-
Strawberry fruit is highly favored by consumers due to its appealing appearance and flavor, volatiles and nutrients. Beyond its biochemical richness, researchers are particularly drawn to the strawberry as an exceptional model for studying fruit development and ripening. It has been extensively documented that the development and ripening of strawberry fruit are finely controlled by plant hormones, with auxin and gibberellic acid (GA) primarily involved in early development, while abscisic acid takes dominance during ripening stages[1−6].
HTML
-
The function of ethylene, an important fruit ripening hormone, in strawberry is still unclear. Strawberry is conventionally designated as a non-climacteric fruit, as it produces relative low levels of ethylene during fruit ripening[7]. However, this observation does not take into account the tissue heterogeneity of a harvest fruit. Harvesting strawberry fruit is typically accomplished by snapping off or twisting the pedicle. As a result, a harvested strawberry fruit is usually composed of a calyx (with a short pedicle and typically five smaller bracts and five larger sepals), a fleshy fruit (which originates from the receptacle), and true fruit in the forms of achenes[8]. In laboratory experiments designed to measure ethylene production in harvested strawberries, researchers often leave the calyx attached to the fruit to minimize the impact of wounding ethylene. It is indeed observed that, fruits at the big green (BG), turning (T), and half red (HR) stages exhibit increased ethylene production when the calyx had been removed, while the small green (SG) and red (R) stages remained unaffected (Fig. 1a). Wounding-induced ethylene production is closely associated with the transcriptional upregulation of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) genes in the fruit[9,10]. Since the incision is made on the receptacle, certain wounding-responsive ACS genes may be locally upregulated specifically in the receptacle. However, keeping the calyx can complicate ethylene measurement, given the tissue heterogeneity between the calyx and the fruit, and the divergent ethylene levels. Further analysis of the SG and R stages, where wounding did not induce additional ethylene production, revealed that the calyx produced 5 fold more ethylene than the fruit (Fig. 1b). The calyx produced a steady level of ethylene during fruit growth and ripening (Fig. 1b). Given its significant mass contribution to the whole fruit, particularly at the small green stage (> 20%, Fig. 1c), the influence of ethylene produced by the calyx should be taken into account. Notably, contradictory results have been reported regarding the pattern of ethylene production during strawberry fruit ripening, with some studies showing continuous increase, some indicating the opposite trend, and a few observing a peak in ethylene production[11−13]. The underlying causes of these discrepancies remain uncertain and could be attributed to differences in cultivars or measurement methods, including the potential influence of the calyx. Since ethylene from the calyx may diffuse to the fruit, it is possible that the angle increase between sepals as the fruit enlarges (Fig. 1c), leads to less concentrated ethylene around the calyx-fruit junction as the fruit grows.
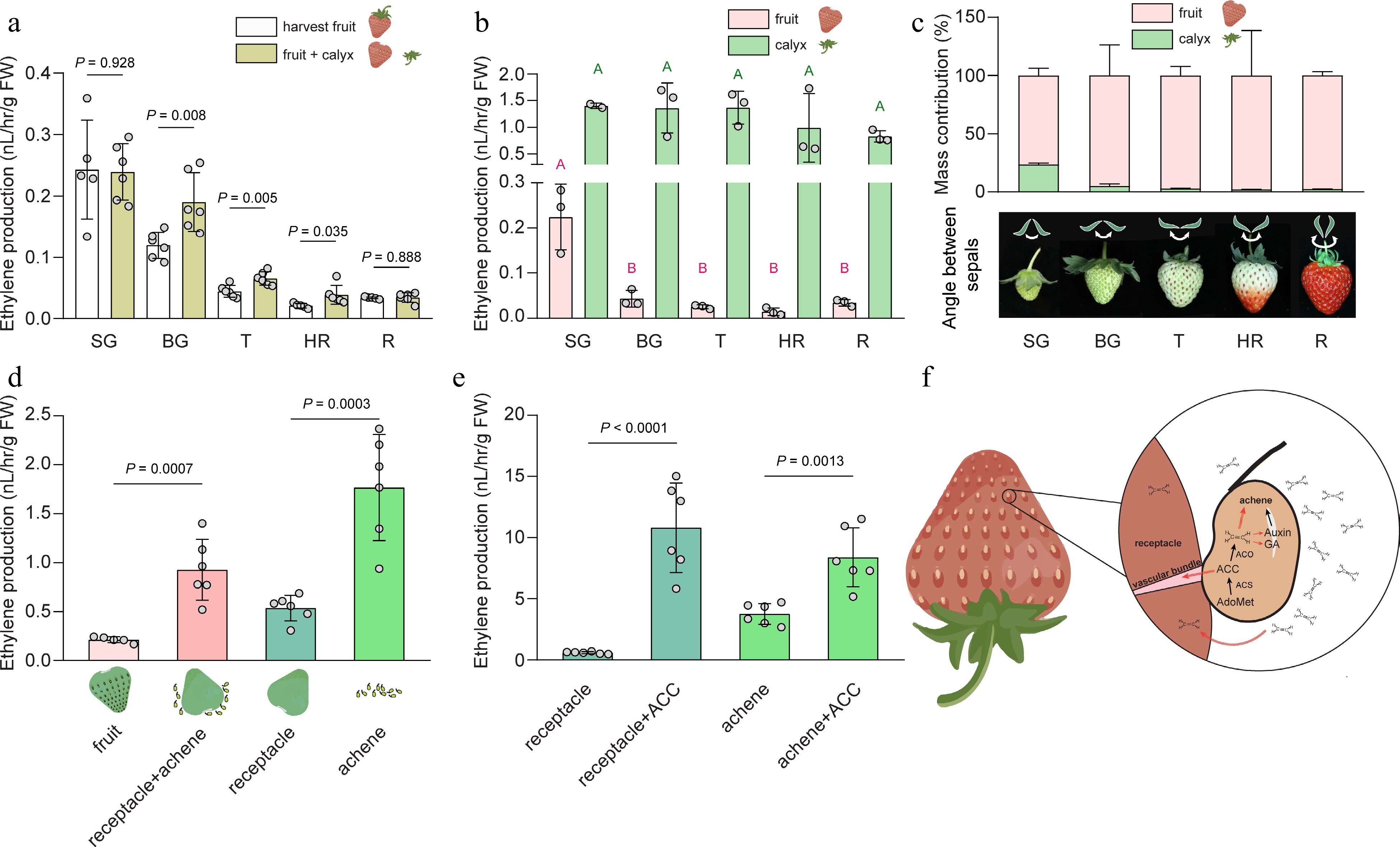
Figure 1.
Ethylene production of the heterogeneous tissues in strawberry fruit during growth and ripening. (a) Ethylene production of harvest strawberry fruit or fruit with detached calyx at five developmental stages. P values were determined using two-tailed t-tests. n ≥ 5. (b) Ethylene production of fruit and the corresponding calyx at five stages during strawberry growth and ripening. Different uppercase letters indicate significant difference at p < 0.05, determined using one-way ANOVA followed by Tukey's posthoc test. n = 3. (c) Upper panel: percentage of mass contribution of calyx and fruit respectively to harvest fruit. Three biological replicates were analyzed, in each biological replicate, five fruit were used. Lower panel: an illustration showing that the angle between sepals increases as fruit develops. (d) Ethylene production of fruit, achenes and receptacle, receptacle, or achenes derived from fruit at SG stage. P values were determined using two-tailed t-tests. n ≥ 5. (e) Ethylene production of receptacle and achenes from SG fruit treated with or without 100 μM ACC. P values were determined using two-tailed t-tests. n = 6. Average ± standard deviation is shown. SG, small green; BG, big green; T, turning; HR, half red; R, red. (f) A diagram showing hypothesized roles of ethylene in strawberry. Ethylene is largely synthesized in the achene by ACC synthase (ACS) and ACC oxidase (ACO). Ethylene may regulate the development and maturation of achene directly and/or indirectly via actions on auxin and gibberellic acid (GA). The ethylene emitted from the achene may diffuse to the receptacle to regulate the growth and ripening of the receptacle. In addition, ACC, the ethylene biosynthesis precursor, might be transported to the receptacle from achenes via the vascular bundles. Red dashed arrows indicate hypothesized roles of ethylene in strawberry fruit. AdoMet, S-adenoysl-methionine. ACC, 1-aminocyclopropane-1-carboxylic acid. Cultivated octoploid strawberry (Fragaria × ananassa 'Yuexin') were used in all experiments. For ethylene measurement, fruit tissues were incubated in 5, or 100 or 300 mL sealed vials for 4 h before headspace withdrawal. The ethylene concentration was measured by gas chromatography (6890N GC system, Agilent, CA, USA) equipped with a flame ionization detector.
To investigate the levels of ethylene production in the receptacle and achenes, we conducted an experiment wherein the removal of achenes led to a significant increase in ethylene production. Achenes produced almost four times the amount of ethylene as the receptacle alone, indicating a substantial contribution of achenes to ethylene production (Fig. 1d). However, this higher level of ethylene production in achenes might be induced by removal impact. In addition, feeding with the ethylene biosynthetic precursor, ACC, led to a significant increase in ethylene levels in both achenes and receptacles. The effect was surprisingly prominent in the receptacles, since this tissue normally produces low levels of ethylene but feeding ACC boosted the level to be as high as in a ripening climacteric fruit (Fig. 1e). This observation raises an intriguing possibility that receptacles may possess a constitutive ACO activity, which may contribute to the oxidation of ACC into ethylene. And the active ACO may be enriched around the connection between achene and the receptacle, possibly in the vascular bundles. Thus the limitation of ethylene production in receptacles may be controlled by ACC homeostasis or transport from other tissues including achenes. Further studies are required to validate this hypothesis and elucidate the underlying mechanisms of why ACO is active in the receptacle, or how ACO activity is induced by wounding, and whether ACC synthesis, transport and metabolism is controlled. A recent study showed that the transcriptional levels of two highly expressed ACO genes in the receptacle of Fragaria vesca, FvACO4a and FvACO4b, exhibited completely opposite trends during fruit ripening[5], but their tissue specific distribution remains unknown.
-
The physiological functions of ethylene, particularly when more abundantly produced by the calyx and achenes in the fruit, raise questions about its role in strawberry development. While it has been proposed that sepals, as seen in tomato[14−16], contribute to flower growth and fruit set, the role of ethylene in the well-developed strawberry calyx, which maintains a relatively constant level of ethylene before fruit set, remains unclear.
The achenes, dotted on the surface of the receptacle, play a crucial role in receptacle enlargement. Previous studies, such as one involving 1 mM ACC feeding, demonstrated a considerable enhancement in ethylene production and an overall increase in fruit weight[11]. Three hypotheses illuminate ethylene's potential roles in achenes and its impact on strawberry fruit development and ripening:
1) Ethylene levels in the achene may change during achene development, akin to climacteric fruits. It could promote achene color formation by inducing anthocyanin biosynthesis, supported by observations of less colored achenes in ethylene-insensitive transgenic plants expressing the Arabidopsis etr1-1 gene in strawberry[17]. Ethylene might be essential for achene maturation.
2) Ethylene may engage in crosstalk with other hormones, such as auxin and GA, in the achene. Since auxin and GA are pivotal for receptacle development, and achenes serve as reservoirs for these hormones inducing receptacle development[3,6], exploring the effect of ethylene on the biosynthesis or transport of auxin and GA in achenes remains intriguing.
3) Ethylene-regulated physiological functions in achenes could impact receptacle development and ripening, either directly by releasing ethylene to trigger a response in the receptacle or by mobilizing the ethylene precursor, ACC, to the receptacle through vascular bundles (Fig. 1f). Alternatively, indirect effects could be achieved by influencing achene development, subsequently influencing receptacle growth.
-
Understanding the role of ethylene in strawberry fruit necessitates precise measurement of ethylene production in the heterogeneous tissues within the fruit. However, detecting the gaseous hormone ethylene in the achene and receptacle of the strawberry in a non-destructive manner poses a significant challenge. Gas chromatography-based measurements, which require headspace collection, face difficulties in separating tissues from the strawberry fruit. Electrochemical sensors, including amperometric, chemoresistive, and capacitive sensors[18], are not suitable for detecting ethylene within strawberry fruit due to the minuteness of the tissues.
Genetically encoded biosensors present a viable strategy for ethylene detection within strawberry tissues[19]. Another example is the development of an artificial metalloenzyme biosensor a few years ago[20] which could be applied to investigate ethylene in strawberry. The detection of ACO activity, ACO protein, or ACC content serves as an indicator of ethylene levels. Additionally, ethylene-responsive elements, such as the EIN3 binding site promoter-derived reporters (e.g., GUS or GFP), offer valuable tools for this purpose[21].
In the pursuit of studying the function of ethylene in strawberry fruit, the use of genetically modified plants with mutations in ethylene biosynthesis or signaling is desirable. Previous attempts involved creating ethylene-insensitive transgenic plants by overexpressing Arabidopsis etr1-1[17]. However, these transgenic plants exhibited only partial insensitivity to ethylene. Employing CRISPR/Cas9 base editing technology to generate etr1-1 mutants in diploid strawberry (Fragaria vesca) could provide stronger evidence for the role of ethylene in strawberry fruit. Furthermore, utilizing stronger mutant like ein2 would contribute to more conclusive findings. Mutants such as eto and ctr1 would also be valuable in enhancing our understanding of the function of ethylene.
-
Cultivated octoploid strawberry (Fragaria × ananassa 'Yuexin') planted at Zhejiang Academy of Agricultural Sciences in Haining, Zhejiang Province (China), were used in all the experiments. Fruits at five developmental stages were harvested, including small green (SG), big green (BG), turning (T), half red (HR), red (R). The receptacles and calyx were gently separated using a scaple, and achenes were carefully datached from the fruit by tweezers. Their corresponding weight were recorded for ethylene production and mass contribution analysis. For ACC feeding assay, 1 mL of 0 or 100 μM ACC solution was added in vials before tissue incubation. Fruit tissues were incubated in 5, 100, or 300 mL vials for 4 h before headspace withdraw with a syringe. The ethylene concentration was measured by gas chromatography (6890N GC system, Agilent, CA, USA) equipped with a flame ionization detector.
-
The authors confirm contribution to the paper as follows: study conception and design: Li D; cexperiments: Chen H, analyzed the data analysis and figures illustrated: Li D, Chen H ; draft the manuscript: Li D, Chen H, Chen K. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This work was supported by the National Natural Science Foundation of China (U23A20215) and the 111 Project (B17039). We thank Prof. Hongli Lian at Shanghai Jiaotong University and Dr. John Vaughan-Hirsch at KU Leuven for their valuable comments.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Chen H, Li D, Chen K. 2024. Spark to blaze: the role of ethylene in achenes and the ripple influences on strawberry fruit growth and ripening. Fruit Research 4: e015 doi: 10.48130/frures-0024-0008 |












