-
In nature, plants can coexist with multiple microorganisms, and these symbiotic relationships are an integral element of natural processes[1,2]. For example, grasses can form co-symbiotic associations with Epichloë endophyte that are present in their aerial parts and arbuscular mycorrhizal fungi (AMF) that colonize their roots[1,3]. Epichloë (Ascomycetes, Clavicipitaceae) endophytic fungi are symbiotic with the majority of cool-season grasses[3]. They primarily reside in the leaf sheaths and stems, and transmit through seeds[4−7]. AMF can infect grass roots horizontally by infecting neighboring plant roots through mycelial growth[8−10]. Thus, Epichloë endophyte and AMF can form a mutualistic symbiosis with grasses simultaneously[11,12]. Host grasses provide habitat and carbon sources for Epichloë endophyte and AMF growth[13,14]. Host grasses also enhance resistance to abiotic stress through Epichloë endophyte and improve disease and insect resistance through the production of alkaloids[14−16]. AMF enhances the uptake of limited mineral nutrients, particularly phosphorus[17−19].
Under suitable nutrition conditions, Epichloë endophytes enhance the resistance of the host grasses[20−23]. AMF helps plants to absorb limited trace elements[24,25]. Epichloë endophytes and AMF promote plant growth, enhance photosynthesis, and contribute to the accumulation of photosynthates[5,19,26]. This accumulation provides a sufficient carbon source for both Epichloë endophytes and AMF, facilitating mutualistic symbiosis among the host, Epichloë endophytes, and AMF, increasing the infection rates of both fungi[5,26,27]. The plant serves as the sole carbon source for the Epichloë endophytes and AMF, and they compete for the photosynthesis products[28,29]. The competition can reduce fungi's ability to provide benefits to the plant, and the costs of symbiosis outweigh the benefits, with antagonistic interactions occurring[28,29].
Numerous studies have shown that Epichloë endophytes can enhance the salt tolerance of host plants[30−32]. Song et al.[31] studied the salt tolerance of wild barley and found that under high salinity stress, Epichloë endophytes can significantly promote the growth of wild barley. Many studies have indicated that AMF enhances plant salt tolerance, although its effects are related to specific species[33,34]. Only a few studies have examined the effects of interactions with both Epichloë endophytes and arbuscular mycorrhizal fungi (AMF)[35−40]. Moreover, there is little research on the interactions between Epichloë endophytes and AMF under salt stress, as well as their effects on the host.
In this study, we investigated the effects of interactions between Epichloë bromicola and different AMF on the growth of wild barley under salt stress, and the impact of symbiotic duration on these effects. We explored the effects of the interactions between E. bromicola and AMF on wild barley growth under salt stress. This study provides support for the application of E. bromicola and AMF in different habitats.
-
Epichloë bromicola infected (E+) and uninfected (E−) wild H. brevisubulatum plants were collected from the Linze Experimental Station of Lanzhou University (100°06' E, 39°11' N), Gansu Province, China. Seeds from E+ and E− plants were separately planted in a common garden at the Yuzhong Experimental Station (103°36' E, 36°28' N), Lanzhou University, China. After assessing the E. bromicola infection rates (aniline blue and molecular detection), the samples were stored in a refrigerator at 4 °C (at the Keith Clay Laboratory, Indiana University, USA).
AMF material
-
The AMF strains were provided by the Bever Laboratory at Indiana University, USA. The strains include spores of Glomus mosseae and Glomus claroideum (BEG23), which were isolated from natural prairie soil in northern Indiana, USA.
The soil (collected from Indiana University) and sand were screened to 2 mm and then sterilized under high pressure at 121 °C for 2 h.
Pot experiment
-
A pot experiment was conducted from February to August 2014 in the Indiana University Greenhouse. The treatments included inoculation with different mycorrhizal species: non-inoculated (M−). If there are specific locations requiring attention, please let us know, and we will address them promptly. ), Glomus mossease (= Funneliformis mosseae, GM), Glomus claroideum (GC), and a mix of G. claroideum and G. mosseae (Gmix). We inoculated approximately 10% of the total soil volume with AMF, at a depth of about 2 cm below the soil surface. For the non-inoculated control, an equal amount of sterilized soil was added using the same method.
Seeds harvested from the E+ and E− plants were disinfected with 75% ethanol for 1 min. The sterilized seeds were sown in pots (3 seeds/pot) filled with sterilized vermiculite. All plants were germinated in the greenhouse (14 h of light) and watered daily. Seedlings of similar size and growth were selected. Each seedling was then transplanted into a separate pot.
The effect of NaCl (NaCl concentrations: 0 mM, 100 mM, 300 mM, labeled as S1, S2, S3) was assessed 7 d after transplanting seedlings, with five replicates per concentration. NaCl was added to the medium in 50 mM increments every 2 d until concentrations of 100 mM and 300 mM were reached.
In each of the first 4 months, harvests were conducted (labeled as H1, H2, H3, and H4) after salt stress treatment; 120 pots containing all treatments (AMF × E. bromicola × salt stress) were randomly selected for harvest. The number of tillers and spikes was measured at each harvest. The roots were washed with water and dried using towels. Wild barley was divided into aboveground and belowground parts. From each plant, the oldest three tillers and the roots were separately collected, each sample weighing approximately 0.05 g. These samples were quickly frozen in liquid nitrogen and stored at −80 °C. The aboveground and belowground biomass were measured and the root-shoot ratio was calculated. After drying, the wild barley was ground to a powder using a ball mill, and the contents of C, N, P, Na+, and K+ were measured separately.
Organic carbon content was determined using the external heating method for chromic acid oxidation titration[41]. Total nitrogen was measured using the Kjeldahl method[42]. Total phosphorus in both aboveground and belowground parts was determined using the molybdenum blue colorimetric method[43], while Na+ and K+ contents were measured using flame photometry[44].
gDNA extraction
-
DNA was isolated from 0.05 g of tiller and root samples using MoBio DNeasy PowerPlant DNA Kit (MO BIO Laboratories, a QIAGEN Company) following the manufacturer's instructions. DNA concentration and purity were determined using NanoDrop (Thermo Fisher Scientific, USA). The A260/A280 purity ratio was used to assess the purity of the extracted nucleic acid sample.
The DNA extracts were used for validation of relative qPCR with specific primers (Table 1) to quantify fungal colonization[29,45,46]. The specificity of the primers was verified using an optimized PCR amplification reaction in a total of 25 μL and 2.5% agarose electrophoresis.
Table 1. Sequences and function of specific qPCR primes for E. bromicola, G. mosseae, and G. claroideum
Primers 5' to 3' Function perA.RTF AACATCGAGCACTCTCATTGC E. bromicola peramine alkaloid
synthesis gene forward primerperA.RTR CCGTTTCCGATGGTGCCATTG E. bromicola peramine alkaloid
synthesis gene reverse primerGmPT.RTF ACGTGAAGTCGATGAACCAG G. mosseae specific sequence
forward primersGmPT.RTR CATGACACCGCAGTACCAAC G. mosseae specific sequence
reverse primersLSU.RTF GCGAGTGAAGAGGGAAGAG G. claroideum specific sequence
forward primersLSU.RTR TTGAAAGCGTATCGTAGATGAAC G. claroideum specific sequence
reverse primersStandard curve
-
The purified PCR products were cloned into TOPO vectors and transformed into competent E. coli cells. After cultivation, plasmid DNA was extracted and purified using the QIAprep Spin Miniprep Kit. The purified plasmid DNA was then serially diluted to create a standard curve for the concentration of wild barley E. bromicola DNA.
Glomus mosseae (GM) and Glomus claroideum (GC) spores were extracted separately from soil samples, and DNA was extracted using the MoBio DNeasy PowerSoil Kit (MO BIO Laboratories, a QIAGEN Company) according to the manual. After purification, the DNA was used to prepare standard samples and build a standard curve for the DNA concentration of both AMF species.
Sequencing and bioinformatics
-
Epichloë bromicola qPCR system: reaction volumes were 10 μL with 5 μL Power SYBR™ Green Master Mix (Thermo Fisher Scientific), 1 μL DNA template, 0.25 μL of each primer, and 3.5μL ultrapure water mix. The reactions were carried out using the Applied Biosystems 7500 Fast Real-Time PCR (USA). The reaction program was as follows: 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 62 °C for 1 min, 72 °C for 15 s, and a final extension at 72 °C for 5 min. A melting curve analysis was also performed simultaneously. The copy numbers in the DNA template (wild barley + E. bromicola) were calculated based on the standard curve, representing the molecular biomass of the species.
AMF qPCR system: reaction volumes were 20 μL with 10 μL Power SYBR™ Green Master Mix (Thermo Fisher Scientific), 2 μL DNA template, 0.5 μL of each primer, and 7 μL ultrapure water. The reactions were carried out using the Applied Biosystems 7500 Fast Real-Time PCR (USA). The reaction program was as follows: 94 °C for 3 min, 30 cycles of 92 °C for 45 s, 72 °C for 1 min, 72 °C for 15 s, and a final extension at 72 °C for 5 min. A melting curve analysis was also performed simultaneously. The copy numbers in the DNA template (wild barley + AMF) were calculated based on the standard curve, representing the molecular biomass of the two AMF species.
Data processing
-
The data were analyzed and plotted using SPSS 22.0 (IBM, USA) and OriginPro 2024b. Significant differences between treatments were determined using Fisher's LSD test, with significance set at the 95% confidence level. The means of different treatments presented in the figures and text represent the average value of five replicates per treatment ± standard deviation.
-
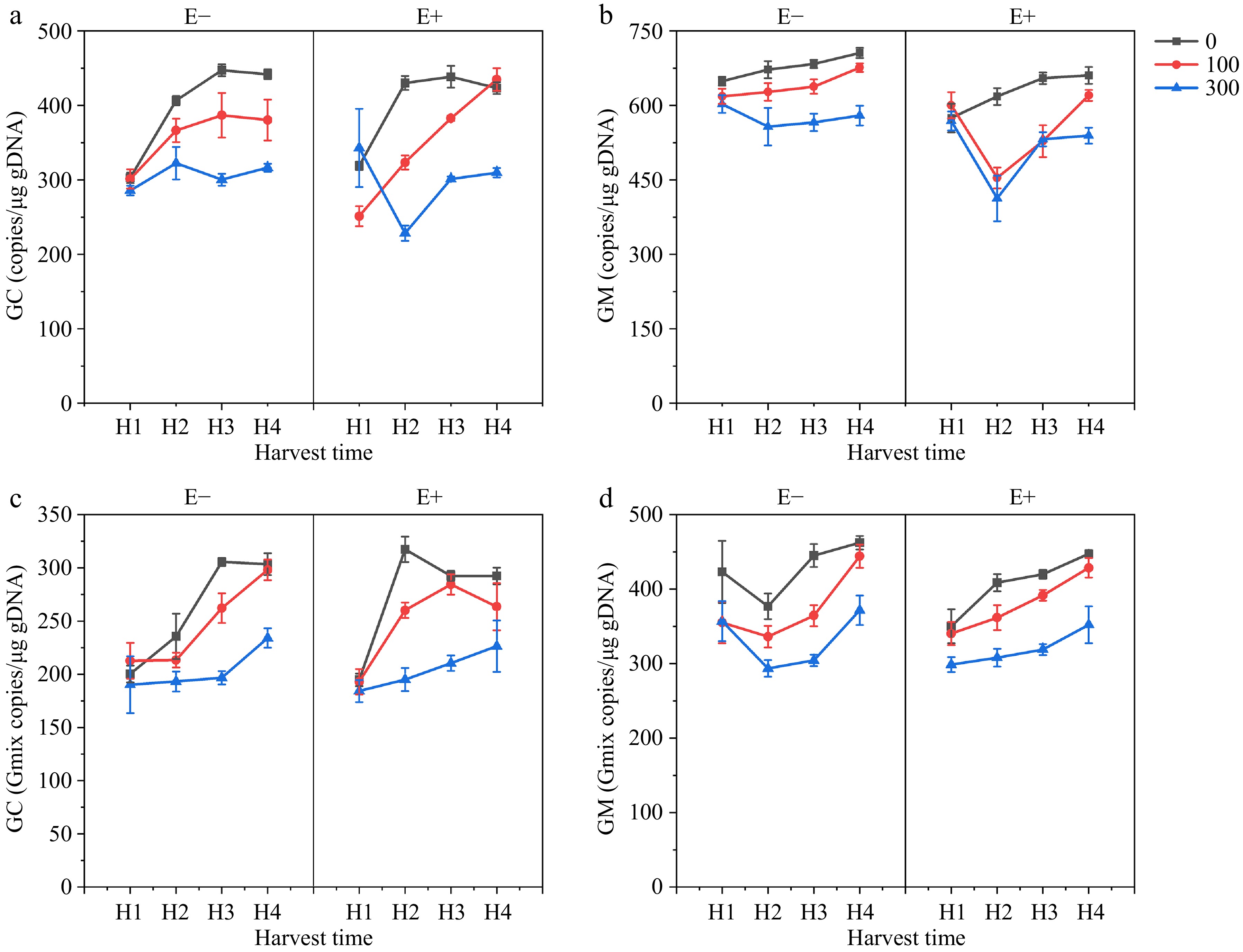
We compared the effects of E. bromicola and AMF on the growth parameters of wild barley under different salt concentrations and harvest times through greenhouse pot experiments. The results revealed that E. bromicola significantly increased the root biomass, root-to-shoot ratio, and spikelet formation ability of wild barley under 300 mM salt conditions (Fig. 1). However, the effects of E. bromicola were not significant under 0 and 100 mM stress conditions (Fig. 1). The effect of E. bromicola gradually weakened with increased symbiotic duration. Under 300 mM salt stress, AMF infection hindered the growth of wild barley, with different AMF strains having varying levels of impact. As the symbiotic duration increased, the hindering effect of different AMF strains on wild barley became more pronounced. Regardless of E. bromicola fungal infection, GC mycorrhizal fungi had no significant impact on the growth of wild barley (Figs 1, 2), indicating that the contributions of E. bromicola and GC mycorrhizal fungi to the growth of wild barley are independent of each other. Under 0 and 100 mM salt conditions, GM mycorrhizal fungi showed a tendency to promote various growth parameters of wild barley in the absence of E. bromicola fungal infection. However, in E+ plants, infection with GM mycorrhizal fungi led to a decrease in all growth parameters, indicating an antagonistic interaction between E. bromicola and GM mycorrhizal fungi (Figs 1 & 2; Supplementary Table S1).
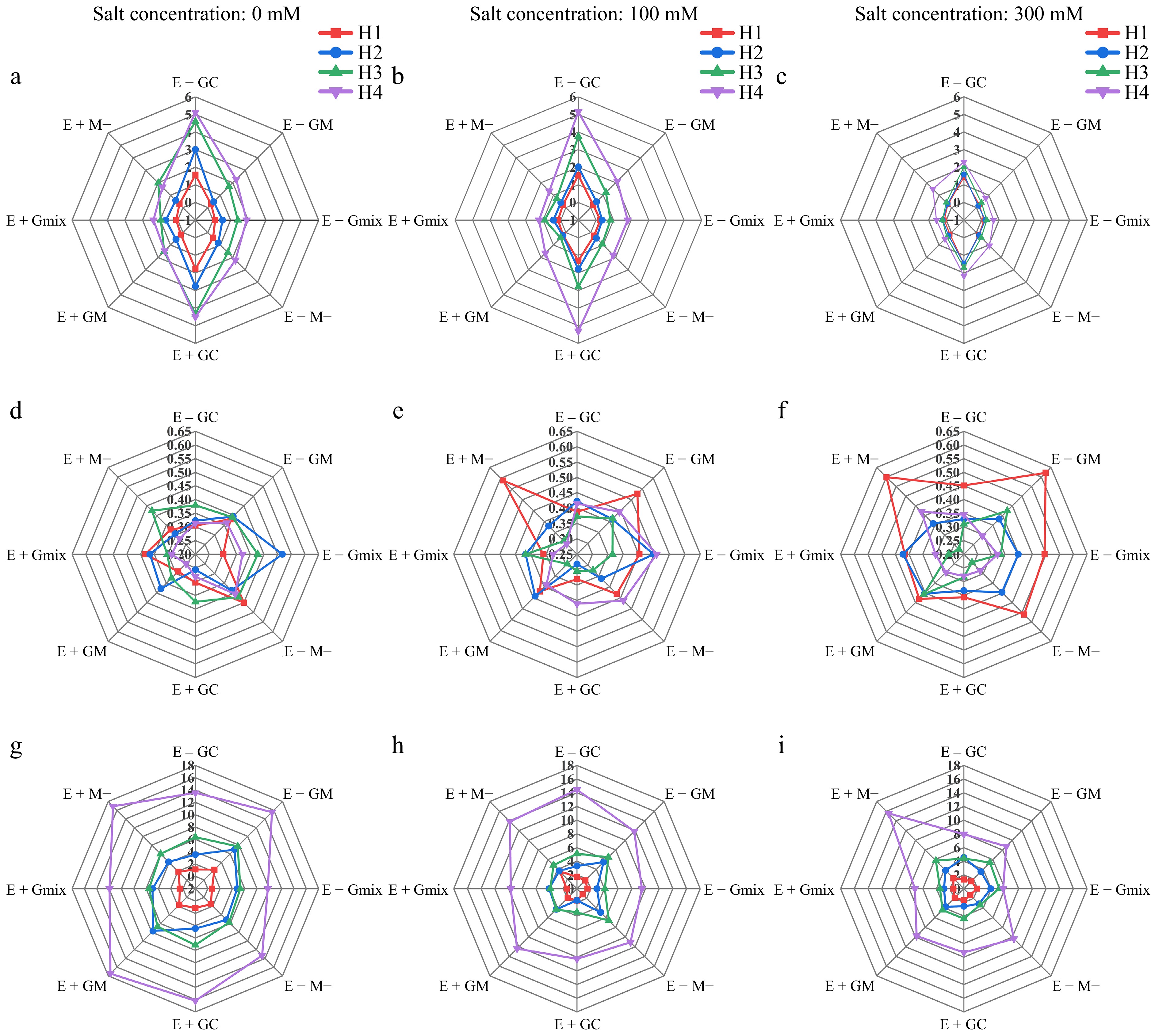
Figure 1.
(a)−(c) Root biomass, (d)−(f) root/shoot ratio, and (g)−(i) seedhead numbers of wild barley at four harvest times (H1, H2, H3, H4) under different treatments of E. bromicola, arbuscular mycorrrhizal fungi, and salt concentrations. The unit of biomass is 'g' .
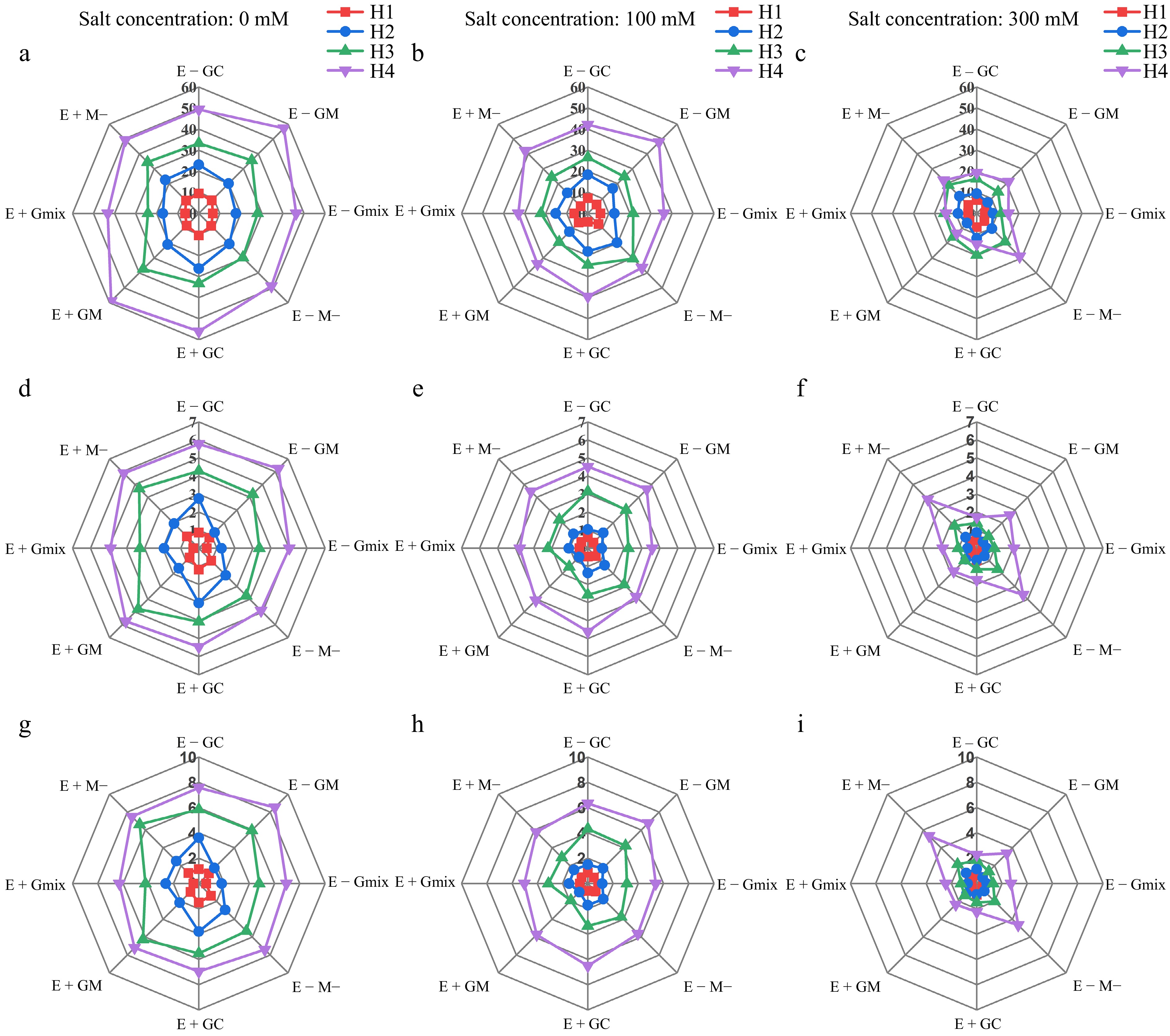
Figure 2.
(a)−(c) Tiller numbers, (d)−(f) shoot biomass, and (g)−(i) total biomass of wild barley at four harvest times (H1, H2, H3, H4) under different treatments of E. bromicola, arbuscular mycorrrhizal fungi, and salt concentrations. The unit of biomass is 'g'.
Effects of Epichloë bromicola and AMF on nutrient content
-
The nutrient elements in the aboveground and belowground parts of wild barley were measured at different harvest times under salt stress (Figs 3 & 4; Supplementary Tables S1 & S2). Under 300 mM salt concentration, inoculation with GM resulted in higher aboveground nitrogen (N) content in wild barley, and infection with E. bromicola enhanced the effects (Fig. 3a). The effect of GC was not significantly influenced by E. bromicola, and an antagonistic effect was observed between the Gmix treatment and E. bromicola. AMF increased the total nitrogen (N) content in the aboveground parts (Fig. 3c, d) and the total phosphorus (P) content in the belowground parts (Fig. 3e, f) of wild barley, with stronger effects observed at 300 mM salt concentration. The three types of mycorrhizal treatments alleviated the nutrient stress caused by salt stress and enhanced nitrogen absorption. This indicates that when E. bromicola and GM infect wild barley together, they reduce the impact of salt stress by enhancing the host plant nitrogen absorption (N).
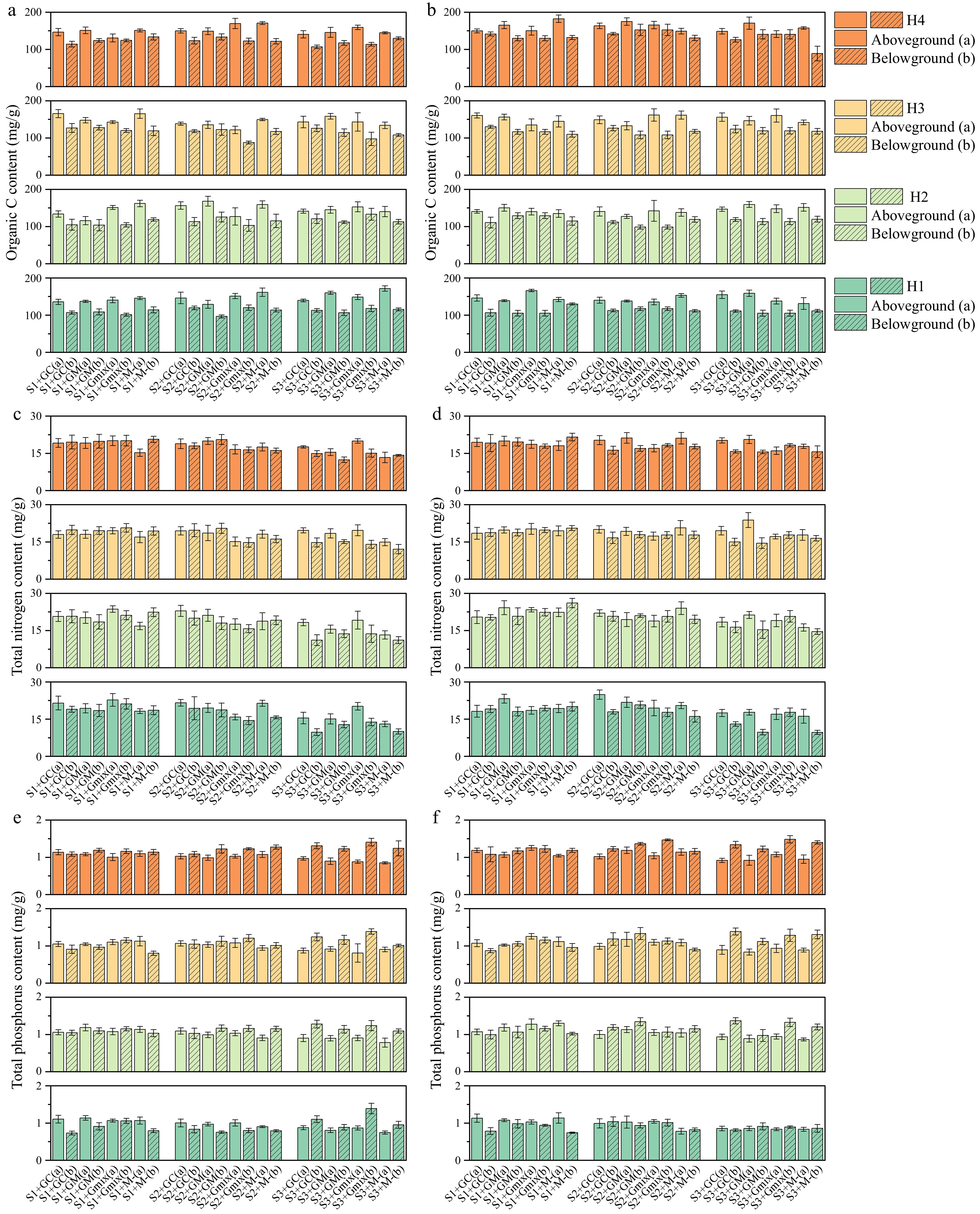
Figure 3.
(a), (b) Organic carbon (C) content; (c), (d) total nitrogen (N) content; and (e), (f) total phosphorus (P) content of wild barley (aboveground and belowground) at four harvest times (H1, H2, H3, H4) under different treatments of E. bromicola (uninfected: a, c, d; infected: b, d, e), arbuscular mycorrrhizal fungi (GC, GM, GMix, M−), and salt concentrations (S1, S2, S3).
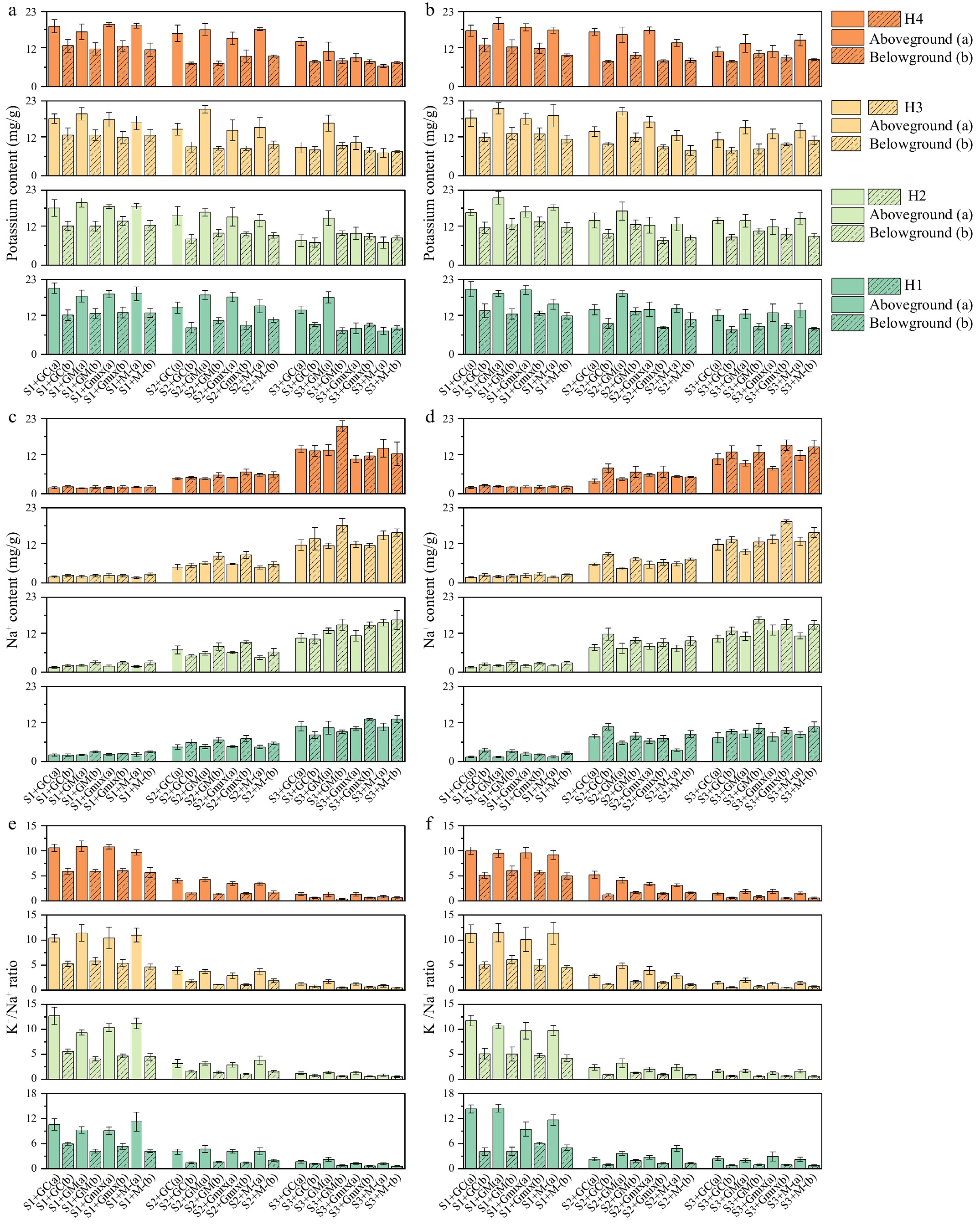
Figure 4.
(a), (b) Potassium (K+) content; (c), (d) Na+ content; and (e), (f) K+/Na+ ratio of wild barley (aboveground and belowground) at four harvest times (H1, H2, H3, H4) under different treatments of E. bromicola (uninfected: a, c, d; infected: b, d, e), arbuscular mycorrrhizal fungi (GC, GM, GMix, M−), and salt concentrations (S1, S2, S3).
Under 0 and 100 mM salt conditions, the three mycorrhizal treatments did not have a significant effect on phosphorus (P) content in the roots (Fig. 3e, f). Both GC and Gmix mycorrhizal treatments increased the phosphorus (P) content in the roots under 300 mM salt conditions (Fig. 3e, f). However, the phosphorus (P) content did not change significantly after GM mycorrhizal infection (Fig. 3e, f). At 300 mM salt concentrations, E+ plants exhibited higher phosphorus (P) content in the roots, particularly in the AMF-free treatments (Fig. 3e, f). However, after inoculation with GM mycorrhizal fungi, this advantage was significantly reduced, demonstrating an antagonistic effect between E. bromicola and GM mycorrhizal fungi.
With E. bromicola, E+ plants exhibited lower Na+ content, and AM fungi also contributed to reducing Na+ levels. GM and Gmix treatments showed an additive effect with E. bromicola, acting synergistically to mitigate Na+ toxicity and osmotic stress on the host plant. There was no interaction between GC and E. bromicola. GM significantly increased the potassium (K+) content and the K+/Na+ ratio in wild barley plants under salt stress (Fig. 4). At 300 mM salt concentrations, the K+/Na+ ratio was increased by GM, compared to AMF-free treatments (Fig. 4e, f). However, the Gmix fungal treatment had no significant effect on either K+ content or the K+/Na+ ratio (Fig. 4a, b).
E. bromicola and AMF had a significant effect on the organic C content in the aboveground part of wild barley, but AMF did not significantly affect the belowground organic C content (Fig. 3a, b). However, Epichloë fungi significantly increased the organic C content in the wild barley roots, resulting in these roots having a higher organic C content than the E- plants (Fig. 3a, b). Compared to E− plants, E+ wild barley roots had higher organic C content (Fig. 3a, b). There were no interactions between E. bromicola and AMF, salinity, or sampling time (Supplementary Table S2). Sampling time had a significant effect on both above and belowground organic C content, with an increasing trend over time in both parts of wild barley (Fig. 3a, b; Supplementary Table S2). Additionally, sampling time and salinity showed an interactive effect on belowground organic C content, where increased salinity reduced the variation in belowground organic C content induced by sampling time (Supplementary Table S2).
qPCR to measure the infection rates of Epichloë bromicola and AMF
-
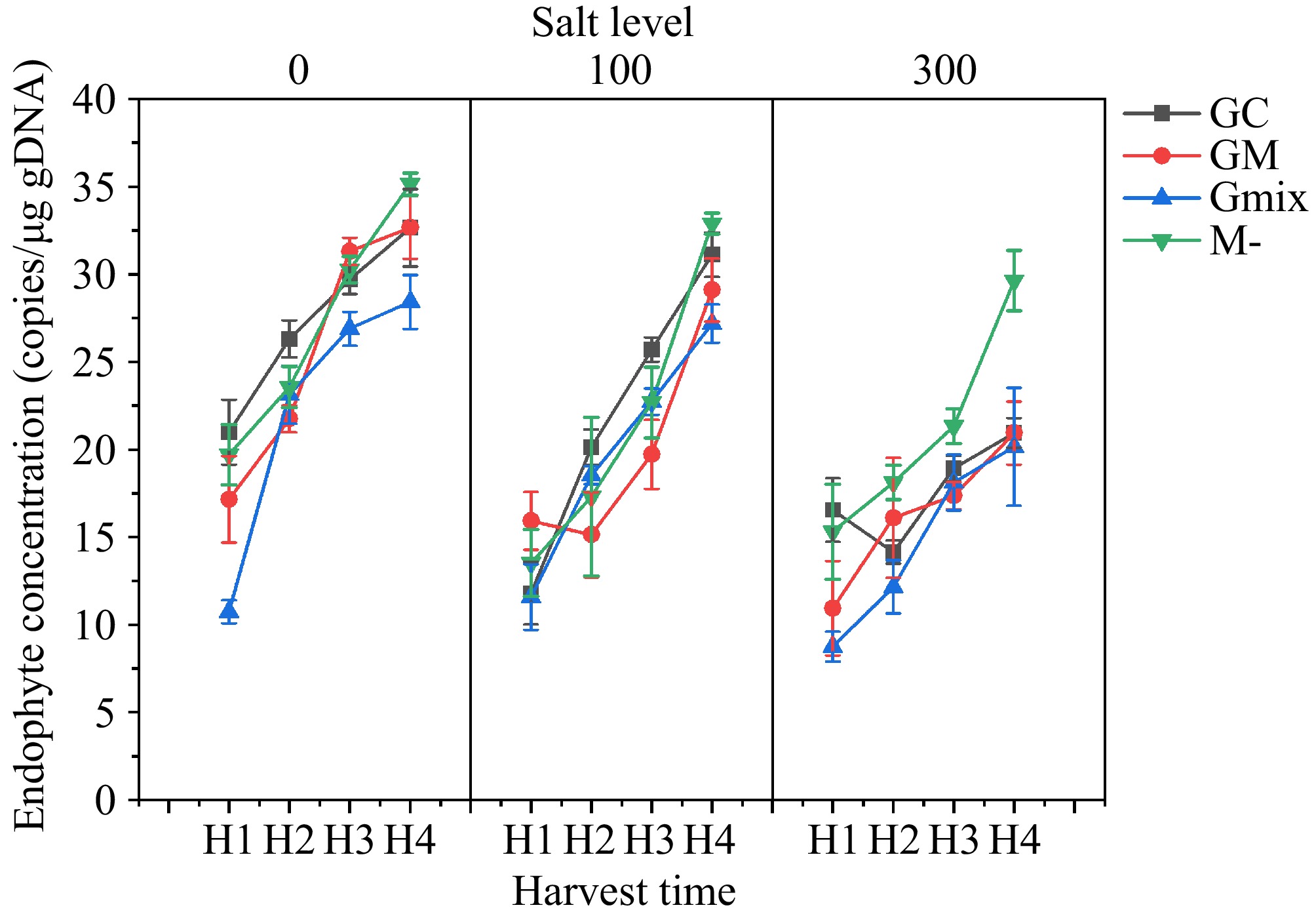
Quantitative fluorescence PCR was used to determine the infection rates (molecular biomass) of E. bromicola and different AMF (GC, GM, Gmix). The results showed that the content of E. bromicola was not affected by GC fungi but was significantly reduced when co-infected with GM and Gmix (Fig. 5), indicating an antagonistic effect between GM, mixed infections, and E. bromicola. E. bromicola showed no significant influence on the content of GC but significantly reduced the content of GM. The content of AMF decreased with increasing salt concentration, but regardless of the salt concentration, the content of AMF increased with symbiotic duration (Fig. 6).
-
Due to the salt and alkali tolerance and strong adaptability of Epichoë endophytes, wild barley is an excellent forage grass. It is important to study the effects of co-infection by E. bromicola and arbuscular AMF under different stress conditions[12,16,47]. Understanding the dynamic interactions among wild barley, E. bromicola, and AMF is crucial for the promotion and application of wild barley and for the development of the forage industry[30−32]. However, there is a gap in research regarding the interactions between E. bromicola and AMF and their effects on the host under salt stress. Only Fang[48] studied the interactions among wild barley, E. bromicola, and AMF under salt and phosphorus stress. The results showed that both fungi, whether individually or jointly inoculated, could enhance wild barley's resistance to salt stress and promote growth. The study did not consider either different AMFs under salt stress or the dynamic changes in the wild barley - E. bromicola-AMF symbiotic system.
Currently, the interaction mechanisms between Epichoë fungi and AMF remain unclear. In the early stages of symbiosis, the symbiotic relationship can consume significant amounts of plant photosynthates and other products, which affect plant growth[49]. However, from a long-term perspective, symbionts can establish mutualistic interactions with host plants[50]. The density of E. bromicola fungal hyphae may alter the nutrient requirements of the host plant, thereby affecting the growth of AMF[51,52]. For instance, some studies have shown that E. bromicola can enhance the host plant's ability to absorb and store phosphorus, thereby reducing the contribution of AMF infection to the host[53−55].
Malinowski & Belesky[56] found that Festuca arundinacea plants with endophytic fungi had significantly longer root hairs but a significantly reduced root diameter compared to non-inoculated plants. This indicates that endophytic fungi can increase the root surface area of the host plant, enhancing nutrient uptake and thereby reducing the host's reliance on AMF[23]. Many cool-season grasses, such as Festuca arundinacea, typically have lower AMF infection rates and rely more on endophytic fungi for nutrient acquisition than other plants[57]. The density of endophytic fungal hyphae is closely related to the concentration of secondary metabolites, such as alkaloids[58,59], and the production of alkaloids directly leads to a reduction in AMF infection rates[60]. Compared to AMF, endophytic fungi have an advantage in terms of infection timing[6,7,9,10]. E. bromicola fungi spread vertically and present in seeds before germination, whereas AMF spread horizontally through the soil and infect plants after seed germination[6,7,9,10]. With the differences in infection space, the timing of infection may be another key factor contributing to the asymmetry in interactions between E. bromicola and AMF[11].
The present study was funded by the Natural Science Foundation of China (31372366, 31971756), the Inner Mongolia Seed Industry Science and Technology Innovation Major Demonstration Project (2022JBGS0040).
-
The authors confirm contribution to the paper as follows: analysis and interpretation of results: Li J, Chai Q; draft manuscript preparation: Li J; data collection: Chai Q; manuscript review: Chen Z, Malik K; study conception and design: Li C. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during thecurrent study are not publicly available due the fact that the results of this study are the basic data for subsequent studies, but are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Jinchen Li, Qing Chai
- Supplementary Table S1 Results (F-values) of Repeated measures ANOVA on the effects of time (T), E. bromicola (E), AMF, salt (S) and the interactions on tiller, shoot biomass, root biomass, total biomass, root / shoot ratio and number of seedheads during the experimental period.
- Supplementary Table S2 Results (F-values) of Repeated measures ANOVA on the effects of time (T), E. bromicola (E), AMF, salt (S) and the interactions on organic C content, total N content, total P content, Na+ content, K+ content and K+/Na+ ratio in shoot and root biomass of wild barley during the experimental period.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Li J, Chai Q, Chen Z, Malik K, Li C. 2025. Interactions of Epichloë endophyte and arbuscular mycorrhizal fungi on wild barley under salt stress. Grass Research 5: e007 doi: 10.48130/grares-0025-0004
Interactions of Epichloë endophyte and arbuscular mycorrhizal fungi on wild barley under salt stress
- Received: 24 October 2024
- Revised: 17 December 2024
- Accepted: 08 January 2025
- Published online: 04 March 2025
Abstract: The mutualistic system involving grasses, endophytic fungi, and arbuscular mycorrhizal fungi (AMF) has been extensively studied. Several studies have focused on the effects of Epichloë endophytic fungi on AMF colonization and the impact of their simultaneous symbiosis on the host under different nutritional conditions, while environmental factors, such as salt stress, have received less attention. This study, using wild barley (Hordeum brevisubulatum) as the experimental material, investigated the effects of simultaneous symbiosis between Epichloë bromicola fungi and three different AMF treatments on the growth, nutrient absorption, and biomass (aboveground and belowground) allocation of wild barley under salt stress. Moreover, the nutrient and biomass changes of the salt effects were explored in these two types of fungi on wild barley during different stages of symbiosis establishment. Under high salt stress, AMF colonization inhibited the growth of wild barley. E. bromicola and Glomus claroideum (GC) mycorrhizal fungi on the growth of wild barley are independent. In E. bromicola-infected (E+) plants, colonization by Glomus mossease (GM) and G. claroideum + G. mossease (Gmix) mycorrhizal fungi led to growth parameters reductions, indicating an antagonistic interaction between E. bromicola and G. mossease.
-
Key words:
- Salt stress /
- Plant growth /
- Epichloë endophyte /
- Arbuscular mycorrhizal fungi