type plants, respectively, indicating that stomatal closure may only be partially dependent on ABA[55]. Additionally, research has shown that ABA is a highly mobile molecule that affects plant growth through short- and long-distance signal transduction[56−58]. The stomatal response of plants to ABA decreases under low VPD, which may be related to the ease of ABA to be decomposed in leaves under low VPD[38]. The ABA level in the leaves of broad bean plants is lower when they are grown under low VPD (0.23 kPa) than under high VPD (1.17 kPa)[59]. Additionally, when plants grown under high VPD are transferred to low-VPD environments, the ABA level decreases sharply, which confirms that low VPD promotes the decomposition of ABA. Additionally, previous studies have shown that protein kinase OST1 is partially independent of ABA, and OST1 rather than the ABA concentration plays a role in the VPD-induced stomatal response[60]. The ABA concentration of guard cells or phloem companion cells is increased to activate protein kinase OST1 and induce stomatal closure under high VPD. Stomatal closure is also induced by a passive hydraulic regulation mechanism[60].
There is a positive correlation between water transport and stomatal conductance and size[61,62]. Higher stomatal conductance and size result in higher water transpiration and water transport[63,64], as well as higher CO2 absorption and photosynthesis[65,66] under the same environmental conditions. Higher stomatal conductance and size also increase the transpiration of leaves, which promotes the passive transport of water[48]. Increases in photosynthesis increase the accumulation of photosynthetic products in leaves, reduce the water potential in leaves, and increase the transport of water to leaves[67]. Stomatal conductance and size are higher under low VPD (0.95 kPa) than under high VPD (2.22 kPa), which results in increases in CO2 absorption, Pn, and water transport under adequate irrigation[12].
-
Photosynthesis plays a key role in primary metabolism and is affected by many environmental factors such as temperature, light, VPD, and soil moisture[68−70]. VPD represents the atmospheric water deficit and is one of the most important environmental factors affecting photosynthesis[71]. Vegetables growing under high VPD usually have a lower Pn under adequate irrigation[14,15]. CO2 diffuses from the atmosphere to chloroplast carboxylation sites for photosynthesis through stomata and mesophyll[72]. Many studies have shown that the reduction in Gs under high VPD (ranging from 2–6 kPa) leads to a reduction in the intercellular CO2 concentration (Ci), thereby limiting the Pn of leaves[14,73]. Studies of the effect of VPD on Gm have shown that Gm decreases or does not significantly vary with increases in VPD[74−77]. VPD can also regulate the movement of chloroplasts. Chloroplasts are located closer to the cell membrane under low VPD (1.48 kPa) than under high VPD (2.55 kPa), which reduces the distance between the cell membrane and the outer membrane of chloroplasts and the distance between adjacent chloroplasts; this results in reductions in cytoplasmic resistance in the process of CO2 transport and improves the efficiency of CO2 carboxylation[31]. The surface of chloroplasts exposed to the intercellular space is higher under low VPD (1.48 kPa) than under high VPD (2.55 kPa), which increases Pn[31]. Additionally, chloroplast movement is closely related to light intensity and light quality under different VPD conditions. Therefore, the effects of light intensity and light quality on chloroplast movement under different VPD conditions require further study.
Reductions in VPD can increase the Pn in various plant species, including tomatoes[14,15], ferns[78], and white birches[79]. Similarly, reductions in VPD (1.20 kPa) increase the Pn, total diffusion conductance, Gs, Gm, Ci, and intracellular CO2 concentration (Cc) in different tomato cultivars, and the increases are more pronounced under deficit irrigation than under adequate irrigation[4]. This indicates that high VPD (2.55 kPa) inhibits photosynthesis, increases resistance to CO2 diffusion, and reduces the available CO2 concentration in leaves[31]. Deficit irrigation can exacerbate the inhibition of high VPD (ranging from 2–5 kPa) on plant growth[4]. Photosynthesis can use light energy to convert inorganic CO2 into organic matter, which provides material and energy needed for plant growth. The internal regulation of leaf photosynthesis is mainly affected by the concentration of CO2 and the carboxylation metabolic activity. CO2 from the atmosphere needs to pass through stomata and mesophyll tissue to reach chloroplast carboxylation sites. The diffusion resistance of CO2 in these two parts is called stomatal resistance and mesophyll resistance[80]. The reciprocal of stomatal resistance and mesophyll resistance is Gs and Gm, respectively. In photosynthesis, changes in Gs and Gm indicate changes in CO2 diffusion from the atmosphere to chloroplast carboxylation sites. Generally, Gs and Gm are strongly correlated, and the ratio between them reflects the degree of environmental stress[66]. High-VPD environments lead to significant increases in Gm/Gs in tomato[81]. Regulating Gs is less costly for vegetables than regulating Gm. The stomatal opening is passively regulated by hydraulic changes. The regulatory process can take as little as a few minutes and as long as several hours. This process does not involve changes in metabolic processes[82,83]. In areas lacking water resources, rapid responses of Gs can effectively reduce water loss, thereby alleviating the tension between water molecules in the xylem and reducing the risk of xylem embolism[84]. Gs is also affected by stomatal density and size. The reduction in stomatal size and density under high VPD indicates reduced investment in stomatal formation[14,85]. Changes in Gm increase the need for photosynthates to be distributed to leaf tissue to a greater degree compared with changes in Gs[86,87], which means that the cost of regulating Gm is higher than the cost of regulating Gs, and the presence of excess photosynthates in leaf tissue does not promote plant growth[31,88,89]. The Gs of vegetables decreases under high VPD, which leads to decreases in Ci. Decreases in Gm limit the CO2 concentration in chloroplasts, thereby reducing Pn[14,90,91].
High VPD results in decreases in tomato Pn. Decreases in Pn under high VPD are mainly caused by stomatal restriction according to previous quantitative analyses of the relative contributions of stomatal restriction, mesophyll restriction, and biochemical restriction to decreases in photosynthesis[74−76]. This shows that Gs is more sensitive and responds more rapidly to changes in VPD than Gm. Gm does not regulate water loss, and reductions in Gs can effectively reduce water loss[66]. Because the amount of water molecules passing through the stomata is approximately two orders of magnitude higher than the amount of CO2 molecules passing through the stomata, changes in Gs can regulate water use efficiency[92]. High VPD (ranging from 2–5 kPa) reduces Gs and Gm, and reductions in Gs are much larger than reductions in Gm. Therefore, high VPD increases stomatal and mesophyll restriction. Furthermore, high VPD (ranging from 2–5 kPa) aggravates stomatal and mesophyll restriction under deficit irrigation; thus, reducing VPD (1.20 kPa) can greatly increase Gs and Gm and reduce stomatal and mesophyll restriction under deficit irrigation[4].
Comparison of the effects of different VPD conditions on the light response curve and CO2 response curve has revealed that the maximum Pn of protected vegetables under CO2 saturation (Pn-CO2) and light saturation (Pn-I) decreases significantly under high VPD (ranging from 2–5 kPa), especially under deficit irrigation[4]. Pn-CO2 is 1.20 times higher than Pn-I under low VPD (1.20 kPa) and 1.09 times higher than Pn-I under high VPD (ranging from 2–5 kPa). This shows that increases in the atmospheric CO2 concentration under low-VPD treatment can promote photosynthesis compared with high VPD. Low VPD (2 kPa) and high CO2 concentrations significantly increase the Pn and yield of vegetables under adequate irrigation in a greenhouse environment compared with high VPD (ranging from 2–6 kPa)[93]. Low VPD (1.20 kPa) promotes the opening of the stomata, reduces the CO2 diffusion resistance from the atmosphere to leaves, and provides sufficient substrates for photosynthesis, thus eliminating restrictions on photosynthetic raw materials and improving Pn[22,94]. Therefore, increases in CO2 and the regulation of VPD can increase the photosynthetic capacity of vegetable crops in greenhouse cultivation.
-
VPD indicates the dryness of the atmosphere. Changes in VPD affect water transport from the roots to the leaves, which affects the absorption and distribution of nutrient elements in vegetable crops[16]. A suitable VPD can promote water transport and nutrient absorption. An excessively high VPD can lead to substantial increases in transpiration, and vegetables wither if the root water and nutrient supply are unable to meet the transpiration demand[95]. Additionally, VPD regulates water transport and the absorption and distribution of nutrients by altering stomatal morphology, which affects Gs and the transpiration rate[16]. Therefore, VPD has a major effect on the absorption and distribution of nutrients in vegetables. The transpiration rate and nutrient concentration decrease under low VPD[96−98], which might stem from the positive correlation between the transpiration rate and nutrient absorption[99−101]. Furthermore, the dilution effect caused by the increase in photosynthetic carbon assimilation might also contribute to reductions in nutrient concentrations[102]. However, nutrient accumulation increases under low VPD, which might stem from increases in the root absorption surface area and xylem vessel cross-sectional area, which promotes the absorption and transport of water and nutrients[103,104]. Additionally, nutrient accumulation is the product of nutrient concentration and dry mass. Nutrient accumulation under adequate irrigation might increase when the magnitude of increases in dry mass is far greater than the magnitude of decreases in nutrient concentrations under low VPD[26,105]. Furthermore, increases in atmospheric humidity during the daytime increase the water flux at night, which might contribute to increases in nutrient accumulation[106].
The transpiration rate of protected vegetables decreases but nutrient accumulation increases under low VPD (0.90 kPa), which indicates that increases in root morphology indirectly compensate for the effects of reduced transpiration on nutrient absorption under adequate irrigation. High dry mass also promotes nutrient accumulation under low VPD (0.90 kPa) under adequate irrigation[26,105]. Additionally, the allocation of nutrients to the stems and roots increases and that to the leaves decreases under low VPD (0.63 kPa) under adequate irrigation. This is because a lower transpiration rate increases the retention of nutrients in the roots and stems[46,107]. Additionally, the nutrient content in substrate decreases, and the nutrient absorption in vegetables increases under low VPD (0.90 kPa) under adequate irrigation[26,105]. Furthermore, less energy is required for nitrate to contribute to osmotic adjustment. Increases in nitrate absorption reduce the energy loss of vegetable crops and increase nitrogen absorption and assimilation efficiency under low VPD (0.90 kPa) and adequate irrigation[105]. In high-temperature environments, moderate potassium application under low VPD (1.50 kPa) and high potassium application under high VPD (ranging from 4−5 kPa at noon) can lead to increases in dry mass and nitrogen, phosphorus, and potassium accumulation and alleviate the inhibition of high temperature on photosynthesis[107]. Therefore, reductions in VPD increase nutrient accumulation and reduce the amount of potassium fertilizer. Potassium ions, which are the main osmotic solute in cells, enter the guard cells through potassium ion channels on the plasma membrane, which causes the water potential of the guard cells to decrease; the water is then absorbed by the guard cells, and the stomata are opened. Low VPD (1.50 kPa) can increase potassium accumulation in leaves, increase the leaf water potential, and promote stomatal opening and CO2 absorption in leaves[108]. Furthermore, changes in potassium accumulation and Gs are consistent under different VPD conditions[107]. The long-distance transport of calcium mainly occurs in the xylem. VPD affects calcium transport through its effects on transpiration[108,109]. Calcium transport in vegetables and calcium accumulation in fruits are closely related to transpiration[110]. The transpiration of leaves is much higher than that of fruits, which makes leaves a competitive pool for the directional flow of calcium accumulation in fruits[111,112]. Reductions in leaf transpiration can increase calcium absorption in fruits, thereby reducing blossom-end rot and increasing yield[113,114]. Therefore, low VPD (0.95) can increase calcium absorption in fruits and calcium accumulation in the pericarp under adequate irrigation[12].
-
An appropriate VPD can significantly increase water use efficiency at the leaf, plant, and yield levels under greenhouse conditions[5,12]. Water use efficiency can be expressed in various ways, and the information provided by these different types of water use efficiency varies[115,116]. The leaf instant water use efficiency (WUEinstant) is defined as the ratio of Pn to the transpiration rate. The WUEinstant in vegetables decreases significantly under high VPD (2.22 kPa) and adequate irrigation, indicating that leaves growing under high VPD lose more water than those growing under low VPD, which results in the production of less dry mass[12]. Furthermore, leaf transpiration is directly related to VPD[17]. Therefore, the decrease in WUEinstant under high VPD mainly stems from the increase in transpiration, followed by the decrease in Pn. The intrinsic water use efficiency (WUEintrinsic) can be determined by the ratio of Pn to Gs. WUEintrinsic is high under high VPD (ranging from 2–6 kPa) under adequate irrigation, which indicates that vegetable growth under high VPD increases water use capacity[22,117]. Many studies have shown that WUEintrinsic and Gm/Gs are significantly positively correlated[117,118]. If Gs and Gm are independent of each other, Gm affects WUEintrinsic, but if Gs and Gm are non-independent, Gm does not have a major effect on increases in WUEintrinsic[66,119]. Crop water use efficiency (WUEcrop) and crop water productivity (WPcrop) have been estimated by calculating the ratio of crop dry mass and yield to crop evapotranspiration, respectively[115]. Crop evapotranspiration is controlled by the atmospheric evaporation capacity and crop growth. At the initial stage of crop growth, VPD regulation has no significant effect on the daily evapotranspiration of vegetable crops because the area of leaves is small. As the leaf area increases, crop evapotranspiration per plant increases under different VPD conditions. The water-saving effect on crop luxury transpiration increases gradually with growth stage under low VPD (1.20 kPa) under adequate irrigation[5]. Water consumption during crop growth includes irrigation water consumption and humidification water consumption. The cumulative irrigation water consumption of vegetable crops is lower under low VPD (1.20 kPa) than under high VPD (ranging from 2–5 kPa) under adequate irrigation[5]. Because water is needed to regulate VPD, both substrate irrigation water and air humidification water are used to regulate crop growth under low VPD. The humidification water consumption of each crop is related to planting density; thus, the water use efficiency of vegetable crops varies with planting density under low VPD. When six tomatoes were planted per square meter, no significant differences in WUEcrop and WPcrop were observed under different VPD conditions under adequate irrigation. When nine plants were planted per square meter, WUEcrop and WPcrop were significantly higher under low VPD (1.20 kPa) than under high VPD (ranging from 2–5 kPa) under adequate irrigation. When the water consumption for humidification and planting density are not considered, the WUEcrop and WPcrop are 65% higher under low VPD (1.20 kPa) than under high VPD (ranging from 2–5 kPa) under adequate irrigation[5]. Increases in WUEcrop and WPcrop are far greater under deficit irrigation than under adequate irrigation[4]. Moderate deficit irrigation can increase WPcrop[120]; however, reducing VPD under deficit irrigation can make up for the loss of yield caused by deficit irrigation and greatly increase WPcrop[4].
Yield is a key factor in agricultural production that not only affects WPcrop but also farmer income and market demand. Many studies have shown that reducing VPD can increase the yield of vegetable crops. The yield of different tomato cultivars is higher under low VPD than under high VPD, which indicates that tomato yield can be increased in several tomato cultivars by reducing VPD[5,12]. When using a fog generation system to keep the VPD lower than 1.20 kPa (low VPD) in a greenhouse, the tomato yield is 14.6% (Jinpeng) and 16.7% (Fenguan) higher under low VPD (1.20 kPa) than in the natural environment (ranging from 2–5 kPa) under adequate irrigation[5]. When low VPD (0.95 kPa) and high VPD (2.22 kPa) are maintained in a climate chamber, the tomato yield is 62.11% (Jinpeng) and 56.36% (Zhongza) higher under low VPD under adequate irrigation[12], which indicates that the positive effect of VPD on vegetable crop yield was not only related to cultivars but also related to the magnitude of and temporal stability in VPD. Similarly, reductions in VPD significantly increase the yield and fruit dry mass of melon and cucumber under adequate irrigation, which might be related to the increase in photosynthetic capacity and water transport capacity under low VPD (1.50 kPa)[2]. Increasing CO2 application while decreasing VPD significantly increases the yield of vegetable crops under adequate irrigation. This might stem from the fact that an increase in CO2 application increases the photosynthetic capacity and accumulation of photosynthetic products in vegetable crops, thus improving the water transport capacity and yield of tomato[93]. Therefore, reducing VPD is effective for increasing the yield of different vegetable crops, but the optimal VPD range for different crops at different growth stages needs to be determined. The fruit yield and dry mass are related to soil water conditions. The fruit yield and dry mass are higher under adequate irrigation than under deficit irrigation[120]. Decreasing VPD can lead to increases in fruit yield and dry mass. The positive effect of low VPD (1.20 kPa) on fruit yield and dry mass is more pronounced under deficit irrigation than under adequate irrigation, which indicates that low VPD can make up for the negative effect of deficit irrigation on plant growth to a certain extent[4]. Reductions in VPD increase the yield of vegetable crops. On the one hand, reductions in VPD reduce transpiration water consumption and increase water accumulation in fruit, thus improving yield. On the other hand, reductions in VPD drive cell expansion, thus increasing single fruit mass. Furthermore, decreases in VPD also increase nutrient element accumulation in reproductive organs, reduce blossom-end rot and fruit cracking, and thus increase yield[12].
The quality of vegetable crops can be divided into appearance quality, nutritional quality, and flavor quality[120,121]. Appearance quality usually includes single fruit mass, fruit transverse and longitudinal diameter, fruit shape index, and fruit color. Low VPD (1.60 kPa) can increase single fruit mass and transverse and longitudinal diameter, which stems from increases in the water content in fruit under low VPD[122]. No significant differences in the fruit shape index (approximately 0.85) of tomato under different VPD conditions have been observed. However, high VPD (2.20 kPa) can improve fruit color[122]. Lycopene, an important antioxidant, plays a key role in enhancing the nutritional quality of tomato. Low VPD can increase the lycopene content and antioxidant activity in several tomato cultivars[13,122]. The content of sugar and acid and the ratio of sugar to acid in fruit are key factors affecting tomato flavor quality. The content of soluble solids and soluble sugar and the ratio of glucose to fructose are higher in tomato under high VPD (2.20 kPa) than under low VPD (1.60 kPa)[15,122,123]. Nutrient absorption in tomato fruit and fruit flavor quality are increased under drought stress because plants redistribute sucrose to fruits under drought stress[124,125]. Water evaporation in the leaves is strong under high VPD (2.22 kPa), the water flowing from the xylem to the fruit decreases[12], and the solute concentration of phloem sap increases; this increases the concentration of sugar and acid in fruit and improves fruit quality[126]. Drought stress promotes the accumulation of starch in developing fruits[127] and the conversion of starch into hexose in mature fruits, thus increasing the content of soluble solids and soluble sugar in fruits[128]. The content of titratable acid in tomato does not vary under different VPD conditions, but the ratio of sugar to acid is significantly higher under high VPD than under low VPD[15]. In short, high VPD can improve the color and flavor quality of tomato fruit but reduce the single fruit mass and nutritional quality of tomato fruit.
-
Global VPD has increased in recent decades and is expected to continue to rise in the future. Under high VPD, the atmospheric transpiration and water transport of the SPAC increase, the stomatal conductance and photosynthesis decrease, and nutrient accumulation is hindered. Although the results of previous studies vary among species, crop yield and water use efficiency decrease under high VPD in the long term, and crop mortality increases. In nature, high-VPD environments are usually concurrent with environmental stresses such as high light intensity and high soil evapotranspiration, which exacerbates the effect of environmental stress on plant growth. Changes in plants in response to environmental stress are not only caused by changes in VPD but also might be related to other environmental factors. Therefore, future studies are needed to clarify how VPD can be modified in climate chambers to enhance crop growth and yield. The mode of chloroplast movement varies under different VPD conditions, and chloroplast movement is also closely related to light intensity and light quality. Therefore, additional studies are needed to clarify the effect of light intensity and light quality on chloroplast movement under different VPD conditions. Additionally, cultivation media affect soil water retention, which affects crop growth under VPD regulation. Therefore, the effects of VPD regulation on crop growth under different cultivation media require further study. Root pressure plays an important role in water transport. Therefore, additional studies are needed to characterize the effects of VPD regulation on crop root pressure. The regulatory effects of VPD on stomata are affected by many factors, such as passive hydraulic regulation and hormone signals; thus, the specific regulatory mechanism of stomatal movement requires clarification. More VPD gradients need to be established to characterize the optimal VPD range of different cultivars in different growth periods.
This work was supported by the Post Expert of National Bulk Vegetable Industry Technology System in China (CARS-23-C05), Technology Innovation Guidance Special Project of Shaanxi Province (Fund) (2021QFY08-04), and Science and Technology Innovation-driven Projects of Shaanxi Province – Technological Research and Development of Advantageous Industries (NYKJ-2020-YL-08).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Yu X, Zhang Y, Zhao X, Li J. 2023. Systemic effects of the vapor pressure deficit on the physiology and productivity of protected vegetables. Vegetable Research 3:20 doi: 10.48130/VR-2023-0020
Systemic effects of the vapor pressure deficit on the physiology and productivity of protected vegetables
- Received: 13 January 2023
- Accepted: 12 April 2023
- Published online: 03 July 2023
Abstract: In previous decades, the global temperature has risen, and the saturation vapor pressure deficit (VPD) has increased. VPD is an important environmental factor affecting crops, especially their yields. However, the effects of various VPD conditions on water transport dynamics, anatomical structure, stomatal morphology, photosynthetic physiology, nutrient absorption, yield, and quality remain unclear. Many studies have shown that atmospheric transpiration is enhanced, water transport dynamics in the soil-plant-atmosphere continuum and water potential gradient are increased, and crop water potential is reduced under high VPD. Crops have undergone a series of changes that have enhanced their adaptation to high-VPD environments. Mesophyll thickness and conductance and stomatal size and conductance have decreased, and this has led to reductions in the photosynthetic rate and nutrient accumulation. High VPD seriously reduces the yield and water use efficiency of protected vegetables but improves fruit color and flavor quality. Reductions in VPD can improve water and nutrient transport in protected vegetables, alter the anatomical structure of crops, promote crop photosynthesis, and increase fruit yield, nutritional quality, and water use efficiency. Comprehensive analysis of the effect of VPD on the physiology and productivity of protected vegetables will provide insights that will aid the cultivation of protected vegetables with high quality and yield.












