-
Water scarcity is a major constraint on crop production worldwide. Studies have shown that previous stress conditions can influence the response and adaptation of plants exposed to subsequent environmental stresses[1,2]. Research has shown that plants may recover during a second drought period or even exceed the quality of those under continuous watering control[3,4]. For example, Abid et al. found that pre-drought priming facilitated a sustenance in grain development during post-anthesis drought stress in wheat (Triticum aestivum) plants[5]. In another study, false wheatgrass (Leymus chinensis) that was subjected to pre-drought priming exhibited a greater number of tillers and higher biomass after the 70-d rewatering stage compared to the well-watered control treatment[6]. Zhao et al. found that a first instance of moderate drought stress could stimulate the growth of the maize (Zea mays) root system and significantly increase root-to-shoot ratio, which can enhance the drought resistance of maize to subsequent instance of drought and promote the maintenance of total biomass at the control level[7]. Although many studies have shown that drought preconditioning can improve drought tolerance and subsequent crop growth, others have suggested that the improvement of drought tolerance by drought preconditioning comes at the expense of reduced biomass. For example, the performance of Festuca arundinacea and Lolium perenne under drought stress was improved to a varying extent by previous exposure to water deficit; however, both shoot and root biomass decreased concomitantly with an increasing number of drought episodes[8]. Therefore, it was concluded that the recovery efforts of plants strongly depend on pre-drought intensity, duration, and species[9]. Looking for suitable drought preconditioning modes is crucial for improving drought tolerance and increasing crop yield.
Tillering is a key trait that controls the density of monocotyledonous grass plants and affects their ability to establish and recover from stress[10,11]. In addition, tiller development in grasses is important agriculturally, since increased tiller buds and consequently bud outgrowth, can result in increased yields[12]. The tillering of grass species occurs at non-elongated internodes at the base of the parent stem, known as the crown. The tiller development of monocotyledons and the lateral branch growth of dicotyledons include two stages: 1) axillary bud initiation and 2) subsequent elongation under suitable conditions[13,14]. Oryza sativa homeobox 1 (OsH1) and MONOCULM 1 (MOC1) were considered key regulatory factors for meristem initiation and tiller bud formation, respectively, in rice (O. sativa)[15,16]. In the osh1 mutant, axillary meristem formation was impaired, resulting in the failure of tiller formation[17]. The rice lax panicle1 (lax1) and lax panicle2 (lax2) mutants have altered axillary meristem formation, as LAX1 and LAX2 regulate the branching of the aboveground parts of a rice plant throughout its development, with the exception of the primary branch in the panicle[15]. Similarly, a loss-of-function moc1 mutant dramatically reduced tiller number, whereas MOC1-overexpressing lines had increased tiller number[13]. For genetic factors that are involved in axillary bud outgrowth, FINE CULM1 (FC1), an ortholog of the maize TEOSINTE BRANCHED1 (TB1) gene in rice, was reported to be specifically expressed in axillary buds and suppressed tiller bud outgrowth[18,19]. Once established, whether the axillary bud can continue to grow into a tiller or remain dormant depends on the conditions to which plants are subjected. The bud dormancy-activation transition was associated with the expression of the dormancy-associated gene 1/auxin repressed protein (DRM1/ARP)[20]. In addition, the resumption of outgrowth after bud dormancy is related to cell division and proliferation genes, like proliferating cell nuclear antigen (PCNA) and Cyclin D2 (CycD2)[21,22]. Environmental factors including water, light, and nutritional conditions affect the development of tillers, with one such example being the drought-mediated inhibition of axillary bud initiation and elongation[23−25]. Despite there being knowledge of genes controlling axillary bud formation and activation in dicotyledonous plant species, little is currently known about how these genes play a role in promoting grass tiller development under drought preconditioning.
Hormones including cytokinin (CK), auxin, strigolactones (SLs), and others regulate tiller development. Cytokinin promotes tiller and branch development, while auxins and SLs inhibit it[26−29]. In several studies, a negative association has been reported between abscisic acid (ABA) and axillary bud development[30,31]. Liu et al. found that ABA treatment reduced the number of basal tillers in both high-tillering mutant tillering20 and wild-type rice plants[30]. Furthermore, previous studies have shown that changes in hormones, such as ABA and jasmonic acid (JA), play an important role in promoting plant growth through drought preconditioning in tall fescue, Arabidopsis (Arabidopsis thaliana), and olive tree (Olea europaea)[32−34]. Liu & Finlayson found that application of JA to buds in situ promoted growth[35]. Although there is currently some understanding of hormones that control the formation and activation of axillary buds in dicotyledonous and monocotyledonous plants, little is known about how hormones specifically function in regulating tiller development in perennial herbaceous plants subjected to drought preconditioning.
The objective of this study was to understand the mechanisms underlying drought preconditioning and their effects on tiller development upon rewatering in perennial grasses, such as perennial ryegrass, which are widely cultivated as forage- or turfgrasses. This study explores the mechanism by which drought preconditioning promotes the tiller development of perennial ryegrass through changes in gene expression levels and hormone regulation.
-
The perennial ryegrass cultivar, 'Pinnacle', was used in this study. Seedlings were germinated on wet filter paper and placed in Petri dishes. Four plants (one shoot for each plant to ensure the same plant density) were planted in a 10-cm diameter, 25-cm height tube filled with fritted clay. Three tubes were prepared for each treatment. Plants were maintained in an environmentally controlled walk-in growth chamber. The conditions were set at 23/18 °C (day/night), 70% relative humidity, 14/10 h (day/night) photoperiod, and 640 µmol·m−2·s−1 of photosynthetically active radiation. Plants were fertilized with half-strength Hoagland's nutrition solution twice weekly and maintained well-irrigated with daily irrigation prior to drought treatment[36].
Plants were acclimated in a growth chamber for 10 d before treatment, after which three types of treatments were carried out in the study (Fig. 1). Well-watered control (W), plants grew under consistent watering through 24 d; drought preconditioning (D5 + W3) plants underwent drought preconditioning (5 d drought + 3 d rewatering), followed by a 5-d period of water-withholding and subsequent rewatering for 11 d; drought–rewatering (D13 + W) plants underwent a water-withholding period of 13 d and were then rewatered for 11 d. Plants were irrigated without nutrition during 0−13 d in W treatment, one fertilization with half-strength Hoagland's nutrient solution was applied for both W and D5 + W3 treatment at 5 d. Nutrient deficiency was not observed during the study. After 13 d, plants were fertilized and irrigated in three groups.
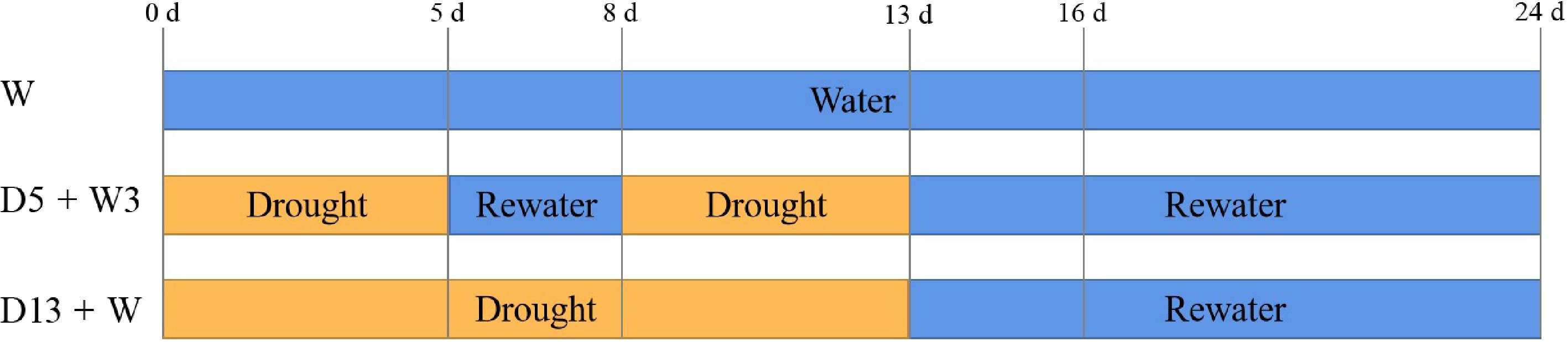
Figure 1.
Diagram of well-watered control (W), drought preconditioning (D5 + W3), and drought–rewatering (D13 + W) treatments. W plants grew under consistent watering through 24 d of treatment; D5 + W3 plants underwent drought preconditioning (5 d drought + 3 d rewatering), then water-withholding for 5 d and rewatering for 11 d; D13 + W plants grew under water-withholding for 13 d and were then rewatered for 11 d. The measurement time points are indicated by the indicators, as follows: 0 d, 5 d, 8 d, 13 d, 16 d, and 24 d.
Tillers and tiller buds observation
-
The number of tillers and tiller buds were calculated at 0 d, 5 d, 8 d, 13 d, and 24 d. For the calculation of tiller buds, leaf sheaths that enclose the culm were carefully removed using microscope forceps under an Olympus SZX16 stereo microscope (Olympus, Tokyo, Japan). A tiller bud that was longer than 1 cm was designated as one tiller[25]. Photos of whole seedlings were taken using a digital single-lens reflex camera (Nikon D5100, Bangkok, Thailand).
RNA extract and first cDNA synthesis
-
Crowns were sampled at 13, 16, and 24 d and stored in an −80 °C freezer for gene expression analysis. Each treatment contained three biological replicates. About 0.10 g of samples were used for RNA extraction using the E.Z.N.A.® Plant RNA Kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer's instructions. First-strand cDNA was synthesized using the PrimeScript RT reagent kit with a gDNA Eraser (Perfect Real Time) (Takara, Otsu, Japan) after being verified for RNA quality and integrity.
qRT-PCR analysis
-
Relative expression analysis was carried out on a Roche LightCycler 480 II machine (Roche Diagnostic, Rotkreuz, Switzerland). The SYBR Green I Master reaction system (Roche Diagnostic, Rotkreuz, Switzerland) was utilized for the reaction. The qRT-PCR procedure was set as follows: pre-denaturation at 95 °C for 10 min, followed by 40 cycles of three-step amplification (denaturation at 95 °C for 10 s, annealing at 60 °C for 15 s, and extension at 72 °C for 20 s). Data were collected at 65 °C in each cycle. Relative expression level was calculated by the 2−ΔΔCᴛ method using LpeIF4A (eukaryotic initiation factor 4 alpha) as a reference gene[37]. Three biological samples and three technical replicates were implemented for each treatment at 13, 16, and 24 d. The primers tested in the study can be found in Supplemental Table S1.
Measurement of hormones
-
Hormones were extracted using the modified methods of Pan et al. and Zhuang et al.[38,39]. Fresh crown samples (0.5 g) were collected from plants exposed to the W, D5 + W3, and D13 + W treatments at 13 d and 16 d. Hormones were extracted and purified in diochloromethane and an extract buffer comprised of isopropanol and hydrochloric acid. The resulting solution was concentrated in a nitrogen evaporator and dissolved in methyl alcohol containing 0.1% methanoic acid. After filtration, the solution was used for hormone measurement. Plant hormone samples were analyzed by high-performance liquid chromatography–tandem mass spectrometry (HPLC/MS/MS) using the Agilent 1290 HPLC (Agilent, Santa Clara, CA, USA) and SCIEX-6500 QTRAP (AB Sciex, Foster, CA, USA), following the equipment setup as described by Pan et al.[38].
Statistical analyses
-
Data in all experiments were analyzed using the general linear model test using the analysis of variance program of SAS 9.0 (Cary, NC, USA). Mean values between different treatments at certain time points were defined as significant when the value of Fisher's protected least significant difference (LSD) was lower than a 0.05 probability.
-
In order to investigate the effects of drought preconditioning on the tiller development of perennial ryegrass, three treatments of W, D5 + W3, and D13 + W were used (Fig. 1). Tiller bud initiation and elongation were observed closely (Fig. 2a). During the 13-d period of D5 + W3 treatment, the number of tillers gradually increased from 2 to 9; after 11 d rewatering, tiller number rapidly increased to 32 (Fig. 2b). Meanwhile, tiller bud gradually increased from 1 to 8 during the 13-d period of D5 + W3 treatment, and rapidly increased to 27 after 11 d rewatering (Fig. 2c). Both tiller number and tiller bud number in plants subjected to D5 + W3 treatment was significantly higher than those of W at 24 d (Fig. 2b & c). The tiller number of the D13 + W treatment was remarkably lower than that of the W treatment at 8 d, while tiller bud number showed no difference until 13 d. The tiller number and tiller bud number of the D13 + W treatment were significantly lower than those of the W treatment at 24 d (Fig. 2b & c). The bud/tiller ratio of the D5 + W3 treatment was significantly higher than that of the W treatment, while it was significantly lower in the D13 + W treatment after 13 d drought stress (Fig. 2d). Compared to the W and D13 + W treatments, plants subjected to D5 + W3 grew more vigorously, as shown in Fig. 2e.
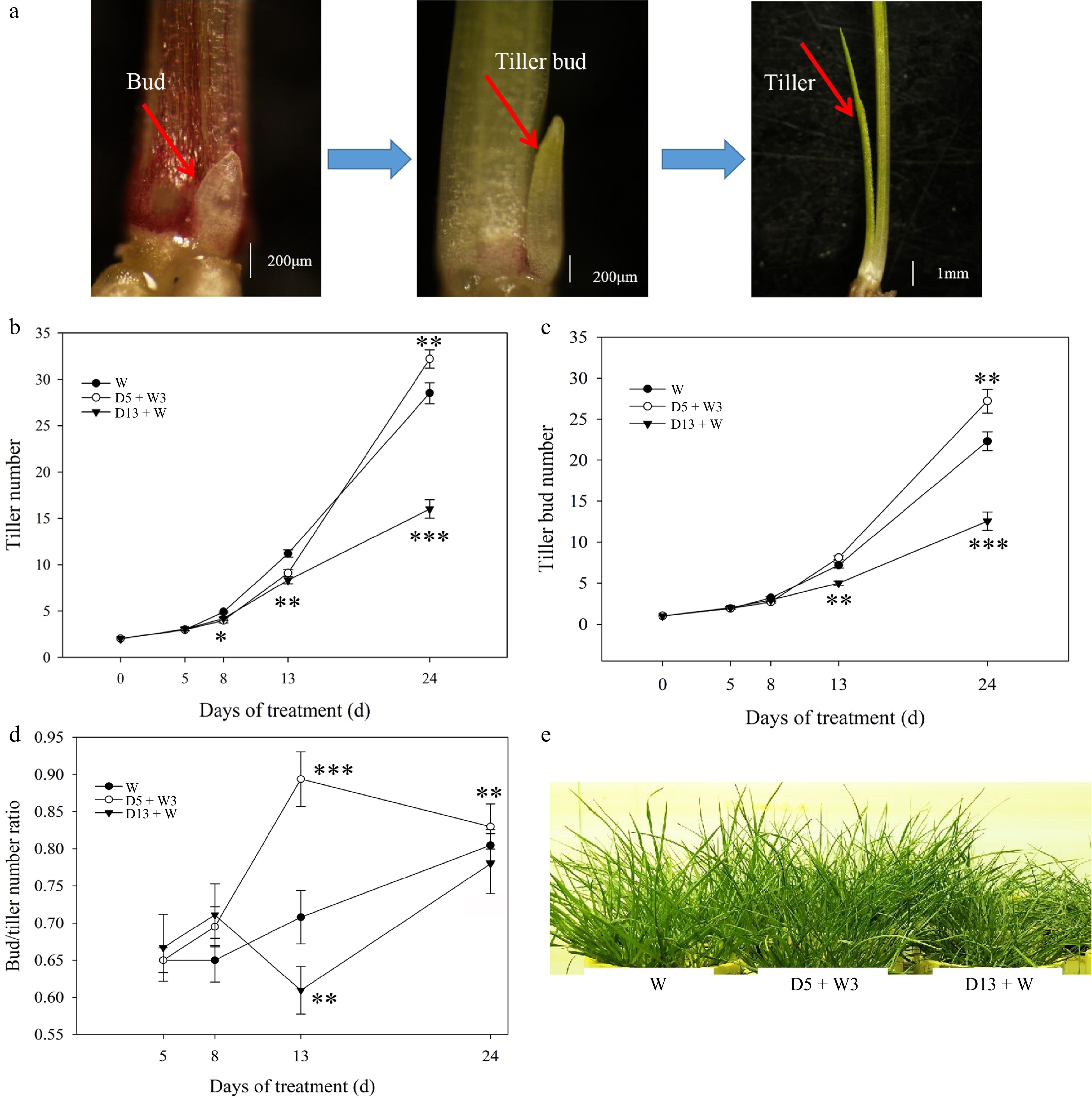
Figure 2.
Effect of drought preconditioning on tiller development in perennial ryegrass. (a) The process of tiller bud initiation and elongation. (b) Tiller number. (c) Tiller bud number. (d) Bud/tiller ratio. (e) Phenotype of plants at 24 d. A tiller bud longer than 1 cm was designated as a tiller. Tiller buds were observed after removing the leaf sheath. The bud/tiller ratio was calculated as the total number of buds divided by the total number of tillers. Ten plants were measured for each treatment. Values were calculated as means ± SE. *, indicates significant differences at p < 0.05, while ** indicates significant differences at p < 0.01, and *** indicates significant differences at p < 0.001.
Effects of drought preconditioning treatment on genes that regulate tiller bud initiation
-
The relative expression level of four genes that are involved in axillary meristem initiation and bud initiation was detected (Fig. 3). LpH1 was down-regulated under the D5 + W3 and D13 + W treatments at 13 d. After rewatering, the expression level of LpH1 in the D5 + W3 treatment returned to normal, whereas the gene was up-regulated in the D13 + W treatment at 13 d. LpMOC1, LpLAX1, and LpLAX2 showed a similar expression pattern at 13 d and 24 d. All three genes were up-regulated at 13 d under the D5 + W3 and D13 + W treatments compared with the W treatment, and its expression level was highest in the D13 + W treatment. Alternatively, the three genes had the highest expression level under the D5 + W3 condition at 24 d. It was noteworthy that the expression levels of both LpMOC1 and LpLAX2 were down-regulated at 16 d under the D5 + W3 and D13 + W conditions compared with the levels of these genes under the W condition. The expression level of LpLAX1 was up-regulated at 16 d under the D13 + W condition but was not changed in the D5 + W3 treatment.
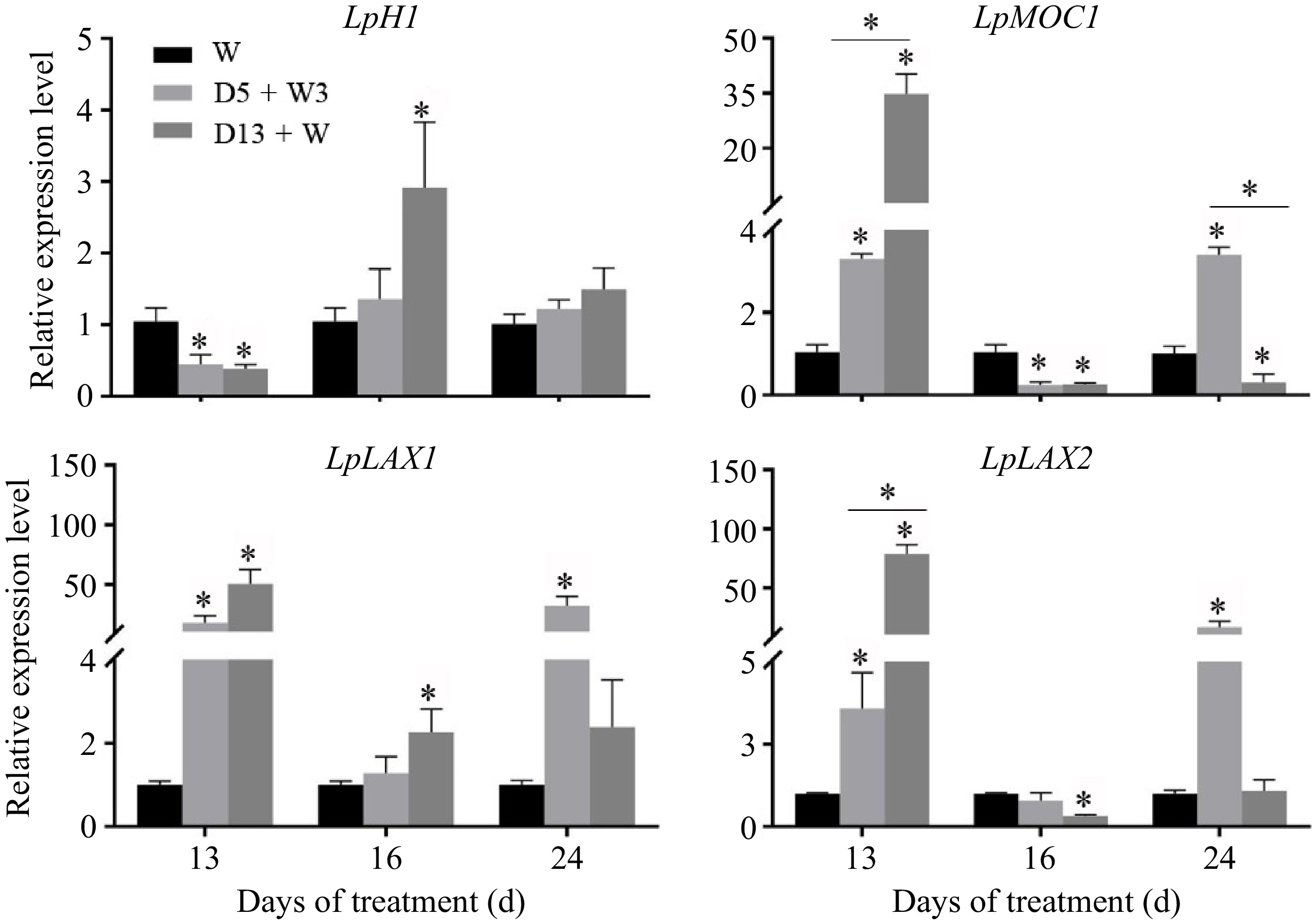
Figure 3.
qRT-PCR analysis of expression level of genes involved in axillary bud initiation in crowns of perennial ryegrass. LpH1, homeobox 1 protein; LpMOC1, monoculm 1; LpLAX1, lax panicle 1; LpLAX2, lax panicle 2. Values were calculated as means ± SE. *, indicates significant differences at p < 0.05.
Effects of drought preconditioning treatment on genes that regulate tiller bud dormancy, bud activity, and bud outgrowth
-
The relative expression levels of LpDRM1 and LpARP were significantly up-regulated at 13 d in the D13 + W treatment and were much higher than those expressed in the D5 + W treatment (Fig. 4). The expression levels of these genes returned to the control level at 16 d. Two cell cycle genes, LpPCNA and LpCycD2, exhibited a pattern of remarkable down-regulation under the 13 d drought stress treatment, while the D5 + W3 treatment significantly delayed cell division gene expression reduction, as indicated by notably higher expression of LpCycD2 compared with that of the D13 + W treatment. Expression levels of both LpPCNA and LpCycD2 were up-regulated at 16 d (rewatering for 3 d) under the D5 + W3 and D13 + W conditions as compared with the W conditions.
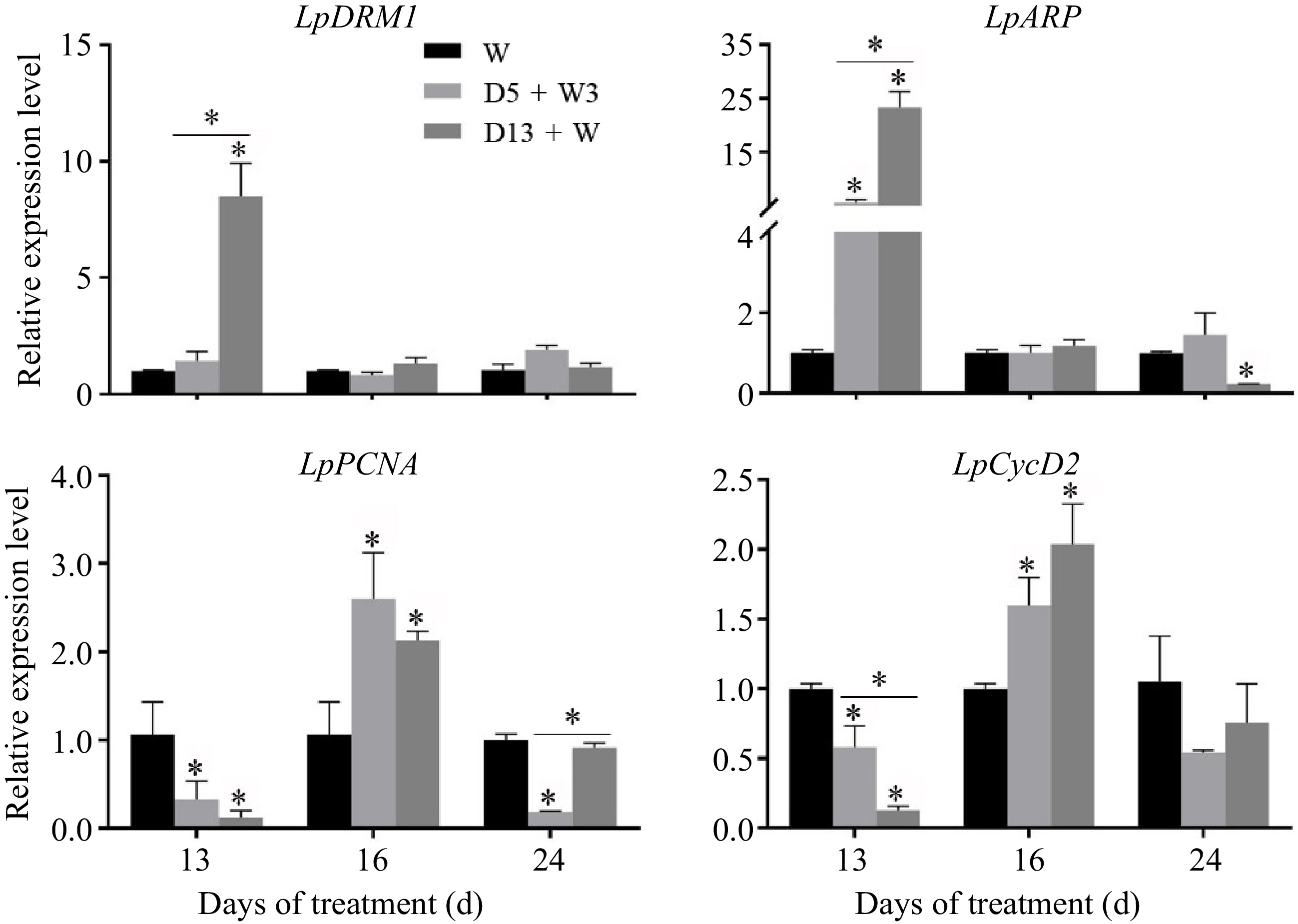
Figure 4.
qRT-PCR analysis of expression level of genes involved in bud dormancy (LpDRM1 and LpARP) and bud activity (LpPCNA and LpCycD2) in crowns of perennial ryegrass. LpDRM1, dormancy-related protein-1; LpARP, auxin-repressed protein; LpPCNA, proliferating cell nuclear antigen; LpCycD2, cyclin D2. Values were calculated as means ± SE. *, indicates significant differences at p < 0.05.
The relative expression level of LpTB1 was significantly up-regulated under the D5 + W3 and D13 + W treatments at 13 d (Fig. 5). Its expression level in plants that underwent the D5 + W3 treatment was lower than in plants that endured consistent drought for 13 d. After rewatering for 3 d, for a total treatment period of 16 d, the expression level of LpTB1 was down-regulated and exhibited a much lower level in the D5 + W3 treatment as compared to the D13 + W treatment. LpTB1 showed the highest expression level overall in the D5 + W3 treatment at 24 d.
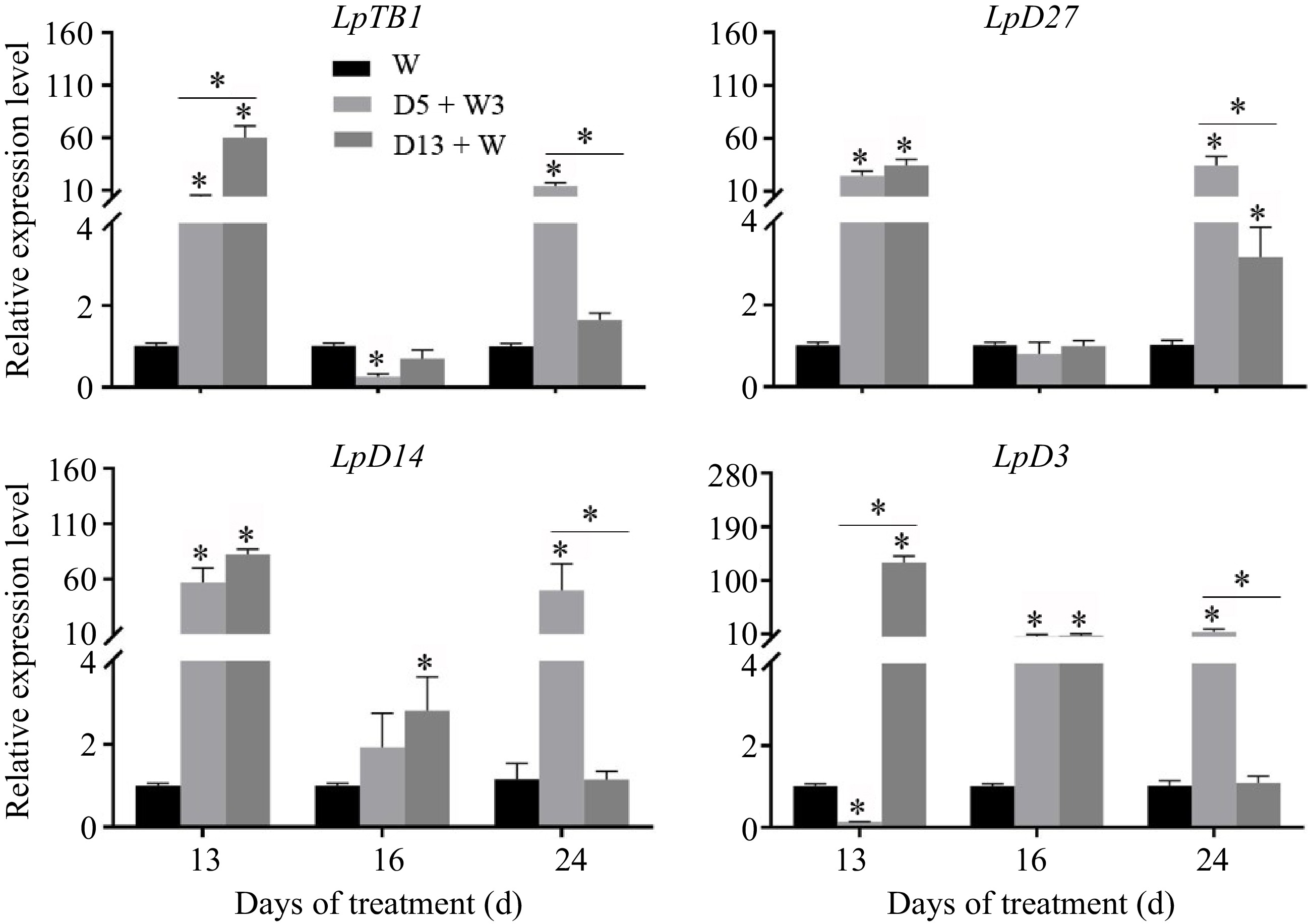
Figure 5.
qRT-PCR analysis of expression level of genes involved in repressing axillary bud outgrowth in crowns of perennial ryegrass. LpTB1, teosinte branched 1; LpD27, dwarf 27; LpD14, dwarf 14; LpD3, dwarf 3. Values were calculated as means ± SE. *, indicates significant differences at p < 0.05.
LpD27, which is a putative strigolactone synthesis gene, exhibited a high up-regulation pattern at 13 d under the D5 + W3 and D13 + W treatments (Fig. 5). The expression level of this gene was restored to a normal level after 3 d of rewatering. The SL receptor, LpD14, was up-regulated at 13 d in both treatment groups (D5 + W3 and D13 + W) and was expressed at a higher level at 16 d under the D13 + W condition. The signal transduction gene, LpD3, was up-regulated at 13 d under the D13 + W condition, whereas it was down-regulated under the D5 + W3 condition. At 16 d of treatment, LpD3 was maintained at higher levels in both treatment groups (D5 + W3 and D13 + W). LpD27, LpD14, and LpD3 showed the highest expression levels in the D5 + W3 treatment at 24 d.
Effects of drought preconditioning treatment on hormone content in crowns
-
To test the effects of drought preconditioning treatment on hormones, the contents of endogenous hormones in crowns were quantified by HPLC-MS/MS (Fig. 6). Abscisic acid (ABA) content was elevated under D13 + W at 13 d, while D5 + W3 showed no difference from the W. The jasmonic acid (JA) content and cis-12-oxophytodienoate (cis-OPDA) content were down-regulated in D13 + W treatment at 13 d, while there was no difference between the D5 + W3 treatment and the W. The content of indole-3-acetic acid (IAA) was down-regulated under D5 + W3 and D13 + W treatments at 13 d, however, it was significantly higher in D5 + W3 compared with that in D13 + W treatment. Regarding cytokinin [isopentenyladenine (iP), isopentenyladenine riboside (iPR), cis-Zeatin (cZ), trans-Zeatin (tZ), cis-Zeatin riboside (cZR), and trans-Zeatin-riboside (tZR)], drought stress reduced the content of cytokinins (iP, cZ, tZ, and cZR) in D5 + W3 and D13 + W at 13 d, while drought preconditioning slowed down the decrease in iP under D5 + W3 treatment. In addition, drought preconditioning up-regulated tZR content in D5 + W3 treatment at 13 d, while there was no difference between the D13 + W treatment and the W. There was no significant difference in the content of iPR in both D5 + W3 and D13 + W compared to W both at 13 d. After rewatering, the content of cZ was significantly increased in D5 + W3 and D13 + W treatments at 16 d. The content of tZR was significantly decreased in both D5 + W3 and D13 + W treatments at 16 d, while tZR content showed a higher level in D5 + W3 compared with that in D13 + W. Other hormone contents, including ABA, JA, cis-OPDA, IAA, iP, iPR, tZ, and cZR, showed no significant difference in D5 + W3 and D13 + W treatments compared with that in W at 16 d.
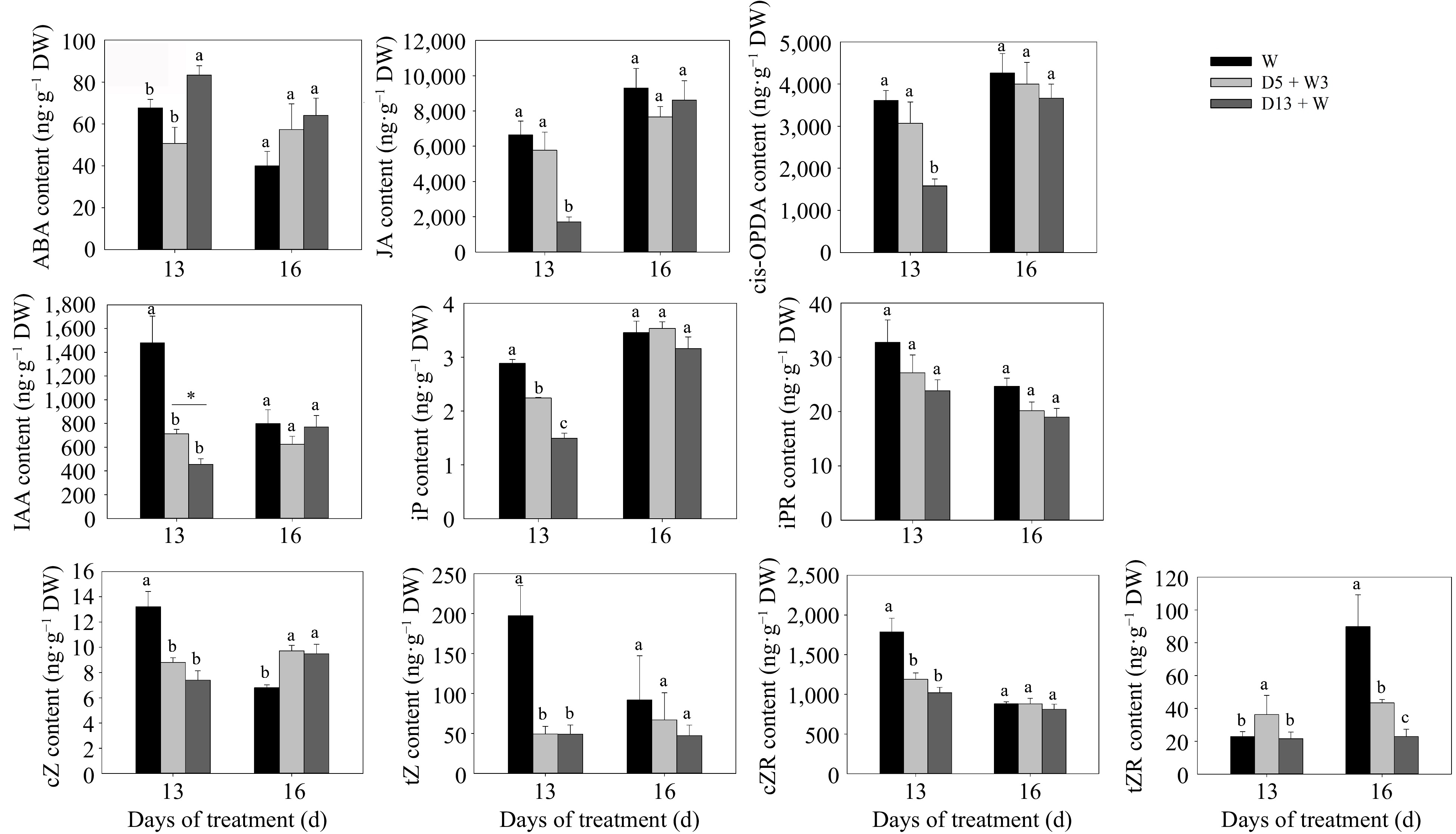
Figure 6.
Hormone content in crowns of perennial ryegrass under drought preconditioning. Abscisic acid (ABA), jasmonic acid (JA), cis-12-oxophytodienoate (cis-OPDA), indole-3-aceticacid (IAA), isopentenyladenine (iP), isopentenyladenine riboside (iPR), cis-Zeatin (cZ), trans-Zeatin (tZ), cis-Zeatin riboside (cZR), and trans-Zeatin-riboside (tZR). Values were calculated as means ± SE. Different lowercase letters above the columns indicated significant differences at p < 0.05 at the same day. *, indicates significant differences at p < 0.05.
Effects of drought preconditioning treatment on genes related to hormones
-
The expression level of four genes that are involved in ABA biosynthesis (LpZEP and LpNCED) and ABA degradation (LpCYP707A5 and LpCYP707A6), was determined (Fig. 7a). LpZEP was up-regulated in the D13 + W treatment but showed no significant difference in D5 + W3 compared with that in W at 13 d. The expression level of LpZEP in D5+W3 and D13+W treatments returned to the control level at 16 d. The expression level of LpNCED was up-regulated in the D5 + W3 and D13 + W treatments at 13 d; however, it was significantly lower in D5 + W3 than in D13 + W. LpNECD under D5 + W3 treatment was down-regulated at 16 d. Both LpCYP707A5 and LpCYP707A6 were significantly up-regulated in the D5 + W3 treatment compared with that in W at 13 d, while no significant difference was detected between D13 + W treatment and W. Expression level of LpCYP707A5 and LpCYP707A6 in D5 + W3 and D13 + W treatments showed no significant difference with that in well-watered control level at 16 d.
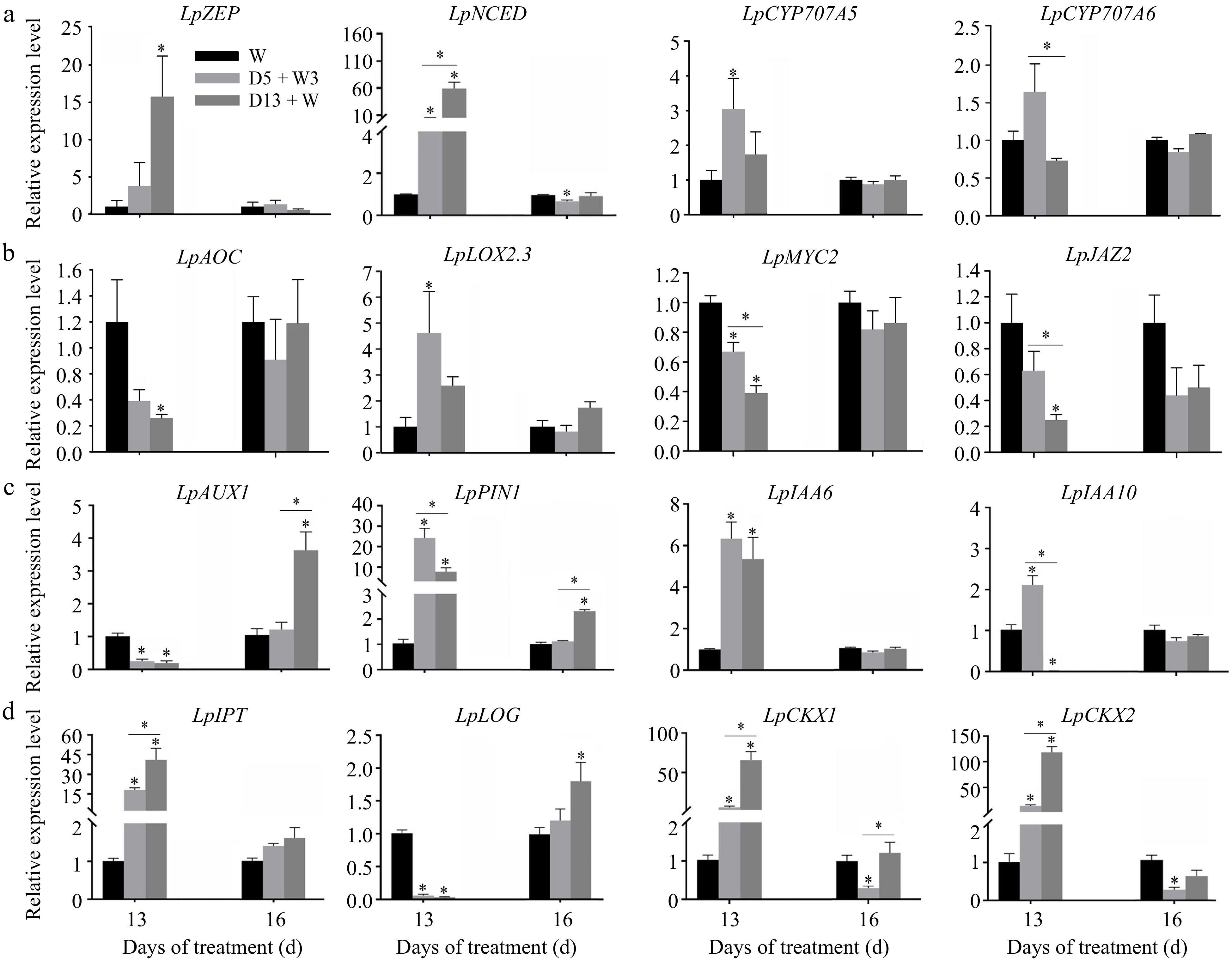
Figure 7.
qRT-PCR analysis of expression levels of genes related to ABA synthesis genes (LpZEP and LpNCED), ABA degradation genes (LpCYP707A5 and LpCYP707A6), JA synthesis genes (LpAOC and LpLOX2.3), JA signaling pathway genes (LpMYC2 and LpJAZ2), auxin transporter genes (LpAUX1, LpPIN1) and auxin-responsive genes (LpIAA6, LpIAA10), and CK biosynthesis genes (LpIPT, LpLOG) and catabolism genes (LpCKX1 and LpCKX2) in crowns of perennial ryegrass. a, LpZEP, zeaxanthin epoxidase; LpNCED, 9-cis-epoxycarotenoid dioxygenase; LpCYP707A5, abscisic acid 8'-hydroxylase 1; LpCYP707A6, abscisic acid 8'-hydroxylase 2. b, LpAOC, chloroplast allene oxide cyclase; LpLOX2.3, lipoxygenase 2.3; LpMYC2, transcription factor MYC2; LpJAZ2, Jasmonate ZIM domain-containing protein 2. c, LpAUX1, auxin transporter protein 1; LpPIN1, probable auxin efflux carrier component 1; LpIAA6, auxin-responsive protein IAA6; LpIAA10, auxin-responsive protein IAA6. d, LpIPT, adenylate isopentenyltransferase 1; LpLOG, cytokinin riboside 5'-monophosphate phosphoribohydrolase LOG; LpCKX1, cytokinin dehydrogenase 1; LpCKX2, cytokinin dehydrogenase 2. Values were calculated as means ± SE. *, indicates significant differences at p < 0.05 on the same day.
The expression level of genes involved in JA biosynthesis and transport were determined (Fig. 7b). The expression level of the JA biosynthesis gene, LpAOC, was significantly down-regulated in the D13 + W treatment at 13 d, but no significant difference was detected between D5 + W3 treatment and W. Another JA biosynthesis gene, LpLOX2.3, was significantly up-regulated at 13 d in the D5 + W3 treatment. LpMYC2, a key transcription factor that regulates corresponding genes downstream of JA, was significantly down-regulated in the D5 + W3 and D13 + W treatments, while LpMYC2 was higher in the D5 + W3 treatment than in the D13 + W treatment. LpJAZ2, which is a repressor of JA, shared a similar expression pattern with LpMYC2. The relative expression levels of LpAOC, LpLOX2.3, LpMYC2, and LpJAZ2 returned to a normal level at 16 d, both in the D5 + W3 and D13 + W treatments compared with that of the W treatment.
The expression level of genes involved in auxin signaling and transport was determined (Fig. 7c). LpAUX1 was significantly down-regulated at 13 d both in the D5 + W3 and D13 + W treatments. Expression of LpAUX1 in the D5 + W3 treatment showed no significant difference compared with that of the W treatment at 16 d, and the highest expression level was detected under the D13 + W treatment. LpPIN1, which was expected to act as an auxin transporter, was remarkably up-regulated in the D5 + W3 and D13 + W treatments at 13 d, with a higher level having been expressed in the D5 + W3 treatment. After 3 d of rewatering, the expression level of LpPIN1 returned to the normal level in the D5 + W3 treatment, while a higher expression level was observed in the D13+W treatment as compared to that of the W treatment. LpIAA6 showed a pattern of up-regulation under the D5 + W3 and D13 + W conditions compared with that in the W condition at 13 d. LpIAA10 demonstrated an up-regulated expression level at 13 d under the D5 + W3 treatment; however, the expression level was down-regulated under the D13 + W treatment. No difference was detected in the expression levels of LpIAA6 and LpIAA10 among the D5 + W3, D13 + W, and W treatments at 16 d.
The relative expression levels of two cytokinin biosynthesis (LpIPT and LpLOG) and two degradation (LpCKX1 and LpCKX2) genes were determined (Fig. 7d). LpIPT was significantly up-regulated in the D5 + W3 and D13 + W treatments and had a much higher level in the D13 + W treatment at 13 d, while LpLOG was down-regulated under both conditions. The expression level of LpIPT in the D5 + W3 and D13 + W treatments returned to the control level at 16 d. The expression level of LpLOG was significantly up-regulated in the D13 + W treatment at 16 d, while in the D5 + W3 treatment, it returned to a normal level. LpCKX1 and LpCKX2, showed the same expression pattern in the study, with both genes having been up-regulated at 13 d but to a much higher extent under the D13 + W condition as compared with that of the D5 + W3 treatment. The relative expression levels of both genes were down-regulated under the D5 + W3 treatment at 16 d.
-
Plants existing in natural environments have to cope with climate change and different stresses, including drought stress, the duration of which can range from seconds to months. Reports have indicated that drought stress inhibits tiller formation and growth[10,25]. In contrast with the rapid progress made in understanding the regulation of tiller and branch development in plants, the mechanisms associated with tiller growth under drought preconditioning are not well-understood. This study identified a drought preconditioning treatment combination (D5 + W3) that promotes tiller development in perennial ryegrass (Fig. 2). After observing the initiation and elongation of tiller buds, it was determined that both tiller and tiller bud number were significantly higher in the D5 + W3 treatment when compared with those of the W treatment at 24 d (Fig. 2a). Moreover, this study showed that tiller bud outgrowth was more sensitive to drought stress than tiller bud initiation, since bud number was not affected until 13 d of drought stress (D13 + W), whereas tiller number was significantly inhibited by 8 d of D13 + W treatment (Fig. 2b−d). The results are consistent with the findings of Zhuang et al.[25]. The D5 + W3 drought preconditioning treatment alleviated the sensitivity of tiller bud initiation and outgrowth to drought, as indicated by D5 + W3-treated plants having a similar number of tiller buds as the W treatment, as well as more tillers than the D13 + W group in the first 13 d (Fig. 2b & c).
There has been ample evidence that the genes that regulate the initiation and growth of plant axillary buds control lateral branches. It is known that MOC1, LAX1, and LAX2 positively regulate the formation of axillary buds in monocotyledons[15,17,40,41]; however, in this study, the expression of LpMOC1, LpLAX1, and LpLAX2 was up-regulated in the D13 + W treatment at 13 d (Fig. 3), which contrasted a decrease in bud number. This suggested that a negative feedback system exists during tiller bud initiation. Interestingly, up-regulation of gene expression levels (LpMOC1, LpLAX1, and LpLAX2) in D5 + W3 was consistent with the increase in bud number per tiller after preconditioning at 13 d (Fig. 2d). Moreover, the mRNA of LpMOC1, LpLAX1, and LpLAX2 accumulated to a remarkably high level in plants receiving the D5 + W3 treatment, while it returned to the level of the W treatment in D13 + W-treated plants at 24 d (Fig. 3). This indicates that LpMOC1, LpLAX1, and LpLAX2 accelerate tiller bud production under drought preconditioning. Though expression of LpH1 was down-regulated in both the D5 + W3 and D13 + W treatments at 13 d, no significant difference was detected between the two groups. This suggests that LpH1 did not play a major role or had very little function in the promotive effects of drought preconditioning. Once established, whether a tiller bud can grow into a tiller or will remain dormant depends on many factors. The DRM1/ARP genes were reported to be marker genes for bud dormancy and are responsive to a diverse range of abiotic factors, such as drought[20,42]. LpDRM1 and LpARP showed an up-regulated expression pattern during 13 d of drought stress compared to the W treatment (Fig. 4), which indicates that tiller buds were undergoing activation-dormancy transition. Alternatively, the expression levels of LpDRM1 and LpARP in the D5 + W3 treatment were significantly lower than those of the D13 + W treatment at 13 d (Fig. 4), indicating that drought preconditioning (D5 + W3) alleviated inductive expression of bud dormancy genes. Studies have shown that the decreased expression level of DRM1/ARP is associated with an increased branching phenotype[43]. Though tiller number was not increased at 13 d in the D5 + W3 treatment, we believed that LpDRM1 and LpARP played a role in the tiller formation process. It was reported that the expression of cell cycle marker genes is expressed in an inverse pattern as compared with the DRM1/ARP genes[44,45]. In this study, LpPCNA and LpCycD2, exhibited an opposite expression pattern to LpDRM1 and LpARP at 13 d (Fig. 4). In addition, there was significant up-regulation of the LpPCNA and LpCycD2 genes, which occured after 3 d of rewatering. Since PCNA was dramatically down-regulated in inhibited tillers compared to normal tillers[46] while CycD2 was associated with quiescent axillary meristem cells in the cell cycle[22], it is possible that tiller development was promoted by alleviating the down-regulation of cell cycle genes in plants preconditioned to drought stress. As mentioned in investigations of other plants, SLs had negative effects on tiller bud outgrowth. Results of this study showed that drought preconditioning might not function by changing the SL content, as expression of LpD27 showed no difference between the D5 + W3 and D13 + W treatments at 13 d. Expression of LpD3 was responsive to drought preconditioning, since the plants accumulated much less LpD3 mRNA in D5 + W3-treated plants than in D13 + W-treated plants. Consistently, it was reported that mutants of the SL signal transduction gene, d3/max2, exhibited a phenotype of greater lateral branching and tillering in many species[47]. TB1, as a transcription factor in the TCP family, inhibits the extension of rice lateral buds and negatively regulates rice tiller numbers[48]. As in the case of maize, up-regulation of TB1 occurred during the domestication of multi-tillering teosinte to a single-stalk modern maize[49]. In this study, the expression level of LpTB1 in the D5 + W3 treatment was significantly lower than that of the D13 + W treatment at 13 d (at the end of preconditioning) (Fig. 5). After 3 d of rewatering, the expression level of LpTB1 rapidly decreased and was lower than that of the W group in the D5 + W3 treatment (Fig. 5). Since there is some evidence that TB1 acts downstream of SL[48], it is reasonable that LpD3 decreased at 13 d and LpTB1 decreased at 16 d under the D5 + W3 condition as compared to the W condition. From this study, low expression of LpTB1 and LpD3 may be an important reason as to why tiller formation increased under the D5 + W3 treatment.
Significant changes were observed in ABA, JA, auxin, and CK levels in crown samples at 13 d. Our results showed that up-regulation of ABA content at 13 d in the D13 + W treatment was consistent with the expected up-regulation of ABA following drought stress[50,51]. The content of ABA under the D5 + W3 treatment showed no significant difference as compared with the W treatment at 13 d, which indicates that pre-conditioning had effects. The expression of ABA synthesis (LpZEP and LpNCED) and degradation (LpCYP707A5 and LpCYP707A6) genes showed a high correlation with ABA content (Fig. 7a). Research has shown that the number of tillers decreases in transgenic lines overexpressing OsNCED1 (a gene encoding the ABA biosynthetic enzyme)[52]. Therefore, it is reasonable that the content of ABA was not up-regulated in plants that underwent drought preconditioning. It was reported that application of JA to buds in situ promoted growth[35]. The content of JA and cis-OPDA in the D5 + W3 treatment showed no significant difference as compared with the W treatment after preconditioning at 13 d, which may have resulted from the neutralized expression of LpAOC and LpLOX2.3 (Fig. 6). The reduction of JA in the D13 + W treatment at 13 d might have been caused by a lower expression of the major JA biosynthesis gene, LpAOC (Fig. 7b), as the activity of the AOC1 promoter determines the endogenous levels of JA[53,54]. Since AOC1 is known to be regulated directly by MYC2[32], this may explain why LpMYC2 showed a similar expression pattern with LpAOC1 at 13 d and was up-regulated in the D5 + W3 treatment (Fig. 7b). JAZ2 acts as a repressor of JA signaling and is activated by the transcription factors, MYC2, MYC3, and MYC4[55]. It was noteworthy that LpJAZ2 showed the same expression pattern as LpMYC2 (Fig. 7b). Gupta et al. discovered that tomato plants overexpressing LeMYC2 have reduced internode distance and greater branching, while the opposite morphology was found in RNAi transgenic lines. It is reasonable that the down-regulation of LpMYC2 was attenuated in plants that underwent drought preconditioning[56]. The content of IAA in the D5 + W3 and D13 + W treatments at 13 d was lower than that of the W treatment, and it was significantly higher in the D5 + W3 in comparison to the D13 + W treatment (Fig. 6). Auxin was reported to play an indirect negative role in regulating branch and tiller development[57], and although auxin content was affected by drought preconditioning, whether it played a vital role in promoting tiller growth under the conditions implemented in this study remains unknown. Previous studies showed that the highly expressed polar auxin transport gene, OsPIN1b, inhibited the growth of axillary buds and that a decrease in the expression of OsPIN1b accelerated the outgrowth of axillary buds in rice[58]. In this study, genes involved in auxin transport (LpAUX1, LpPIN1) and auxin responsiveness (LpIAA6, LpIAA10), especially LpPIN1 and LpIAA10, showed differences in expression levels between the D5 + W3 and D13 + W treatments at 13 d; however, it was inconsistent with the phenotype of increased tillers. These data indicate that auxin was not the main hormone that functioned in the promotion of tiller growth under drought preconditioning. For CK, a relative higher content of iP and tZR was detected in plants receiving the D5 + W3 treatment compared with that of the D13 + W treatment at 13 d (Fig. 6); however, the expression level of the CK biosynthesis genes, LpIPT and LpLOG, cannot explain the higher CK level exhibited in the D5 + W3 treatment (Fig. 7), while the expression change of the cytokinin degradation genes, LpCKX1 and LpCKX2, might be the main reason (Fig. 7). The results of the present study indicate that changes in ABA, JA, and CK (iP and tZR) content, as well as changes in the expression of tiller development genes at drought and rewatering time points during the D5 + W3 treatment, promote tiller bud outgrowth (Fig. 8); however, further research is needed on how these hormones interact with each other to promote tiller growth after drought preconditioning.
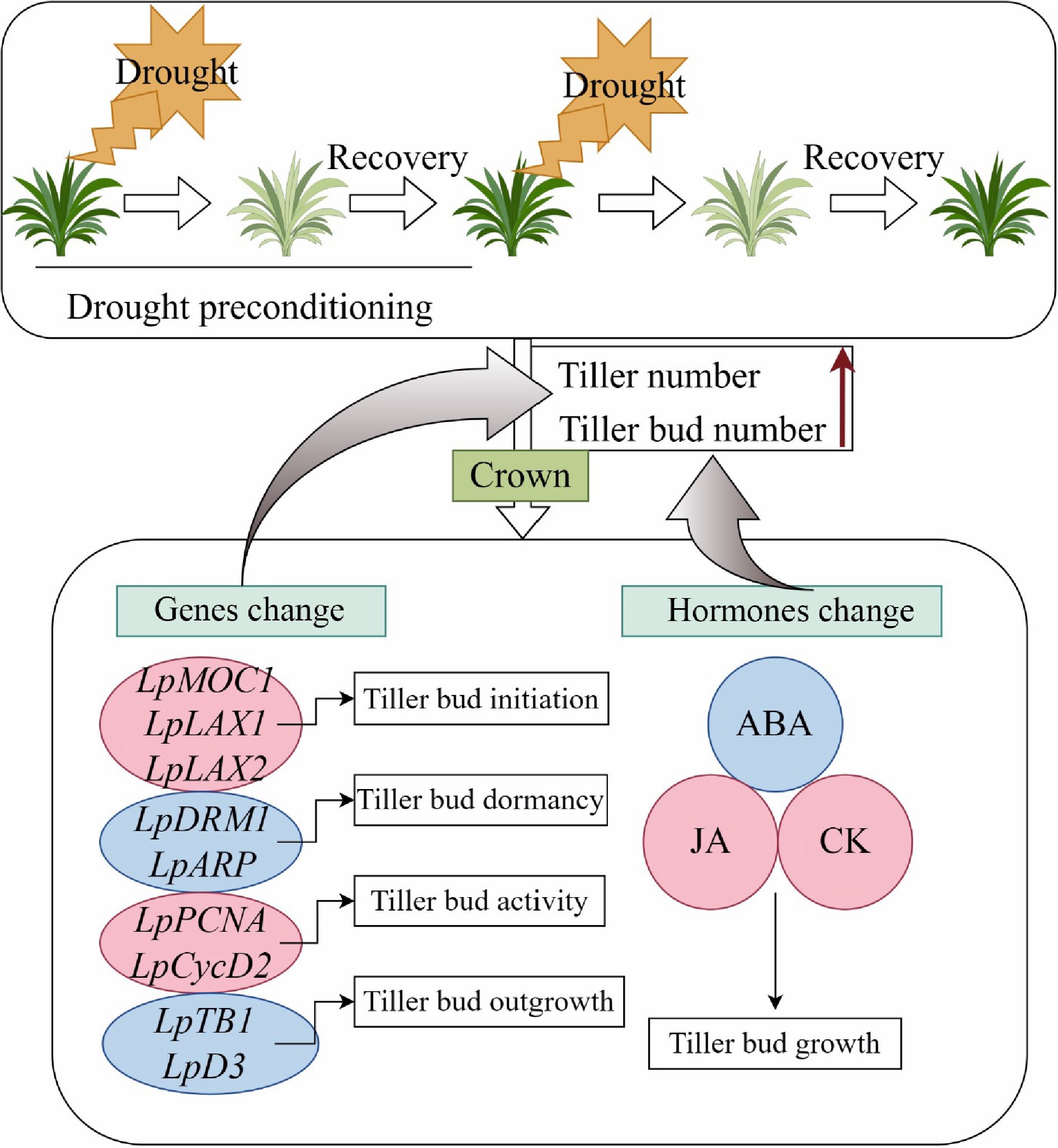
Figure 8.
A proposed model of the genes and hormones responding to drought preconditioning in crowns of perennial ryegrass. The black box above describes the D5 + W3 treatment, which significantly increased the number of tillers and tiller buds at the conclusion of the experiment. The changes in tiller bud initiation, dormancy, activity, and outgrowth-related genes, as well as hormones, were summarized in the black box below. The red circle is indicative of a significant increase, while the blue circle indicates a significant decrease.
-
In conclusion, drought preconditioning could promote tiller development involving the regulation of both tiller bud initiation and outgrowth. Several genes, such as bud dormancy genes (LpDRM1 and LpARP), bud activity genes (LpPCNA and LpCycD2), axillary bud initiation genes (LpMOC1, LpLAX1, and LpLAX2) and axillary bud outgrowth genes (LpTB1 and LpD3) were found to be related to short-term or mild drought preconditioning (D5 + W3 treatment) and enhanced tiller development. In addition, low ABA, high JA, and high CK content were positively associated with short-term or mild drought preconditioning, promoting the initiation and outgrowth of tiller buds.
-
The authors confirm contribution to the paper as follows: investigation, methodology, data curation, writing - original draft preparation: Ding Y; investigation, conceptualization, methodology, data curation: Niu K; investigation, methodology, software: Liu T; data curation, software: Zhang X; investigation, data curation: Gao Y; resources, review & editing: Rossi S; methodology, resources: Yang Z; resources, conceptualization, funding acquisition, supervision: Huang B; conceptualization, methodology, supervision, data curation, funding acquisition, writing - review & editing: Zhuang L. All authors reviewed the results and approved the final version of the manuscript.
-
The sequences of the genes tested in the study are from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus database (accession number: PRJNA917112). The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
This research was funded by the National Natural Science Foundation of China (Grant No. 32171689), Innovation and Promotion of Forestry Science and Technology of Jiangsu Province (LYKJ[2021]29).
-
The authors declare that they have no conflict of interest. Bingru Huang is the Editorial Board member of Grass Research who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and the research groups.
-
# Authors contributed equally: Yunjia Ding, Kuiju Niu
- Supplemental Table S1 Primer sequences used in RT-qPCR.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Ding Y, Niu K, Liu T, Zhang X, Gao Y, et al. 2024. Transcriptional reprogramming of genes related to bud dormancy, outgrowth, and hormones for tiller development due to drought preconditioning in Lolium perenne. Grass Research 4: e001 doi: 10.48130/gr-0023-0028
Transcriptional reprogramming of genes related to bud dormancy, outgrowth, and hormones for tiller development due to drought preconditioning in Lolium perenne
- Received: 23 October 2023
- Revised: 26 November 2023
- Accepted: 28 November 2023
- Published online: 05 January 2024
Abstract: Drought-preconditioned plants may recover quickly from mild stress upon rewatering, but the mechanisms of tiller regeneration from stressed plants are not well understood. The objective of this study was to elucidate the mechanism underlying the promotive effects of mild drought stress or drought preconditioning on tiller development during rewatering. Perennial ryegrass plants were subjected to three treatments: 1) well-watered control of plants irrigated regularly (W); 2) drought preconditioning of plants exposed to mild drought stress through the withholding of irrigation for 5 d and then rewatering for 3 d (D5 + W3), then withholding water for 5 d and rewatering for 11 d; or 3) by withholding irrigation for 13 d and rewatering for 11 d (D13 + W). Both bud initiation and outgrowth were accelerated in plants that underwent drought preconditioning (D5 + W3 treatment). Transcriptional profiling revealed an alteration in genes related to bud dormancy (LpDRM1 and LpARP), bud activity (LpPCNA and LpCycD2), axillary bud initiation (LpMOC1, LpLAX1, and LpLAX2), and bud outgrowth (LpTB1 and LpD3) in the crowns of the W-, D5 + W3-, and D13 + W- treated plants, suggesting that these factors played significant roles in the promotion of tiller bud initiation and outgrowth processes. In addition, abscisic acid (ABA), jasmonic acid (JA), and cytokinin (CK) participated in the enhancement of axillary bud initiation and outgrowth. This research enriched our knowledge of the mechanisms of drought preconditioning. The methods of water management employed in this study are of great importance for increasing crop production in circumstances of water shortage.













