-
Baphicacanthus cusia (Nees) Bremek ('Ma-Lan' in Chinese; Fig. 1), a member of the genus Baphicacanthus with the family Acanthaceae, has a long history of medicinal use and recorded in some ancient books of traditional Chinese medicine (TCM), such as Compendium of Materia Medica (Bencao Gangmu in Chinese). B. cusia is widely distributed at 21° to 26° N latitude and 99° to 120° E longitude with an elevation below 1,000 m altitude. In China, the wild B. cusia is mainly distributed in Guangxi, Yunnan, Fujian, Guangdong, Guizhou, Hainan, and other places[1]. In Fujian Province, Xianyou County is the main cultivation area[2,3]. The Chinese medicine processed using the plant grown in this place is called 'Dao-Di' (in Chinese) medicinal materials, which indicates better quality. In some studies, it is believed that Baphicacanthus cusia (Nees) Bremek and Strobilanthes cusia (Nees) Kuntze are the same plant[1,4,5]. Based on abundant evidence, Huang suggested that Strobilanthes cusia (Nees) Kuntze should be 'Ban-Lan' in Chinese, and Baphicacanthus cusia (Nees) Bremek should be 'Ma-Lan' in Chinese. The same issue involves isatidis folium ('Da-Qing-Ye' in Chinese)[6,7]. Thus, normalizing the name is an issue that needs tackling. In the present paper, the studies of the plant named B. cusia are mainly discussed.
Based on the TCM theory, B. cusia has the activity to clear heat, detoxify, cool blood, and remove freckles. The whole plant of B. cusia can be used as a source of medicine. The above-ground parts, including stems and leaves, are processed as medicine named indigo naturalis ('Qing-Dai' in Chinese), which is a kind of Daodi herb in Fujian and is known as 'Jian Qing-Dai' in Chinese. The below-ground parts, roots of B. cusia, is a source of medicine named 'Nan-Ban-Lan-Gen' in Chinese. Both are officially listed in the Chinese Pharmacopoeia. In TCM, indigo naturalis are widely used in traditional Chinese medicine formulae (Table 1). Besides, modern pharmacology has found that indigo naturalis effectively treats human leukemia[8]. And 'Nan-Ban-Lan-Gen' are also widely used in China. Due to the application of indigo naturalis and 'Nan-Ban-Lan-Gen' in the medicinal industry, the growing demand for B. cusia has led to an expansion of cultivation efforts. With the rapid development of detection technologies and the utilization of multi-omics approaches, more compounds have been identified, and more detailed metabolic pathways elaborated. Thus, this review focuses on the chemical compositions and the major biological activity—antitumor of B. cusia. Moreover, the metabolism and synthesis of the major bioactive compounds, and their related genes are also discussed.
Table 1. Traditional Chinese Medicine prescriptions containing indigo naturalis (Qing-Dai).
Prescription Formula Chinese ancient books Baidai Powder Bark of Phellodendron chinense, 2 qian (6 g); Leaves and/or stems of Baphicacanthus cusia, Polygonum tinctorium, Indigofera tinctoria or Isatis indigotica, 2 qian (6 g) Dongtian Aozhi Bijing Pill Calomel, 1.5 qian (4.5 g); Talcum, 1.5 qian (4.5 g);
Tubers of Arisaema erubescens, A. heterophyllum or A. amurense, 1 qian (3 g);
Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 5 fen (1.5 g)Puji Fang Biyu Tongshen Powder Bark of P. chinense, 5 qian (15 g);
Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 1 fen (0.3 g);
Camphor (Branches, stems, leaves and roots of Cinnamomum camphora), a modicum (~ 0.1 g)Puji Fang Chaihu Qingdai Decoction Roots of Bupleurum chinensie or B. scorzonerifolium, 5 fen (1.5 g);
Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 5 fen (1.5 g);
Rhizomes of Cyperus rotundus, 1 qian (3 g); Rhizomes of Ligusticum chuanxiong, 1 qian (3 g);
Pericarps of Citrus reticulata or its cultivated varieties, 8 fen (2.4 g);
Rhizomes of Coptis chinensis, C. deltoidei or C. teeta, 8 fen (2.4 g);
Fruits of Cardenia jasminoides, 8 fen (2.4 g);
Roots and rhizomes of Glycyrrhiza uralensis, G. inflata or G. glabra, 8 fen (2.4 g)Miscellaneous Diseases Chaya Niuhuang Qingdai Powder Gallstones of Bos taurus, 5 fen (1.5 g);
Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 5 fen (1.5 g);
Borax, 2 qian (6 g); Cinnabar, 1 qian (3 g);
Naturally deposited solids in healthy human urine, 2 fen (0.6 g);
Skeletal fossils of ancient mammals, 2 fen (0.6 g); Borneol, 3 fen (0.9 g)Yizong Jinjian Chunbi Biyu Powder Refined nitrokalite, 1 fen (0.3 g); Camphol, 1 qian (3 g);
Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 1 qian (3 g)General Records of Saints Daibai Powder Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 1 qian (3 g);
Bark of P. chinense, 2 qian (6 g); Borneol, 1 qian (3 g)Integrative Medicine Dermatology Daiehuang Powder Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 2 qian (6 g);
Bark of P. chinense, 2 qian (6 g); Bassanite, 2 liang (60 g);
Liuyi Power (Talcum : Roots of G. uralensis = 6 : 1), 24 qian (72 g)Drug dowry secret Daige Powder Shell of Meretrix meretrix or Cyclina sinensis, 10 liang (300 g);
Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 1 liang (30 g)Medical Theory Danggui Longhui Pill Roots of Angelica sinensis, 1 liang (30 g);
Roots and rhizomes of Gentiana scabra, G.triflora or G.manishurica, 1 liang (30 g);
Fruits of Gardenia jasminoides, 1 liang (30 g);
Rhizomes of C. chinensis, C. deltoidea or C. teeta, 1 liang (30 g);
Bark of Phellodendron chinense, 1 liang (30 g); Roots of Scutellaria baicalensis, 1 liang (30 g);
Roots and rhizomes of Rheum palmatum, R. tanguticum or R.officinale, 5 qian (15 g);
Concentrated dried juices of Aloe barbadensis or A.ferox, 5 qian (15 g);
Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 5 qian (15 g);
Roots of Aucklandia lappa, 1 fen (0.3 g);
Secretions from mature male sachets of Moschus berezovskii, M. sifanicus or M. Moschiferus,
5 fen (1.5 g)Danxi Xinfa Daihong Powder Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica;
Rhizomes of Coptis chinensis, C. deltoidei or C. teeta; Flowers of Carthamus tinctorius;
with each equal divisionFamous selection and sequel Daihuang Powder Bark of P. chinense, 1 liang (30 g);
Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 2 qian (6 g);
Rhizomes of Coptis chinensis, C. deltoidei or C. teeta, 1.5 qian (4.5 g);
Roots of A. dahurica or A. dahurica, 1.5 qian (4.5 g);
Roots of Paeonia lactiflora or P. veitchii, 1 qian (3 g); Tea, 1 qian (3 g);
Secretions from mature male sachets of M. berezovskii, M. sifanicus or M. Moschiferus,
2.5 fen (0.75 g)Continued name family selection Gualou Qingdai Pill Seeds of Trichosanthes kirilowii or T. rosthornii, 1 liang (30 g);
Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 3 qian (9 g)Danxi Xinfa Hezi Qingdai Pill Fruits of Terminalia chebula;
Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica;
Seeds of Armeniaca vulgaris, A. vulgaris, A. sibirica or A. mandshurica;
Egg-banding of Notarchus leachii cirrosus; Rhizomes of Cyperus rotundus;
Seeds of T. skirilowii or T. rosthornii; Rhizoma Pinelliae Fermentata;
with each equal divisionMiscellaneous Disease Origin Rhinoceros Candle Hupo Biyu Powder Talcum, 6 liang (180 g); Roots and rhizomes of G. uralensis, G. inflata or G. glabra, 1 liang (30 g);
Amber, 5 qian (15 g);
Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 8 fen (2.4 g)Yizong Jinjian Jiujing Pill Above ground part of Mentha haplocalyx, 3 qian (9 g);
Larvae of Bombyx mori infected or artificially inoculated with Beauveria bassiαna, 3 qian (9 g);
Roots of Saposhnikovia divaricate, 3 qian (9 g); Tubers of Gastrodia elata, 3 qian (9 g);
Buthus martensii, 3 qian (9 g); Roots and rhizomes of G. uralensis, G. inflata or G. glabra, 3 qian (9 g);
Dried exudate from stems of Bambusa tertilis or Schizostachyum chinense, 3 qian (9 g);
Stems and branches containing hooks from Uncaria rhynchophylla, U. macrophylla, U. hirsute, U. sinensis or U. sessilifructus, 3 qian (9 g);
Mixture of prepared tubers of A. erubescens, A. heterophyllum or A. amurense and bile from Bos spp., Ovis spp. or Sus spp., or fermented mixture of tuber power of A. erubescens, A. heterophyllum or A. amurense and bile from Bos spp., Ovis spp. or Sus spp., 4 qian (12 g);
Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria, or I. indigotica, 4 qian (12 g);
Tubers of A. erubescens, A. heterophyllum or A. amurense, 4 qian (12 g);
Tubers of Typhonium giganteum, 2 qian (6 g); Cinnabar, 2 qian (6 g); Amber, 2 qian (6 g);
Secretions from mature male sachets of M. berezovskii, M. sifanicus or M. Moschiferus, 8 fen (2.4 g);
Gallstones of Bos taurus, 5 fen (1.5 g); Pearl, 5 fen (1.5 g)National Traditional Chinese Medicine Prescription Collection Kexue Recipe Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 2qian (6 g);
Seeds of T. skirilowii or T. rosthornii, 3 qian (9 g);
Egg-banding of Notarchus leachii cirrosu, 3 qian (9 g);
Fruits of G. jasminoides, 3 qian (9 g); Fruits of Terminalia chebula, 2 qian (6 g)Danxi heart method Lijing Pill Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 1 qian (3 g);
Calomel, 1 qian (3 g); Power of seeds of Ipomoea nil or I. biflora, 5 qian (15 g);
Dried exudate from stems of B. tertilis or S. chinense, 2 qian (6 g)Children's medicine certificate straight recipe Qingdai Shigao Decoction Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 1.5 qian (4.5 g);
Fresh roots of Rehmannia glutinosa, 2 liang (60 g); Gypsum, 8 qian (24 g);
Rhizome of Cimicifuga heracleifolia, C. dahurica or C. foetida, 6 fen (1.8 g);
Roots of S. baicalensis, 2 qian (6 g); Processing fruits of Gardenia jasminoides, 3 qian (9 g);
Allium fistulosum, 3 piecesRedefinition of popular typhoid theory Yangdu Neixiao Powder Secretions from mature male sachets of M. berezovskii, M. sifanicus or M. Moschiferus, 2 qian (6 g);
Borneol, 2 qian (6 g);
Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 2 qian (6 g);
Tubers of Bletilla striata, 3 qian (9 g);
Tubers of A. erubescens, A. heterophyllum or A. amurense, 3 qian (9 g);
Prepared scales of Manis pentadactyla with Rhizomes of Curcuma longa, 3 qian (9 g);
Camphor (Branches, stems, leaves and roots of Cinnamomum camphora), 3 qian (9 g);
Verdigris, 3 qian (9 g); Chalcanthite, 3 qian (9 g)Drug dowry secret Yanhou Biyu Powder Leaves and/or stems of B. cusia, P. tinctorium, I. tinctoria or I. indigotica, 1 liang (30 g);
Mirabilite, 1 liang (30 g); Pollen of Typha angustifolia, T. orientalis or similar plants, 1 liang (30 g);
Roots and rhizomes of G. uralensis, G. inflata or G. glabra, 1 liang (30 g)Yuyao Yuanfang Note: Li, fen, qian, and liang are units of measurement in ancient China. 1 liang = 10 qian = 100 fen = 1000 li = 30 g. -
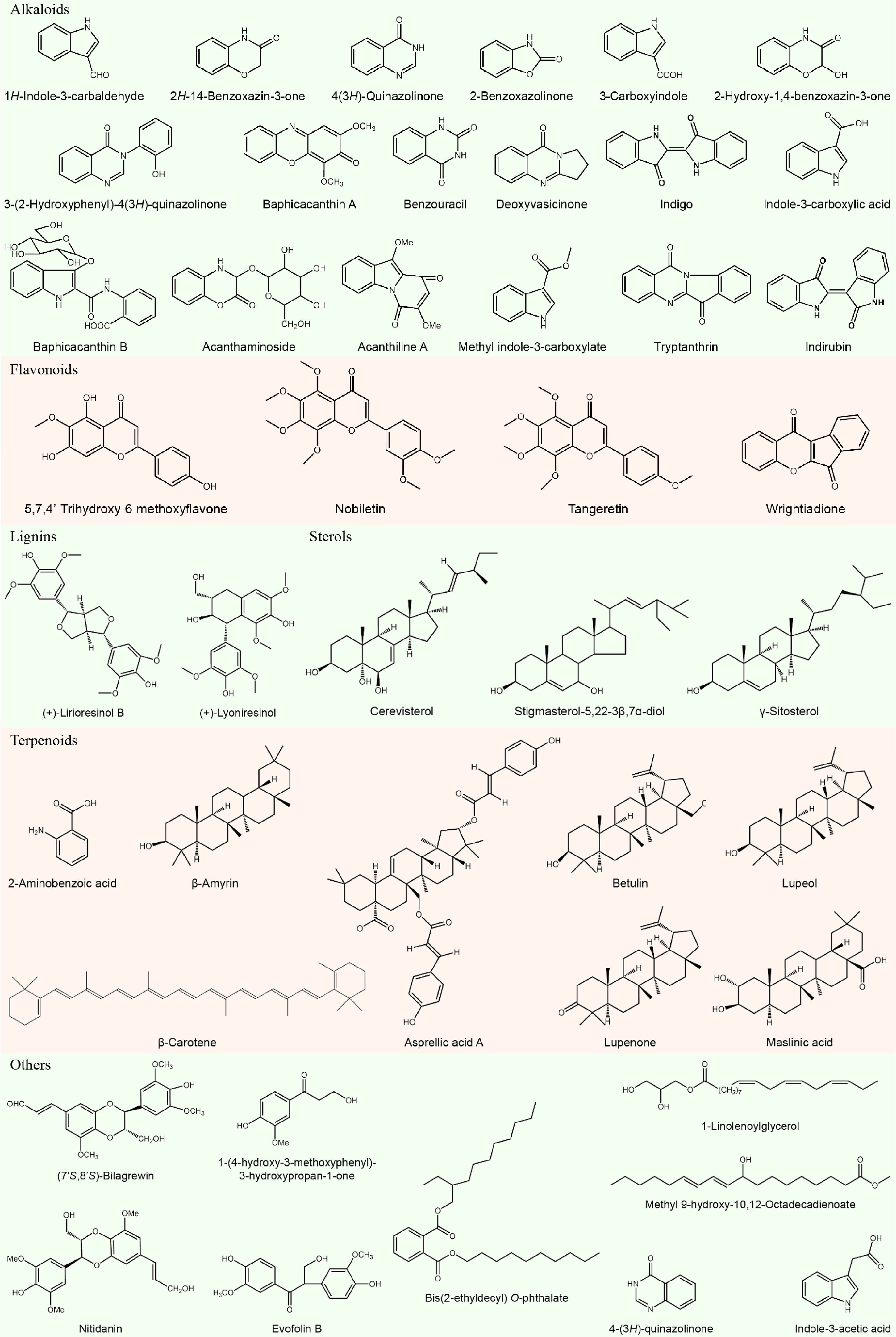
Because of a traditional Chinese medicine, research on the chemistry of B. cusia began decades ago. Studies showed that the plant and corresponding medicinal materials include indole alkaloids (indirubin, indigo, and tryptanthrin[9]), sterols (β-sitosterol, γ-sitosterol, stigmasta-5,22-diene-3β,7β-diol, and stigmasta-5,22-diene-3β,7α-diol[9,10]), triterpenoids (lupeol, lupenone, and botulin[11]), lignans ((2R,3R)-(+)-lyoniresinol[12]) and flavonoids (5,7,4'-trihydroxy-6-methoxyflavone and 5,7,4'-trihydroxy-6-methoxyflavone-7-O-β-D-glucopyranoside[13]) (Fig. 2). With the deepening of research and the development of detection technology, more and more new compounds are constantly being discovered from B. cusia. For example, Liu et al. firstly found monoterpenoids (−)-loliolide and (+)-isololiolide[13]; Feng et al. obtained two new alkaloids, baphicacanthin A and baphicacanthin B, from the roots of B. cusia[14]; Zhu et al. identified four stereoisomeric indole alkaloids, (±)-baphicacanthcusines A−D, and one new indole alkaloid, baphicacanthcusine E, together with nine known compounds from the leaves[15]; Xu et al. identified 11 compounds form leaves of B. cusia for the first time, such as 1,3-dihydro-2H-indol-2-one, 2-hydroxy-N-(2-hydroxyphenyl)propionamide, (3R,6R,7E)-3-hydroxy-4,7-megastigma-dien-9-one, 3β-hydroxy-5α,6α-epoxy-7-megastigmen-9-one, calendin, 3β-hydroxy-β-ionone, hispidulin, bungein A, bis(2-ethylhexyl)benzene-1,2-dicarboxylate, nonacosane and p-hydroxyacetophenone[5]; and Bai et al. firstly isolated 18 compounds from roots of B. cusia, including indole-3-acetic acid, methyl indol-3-ylacetate, acanthiline A, hyptatic acid-A, 2α-hydroxy oleanolic acid, β-amyrin, nobiletin, tangeretin, wrightiadione, (+)-evofolinB, nitidanin, (7'S,8'S)-bilagrewin, (+)-lyoniresinol, cerevisterol, 1-(4-hydroxy-3-methoxyphenyl)-3-hydroxypropan-1-one, O-phthalic acid bis-(2-ethyl decyl)-ester, 1-linoleoylglycerol, and 10,12-octadecadienoicacid-9-hydroxymethylester[16]. However, studies on chemical compositions are focused on several secondary metabolites with clearly biological activity, such as flavonoids and indole alkaloids[4,17]. Further studies of isolation, purification, identification, and characterization of new compounds from B. cusia are needed.
-
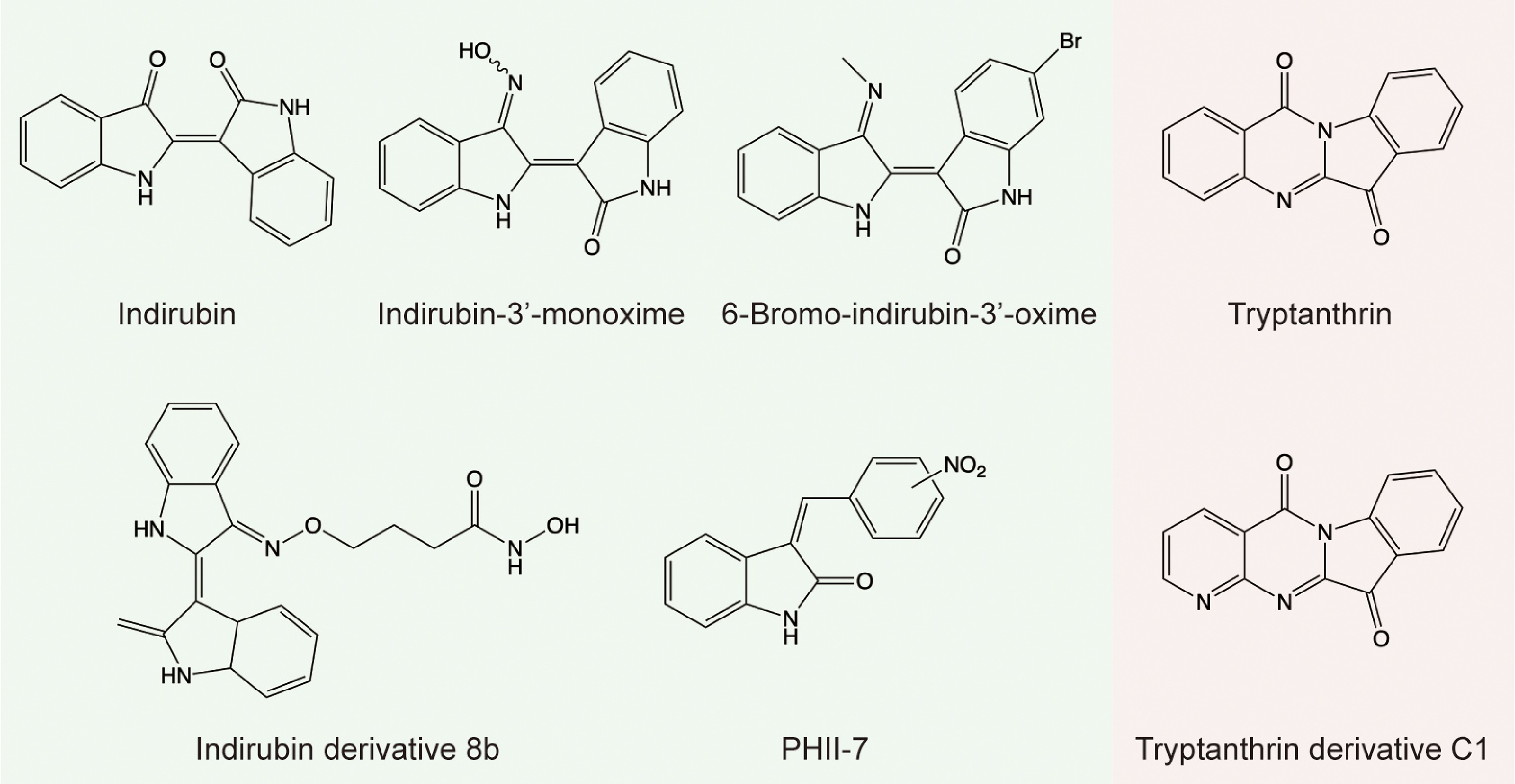
Combination of the TCM theory and modern pharmacological study, some indigo naturalis-containing Chinese medicine, such as the Danggui Longhui Wan[18] and the Realgar-indigo naturalis formula[19], are clinically administrated to treat human leukemia. Indirubin, indigo, and tryptanthrin are believed to be the main bioactive substances in B. cusia. Indirubin exhibits inhibitory activity towards cyclin-dependent kinase (CDK) 1-cyclin B with half-maximal inhibitory concentration (IC50) = 10 μM, and its derivatives inhibit CDK1 efficiently as well, indirubin-3′-monoxime with IC50 = 0.18 μM, 5-chloro-indirubin with IC50 = 0.4 μM, and indirubin-5-sulphonic acid with IC50 = 0.055 μM[18]. Cancer statistics, 2023 depicts that prostate cancer is the most common cancer diagnosed in men and breast cancer in women. The report also shows that lung cancer in men and women will cause the maximum number of deaths[20]. Fortunately, indirubin, indigo, and their derivatives (Fig. 3) exhibit significant antitumor effects against these cancers in vitro and in vivo through multiple pathways (Fig. 4).
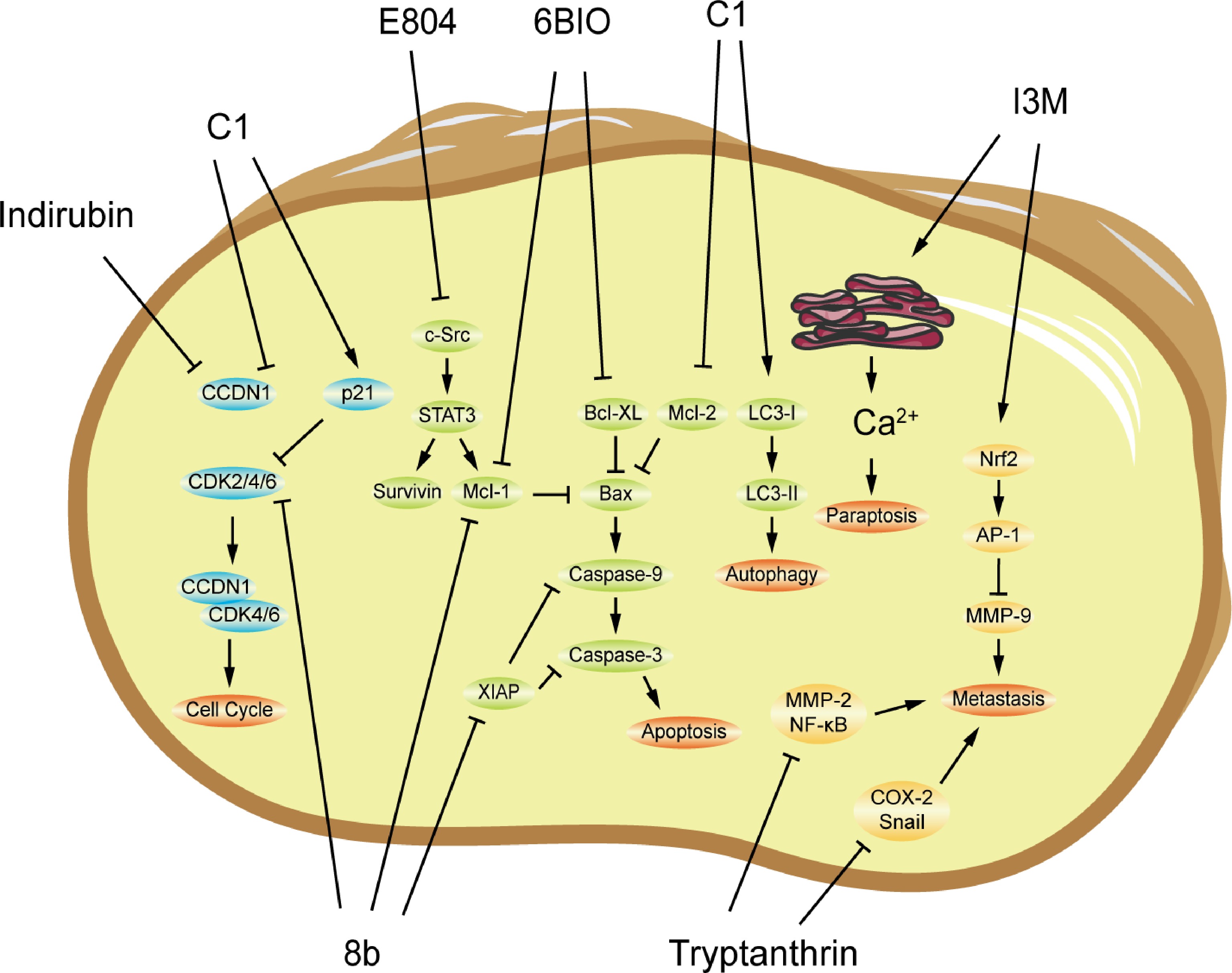
Figure 4.
The possible mechanisms of antitumor activity of indirubin and tryptanthrin and their derivatives. 8b, 6BIO E804, and I3M are indirubin derivatives; and C1 is tryptanthrin derivative. AP-1, activator protein-1; Bax, Bcl-2-associated X protein; Bcl-xL, B-cell lymphoma extra-large; CCND, cyclin D; CDK, cyclin-dependent kinase; COX-2, cyclooxygenase 2; IL, interleukin; LC3, microtubule-associated protein 1 light chain 3; Mcl-1, mantle cell lymphoma 1; MMP, matrix metalloprotein; NF-κB, nuclear factor κ B; Nrf2, nuclear factor-erythroid 2-related factor 2; Src, proto-oncogene tyrosine-protein kinase; STAT, signal transducer and activator of transcription; TNF-α, tumor necrosis factor-α; and XIAP, X-linked inhibitor of apoptosis.
Induction of cell cycle arrest
-
In the CDK-dependent mechanism, cyclin D1 (CCND1) and CDK 4/6 form an active CCND1-CDK4/6 complex, and then phosphorylate retinoblastoma protein (Rb), which is dissociated from the bound E2F transcription factor 1 (E2F1) to initiate transcription, resulting in cell cycle progression[21]. Wei et al. found that indirubin inhibits CCND1 expression and induces cell cycle arrest with an increase in the G0 and G1 phases and a decrease in the S and G2/M phases in human prostate cancer PC-3 cells[22]. Cao et al. design and synthesize a series of indirubin derivatives as dual inhibitors against CDK and histone deacetylase (HDAC)[23]. Compound 8b, one of which possesses remarkable CDK2/4/6 and HDAC6 inhibitory activity, induces S-phase arrest in human breast cancer MCF-7 and non-small cell lung cancer A549 cells. Zou et al. synthesized tryptanthrin derivatives, wherein compound C1 significantly downregulates CCND1 level and upregulates the expression of CDK inhibitor p21, resulting in an increase in the percentage of the A549 cells in the G2/M phase[24].
Induction of programmed cell death
-
Apoptosis is a kind of cell death processes. Signal transducer and activator of transcription (STAT) 3 is a transcription factor and is involved in numerous biological processes, including cell survival[25]. Various factors can activate STAT3, which then regulates downstream genes, such as apoptosis-related genes B cell lymphoma 2 (Bcl-2), B-cell lymphoma extra-large (Bcl-xL), Survivin, and mantle cell lymphoma 1 (Mcl-1). Subsequently, the Bcl-2 family regulates caspases, leading to trigger apoptosis. Targeting STAT3 is an attractive therapeutic strategy for cancer management[26]. In human breast cells, indirubin derivative E804 blocks tyrosyl phosphorylation and DNA-binding activity of STAT3 by directly inhibiting c-Src kinase activity with IC50 = 0.43 μM, and then the expression levels of Mcl-1 and Survivin are reduced, leading to apoptosis[27]. A bromo-substituted indirubin 6-bromo-indirubin-3’-oxime (6BIO), as a strong inhibitor of GSK-3b with IC50 = 0.005 μM, CDK1 with IC50 = 0.320 μM, and CDK5 with IC50 = 0.083 μM, induces apoptosis in various cancer cell lines[28]. In breast cancer cells, 6BIO downregulates Mcl-1 and Bcl-xL expression levels and activates the intrinsic caspase-9 and caspase-3 mediated apoptotic pathway[29,30]. Indirubin derivative 8b downregulates Mcl-1 and X-linked inhibitor of apoptosis (XIAP) in A549 cells[23]. Tryptanthrin derivative C1 treatment increases Bax/Bcl-2 ratio, with reduced Bcl-2 expression and increased Bax expression[24]. Additionally, the C1 can also induce autophagy in NSCLC with the increased transformation from LC3-I to LC3-II[24]. An indirubin derivative, indirubin-3’-monoxime (I3M), triggers proteasome dysfunction, induces ER stress, and promotes Ca2+ release with transmission to the mitochondria regulated by MCU, resulting in paraptosis[31].
Metastasis inhibition
-
The tumor microenvironment (TME), including surrounding cells, signaling molecules, and extracellular matrix (ECM), is critical to cancer progression. Active transforming growth factor (TGF)-β stored in the ECM complex is released into the TME[32]. The TGF-β signaling pathway has been proven to be a significant inducer of Epithelial-to-mesenchymal transition (EMT) and promotes tumor metastasis in human cancers[33]. ECM is degraded by matrix metalloproteinase (MMP), which results in tumor cell invasion and migration[34]. Indirubin derivative I3M slightly decreases viability of human prostate cancer LNCaP cells, but inhibits phorbol 12-myristate 13-acetate (PMA)-induced invasion of LNCaP cells[35]. I3M activates nuclear factor-erythroid 2-related factor 2 (Nrf2), subsequently stimulates its movement to the nucleus, and consequently reduces activator protein-1 (AP-1) activity, thereby downregulating MMP-9 expression. This results in the reduction of prostate cancer invasion. In MCF-7 cells, tryptanthrin inhibits cell proliferation, migration and invasion, induces poor adherence, and suppresses the TGF-β1-induced transition in vitro[36]. Moreover, tryptanthrin enhances the E-cadherin expression level and depresses expressions of MMP-2 and Snail stimulated by TGF-β1, suggesting that tryptanthrin suppresses the TME-associated EMT induced by TGF-β1. In 4T1 mouse breast cancer models, tryptanthrin also has considerable inhibitory activity with much lower toxic and side effects on organisms compared with the positive drug cyclophosphamide. Tryptanthrin suppresses the expression levels of NOS1, COX-2, and NF-κB in mouse tumor tissues, and upregulates serum expression levels of IL-2, IL-10, and TNF-α in tumor-bearing mice. It is showed that tryptanthrin exerts anti-breast cancer activities by modulating the inflammatory TME in vitro and in vivo.
Multidrug resistance overcoming
-
Most tumors develop resistance during therapy, and multidrug resistance often leads to failure of cancer chemotherapy. New therapeutic strategies are urgently needed to overcome chemotherapy resistance. Indirubin derivative PHII-7 significantly inhibits cell growth (IC50 = 6.07 μM against MCF-7 cells; IC50 = 5.51 μM against adriamycin-selected MCF-7 cells), potentiates adriamycin cytotoxicity, and restores chemotherapy sensitivity in MCF-7 cells with adriamycin resistance[37]. It is suggested that the downregulation of the P-glycoprotein level could reverse multidrug resistance. The indirubin derivative 6BIO overcomes tumor necrosis factor α-related apoptosis inducing ligand (TRAIL) resistance and synergizes with TRAIL to abrogate cell proliferation and induce caspase-dependent apoptosis[30]. 6BIO in combination with TRAIL augments caspase-dependent apoptosis. Tryptanthrin and its derivative benzo[b]-tryptanthrin reverse multidrug resistance through the downregulation of multidrug resistance protein 1 (MDR1) level in MCF-7 cells[38,39].
-
Hu et al. applied flow cytometry to estimate the B. cusia genome size[40]. They used the genome size of Oryza Sativa L. spp. japonica as a reference, and found that the B. cusia genome size is 0.99 ± 0.01 Gb. For the first time, this team performed de novo transcriptome sequencing of B. cusia[41,42]. They found 51,381 unique sequences based on 137,216,248, 122,837,394, and 140,240,688 clean reads from leaves, stems, and roots, respectively, and annotated 33,317 unigenes using the databases of Nr, Swiss-Prot, KEGG, and KOG. Within the 51,381 examined unigenes, 6,782 unigenes consist of 8,471 SSR markers, and 1,350 unigenes contain more than one SSR. Tri-nucleotide is the most abundant SSR, and penta-nucleotide is the least frequent SSR. In order to analyze the content of indigo and indirubin in the leaf and root tissues of B. cusia, the team performed de novo RNA-seq of transcriptional profiles of B. cusia leaf and root treated by methyl jasmonate (MeJA)[43]. In response to MeJA treatment, 33,317 unigenes are annotated and contain 8,355 DEGs with 5,999 DEGs in MeJA-treated roots and 2,356 DEGs in the treated leaves. Huang also performed transcriptome sequencing and annotation of B.cusia using the Illumina Hiseq platform[3]. They established a transcriptome database containing 293,666 unigenes.
For the species determination of Nan-Ban-Lan-Gen, Chen et al. analyzed and characterized the complete chloroplast (cp) genome sequence of S. cusia[44]. The genome is 144,133 bp in length and encodes 113 unique genes consisting of 79 protein-coding, 30 transfer RNA, and four ribosomal RNA genes. Combination of single-molecule sequencing and high-throughput chromosome conformation capture technologies, Xu et al. analyzed the chromosome scale genome of S. cusia[45]. The genome size is approximately 865 Mb, containing about 79% of repetitive sequences and 32,148 annotated protein-coding genes.
-
Indirubin and indigo are isomers and are believed to be the most important bioactive components in B. cusia. Thus, exploration and analysis of their biosynthesis are vital. In the past 30 years, indole alkaloids biosynthesis has been believed to be one of the most investigated secondary metabolic pathways in blue-genera plants. This biosynthesis pathway is originally studied in microorganisms[46]. It comprises the shikimate pathway and tryptophan metabolism pathway. The shikimate pathway acts like a bridge for connecting primary and secondary metabolism[47]. The secondary metabolism pathway of indoles in plants begins the tryptophan metabolism pathway (Fig. 5). This pathway of indole alkaloids in plants is still unclear, and the work is still in progress.
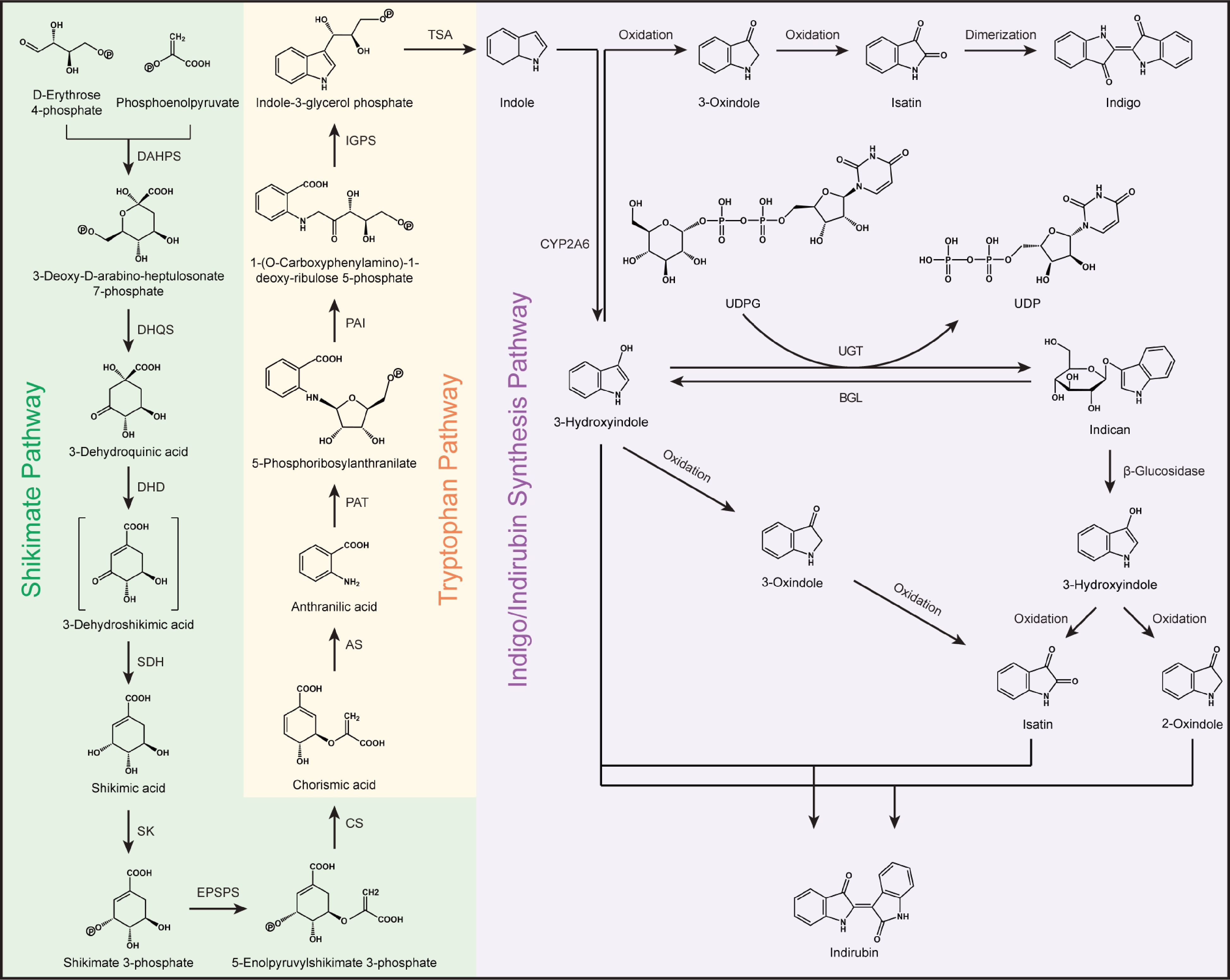
Figure 5.
Possible metabolic pathways of indigo and indirubin in B. cusia. AS, anthranilate synthase; BGL, β-glucosidase; CS, chorismate acid; CYP2A6, cytochrome P450 monooxygenase; DAHPS, 3-deoxy-D-arabinoheptulosonate 7-Phosphate synthase; DHD, 3-dehydroquinate dehydratase; DHQS, 3-dehydroquinate synthase; EPSPS, 5-enolpyruvylshikimate-3-phosphate synthase; IGPS, indole-3-glycerol phosphate synthase; PAI, phosphoribosyl anthranilate isomerase; PAT, phosphoribosyl anthranilate transferase; SDH, shikimate dehydrogenase; SK, shikimate kinase; TSA, tryptophan synthase alpha-subunit; UDPG, uridine diphosphate glucose; UDP, uridine diphosphate; and UGT, uridine diphosphate glucuronide transferase.
Analysis of the synthesis of indole alkaloids in B.cusia is just beginning. Some genes are predicted, identified, and cloned based on mining omics data. In S. cusia, Hu et al. confirmed 18 indole alkaloids-related coding genes[48]. It was found that three UGT and two CYP450 genes are mainly expressed in the stems and leaves, and two UGT, one ASA, one TSB, one BGL, one CS, and one EPSPS genes are significantly expressed in roots. Plant hormones are necessary for plant growth and development[49] and secondary metabolism[50]. Using MeJA-treated B.cusia or S. cusia, comparative transcriptome analyses reveal several genes upregulated by MeJA and involved in the synthesis of indole alkaloids[43,48].
Using the transcriptome data, some genes have been cloned, such as BcASA[51], BcASB[52], BcIGPS[53], BcEPSPS[54], and BcTSA[55]. Transcription factors control expressions of biosynthetic genes, thereby participating in the production of indole alkaloids. Zeng et al. cloned a WRKY transcription factor BcWRKY1, which is 916 bp in length containing three exons and two introns, and has 534 bp of open reading frame ORF encoding 177 amino acids residues[56].
Both in vitro and in vivo experimental approaches are employed to verify the functions of these pathway-related genes. The enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) catalyzes the reaction from enolpyruvyl moiety of phosphoenol pyruvate (PEP) to 5-hydroxyl shikimate-3- phosphate (S3P), thereby producing shikimic acid and chorismite. Yu et al. expressed and identified the recombinant BcEPSPS protein in E. coli strain BL21[54]. After purification, the enzymatic activity assays are performed. Model organisms are an essential intermediate tool to confirm gene functions in vivo experiments. The functions of BcTSA[55] and BcWRKY1[56] are investigated in E. coli and Arabidopsis thaliana, respectively. Besides model organisms, Isatis tinctoria containing indole alkaloids pathway is also used to study the functions of the pathway related genes, such as BcTSA[55] and BcEPSPS[54].
-
Generally, there are three ways to acquire natural products including indigo and indirubin, such as extraction, chemical synthesis, and biosynthesis. The typical extraction method mainly consists of microbial fermentation and organic solvent extraction. Nevertheless, this method is cumbersome in operation and low in extraction purity, with high cost and toxicity[57]. After the chemical structure of indigo is determined, the chemical synthesis method replaces the plant extraction and dramatically increases the yield of indigo pigments[58]. To this day, the majority of indigo is chemically synthesized. Although the chemical synthesis method is simple, efficient, high yield, and high purity[59], the used primary raw materials, catalysts, and a significant amount of produced toxic by-products bring a severe potential threat to human beings and the environment[60].
Since the microbial synthesis of indigo is first reported, the green synthesis of indigo has paid much attention. The first indigo-reducing alkaliphile, Bacillus alkaliphiles, is isolated in the 1960s, and then multiple microorganisms involved in the reduction of indigo during natural fermentation are reported, such as alkaliphilic bacterium IDR2-2T[61], Amphibacillus spp. strain C40[62], and Oceanobacillus spp. strain A21[62]. Escherichia coli is capable of producing indirubin from indican, a primary metabolite of indole, as well[63]. Indole can be catalyzed from indole 3-glycerol phosphate, forming L-tryptophan; tryptophan can also reversibly convert to indole. Thus, the metabolic engineering strategies mainly include enhancement of the production of biomass-derived substrates, L-tryptophan, and indicant[64]. Besides, the indole is oxidized to indoxyl, followed by being oxidized to indigo. Thus, microbial synthesis of indigo and indirubin also depends on the indole oxidative enzymes (Fig. 6).
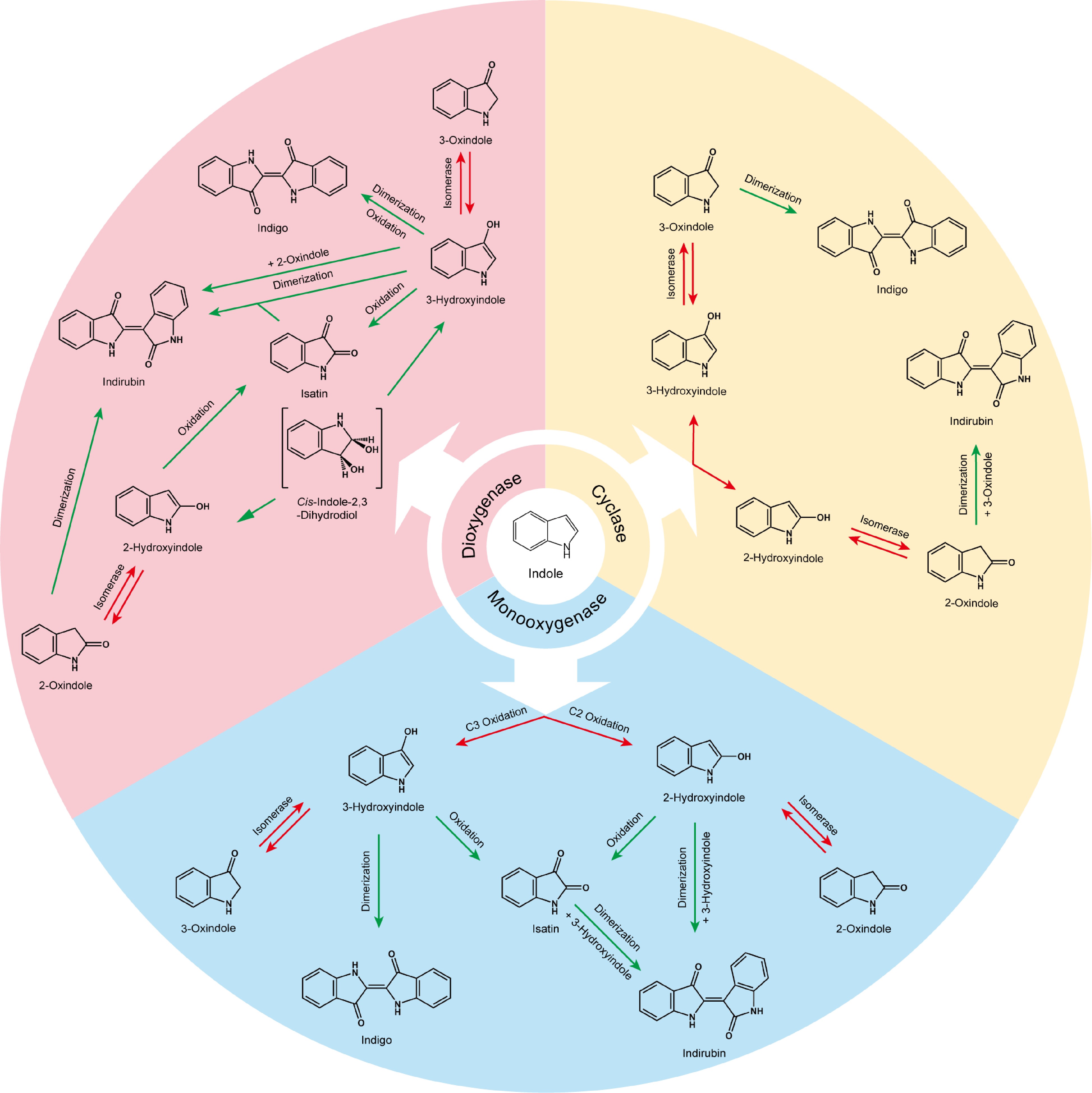
Figure 6.
The pathways of biosynthetic production of indigo and indirubin from indole. Three strategies can be applied for biosynthesis. Monooxygenase oxidizes indole into indole oxide, eventually producing indigo and indirubin; dioxygenase oxidizes indole into cis-indole-2,3-dihydrodiol, then generating indigo and indirubin; and cyclase catalyzes the hydroxylation of indole to produce indigo or indirubin. The red arrow indicates enzymatic reaction; the green arrow indicates spontaneous process.
Baeyer-Villiger monooxygenase (BVMO) can catalyze chemo-, regio-, and enantio-selective Baeye-Villiger oxidations[65]. Phenylacetone monooxygenase (PAMO) is a new variant of the BVMO family[66]. Núñez-Navarro et al. developed two PAMOs, PAMOHPCD and PAMOHPED, by iterative saturation mutagenesis[67]. The E. coli with PAMOHPCD expression and L-tryptophan supplement produces approximately 3,000 mg/L of indigo and 130.0 mg/L of indirubin. Flavin-containing monooxygenases (FMOs) belong to a family of molecular oxygen-dependent enzymes and are involved in oxidative processes. Recombinant E. coli DH5α cells harboring an FMO gene cloned from Methylophaga aminisulfidivorans MPT produce about 5 mg/L of indirubin and 920 mg/L of indigo in tryptophan medium[68,69]. Then, in optimal tryptophan medium by adding cysteine, the cells produce 223.6 mg/L of indirubin[70]. Toluene 4-monooxygenase (T4MO) is a multicomponent hydroxylating enzyme involved in the conversion of toluene to hydroxytoluene[71]. T4MO can also catalyze the production of indigo and indirubin[72]. The tmoABCDEF operon encoding T4MO components is cloned from Pseudomonas sp. M4 and expressed in E. coli. When the developed two-phase (dioctyl phthalate/aqueous medium) culture system combines with the optimized culture conditions, the strain yields 102.4 mg/L of indirubin[73]. Styrene monooxygenase (SMO) catalyzes the conversion of styrene into (S)-styrene oxide in a highly enantioselective manner[74]. As the key rate-limiting enzyme in the indigo biosynthesis pathway, SMO can catalyze the oxidation of indole efficiently and consequently promote indigo biosynthesis[75]. Recently, an engineering E. coli is constructed with co-expressed styrene monooxygenase StyAB from Pseudomonas putida and the chaperone groES-groEL[76]. The yield of indigo is increased to 550 mg/L. When the StyAB gene is co-expressed with the malate dehydrogenase (mdh) gene selected for NADH regeneration, the yield of indigo is up to 787.25 mg/L after 24 h of fermentation[77]. Rieske-type oxygenases are a class of non-heme iron enzymes involved in the degradation of aromatic compounds[78]. In E. coli, tryptophanase oxidizes tryptophan into indole, and then heterologous oxygenases such as naphthalene dioxygenase (NDO), a Rieske non-heme iron dioxygenase, convert indole into precursors of indigo and indirubin[79]. Zhang et al. cloned an NDO gene from Comamonas sp. MQ and induced it into E. coli, which can produce 57.98 mg/L of indirubin[80].
Besides monooxygenases and dioxygenases, cyclases such as flavin-dependent terpenoid cyclases Xiamycins (Xias) also involve in the production of indigo and indirubin[81]. Yin et al. found that XiaI can produce 1.7 mg/L of indigoids in E. coli BL21 (DE3)[82]. Through introducing other vital enzymes such as flavin-reducing enzyme Fre, tryptophan-lysing and -importing enzymes TnaA and TnaB, and H2O2-degrading enzyme KatE, and optimizing the fermentation parameters, the yield of indigoids reached 101.9 mg/L. Moreover, the productivity of indigo and indirubin in 1 L of the flask is improved to 26.0 and 250.7 mg/L, respectively.
-
B. cusia has been a traditional medicinal plant for thousands of years. In modern medicine, B. cusia still possesses great potential for exploration. For example, the Compound Huangdai Tablet and the Compound Nan-Ban-Lan-Gen Tablets are medicines approved for sale in China. Indigo naturalis is a significant component of the Compound Huangdai Tablet used to treat leukaemia. Indigo naturalis also excels in the treatment of psoriasis. Zhang et al. reviewed the functions and mechanisms of indigo naturalis in the treatment of psoriasis through immune cells, signal pathways, and disease-related mediators[83]. The Compound Nan-Ban-Lan-Gen Tablet contains Nan-Ban-Lan-Gen as the main ingredient and possesses anti-inflammatory and detoxifying properties. Regrettably, the primary source of this medicinal herb is controversial.
The rich and diverse chemical compositions of B. cusia confers many bioactive properties, such as antitumor, anti-inflammation, and neuroprotection. Among the numerous chemical components contained in B. cusia, indirubin, indigo, and tryptanthrin are mainly bioactive compounds. They exhibit inhibitory effects against a variety of human cancers such as glioma[84] and skin cancer[85], besides cancers discussed in the text. Additionally, these bioactive components can also suppress fibroblast[86], inhibit SARS-CoV-2 infection[87], and prevent and treat atherosclerosis[88]. The functions of other compounds are less well-studied. Thus, there is still a significant scope to explore their effects and application. Moreover, considering the practical biological functions of these bioactive compounds, a series of their derivatives are designed and synthesized to improve the effects and reduce toxicity[89]. In addition to these mainly bioactive compounds, the biological functions of other compounds need further investigation. Identification of compound targets using the tools of modern biotechnology such as target fishing hook[90] is of great importance in terms of exploring mechanisms involved.
Although the biological functions of these natural ingredients are very impressive, their metabolic processes, especially the indole alkaloids pathway, in B. cusia have been less studied. Only a few of these pathway-related enzyme genes are cloned from B. cusia, and the studies of their functions are even fewer. However, the decipherment of a whole-genome map[48] and analysis of transcriptome[43] will provide an essential reference for the analysis of the metabolic pathway of indole alkaloids and other secondary metabolites. Multi-omics and comparative omics analysis is also a maturation-researching strategy for elaborating metabolic pathways. Genetic transformation is a technological tool to study plant gene structure and function. The genetic transformation system is relatively immature for B. cusia, leading to difficulty in validating gene function. In addition to model plants, functions of several genes cloned from B. cusia have to be studied in I. indigotica and other plants. Catharanthus roseus also contains a synthesis pathway of indole alkaloids and could be used for genetic transformation[91]. Thus, the establishment of a genetic transformation system for B. cusia is in urgent demand. Based on the refinement of metabolic pathways and genetic transformation system, genetic engineering approaches are employed to enhance the contents of active ingredients. Gene editing has been proved to be an effective strategy. But the efficiency of gene editing varies widely across species[92]. It is quite necessary to develop species-specific gene-editing technology. Moreover, synthetic biology is an effective way to address the scarcity of resource components. The reactions of oxidation and cyclization contribute to the production of bioactive compounds, especially indigo and indirubin, from indole. Exploration and discovery of new indole oxidases and cyclases with high catalytic efficiency are the keys to promoting the large-scale bioproduction and application of indigo and indirubin in the future[82].
-
The authors confirm contribution to the paper as follows: study conception and design: Zhang L, Li QZ; data collection: Feng ZH, Jing S, Shen YP; analysis and interpretation of results: Feng ZH, Jing S, Shen YP, Tong YQ, Xiao CJ; draft manuscript preparation: Feng ZH, Jing S, Shen YP, Tong YQ, Xiao CJ, Xue JP, Zhang H, Li QZ, Zhang L. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
This work was supported by the National Natural Science Foundation of China (grant numbers 82225047 and 82170274), the National Key Research and Development Program of China (grant number 2022YFC3501703), and the Excellent Scientific Research and Innovation Team of University in Anhui Province (grant number 2022AH010029).
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Zhi-Hui Feng, Shuang Jing, Yu-Ping Shen
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Feng ZH, Jing S, Shen YP, Tong YQ, Xiao CJ, et al. 2024. Advances and perspectives in chemical and biological studies of Baphicacanthus cusia: a focus on antitumor constituents. Medicinal Plant Biology 3: e001 doi: 10.48130/mpb-0024-0001
Advances and perspectives in chemical and biological studies of Baphicacanthus cusia: a focus on antitumor constituents
- Received: 29 September 2023
- Revised: 15 December 2023
- Accepted: 27 December 2023
- Published online: 23 January 2024
Abstract: Baphicacanthus cusia (Nees) Bremek has a rich historical significance in China. Its stems and leaves have been used for traditional Chinese medicine or a dye called Qing-Dai in Chinese, while its roots are referred to as Nan-Ban-Lan-Gen in Chinese after processing. Both have been recognized and documented in the Chinese Pharmacopoeia. Modern pharmacological studies have revealed that B. cusia has numerous bioactive properties, such as antitumor, antiviral, anti-inflammatory, antioxidant, hepatoprotective, and neuroprotective properties. Moreover, B. cusia has been employed in clinical settings for its antitumor activity. The pivotal bioactive compounds in B. cusia are indirubin, indigo, and tryptanthrin. These potent substances and their derivatives demonstrate antitumor effects through inducing cell cycle arrest, triggering programmed cell death, inhibiting metastasis, and overcoming multidrug resistance. Nonetheless, the metabolic pathway of these compounds, particularly indole alkaloids, in B. cusia remains inadequately investigated. To increase the production of indole alkaloids such as indirubin and indigo, various biosynthetic approaches are employed. Consequently, this review discusses the bioactive compounds present in B. cusia, and elucidates their antitumor mechanisms, metabolism, and biosynthesis pathway. Existing studies indicate that B. cusia is a crucial medicinal plant harboring numerous biologically active substances, and its exploitation and application hold significant potential for substantial economic gains.
-
Key words:
- Baphicacanthus cusia /
- Active components /
- Antitumor /
- Metabolism /
- Biosynthesis