-
Citrus is the world's most economically important fruit crop, and the majority of citrus is grown in mountainous regions with barren soil[1]. Citrus productivity can be greatly affected by environmental changes, such as brief periods of flooding, drought, or cold[2−4]. Moreover, citrus is susceptible to alkaline soils and exhibits leaf/shoot chlorosis, limiting its geographical distribution[5,6]. Citrus is cultivated through grafting, and rootstock can modify scion architecture and act as the core source of resistance to various stresses, allowing the upper section growth of scion cultivars to thrive[7,8]. With growing interest in perennial crops as valuable components of sustainable agriculture, rootstocks provide an approach for improving and expanding citrus perennial cultivation under various environmental conditions[9].
Generally, rootstock selection and use are mainly determined by compatibility, orchard soil conditions, and local citriculture practice[10]. Germplasm with polyembryonicity, which can develop one or more somatic embryos that are genetically identical to the mother tree, is often selected for citrus rootstock due to genetically uniform rootstocks which can feasibly be prepared solely by sowing seeds[11,12]. Many rootstocks are used in citrus cultivation. Trifoliate orange (Poncirus trifoliata (L.) Raf) is widely used in citrus breeding owing to its cold hardiness and disease resistance[13]. However, trifoliate orange is sensitive to alkalinity and mineral deficiency and is incompatible with some citrus cultivars[5,14−16]. Citrange (Citrus sinensis × P. trifoliata) is drought tolerant yet susceptible to salt and alkalinity[17,18]. Red tangerine (Citrus reticulata Blanco) is resistant to B-deficiency and citrus exocortis viroid (CEVd); however, the fruit quality of scion degrades when red tangerine is used as a rootstock[19]. Citrus junos Sieb. Ex Tanaka is an iron-deficient, alkaline-, cold- and acid-tolerant citrus rootstock native to southwest China[5,17,20]. Abiotic stresses can alter osmotic equilibrium and induce oxidative stress in plants through excessive generation of reactive oxygen species (ROS)[21,22]. Plants neutralize these ROS through different mechanisms, which can be classified as non-enzymatic and enzymatic antioxidant systems, including antioxidant enzymes superoxide dismutase (SOD), peroxidases (POD), and catalase (CAT)[23−25]. Tolerant species or genotypes exhibit higher antioxidant enzyme activities than sensitive genotypes. Investigating tolerance to different abiotic stresses is critical for identifying the genetic resource for abiotic stress tolerance. Although rootstock can influence the agronomic performance of citrus trees, some widely used rootstocks may still demonstrate graft incompatibility in the orchard[9, 10]. Graft compatibility of intergeneric and intrafamilial species represents a tremendous agronomic potential for genetic improvement and improved crop management by combining unique traits from wild relative rootstocks with commercial citrus scion varieties[7].
In our previous citrus rootstock breeding effort, we reported a novel rootstock cultivar, C. junos cv. Shuzhen No.1, with vigorous growth, spherical crown, upright and dense hard branches, cold resistance, and robust adaptation to basic soil conditions[26, 27]. Therefore, this study compared the differences in seed germination characteristics, abiotic tolerance (drought, flooding, alkaline, and freezing), and grafted plant performance between 'Shuzhen No.1' and other common citrus rootstocks. This study determined the polyembryony and seedling emergence traits of five citrus rootstocks, comprehensively evaluated the tolerance responses of different genotypes of citrus rootstock, and provided information on the performances of five citrus varieties on rootstocks. Our findings provide insights into rootstock selection and promote the utilization of the new citrus rootstock 'Shuzhen No.1'.
-
Mature fruits of 'Shuzhen No.1' (abbreviated CjSz), trifoliate orange (Pt), red tangerine (Ct), citrange (Cp), and 'Ziyang Xiangcheng' (CjZy) were harvested for collecting seeds from the Citrus Germplasms Repository of Sichuan Province, Chengdu, China. Isolated seeds were surface sterilized using 0.5 M NaOH as described previously[17]. Uniform-sized seedlings were selected and grown in a growth chamber in perlite-filled pots. All seedlings were cultured for approximately six months with normal watering and fertilization.
Determination of polyembryony and seedling emergence traits
-
Two hundred viable seeds (not replicated) were selected from each rootstock, and the following parameters were assessed: (1) cumulative seedling number, (2) percentage of single seed emergence, and (3) polyembryony. CitRWP plays a principal role in regulating somatic embryogenesis in citrus nucellar tissues, and its alleles were divided into two types and polyembryonic alleles with a MITE insertion[11,12]. The absence or presence of the MITE insertion was evaluated with genomic PCR using the following primer set: forward 5ʹ-GTTACTTGGAGACGGCCTAACG-3ʹ and reverse 5'-TCGATCATGTAATGCTGACTC-3ʹ[11].
Stress treatments
-
Abiotic stress treatments included flooding (roots submerged in water with only stem and leaves exposed to the environment for 6 and 7 weeks), drought (20% soil water content for 1 and 2 weeks), alkalinity (watering distilled water with pH 8.0 and 9.0 for 8 weeks), and freezing (–10 °C for 1 and 2 h) were performed. Three biological replicates (five seedlings per replicate) were set randomly for each treatment. All leaves were sampled from five rootstocks, frozen in liquid nitrogen, and stored at –80 °C.
Determination of physiological and biochemical indexes
-
The total chlorophyll and carotenoid contents were measured using the method reported by Lichtenthaler & Buschmann[28]. Fresh leaves (1 g) were ground in a freezing mortar and pestle with 10 mL of 80% acetone. Following filtering, the pigment solution's optical density (OD) was measured at 470, 645, and 663 nm to determine carotenoid, chlorophyll (Chl) a, Chl b, and total Chl content, respectively. The assessed photosynthetic pigments were presented in mg/g fresh weight (FW). Antioxidant enzyme activities of SOD (EC 1.15.1.1), guaiacol peroxidase (POD, EC 1.11.1.7), and CAT (EC 1.11.1.6) were determined as previously described[29]. Malondialdehyde (MDA) content was measured using the thiobarbituric acid (TBA) method[30]. Soluble sugars and proteins were analyzed as previously described[31]. To minimize the differences between different genotypes, the data were expressed as ratios relative to the values of control groups.
Performances of different graft combinations
-
'Chunjian' (C. reticulata × (C. reticulata × C. sinensis)), 'Buzhihuo' (C. unshiu × C. sinensis), 'Mingrijian' ((C. unshiu × C. hassaku) × C. sinensis), 'Dafen' (C. unshiu), and 'Tarocco' (C. sinensis) were grafted onto four rootstocks, including CjSz, CjZy, Ct, and Pt. Ninety seedlings were cultured for 1 month with normal watering and fertilization, and their survival rates were measured on March 30th, 2019. Tree growth and leaf greenness were assessed in 5–10 grafted trees in each cultivar. Stem thicknesses below and above the graft joint were measured using a vernier caliper on October 30th, 2020. Shoot length and longitudinal and horizontal growth of trees were recorded using a tape measure from summer shoots on October 30th, 2020. Ten mature leaves from summer shoots were selected from each tree to measure soil-plant analysis development (SPAD) with a SPAD-502 chlorophyll meter.
Statistical analysis
-
Microsoft Excel was used to prepare the collected data. Significant differences between grafted combinations were analyzed using Tukey's method, and Pearson correlation and principal component analyses were performed using SPSS 20.0 software. All figures were drawn using GraphPad Prism (v. 7.04).
-
Compared with CjZy, CjSz has a spherical crown and upright and dense hard branches (Fig. 1a, b). CjSz and CjZy had solitary flowers (Fig. 1c, d). However, the lateral petals of CjSz are purple from bud to bloom, with a flower diameter of 2.0 cm (Fig. 1c). The fruits of both CjSz and CjZy were orange in color at maturity (Fig. 1c, d) and both were polyembryonic (Fig. 1c, d). Citrus rootstock seeds germinated 40–45 d after seeding and stopped germinating 95–100 d later (Fig. 1e). CjSz had the greatest emergence rate and polyembryonic ratio among the tested rootstocks, reaching 160.77% and 70.69%, respectively (Fig. 1e, f). The germination potential was 25.50%, which was lower than that of trifoliate orange (29.00%) but higher than that of CjZy, citrange, and red tangerine (Fig. 1e). According to statistics on single seed emergences, CjSz had the most single seed emergences (two, up to 44.83%), slightly lower than CjZy (46.76%) but higher than Pt, Ct, and Cp. The proportion of CjSz was highest among the five tested rootstocks, at 18.72% (three seedlings), 4.39% (four seedlings), 1.48% (five seedlings), and 0.49% (six seedlings). Additionally, the maximum number of seedlings per grain was seven (Fig. 1f). These results are consistent with the MITE insertion detection results (Fig. 1g). Apomixis in citrus is sporophytic and highly stable across commercial varieties. Citrus junos fruits were densely seeded, with most of the seeds being plump and polyembryonic, which can generate large numbers of uniform rootstocks from seeds[12,31].

Figure 1.
Comparison of morphology between two genotypes of Citrus junos. (a), (b) Six year old trees; (c), (d) flowers, fruits and seeds; (e) cumulative number of seedlings; (f) percentage of single seed emergence; (g) MITE insertion in five rootstock germplasms. CjSz: Shuzhen No.1 (Citrus. junos Sieb. Tanaka); CjZy: Ziyang Xiangcheng (C. junos Sieb. Tanaka); Cp: citrange (C. sinensis Osbeck. × Poncirus trifoliate Raf.); Pt: trifoliate orange (P. trifoliate [L.] Raf) and Ct: Red tangerine (C. tangerine Hort. Ex Tanaka). Scale bars = 1 cm.
Leaf photosynthetic pigments under abiotic stresses
-
All genotypes developed leaf chlorosis at the end of the abiotic stress treatments (Fig. 2). Almost all treatments reduced the content of leaf photosynthetic pigments in all rootstocks (Table 1). Comparing the pigment content data among the different rootstocks, it is evident that CjSz demonstrated better adaptability to alkaline and freezing stresses (Table 1, Fig. 2). Specifically, under alkaline treatment with a pH of 8.0, CjSz exhibited the smallest decrease in leaf photosynthetic pigments content. In the case of alkaline treatment with a pH of 9.0, CjSz displayed similar levels of leaf chlorina compared to Cp, followed by CjZy, Pt and Ct. Similarly, under freezing treatment for 1 h, the ratios of Chl a, total Chl and total carotenoids in CjSz were higher than in other rootstocks, although the differences were not statistically significant. More specifically, among the different treatments, CjSz experienced the greatest decrease in Chl a, Chl b, and total Chl under 2 weeks of drought treatment, with ratios of 0.48, 0.64, and 0.53, respectively. Conversely, CjSz demonstrated the least decrease under freezing treatment for 1 h, with ratios of 0.96, 1.05, and 0.99, respectively (Table 1). The total carotenoid content in CjSz experienced the most significant decrease after 2 weeks of drought treatment, while the least decrease occurred under alkaline treatment with a pH of 8.0 (Table 1).
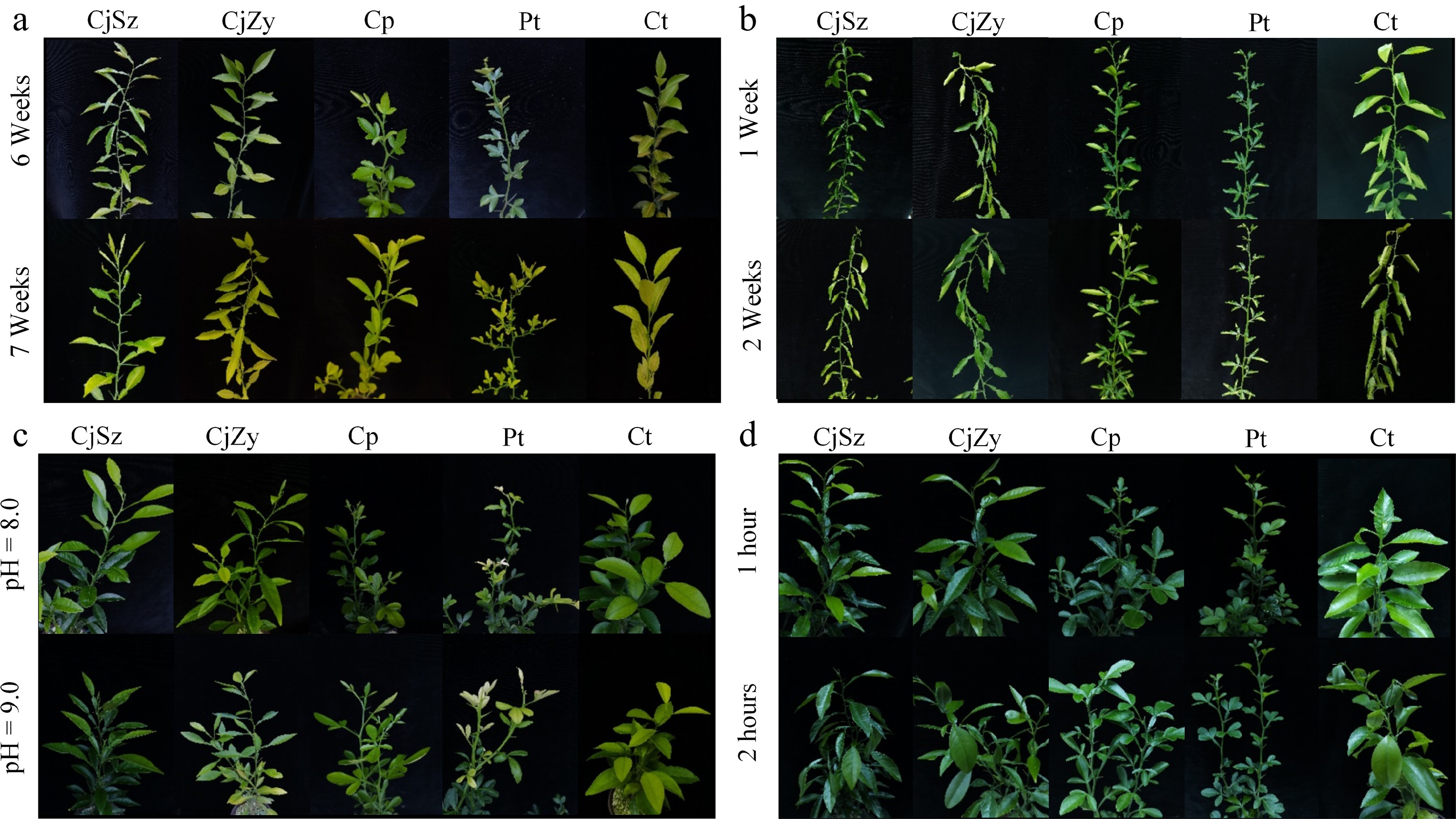
Figure 2.
Growth state of five rootstocks under abiotic stresses. (a) Flooding stress; (b) drought stress; (c) alkaline stress (pH = 8.0 and pH = 9.0); (d) freezing stress.
Table 1. Statistics for chlorophyll a, chlorophyll b, total chlorophyll and total carotenoid compared with controls.
Species Flooding stress Drought stress Alkaline stress Freezing stress 6 weeks 7 weeks 1 week 2 weeks pH = 8.0 pH = 9.0 1 h 2 h Chl a CjSz 0.72 ± 0.12a 0.60 ± 0.04a 0.53 ± 0.08a 0.48 ± 0.15a 0.86 ± 0.26a 0.74 ± 0.35ab 0.96 ± 0.24a 0.83 ± 0.11a CjZy 0.67 ± 0.22a 0.68 ± 0.09a 0.59 ± 0.16a 0.45 ± 0.22a 0.79 ± 0.14a 0.69 ± 0.19ab 0.85 ± 0.17a 0.79 ± 0.19a Cp 0.77 ± 0.26a 0.69 ± 0.26a 0.66 ± 0.28a 0.61 ± 0.16a 0.83 ± 0.26a 0.77 ± 0.20a 0.88 ± 0.12a 0.85 ± 0.18a Pt 0.63 ± 0.06ab 0.63 ± 0.19a 0.63 ± 0.03a 0.60 ± 0.08a 0.72 ± 0.03ab 0.68 ± 0.07ab 0.91 ± 0.20a 0.88 ± 0.05a Ct 0.44 ± 0.10b 0.38 ± 0.09b 0.28 ± 0.05b 0.23 ± 0.07b 0.48 ± 0.04b 0.47 ± 0.07b 0.92 ± 0.06a 0.69 ± 0.15a Chl b CjSz 0.78 ± 0.12a 0.70 ± 0.08a 0.67 ± 0.14a 0.64 ± 0.15a 0.87 ± 0.26a 0.77 ± 0.31a 1.05 ± 0.22a 1.00 ± 0.12a CjZy 0.70 ± 0.20a 0.65 ± 0.09a 0.72 ± 0.09a 0.52 ± 0.20a 0.76 ± 0.11a 0.74 ± 0.13a 0.86 ± 0.11ab 0.92 ± 0.17ab Cp 0.76 ± 0.24a 0.68 ± 0.25a 0.71 ± 0.25a 0.68 ± 0.15a 0.85 ± 0.18a 0.78 ± 0.16a 0.82 ± 0.09b 0.83 ± 0.11b Pt 0.71 ± 0.13a 0.66 ± 0.20a 0.66 ± 0.05a 0.68 ± 0.11a 0.69 ± 0.09ab 0.66 ± 0.08ab 0.86 ± 0.19ab 0.87 ± 0.11ab Ct 0.47 ± 0.09b 0.50 ± 0.12a 0.39 ± 0.04b 0.33 ± 0.06b 0.51 ± 0.04b 0.47 ± 0.05b 1.00 ± 0.10ab 0.64 ± 0.12c Total CjSz 0.74 ± 0.12a 0.63 ± 0.05ab 0.57 ± 0.10a 0.53 ± 0.15a 0.86 ± 0.26a 0.75 ± 0.33a 0.99 ± 0.23a 0.89 ± 0.12a Chl CjZy 0.68 ± 0.21a 0.67 ± 0.09a 0.63 ± 0.14a 0.47 ± 0.21a 0.78 ± 0.13a 0.71 ± 0.17ab 0.85 ± 0.15a 0.84 ± 0.19ab Cp 0.76 ± 0.25a 0.68 ± 0.26a 0.67 ± 0.27a 0.63 ± 0.16a 0.84 ± 0.23a 0.77 ± 0.19a 0.86 ± 0.11a 0.84 ± 0.15ab Pt 0.65 ± 0.08ab 0.64 ± 0.19a 0.63 ± 0.03a 0.63 ± 0.09a 0.71 ± 0.05ab 0.67 ± 0.07ab 0.89 ± 0.19a 0.87 ± 0.07a Ct 0.45 ± 0.10b 0.42 ± 0.10b 0.32 ± 0.04b 0.27 ± 0.07b 0.49 ± 0.03b 0.47 ± 0.06b 0.95 ± 0.08a 0.67 ± 0.14b Total CjSz 0.83 ± 0.12a 0.65 ± 0.10a 0.68 ± 0.11a 0.55 ± 0.18ab 0.96 ± 0.30a 0.71 ± 0.27a 0.96 ± 0.20a 0.95 ± 0.13a Car CjZy 0.62 ± 0.15bc 0.65 ± 0.08a 0.56 ± 0.14a 0.51 ± 0.25ab 0.68 ± 0.11bc 0.69 ± 0.12ab 0.77 ± 0.14a 0.76 ± 0.12bc Cp 0.76 ± 0.24ab 0.67 ± 0.23a 0.63 ± 0.19a 0.69 ± 0.13a 0.79 ± 0.15ab 0.75 ± 0.17a 0.92 ± 0.06a 0.86 ± 0.14ab Pt 0.69 ± 0.08ab 0.62 ± 0.16ab 0.64 ± 0.04a 0.65 ± 0.09a 0.73 ± 0.04b 0.63 ± 0.05ab 0.91 ± 0.23a 0.95 ± 0.05a Ct 0.45 ± 0.12c 0.43 ± 0.13b 0.38 ± 0.05b 0.37 ± 0.10b 0.47 ± 0.03c 0.49 ± 0.05b 0.89 ± 0.14a 0.67 ± 0.14c Note: Chl: chlorophyll, Car: carotenoids. Data shown in the table are expressed as ratios relative to the values obtained on control seedlings. Three biological replicates (five seedlings per replicate) were set randomly for each treatment. Significance was tested for indicators of different rootstocks in the same treatment, and different lowercase letters indicate significant differences at p < 0.05. MDA, soluble protein, and soluble sugar contents and SOD, POD, and CAT activities under abiotic stresses
-
The levels of MDA, and the activities of SOD, POD, and CAT were significantly influenced by abiotic stresses (Table 2). CAT activity decreased under flooding, alkaline, and freezing stress, but slightly increased under drought stress. MDA levels and SOD and POD activities increased under flooding, drought, alkaline, and freezing stresses in all citrus rootstock genotypes (Table 2). However, CAT activity decreased under abiotic stress. Among the genotypes, CjSz exhibited the highest increase in MDA levels during 7 weeks of flooding treatment, and the lowest increase during 1 h of freezing treatment. SOD activity in CjSz showed the greatest increase after 7 weeks of flooding treatment and the smallest increase after alkaline stress treatment at pH 8.0. CjSz had the highest POD activity ratio of 1.62 under freezing stress, and the lowest value of 1.25 under 2 weeks of drought treatment. In comparison to the other four rootstocks, CjSz had the highest SOD ratio value (1.71) under 7 weeks of flooding stress. Additionally, CjSz exhibited the highest POD ratio value under alkaline (1.37 and 1.46) and freezing stresses (1.62). Under drought stress, CjSz had significantly lower MDA and POD ratio values than the other four rootstocks.
Table 2. Mean comparison of malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD).
Species Flooding stress Drought stress Alkaline stress Freezing stress 6 weeks 7 weeks 1 week 2 weeks pH = 8.0 pH = 9.0 1 h 2 h MDA CjSz 1.42 ± 0.30bc 2.02 ± 0.16ab 1.07 ± 0.11d 1.90 ± 0.26b 1.11 ± 0.11b 1.50 ± 0.13c 1.02 ± 0.22b 1.24 ± 0.39b CjZy 1.45 ± 0.17bc 1.81 ± 0.30b 1.79 ± 0.22b 2.10 ± 0.14a 1.47 ± 0.30ab 1.75 ± 0.28bc 1.02 ± 0.13b 1.15 ± 0.27b Cp 1.67 ± 0.20ab 1.84 ± 0.41b 1.31 ± 0.23cd 2.25 ± 0.61a 1.33 ± 0.39ab 1.36 ± 0.20c 1.49 ± 0.29a 1.92 ± 0.63a Pt 1.26 ± 0.16c 1.48 ± 0.34b 1.45 ± 0.31bc 2.03 ± 0.43a 1.84 ± 0.40a 2.41 ± 0.42a 0.91 ± 0.34b 1.01 ± 0.54b Ct 1.75 ± 0.25a 2.42 ± 0.66a 2.30 ± 0.36a 2.29 ± 0.37a 1.36 ± 0.10ab 1.92 ± 0.36b 1.00 ± 0.17b 1.46 ± 0.26ab SOD CjSz 1.60 ± 0.26a 1.71 ± 0.03a 1.53 ± 0.02ab 1.63 ± 0.11a 1.25 ± 0.08a 1.31 ± 0.15ab 1.31 ± 0.06ab 1.35 ± 0.16a CjZy 1.65 ± 0.10a 1.70 ± 0.07a 1.62 ± 0.07a 1.71 ± 0.06a 1.31 ± 0.29a 1.36 ± 0.29a 1.12 ± 0.27b 1.54 ± 0.21a Cp 1.25 ± 0.18c 1.19 ± 0.18b 1.18 ± 0.16c 1.15 ± 0.15b 1.12 ± 0.05ab 1.26 ± 0.02ab 1.25 ± 0.17ab 1.05 ± 0.23b Pt 1.56 ± 0.10ab 1.50 ± 0.46ab 1.69 ± 0.16a 1.51 ± 0.28a 0.94 ± 0.30b 0.94 ± 0.39c 1.39 ± 0.19a 1.56 ± 0.11a Ct 1.36 ± 0.10bc 1.61 ± 0.18a 1.35 ± 0.17bc 1.23 ± 0.18b 1.08 ± 0.07ab 1.04 ± 0.09bc 1.18 ± 0.24ab 1.07 ± 0.08b POD CjSz 1.40 ± 0.31a 1.50 ± 0.10ab 1.25 ± 0.38c 1.45 ± 0.61b 1.37 ± 0.49a 1.46 ± 0.22a 1.62 ± 0.32ab 1.62 ± 0.37a CjZy 1.61 ± 0.33a 1.62 ± 0.38ab 2.23 ± 0.68ab 2.69 ± 0.72a 1.29 ± 0.24a 1.38 ± 0.54a 1.25 ± 0.66b 1.55 ± 0.59a Cp 1.45 ± 0.16a 2.16 ± 0.86a 2.59 ± 1.09a 2.90 ± 0.70a 1.27 ± 0.28a 1.31 ± 0.32a 1.28 ± 0.44b 1.46 ± 0.49a Pt 0.85 ± 0.17b 1.25 ± 0.21b 1.40 ± 0.41bc 1.57 ± 0.50b 0.83 ± 0.22b 1.17 ± 0.32a 1.85 ± 0.28a 1.83 ± 0.23a Ct 1.00 ± 0.26b 1.99 ± 0.65a 2.64 ± 0.67a 2.25 ± 1.20ab 0.80 ± 0.24b 1.06 ± 0.32a 1.21 ± 0.21b 1.25 ± 0.48a CAT CjSz 0.98 ± 0.20ab 0.77 ± 0.10a 1.14 ± 0.14ab 1.00 ± 0.10ab 0.91 ± 0.30a 0.67 ± 0.09c 0.87 ± 0.14a 0.67 ± 0.10c CjZy 0.74 ± 0.03b 0.91 ± 0.09a 1.52 ± 0.28a 0.93 ± 0.08b 0.57 ± 0.17a 1.08 ± 0.17b 0.71 ± 0.09ab 1.56 ± 0.24a Cp 0.90 ± 0.06ab 0.79 ± 0.18a 0.91 ± 0.04b 1.08 ± 0.14ab 0.70 ± 0.14a 1.59 ± 0.10a 0.90 ± 0.08a 0.61 ± 0.03c Pt 1.08 ± 0.11a 0.72 ± 0.02a 0.47 ± 0.06c 1.28 ± 0.08a 1.04 ± 0.14a 0.84 ± 0.15bc 0.53 ± 0.14b 0.85 ± 0.10bc Ct 0.77 ± 0.10 ab 0.66 ± 0.12 a 1.33 ± 0.44 a 1.15 ± 0.15 ab 0.79 ± 0.11 a 0.88 ± 0.11 bc 0.70 ± 0.13 ab 1.09 ± 0.19 b Note: Data showed in the table are expressed as ratios relative to the values obtained on control seedling. Different lowercase letters indicate significant differences at p < 0.05. The regulation of soluble protein and sugar contents in response to abiotic stressors varied among the citrus rootstock genotypes (Table 3). Compared with other rootstocks, CjSz had the lowest ratio of soluble proteins during 7 weeks of flooding stress, but the highest ratio under drought, alkaline, and freezing stresses. CjSz exhibited the greatest increase in soluble sugars under 7 weeks of flooding stress and 1 week of drought stress, whereas other rootstocks showed moderate increases. Specifically, the overall ratio of soluble proteins in CjSz was the highest (2.16) under 2 h of freezing stress and the lowest (1.10) during 7 weeks of flooding stress. However, the ratio of soluble sugars displayed opposite trends.
Table 3. Mean comparison of soluble proteins and soluble sugars.
Species Flooding stress Drought stress Alkaline stress Freezing stress 6 weeks 7 weeks 1 week 2 weeks pH = 8.0 pH = 9.0 1 h 2 h Soluble proteins CjSz 1.11 ± 0.17bc 1.10 ± 0.26d 1.35 ± 0.36a 1.52 ± 0.08ab 1.86 ± 0.18a 2.04 ± 0.17a 2.15 ± 0.18a 2.16 ± 0.17a CjZy 1.01 ± 0.17c 1.46 ± 0.28bc 1.19 ± 0.19a 1.17 ± 0.28b 1.81 ± 0.06a 1.82 ± 0.31a 1.58 ± 0.09c 1.77 ± 0.32b Cp 1.59 ± 0.19a 1.88 ± 0.19a 1.29 ± 0.32a 1.67 ± 0.28a 1.62 ± 0.10b 1.83 ± 0.18a 1.74 ± 0.17bc 1.77 ± 0.17b Pt 1.09 ± 0.07bc 1.24 ± 0.17cd 1.13 ± 0.19a 1.11 ± 0.17b 1.13 ± 0.13c 1.19 ± 0.26b 1.84 ± 0.09b 1.93 ± 0.10ab Ct 1.26 ± 0.02b 1.54 ± 0.12b 1.21 ± 0.55a 1.35 ± 0.51ab 1.23 ± 0.15c 1.30 ± 0.15b 1.28 ± 0.23d 1.36 ± 0.17c Soluble sugars CjSz 2.78 ± 0.48a 3.24 ± 0.40a 2.50 ± 0.62a 1.87 ± 0.71bc 1.77 ± 0.23ab 2.08 ± 0.51b 1.37 ± 0.20a 1.25 ± 0.21b CjZy 1.52 ± 0.45c 1.79 ± 0.10b 1.36 ± 0.37b 1.62 ± 0.13bc 1.42 ± 0.37bc 2.32 ± 0.44ab 1.29 ± 0.13a 1.53 ± 0.23ab Cp 2.80 ± 0.15a 2.83 ± 0.53a 1.96 ± 0.30ab 2.76 ± 0.92a 2.22 ± 0.63a 2.77 ± 0.37a 1.14 ± 0.41a 1.74 ± 0.22a Pt 2.12 ± 0.49b 2.13 ± 0.33b 1.86 ± 0.13ab 1.26 ± 0.43c 0.98 ± 0.16c 1.15 ± 0.16c 1.23 ± 0.28a 1.41 ± 0.32ab Ct 3.06 ± 0.26a 3.16 ± 0.64a 2.44 ± 0.93a 2.25 ± 0.17ab 1.01 ± 0.24c 1.18 ± 0.25c 1.17 ± 0.33a 1.63 ± 0.31a Note: Data shown in the table were expressed as ratios relative to the values obtained on control seedling. Different lowercase letters indicate significant differences at p < 0.05. Comprehensive evaluation of rootstocks under abiotic stresses
-
To provide a comprehensive assessment of the tolerance of different rootstocks, we conducted a principal component analysis to calculate various physiological and biochemical parameters. By comparing the comprehensive evaluation values of all citrus rootstocks, we observed that the ranking of tolerance for each rootstock varied with treatment time. The comprehensive evaluation values were determined using a membership function and weight calculation, which allowed us to assess the performance of each rootstock under each different abiotic stress treatment (Table 4).
Table 4. Comprehensive evaluation of five genotypes citrus rootstocks under different stresses.
Treatment Variety Comprehensive evaluation Membership function Comprehensive evaluation value Order F1 F2 F3 U1 U2 U3 Flooding 6 weeks CjSz 2.778 −0.145 0.154 1.000 0.397 0.677 0.710 1 CjZy −0.703 −1.678 0.998 0.130 0.000 1.000 0.229 4 Cp −1.222 0.620 0.569 0.000 0.596 0.836 0.375 3 Pt −0.270 2.180 −0.108 0.238 1.000 0.577 0.592 2 Ct −0.582 −0.978 −1.614 0.160 0.181 0.000 0.141 5 Weights 0.440 0.388 0.172 Flooding 7 weeks CjSz 2.261 1.481 0.119 1.000 1.000 0.621 0.966 1 CjZy 0.985 −1.106 0.866 0.715 0.000 1.000 0.565 2 Cp −1.878 −0.464 0.269 0.075 0.248 0.698 0.174 5 Pt −2.215 1.047 −0.146 0.000 0.832 0.487 0.249 4 Ct 0.847 −0.957 −1.108 0.684 0.058 0.000 0.468 3 Weights 0.663 0.246 0.091 Drought 1 week CjSz −0.468 1.581 0.303 0.239 1.000 0.504 0.516 2 CjZy −1.545 0.762 −0.946 0.000 0.755 0.000 0.228 4 Cp 0.003 −1.758 −0.943 0.343 0.000 0.001 0.178 5 Pt 2.964 0.349 0.050 1.000 0.631 0.401 0.781 1 Ct −0.954 −0.934 1.535 0.131 0.247 1.000 0.321 3 Weights 0.519 0.302 0.179 Drought 2 weeks CjSz −0.005 1.386 1.251 0.386 1.000 1.000 0.672 2 CjZy −1.787 0.898 −1.024 0.000 0.864 0.000 0.287 4 Cp 0.005 0.146 −0.150 0.388 0.654 0.384 0.476 3 Pt 2.826 −0.236 −0.492 1.000 0.547 0.234 0.747 1 Ct −1.039 −2.194 0.415 0.162 0.000 0.632 0.171 5 Weights 0.535 0.332 0.133 Alkaline pH = 8.0 CjSz 0.492 1.831 0.873 0.674 1.000 1.000 0.810 1 CjZy 2.056 0.273 −0.941 1.000 0.551 0.000 0.761 2 Cp 1.059 −1.087 0.226 0.792 0.160 0.643 0.574 3 Pt −2.749 0.626 −0.598 0.000 0.653 0.189 0.229 5 Ct −0.859 −1.643 0.440 0.393 0.000 0.761 0.301 4 Weights 0.582 0.323 0.094 Alkaline pH = 9.0 CjSz 0.178 1.765 −0.992 0.681 1.000 0.000 0.699 2 CjZy 1.244 0.969 0.689 0.941 0.770 0.990 0.885 1 Cp 1.484 −1.154 0.590 1.000 0.156 0.932 0.676 3 Pt −2.614 0.115 0.706 0.000 0.523 1.000 0.342 4 Ct −0.292 −1.695 −0.993 0.567 0.000 0.000 0.272 5 Weights 0.481 0.372 0.148 Freezing 1 h CjSz −0.127 0.600 1.842 0.330 0.644 1.000 0.568 2 CjZy −1.364 −1.396 −0.133 0.021 0.000 0.284 0.068 5 Cp 2.549 0.457 −0.447 1.000 0.598 0.170 0.701 1 Pt −1.447 1.702 −0.916 0.000 1.000 0.000 0.324 3 Ct 0.389 −1.364 −0.346 0.459 0.010 0.207 0.263 4 Weights 0.473 0.324 0.203 Freezing 2 h CjSz 1.279 0.579 −0.031 0.907 0.650 0.416 0.749 1 CjZy −1.946 1.708 0.603 0.000 1.000 0.714 0.443 4 Cp 0.900 −1.426 1.209 0.800 0.029 1.000 0.567 3 Pt 1.609 0.661 −0.915 1.000 0.676 0.000 0.746 2 Ct −1.843 −1.521 −0.866 0.029 0.000 0.023 0.018 5 Weights 0.516 0.340 0.144 The results showed that CjSz had the highest comprehensive evaluation values during 6 and 7 weeks of flooding (0.710 and 0.966), at pH 8.0 (0.810), and after 2 h of freezing treatment (0.749). On the other hand, Ct exhibited the lowest comprehensive evaluation values during 6 weeks of flooding (0.141), and 2 weeks of drought (0.171), at pH 9.0 (0.272), and after 2 h of freezing treatment (0.018) (Table 4).
Performances of different graft combinations
-
Among the different graft combinations, the success rate of grafting 'Chunjian' and 'Mingrijian' onto Pt rootstocks wad below 80%, with success rates of 70.67% and 79.33%, respectively (Table 5). The highest survival rates of 100% were observed in 'Buzhihuo' was grafted onto CjSz and 'Dafen' grafted onto Ct. In terms of graft joint thickness (Ta), 'Dafen' grafted onto CjSz had the highest value of 20.13 mm, while 'Mingrijian' grafted onto Pt had the lowest value of 6.65 mm. The stem thickness below the graft joint (Tb) was the highest in 'Mingrijian' grafted onto CjSz (28.62 mm) and the lowest in 'Buzhihuo' grafted onto Pt (11.80 mm). The Ta/Tb ratio, which indicates the relative thickness above the blow the graft joint, was the highest in 'Tarocco' grafted onto CjZy and 'Tarocco' grafted onto Ct (0.82 for both), followed by 'Dafen' and 'Buzhihuo' grafted onto Ct (0.81 and 0.80, respectively). The lowest Ta/Tb ratio was observed in 'Mingrijian' and 'Chunjian' grafted onto Pt (0.52 and 0.58, respectively).
Table 5. Survival rate and graft union situation of different graft combinations.
Graft combination Survival rate (%) Diameter of scion (mm) Diameter of rootstock (mm) Ration of scion to rootstock Rootstock Scion CjSz Chunjian 95.67 18.81 ± 0.35ab 26.52 ± 2.75b 0.70 ± 0.03ab Buzhihuo 100.00 18.31 ± 2.34ab 23.96 ± 2.49c 0.77 ± 0.02a Mingrijian 91.33 17.87 ± 1.95b 28.62 ± 2.88a 0.63 ± 0.06c Dafen 90.67 20.13 ± 2.52a 28.40 ± 3.42ab 0.70 ± 0.06ab Tarocco 95.50 20.10 ± 1.68a 30.12 ± 2.74a 0.67 ± 0.01bc CjZy Chunjian 86.33 11.92 ± 0.94b 16.00 ± 0.46b 0.75 ± 0.05ab Buzhihuo 95.50 12.59 ± 1.87b 16.05 ± 1.39b 0.78 ± 0.06ab Mingrijian 81.67 11.26 ± 1.28b 16.01 ± 1.52b 0.71 ± 0.01b Dafen 95.50 14.78 ± 1.99a 20.48 ± 2.29a 0.74 ± 0.12ab Tarocco 90.67 14.82 ± 2.01a 17.66 ± 2.85ab 0.82 ± 0.05a Ct Chunjian 91.33 15.94 ± 1.64ab 21.17 ± 1.71a 0.75 ± 0.03ab Buzhihuo 95.67 14.77 ± 2.50b 18.83 ± 2.51ac 0.80 ± 0.12ab Mingrijian 90.67 15.02 ± 2.48b 20.91 ± 2.27ab 0.72 ± 0.05b Dafen 100.00 16.44 ± 0.81ab 21.60 ± 3.82a 0.81 ± 0.09a Tarocco 87.33 17.37 ± 1.07a 21.28 ± 1.65a 0.82 ± 0.03a Pt Chunjian 70.67 8.19 ± 0.49bc 14.10 ± 2.02abc 0.58 ± 0.05ab Buzhihuo 95.67 6.66 ± 0.66c 11.80 ± 0.44c 0.57 ± 0.07ab Mingrijian 79.33 6.65 ± 0.76c 12.81 ± 1.13bc 0.52 ± 0.06b Dafen 91.67 10.94 ± 0.97a 17.01 ± 1.67a 0.65 ± 0.07a Tarocco 91.33 10.36 ± 1.84ab 16.55 ± 0.68ab 0.62 ± 0.18a The tree growth was significantly affected by different rootstocks (Table 6). The citrus scions grafted onto CjSz rootstock exhibited strong tree vigor, followed by Ct, CjZy, and Pt (Table 6). The leaf greenness, as indicated by the SPAD value, was the highest in trees with 'Tarocco' grafted onto CjZy (86.46) and 'Buzhihuo' grafted onto CjSz (85.79), while it was lowest for 'Mingrijian', 'Chunjian', and 'Buzhihuo' grafted onto Pt (71.43, 75.63, and 75.70, respectively). Overall, the results suggest that CjSz exhibited good graft compatibility with the test scions.
Table 6. The growth situation of different graft combinations.
Graft combination Scion length (cm) Crown breadth Leaf greenness
(SPAD)Rootstock Scion Longitudinal (cm) Horizontal (cm) CjSz Chunjian 97.54 ± 9.53b 87.83 ± 14.42a 89.18 ± 16.32b 82.98 ± 0.74abc Buzhihuo 79.11 ± 10.63d 73.67 ± 5.78b 78.00 ± 17.04c 85.79 ±1.85a Mingrijian 115.78 ± 8.88a 91.33 ± 13.53a 110.38 ± 19.01a 84.97 ± 0.47a Dafen 88.11 ± 5.98c 97.33 ± 20.63a 98.00 ± 18.89ab 81.34 ± 0.14c Tarocco 81.11 ± 12.07cd 61.56 ± 11.59c 65.56 ± 9.19d 84.36 ± 1.55ab CjZy Chunjian 69.67 ± 11.15ab 66.99 ± 4.20a 66.88 ± 15.99a 82.16 ± 2.12b Buzhihuo 55.89 ± 9.71c 52.33 ± 3.71b 61.11 ± 3.20ab 82.93 ± 0.57b Mingrijian 69.67 ± 5.93ab 58.33 ± 14.25ab 71.00 ± 13.91a 79.54 ± 3.83c Dafen 73.56 ± 6.00a 72.17 ± 16.54a 71.33 ± 18.34a 84.93 ± 3.30ab Tarocco 57.56 ± 10.84bc 64.22 ± 11.52a 52.67 ± 20.54b 86.46 ± 0.80a Ct Chunjian 96.11 ± 10.24a 77.44 ± 12.30a 83.33 ± 14.88ab 81.37 ± 1.92ab Buzhihuo 82.44 ± 15.61b 62.56 ± 8.55bc 69.22 ± 14.34b 81.36 ± 1.68abc Mingrijian 97.89 ± 9.34a 76.89 ± 5.43ab 77.67 ± 31.56ab 78.81 ± 1.97bcd Dafen 87.33 ± 5.46ab 89.22 ± 1.26a 92.67 ± 20.85a 83.47 ± 1.70a Tarocco 73.22 ± 13.77c 55.22 ± 12.85c 53.33 ± 16.68c 83.43 ± 1.28a Pt Chunjian 41.18 ± 7.44ab 38.09 ± 12.96ab 42.13 ± 14.27a 75.63 ± 2.67b Buzhihuo 25.56 ± 3.17c 18.89 ± 1.92c 15.56 ± 1.50bc 75.70 ± 2.04b Mingrijian 32.11 ± 7.93bc 20.78 ± 5.23bc 15.11 ± 2.12c 71.43 ± 4.99c Dafen 51.44 ± 4.17a 50.56 ± 10.40a 48.00 ± 6.66a 80.92 ± 4.33a Tarocco 42.13 ± 16.27ab 27.89 ± 10.00bc 33.89 ± 15.79ab 81.71 ± 0.70a -
Grafting is widely used in citrus propagation and provides many agronomical advantages to scion[32,33]. Rootstock is vital for the citrus industry as it provides resistance to multiple stresses[3,34]. The rootstocks used in citrus have certain issues with stress resistance, disease resistance, or grafting compatibility[14−16], rendering them inflexible to the varying soil environment and climatic conditions, and consequently, cannot be widely used in various cultivars[17]. Therefore, citrus rootstock cultivation and evaluation are critical for the industry's healthy, stable, and sustainable development[27].
In this study, abiotic stress altered the physiological, metabolic, and molecular processes[17,35]. Almost all rootstock leaf photosynthetic pigments (Chl a, Chl b, total Chl, and carotenoid) were decreased under abiotic stress treatments (Table 1), creating an imbalance in the photosynthetic machinery[36]. Under stress conditions, MDA, soluble protein, soluble sugar contents, and antioxidant enzyme activities were in an unbalanced equilibrium state[23, 24,37]. Alkaline stress inhibits plant growth far more than salt stress[38]. CjZy is widely used as an alkaline-tolerant citrus rootstock in calcareous soil areas in China[5]. Under alkaline stress, CjSz performed similarly to CjZy. Low temperatures cause the leaves to wilt and dehydrate, reducing the photosynthesis rate[39]. The disruption of photosynthetic mechanisms causes excessive production of ROS, leading to oxidative stress, one of the most damaging consequences of freezing stress. Pt has shown the highest resistance to cold stress[40−42]. CjSz and Pt undergo similar physiological and biochemical changes, indicating that CjSz is also highly resistant to freezing. Flooding is a seasonal stress factor affecting Chinese citrus production areas[2]. Under flooding stress, the MDA content in Ct was the highest, and that in Pt was the lowest. Comprehensive analysis revealed that Cp is the most flood-resistant genotype, correlating with previous research findings[43]. CjSz had reasonable flooding resistance. Cp has been considered drought-resistant citrus rootstocks owing to its higher chlorophyll content and POD activity under drought stress than CjSz. CjSz had less MDA content than Cp but more soluble sugar and soluble protein content. The comprehensive evaluation revealed that CjSz's drought tolerance was second only to Pt and superior to other rootstocks.
Rootstocks significantly affect tree performance in multiple aspects[9]. Several studies have indicated that rootstocks have a significant effect on shoot growth[3,18]. In this study, the effects of rootstocks on the horticultural performance of scion varieties were investigated. Rootstock genotypes influence compatibility[9]. In our study, the graft success rate of CjSz with five citrus cultivars ranged from 91.33% to 100%. Scions on CjSz developed faster than those on Pt, suggests that graft compatibility is related to the genetic relationship between scion and rootstock. The supply of root-derived nutrients, such as water and minerals, to the shoots may be limited due to incompatibility, leading to poor shoot growth and leaf function[44]. Scions on Pt exhibited a much smaller canopy size and lower SPAD value than those on other rootstocks, consistent with the findings in three late-ripening navel oranges[45] and Folha Murcha sweet oranges[46]. These results suggest that Pt can be used for dense planting and that two genotypes of C. junos are preferable for sparse planting, consistent with previous findings[45]. Therefore, scion-rootstock compatibility based on graft success and tree vigor supports that 'Shuzhen No.1' has a high potential for usage as a citrus rootstock.
-
The authors confirm contribution to the paper as follows: conceptualization and supervision: Wang X; methodology: He W; investigation: Chai J, Wang Y, Wu Z, Li M, Lin Y, Luo Y, Yong Zhang, Yunting Zhang, Wang H; bioinformatic analyses: He W, Chai J; data curation: He W, Xie R, Chai J; manuscript preparation: He W; writing—review and editing: He W, Tang H, Wang X. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
This work was financially supported by Sichuan Provincial Postdoctoral Science Foundation, Sichuan Science and Technology Program (2020ZHCG0027), and Shuangzhi Project Innovation Team of Sichuan Agricultural University (Grant No. P202107).
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Wen He, Rui Xie
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
He W, Xie R, Chai J, Wang H, Wang Y, et al. 2024. Comprehensive evaluation of abiotic stress tolerance and graft compatibility of Citrus junos cv. 'Shuzhen No.1'. Fruit Research 4: e006 doi: 10.48130/frures-0023-0042
Comprehensive evaluation of abiotic stress tolerance and graft compatibility of Citrus junos cv. 'Shuzhen No.1'
- Received: 03 September 2023
- Revised: 31 October 2023
- Accepted: 13 November 2023
- Published online: 01 February 2024
Abstract: Citrus is one of the world's most economically important fruit crops cultivated by grafting. To support the growth of scion cultivars, rootstock is the primary source of resistance to various abiotic stresses. Herein, seedlings of two genotypes of Citrus junos Sieb. ex Tanaka (the novel rootstock 'Shuzhen No.1' and commonly used rootstock 'Ziyang Xiangcheng'), as well as three commonly used rootstocks including citrange (Citrus sinensis Osbeck. × Poncirus trifoliata Raf.), trifoliate orange (P. trifoliata), and red tangerine (Citrus tangerine Hort. Ex Tanaka), were used as testing materials. The seed characteristics were evaluated, and the rootstock seedlings were subjected to flooding, drought, alkaline, and freezing treatments. Over time, the contents of chlorophyll, soluble sugar, proline, malondialdehyde, and the activity of superoxide dismutase, peroxidase, and catalase in the leaves under different treatments were examined. Furthermore, five citrus varieties were grafted as scions onto one-year-old seedlings from the four rootstocks. Graft success, shoot growth, and leaf greenness were measured and compared. The physiological and biochemical changes in 'Shuzhen No.1' were found to be similar to those in 'Ziyang Xiangcheng'. 'Shuzhen No.1' exhibited greater tolerance to flooding, alkaline, and freezing stress compared to the other four widely used citrus rootstocks, as indicated by physiological and biochemical indexes and principal component analysis. Moreover, the five citrus varieties grafted onto 'Shuzhen No.1' demonstrated vigorous growth and tree vigor. These findings provide valuable insights for the application of 'Shuzhen No.1' and future research on citrus rootstock.
-
Key words:
- Citrus rootstock /
- Shuzhen No.1 /
- Seed trait /
- Abiotic stress /
- Antioxidant system /
- Scion–rootstock combination.












