-
Bletilla Rchb. f. is one of the most economically valuable groups of orchids in the world. Due to its ornamental significance, the genus Bletilla occupies an important place in the worldwide horticultural market. Furthermore, in China, Japan, South Korea, and other Asian countries, it is highly valued for its medicinal use[1].
There are eight species in the genus Bletilla, including Bletilla chartacea (King & Pantl.) Tang & F.T. Wang, Bletilla cotoensis Schltr., Bletilla foliosa (King & Pantl.) Tang & F.T. Wang, Bletilla formosana Schltr., Bletilla guizhouensis J. Huang & G.Z. Chen, Bletilla morrisonensis Schltr., Bletilla ochracea Schltr., and Bletilla striata Rchb.f.[2,3]. The distribution area spans from northern Myanmar in Asia to Japan via China[4]. Five species are native to China, namely, B. foliosa, B. formosana, B. guizhouensis, B. ochracea, and B. striata. In China, people have assigned various names to Bletilla based on its morphology and efficacy, such as baiji (白及/白芨), baigen (白根), baige (白给), baijier (白鸡儿), baijiwa (白鸡娃), diluosi (地螺丝), gangen (甘根), junkouyao (皲口药), lianjicao (连及草), and yangjiaoqi (羊角七)[5]. These diverse appellations highlight the importance of this genus in Chinese folk biological culture.
The medicinal material known as 'baiji' in traditional Chinese medicine (TCM) is usually the dried tuber of B. striata, which is also the authentic product included in the Chinese Pharmacopoeia[6]. According to the Chinese Pharmacopoeia (2020), TCM baiji is sliced, dried, and crushed into a powder that can be used topically or internally, with a recommended dosage of 3–6 g at a time, offering astringent, hemostatic, detumescence, and myogenic effects. It is often used for conditions such as hemoptysis, hematemesis, traumatic bleeding, sores, and skin chaps[7]. Although only B. striata is the authentic product of TCM baiji, the other four Bletilla species native to China are also used as substitutes, and this practice is widespread[8].
Modern research indicates that Bletilla contains a variety of chemical components, including benzol, dihydrophenanthrene, phenanthrene, and quinone derivatives. These components confer pharmacological effects on Bletilla, such as hemostasis, anti-tumor activity, and promotion of cell growth[9]. Due to its outstanding medicinal value, Bletilla can be found in nearly every corner of the traditional medicine market (Fig. 1). However, habitat destruction and uncontrolled mining have led to a significant reduction in the native populations of Bletilla, making its protection an urgent priority. Therefore, this paper provides a comprehensive review of relevant research up to August 2023, covering botanical characteristics, resource distribution, ethnobotanical uses, chemical components, pharmacological effects, clinical applications, and safety evaluations of Bletilla. The aim is to raise awareness and promote the protection and sustainable use of this genus.
-
The morphology of different Bletilla species is highly similar. The primary taxonomic feature distinguishing each species is the characteristics of the flower, particularly the lip of the flower, including its size, shape, and the number and shape of longitudinal ridges on the lip plate (Table 1, Fig. 2)[10−14].
Table 1. The morphological differences among five species of Bletilla plants native to China.
Morphological feature Bletilla striata Bletilla formosana Bletilla ochracea Bletilla foliosa Bletilla guizhouensis Plant height (cm) 18−60 15−80 25−55 15−20 45−60 Rhizome shape Compressed Compressed Somewhat compressed Subglobose Compressed Rhizome diameter (cm) 1−3 1−2 About 2 1−1.5 3−4 Stem characteristics Stout Enclosed by sheaths Stout Stout, short Thin Leaf shape Narrowly oblong Linear-lanceolate Oblong-lanceolate Elliptic-lanceolate Narrowly lanceolate Leaf size (cm) 8−29 × 1.5−4 6−40 × 0.5−4.5 8−35 × 1.5−2.8 5−12 × 0.8−3 25−45 × 1.2−4.5 Flower color Purplish red or pink Pale purple or pink Yellow Pale purple Deep purple Flower size Large Medium Medium Small to medium Large Inflorescence structure Branched or simple Branched or simple Simple Simple Branched Pedicel and ovary length (mm) 10−24 8−12 About 18 7−9 13−17 Sepal shape Narrowly oblong Lanceolate Lanceolate Linear-lanceolate Oblong-elliptic Petal shape Slightly larger than sepals Slightly narrower than sepals Oblique Lanceolate Oblong-elliptic Lip shape Obovate-elliptic Broadly elliptic Narrowly rhombic-obovate Narrowly oblong Narrowly oblong Lip color White with purplish veins Whitish to pale yellow with small dark purple spots Whitish to pale yellow with small dark purple spots White with purplish spots and purple edge White with deep purple edge Number of lip Lamellae 5 lamellae 5 undulate lamellae 5 longitudinal lamellae 3 fimbriate lamellae 7 longitudinal lamellae Column characteristics Subterete, dilated towards apex Subterete, dilated towards apex Slender, dilated towards apex Cylindric, dilated towards apex Suberect, with narrow wings 
Figure 2.
(a)−(d) Bletilla striata (Thunb. ex Murray) Rchb. f. (e)−(h) Bletilla formosana (Hayata) Schltr. (i)−(l) Bletilla ochracea Schltr. (m), (n) Bletilla sinensis (Rolf) Schltr. (o), (p) Bletilla guizhouensis Jie Huang & G.Z. Chen (Photographed by Wang Meina, Zhu Xinxin, and He Songhua).
The flowers of B. striata are large and purplish-red or pink, with narrowly oblong sepals and petals measuring 25−30 mm in length and 6−8 mm in width. They have acute apices, nearly as long as the sepals and petals. The lip is obovate or elliptic, predominantly white with purplish-red coloration and purple veins, measuring 23−28 mm in length, slightly shorter than the sepals and petals. The lip disc exhibits five longitudinal folds extending from the base to near the apex of the middle lobe, with waviness occurring only above the middle lobe[11]. In China, B. striata is found in regions such as Anhui, Fujian, Guangdong, Guangxi, Gansu, Guizhou, Hubei, Hunan, Jiangsu, Jiangxi, Shaanxi, Sichuan, and Zhejiang. It also occurs in the Korean Peninsula and Japan, thriving in evergreen broad-leaved forests, coniferous forests, roadside grassy areas, or rock crevices, at altitudes ranging from 100−3,200 m[12].
B. ochracea's flowers are medium to large, featuring yellow or yellow-green exteriors on the sepals and petals, while the insides are yellow-white, occasionally nearly white. The sepals and petals are nearly equal in length, oblong, measuring 18−23 mm long and 5−7 mm wide, with obtuse or slightly pointed apices, often adorned with fine purple spots on the reverse side. The lip is elliptic, typically white or light yellow, measuring 15−20 mm in length and 8−12 mm in width, with three lobes above the middle. The lip disc is characterized by five longitudinally ridged pleats, with undulations primarily occurring above the middle lobe[13]. B. ochracea is native to southeastern Gansu, southern Shaanxi, Henan, Hubei, Hunan, Guangxi, Guizhou, Sichuan, and Yunnan, thriving in evergreen broad-leaved forests, coniferous forests, or beneath shrubs, in grassy areas or alongside ditches at altitudes ranging from 300−2,350 m[14].
B. formosana's flowers come in shades of lavender or pink, occasionally white, and are relatively small. The sepals and petals are narrowly oblong, measuring 15−21 mm in length and 4−6.5 mm in width, and are nearly equal in size. The sepals have subacute apices, while the petal apices are slightly obtuse. The lip is elliptic, measuring 15−18 mm in length and 8−9 mm in width, with three lobes above the middle. The lip disc exhibits five longitudinal ridge-like pleats, which are wavy from the base to the top of the middle lobe[15]. B. formosana is indigenous to southern Shaanxi, southeastern Gansu, Jiangxi, Taiwan, Guangxi, Sichuan, Guizhou, central to northwest Yunnan, southeast Tibet (Chayu), and Japan. It is typically found in evergreen broad-leaved forests, coniferous forests, road verges, valley grasslands, grassy slopes, and rock crevices, at altitudes ranging from 600−3,100 m[16].
The flowers of B. foliosa are small and lavender, with white sepals and petals featuring purple apices. The sepals are linear-lanceolate, measuring 11−13 mm in length and 3 mm in width, with subacute apices. The petals are lanceolate, also measuring 11−13 mm in length and 3 mm in width, with acute apices. The lip is white, oblong, adorned with fine spots, and features a purple apex. It measures 11−13 mm in length and 5−6 mm in width, tapering near the base and forming a scaphoid shape. The lip is anteriorly attenuated, unlobed, or abruptly narrowing with inconspicuous three lobes and exhibits fringe-like fine serrations along the edge. Three longitudinal ridge-like pleats are present on the upper lip disc[17]. B. foliosa typically grows on hillside forests, with its type specimen collected from Mengzi City, Honghe Hani and Yi Autonomous Prefecture, Yunnan Province, China[17].
B. guizhouensis is a recently discovered species in Guizhou, China. In terms of shape, B. guizhouensis closely resembles B. striata, but it can be distinguished by its ovate-oblong buds, oblong dorsal sepals, obovate lips, and middle lobes of the lips, which are oval in shape. The disc of B. guizhouensis features seven distinct longitudinal lamellae, setting it apart from other known Bletilla species and establishing it as a distinct species[2]. Presently, B. guizhouensis has only been found in Guizhou, China, primarily thriving in evergreen broad-leaved forests at altitudes ranging from 900−1,200 m[3].
Understanding the morphology, habitat, and distribution of Bletilla species is crucial for the conservation and propagation of these resources. To effectively implement plant conservation and breeding programs, a comprehensive understanding of the specific morphological characteristics, growth environments, and native habitats of these plants is essential, as without this knowledge, effective results cannot be achieved.
-
The ethnobotanical uses of Bletilla worldwide primarily fall into two categories: ornamental and medicinal purposes. Bletilla orchids, renowned for their striking and distinct flowers, are commonly cultivated for ornamental purposes across many countries[18]. Valued for their aesthetic appeal, these orchids are frequently grown in gardens and utilized as potted plants. Among the various cultivars, B. striata stands out as the most favored choice for ornamental horticulture due to its ease of cultivation and adaptability to diverse climates[19,20].
Contrastingly, in select Asian countries, Bletilla assumes a crucial role as a medicinal plant. For instance, influenced by TCM, the tuber of Bletilla also serves as a crude drug for hemostatic and anti-swelling purposes in Japan[21]. Likewise, traditional Korean medicine, deeply rooted in TCM principles, extensively documents the versatile use of Bletilla in addressing issues such as alimentary canal mucosal damage, ulcers, bleeding, bruises, and burns[22]. In Vietnam, Bletilla has been used as a medicinal herb for treating tumors and skin fissures, aligning with practices observed in the ethnic communities of southwest China[23].
In China, Bletilla boasts a longstanding medicinal history, with numerous classical ancient Chinese medicine books containing detailed records of its medicinal applications[24−32]. Even in contemporary society, many ethnic groups residing in mountainous areas in China continue to uphold the traditional medical practice of using Bletilla medicinally[31].
Ancient medicinal book records
Morphological description
-
In ancient Chinese medical literature, detailed records of Bletilla's morphology can be traced back to the late Han Dynasty, around 200 AD[24]. The Mingyi Bielu, a historical source, documented, 'Bletilla grows in the valley, with leaves resembling those of Veratrum nigrum L., and its root is white and interconnected. The ideal time for harvesting is September'. As awareness of the medicinal significance of Bletilla grew, successive dynastic-era Chinese medical texts consistently included descriptions of Bletilla's morphology (Table 2). In the Ming Dynasty, Li Shizhen compiled these earlier accounts of Bletilla's plant characteristics in his work, the Compendium of Materia Medica. He even provided an illustrative depiction of this plant genus (Fig. 3)[25].
Table 2. Morphological description of the plants belonging to Bletilla in the ancientChinese medicinal books.
Dynasty (Year) Title Author Original Chinese English translation Late Han
(184−220 AD)Mingyi Bielu / 白给生山谷, 叶如藜芦,
根白相连, 九月采Bletilla grows in the valley, with leaves like Veratrum nigrum L., root is white and connected. September is the time for harvesting. Wei-Jin period
(220−420 AD)WuPu Bencao Wu Pu 白根, 茎叶如生姜, 藜芦,
十月花, 直上, 紫赤色,
根白连, 二月, 八月, 九月采Bletilla, stems and leaves like Zingiber officinale Roscoe and V. nigrum. It blooms in October and is purple and red, the inflorescence is vertical and upward. The roots are white and connected. It can be dug in February, August, and September. the Northern and Southern
(420−589 AD)Bencao Jizhu Tao Hongjing 近道处处有之, 叶似杜若,
根形似菱米, 节间有毛It is everywhere near the road. The leaves are like Pollia japonica Thunb. The roots are like the fruit of Trapa natans L., and internode are many fibrous roots. Tang
(618−907 AD)Su Jing, Zhangsun Wuji, etc Tang materia medica 生山谷, 如藜芦, 根白连, 九月采 Born in the valley, with leaves like V. nigrum, root is white and connected. September is the time for harvesting. Song
(960−1279 AD)Su Song Commentaries on the Illustrations 白芨, 生石山上。春生苗,
长一尺许, 似栟榈及藜芦,
茎端生一台, 叶两指大, 青色,
夏开花紫, 七月结实, 至熟黄黑色。
至冬叶凋。根似菱米, 有三角白色, 角端生芽。二月, 七月采根Bletilla grow on the stone hill. It sprouts in spring and grows about a foot long. The seedlings are like Trachycarpus fortunei (Hook.) H. Wendl. and V. nigrum. The leaves are two finger-size. In summer, it blooms purple flowers and bears fruit in July. The ripe fruit is yellow-black. The leaves wither in winter. The root is like the fruit of T. natans, with three corners, white, and sprouting at the corners. The roots are dug in February and July. Ming
(1368−1644 AD)Li Shizhen Compendium of Materia Medica 一棵只抽一茎, 开花长寸许,
红紫色, 中心如舌, 其根如菱米,
有脐, 如凫茈之脐,
又如扁扁螺旋纹, 性难干Only one stem per herb. The flower is more than one inch long, red and purple, and the center resembles a tongue. Its root is similar to the fruit of T. natans, possessing an umbilicus akin to that of Eleocharis dulcis (N. L. Burman) Trinius ex Henschel. It has spiral veins and is challenging to dry. −, Anonymous. Generally, ancient Chinese medical texts did not make clear distinctions between different Bletilla species. They collectively referred to plants with similar morphological traits as 'baige', 'baigen', 'baiji', 'gangen', 'lianjicao', or 'ruolan'. However, through textual analysis, it has been established that the descriptions of Bletilla in ancient texts before the Ming Dynasty largely align with Bletilla striata in terms of plant height, pseudo-bulb shape, leaf morphology, flower and fruit colors, and other characteristics. While the Bletilla portrayed in attached images may not precisely match B. striata in terms of morphology, considering the textual descriptions, it generally corresponds with B. striata. In writings from the Ming Dynasty and later periods, more specific descriptions of Bletilla emerged, encompassing details about its vascular arrangement, inflorescence, and flower structure, which consistently align with B. striata. Consequently, researchers have corroborated that the original plant of Bletilla described in ancient texts is Bletilla striata[24,33].
Medicinal effect
-
According to the ancient Chinese medicinal books, Bletilla was used to treat a wide variety of conditions, including coughing, bruising, and bleeding, but their most mentioned use in ancient Chinese texts is for skin whitening and freckle removal[25]. Since ancient times, Bletilla species have been used consistently for skin care and whitening, and there are many well-known skincare products related to Bletilla. These Chinese formulae with Bletilla are similar to modern facial masks, face creams, facial cleanser, hand creams and other skin care products[26].
For example, a prescription for 'facial fat (面脂)' in Medical Secrets from the Royal Library (752 AD) is made by boiling Bletilla with other traditional ingredients, and is applied to the face, resulting in skin whitening, freckle and wrinkle removal[27]. The 'Angelica dahurica cream (白芷膏)' in the General Medical Collection of Royal Benevolence (1111−1125 AD) is reputed to whiten facial skin through a seven-day treatment regiment, and contains Bletilla as the main botanical ingredient along with Angelica dahurica[28]. Jingyue Quanshu (1563-1640 AD) also contains a prescription called 'Yurong powder (玉容散)' for facial skin care. 'Yurong powder' is made of a fine powder of Bletilla, Nardostachys jatamansi (D. Don) DC., Anthoxanthum nitens (Weber) Y. Schouten & Veldkamp and other herbs[29]. Washing the face with Yurong powder in the morning and evening every day is said to make a person's face white without blemishes (Fig. 4)[29].

Figure 4.
Yurong powder made of Bletilla and other traditional Chinese medicines in Jingyue Quanshu.
In addition, in ancient Chinese medicine texts, Bletilla is also a well-known medicine for treating hematemesis, hemoptysis and bruises[23]. According to Shennong's Classic of Materia Medica (25−220), grinding the white fungus into fine powder and taking it after mixing with rice soup can be effective for treating lung damage and hematemesis[30]. Among the Prescriptions for Universal Relief (1406), 18 traditional Chinese medicines, such as Bletilla, are used to make 'snake with raw meat cream', which is said to be useful to treat carbuncles and incised wounds[31]. There is also a record of Bletilla powder treating lung heat and hematemesis in the Collected Statements on the Herbal Foundation (1624)[32].
In ancient Chinese medicinal texts, most Bletilla are said to be useful for lung injury and hemoptysis, epistaxis, metal-inflicted wounds, carbuncles, burns, chapped hands and feet, whitening and especially for skin care. In the ancient medicinal texts, Bletilla is used alone or mixed with other traditional Chinese medicines. It is usually used in the form of a powder. The various medicinal effects of Bletilla described in these ancient texts suggest the great potential of this genus in clinical application, especially in the market of skin care products and cosmetics.
Traditional folk application
-
As a skin care herb praised by ancient medical classics, 11 ethnic minorities in China, such as Bai, Dai, De'ang, Jingpo, Lisu, Miao, Mongolian, Mulao, Tu, Wa, and Yi still retain the traditional habit of using Bletilla for skin care in their daily life (Table 3). In addition to B. striata, B. formosana and B. ochracea are also used as substitutes. Although Chinese ethnic groups have different names for Bletilla spp., the skin care methods are basically the same. Dry Bletilla tubers are ground into a powder and applied to the skin[34], and this usage is also confirmed by the records in ancient medical texts[23, 24]. The various local names of Bletilla by different ethnic groups also indirectly suggests which ethnic groups play an important role in the traditional use. For example, Bai people called B. striata baijier (白鸡儿), goubaiyou (狗白尤), and yangjiaoqi (羊角七) (Table 3).
Table 3. The traditional medicinal knowledge of Bletilla in ethnic communities, China.
Ethnic group Latin name Local name Used part Use method Medicinal effect Achang Bletilla striata (Thunb. ex Murray) Rchb. F. Baiji Tuber After the roots are dried, chew them orally or grind them into powder for external application Tuberculosis, hemoptysis, bleeding from gastric ulcer, burns and scalds Bai Baijier, Goubaiyou, Yangjiaoqi Tuber Treatment of tuberculosis hemoptysis, bronchiectasis hemoptysis, gastric ulcer hemoptysis, hematochezia, skin cracking Dai Yahejie Tuber Used for tuberculosis, tracheitis, traumatic injury, and detumescence De'ang Bagerao Tuber Tuberculosis, hemoptysis, bleeding from gastric ulcer, burns and scalds Dong Shaque, Sanjue Tuber Treat hematemesis and hemoptysis Jingpo Lahoiban, Pusehzuo tuber For tuberculosis, bronchiectasis, hemoptysis, gastric ulcer, hematemesis, hematuria, hematochezia, traumatic bleeding, burns, impotence Meng Moheeryichagan, Nixing Tuber For tuberculosis hemoptysis, ulcer bleeding, traumatic bleeding, chapped hands, and feet Miao Bigou, Wujiu, Sigou Tuber Used for hemoptysis of tuberculosis, bleeding of ulcer disease, traumatic bleeding, chapped hands, and feet Molao Dajieba Tuber Treat internal and external injuries caused by falls Tibetan Sanchabaiji Tuber Fresh chopped and soaked with honey; Powdered after sun-dried, then taken with honey and water Mainly used to treat cough, asthma, bronchitis, lung disease and a few gynecological diseases Tu Ruokeye Tuber After the roots are dried, chew them orally or grind them into powder for external application Treatment of tuberculosis, hemoptysis, bloody stool, chapped skin Wa Baiji Tuber After the roots are dried, chew them orally or grind them into powder for external application For tuberculosis, hemoptysis, gastrointestinal bleeding, scald and burn Yao Biegeidai Tuber Treat gastric ulcer, pulmonary tuberculosis, cough, hemoptysis, and hematemesis Yi Daibaij, Tanimobbaili, Niesunuoqi, Atuluobo Tuber Treatment of tuberculosis, hemoptysis, golden wound bleeding, burns, chapped hands and feet Zhuang Manggounu Tuber Treat stomachache and hemoptysis Bai Bletilla formosana (Hayata) Schltr. Baijier, Yangjiaoqi Tuber After the roots are dried, chew them orally or grind them into powder for external application It is used for emesis, hemoptysis due to tuberculosis, and hemoptysis due to gastric ulcer. External application for treatment of incised wound Miao Lianwu Tuber The effect is the same as that of B. striata Lisu Haibiqiu Tuber It can treat tuberculosis, hemoptysis, epistaxis, golden sore bleeding, carbuncle and swelling poison, scald by soup fire, chapped hands and feet Yi Niesunuoqi, Yeruomaoranruo, Atuluobo, Ribumama, Atuxixi, Abaheiji, Binyue, Ziyou Tuber It is used for tuberculosis, hemoptysis, traumatic injury, treatment of frostbite, burn, scald, bed-wetting of children and other diseases Bai Bletilla ochracea Schltr. Baijier, Yangjiaoqi Tuber After the roots are dried, chew them orally or grind them into powder for external application For hematemesis, epistaxis, hemoptysis due to tuberculosis, hemoptysis due to gastric ulcer; External application of golden sore and carbuncle Meng Moheeryichagan, Nixing tuber The effect is the same as that of B. striata The formation of traditional medical knowledge among Chinese people is often directly related to the specific living environment and cultural background[34]. For example, the Bai, Dai, De'ang, Jingpo, Lisu Yi, Wa and other ethnic minorities live in mountainous areas. The cold weather in winter and year-round outdoor manual work makes it difficult to maintain their skin[35, 36]. In the face of this situation, the ethnic people who are concerned about their physical appearance have long ago chosen local Bletilla species for skin care, and have handed down this tradition for many generations[34]. This important traditional skin care tradition is worthy of further in-depth study.
-
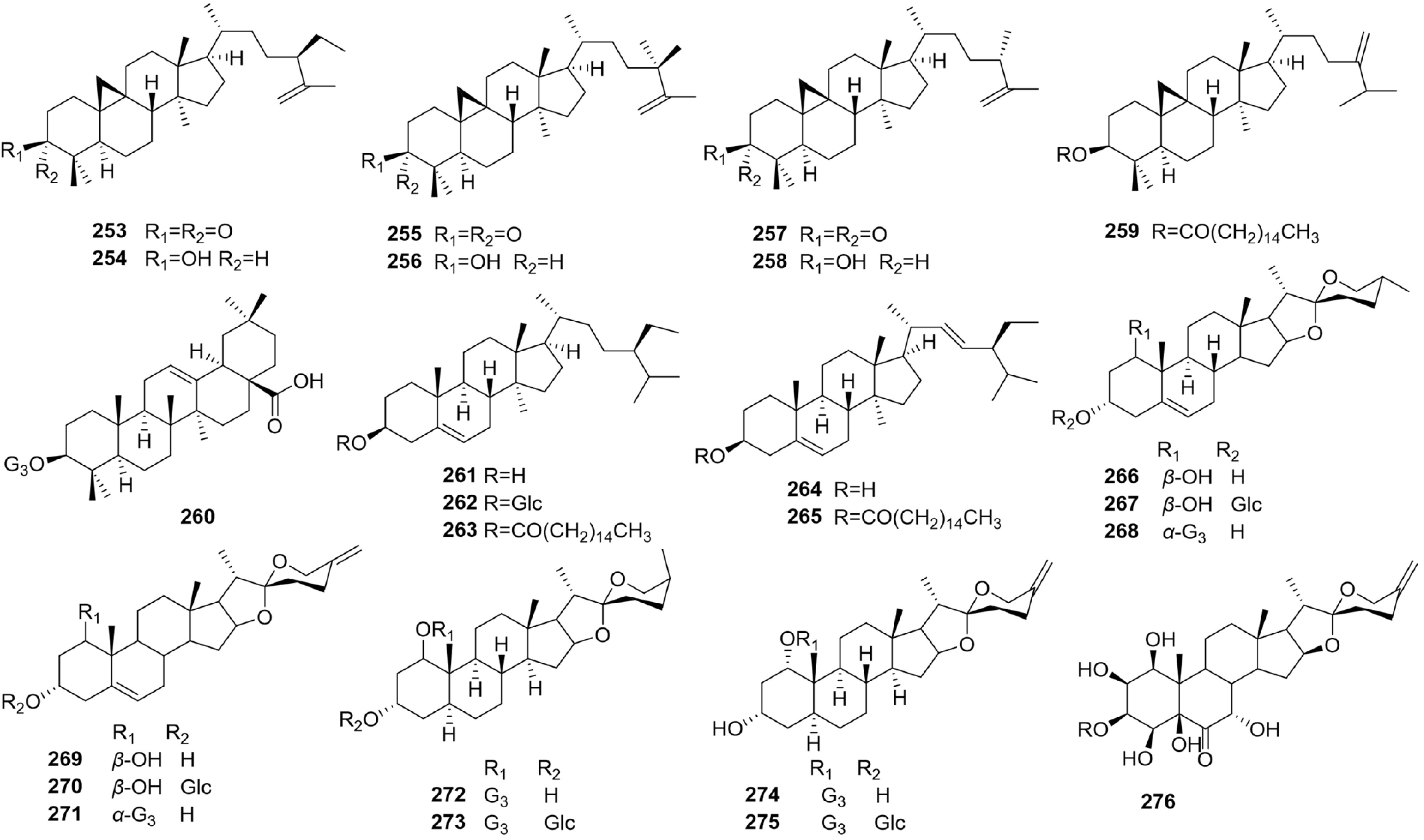
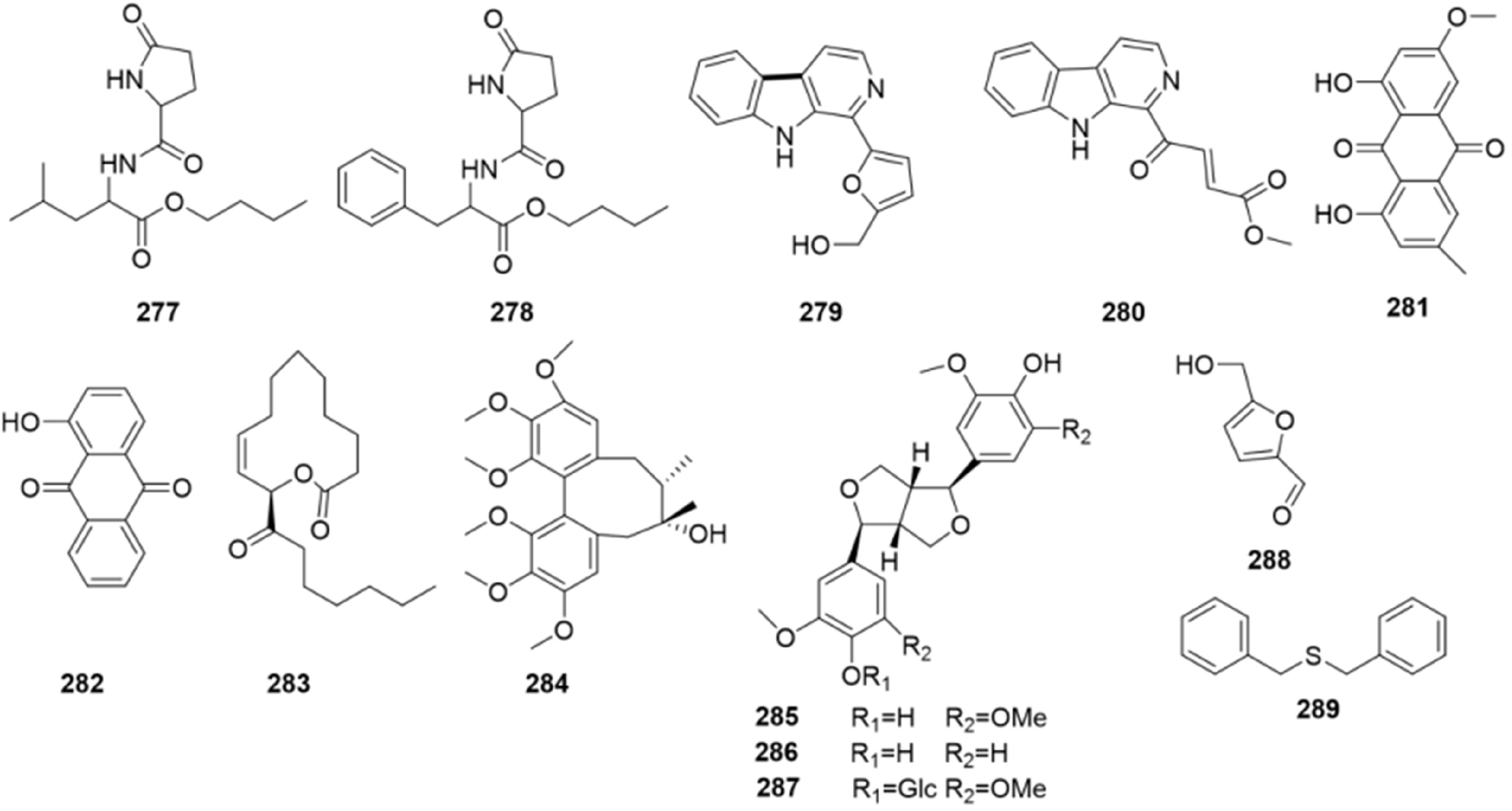
The six main classes of Bletilla chemical components, phenanthrene derivatives, phenolic acids, bibenzyls, flavonoids, triterpenoids, and steroids, have been described previously. Almost three hundred compounds have been isolated from Bletilla, including 116 phenanthrene derivatives, 58 phenolic acids, 70 bibenzyls, 8 flavonoids, 24 triterpenoids and steroid and 13 other compounds (Figs 5−14). Chemical structures of the isolates of Bletilla species most are phenanthrene derivatives, which have been demonstrated to possess various pharmacological activities.
Phenanthrene derivatives
-
The prominent opioids oxycodone, hydrocodone, naloxone, and naltrexone are all phenanthrene derivatives[37]. Currently, phenanthrene derivatives (Fig. 5, 1 to 66) were isolated from B. formosana, B. ochracea, and B. striata. In 2022, 17 phenanthrene derivatives (1–17) were isolated from the ethyl acetate (EtOAc) extracts of B. striata tubers[38]. Then, other phenanthrene derivatives were isolated from Bletilla, such as dihydrophenanthrenes (18–41), phenanthrenes (42–66), biphenanthrenes (Fig. 6, 67–89), dihydro/phenanthrenes with uniquestructures (90–112) and phenanthraquinones (Fig. 7, 113–116). Thus far, this genus has been documented to include these compounds, which have been shown to exhibit pharmacological actions[39−45].
Phenolic acids
-
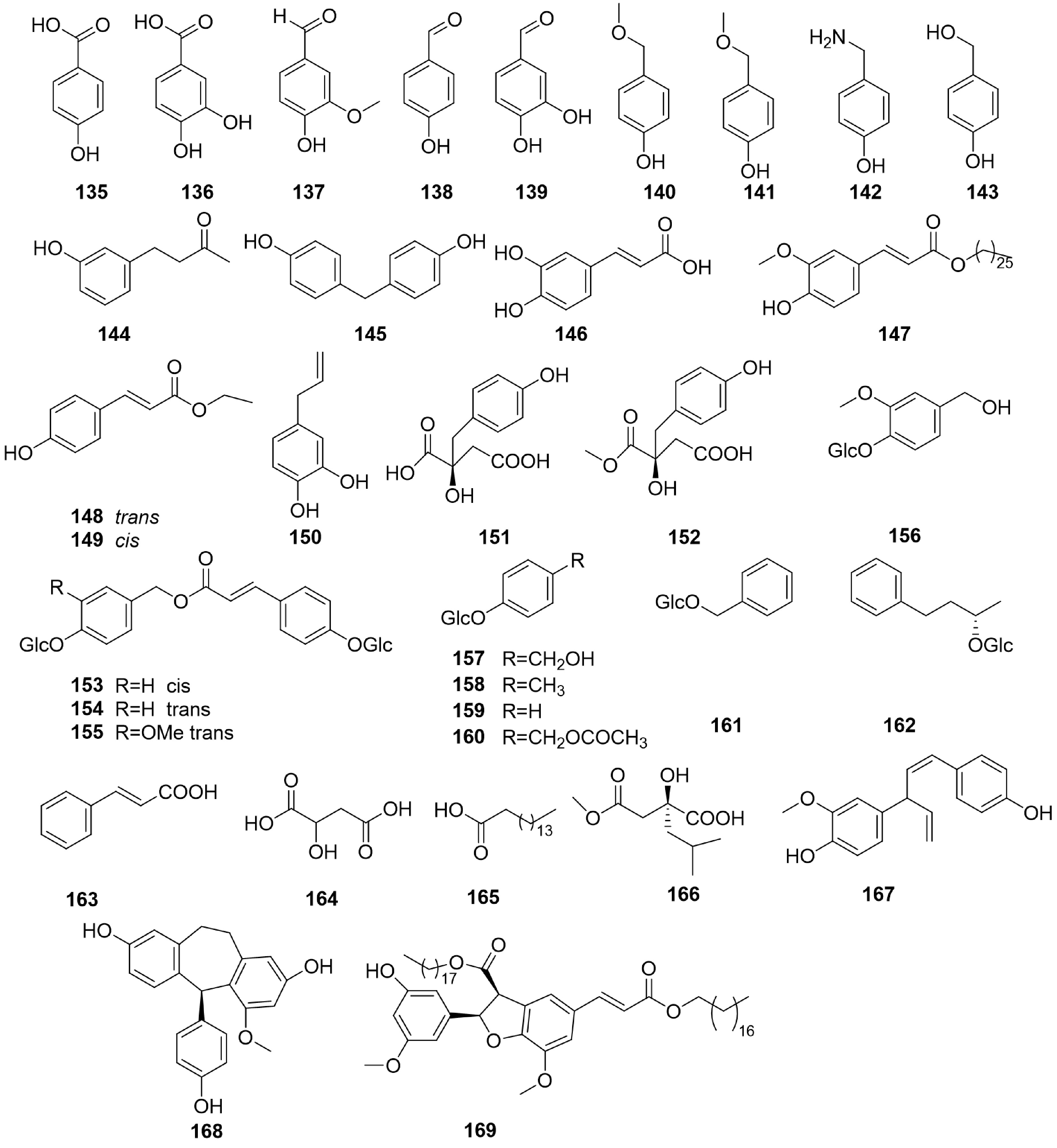
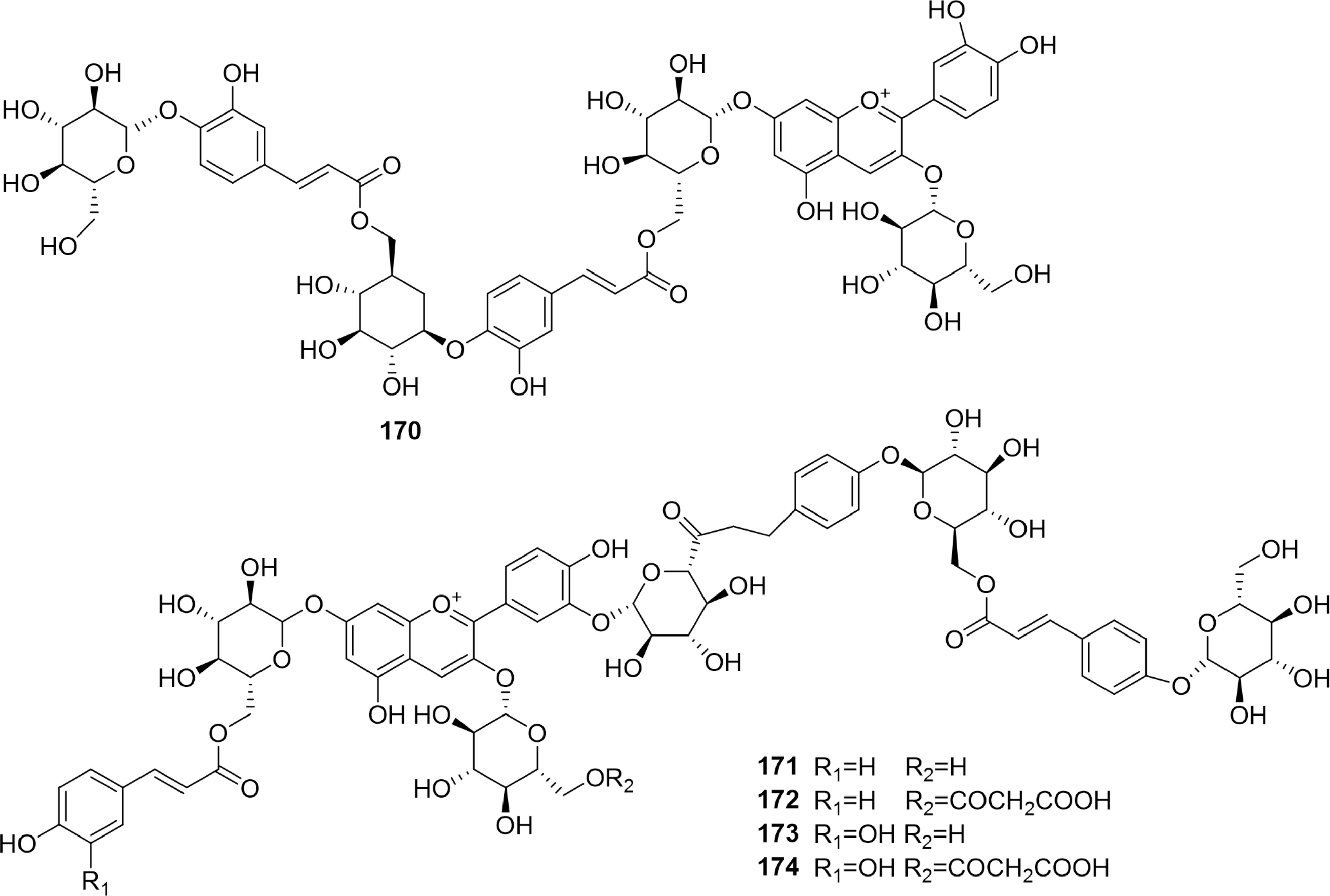
Phenolic acids are carboxylic acids created from the skeletons of either benzoic or cinnamic acids[46−48]. Fifty-eight phenolic acids (Figs 8−10, 117 to 174) were isolated from B. formosana, B. ochracea, and B. striata.
For example, compounds 121, 126, 139, 141, 148, 149, 154, 155 and 157 were isolated from the rhizomes of B. formosana[1,49,50−52]. The structures of these compounds were determined, mostly from their NMR spectroscopy data. Additionally, protocatechuic (136) and vanillin (137) also have been isolated from B. striata[53]. Moreover, some bioactive components such as 2-hydroxysuccinic acid (164) and palmitic acid (165) have been discovered and identified from B. striata[20,54−56].
Bibenzyls
-
The bibenzyls were small-molecular substances with a wide range of sources, which were steroidal ethane derivatives and resembling the structural moiety of bioactive iso-quinoline alkaloids[57].
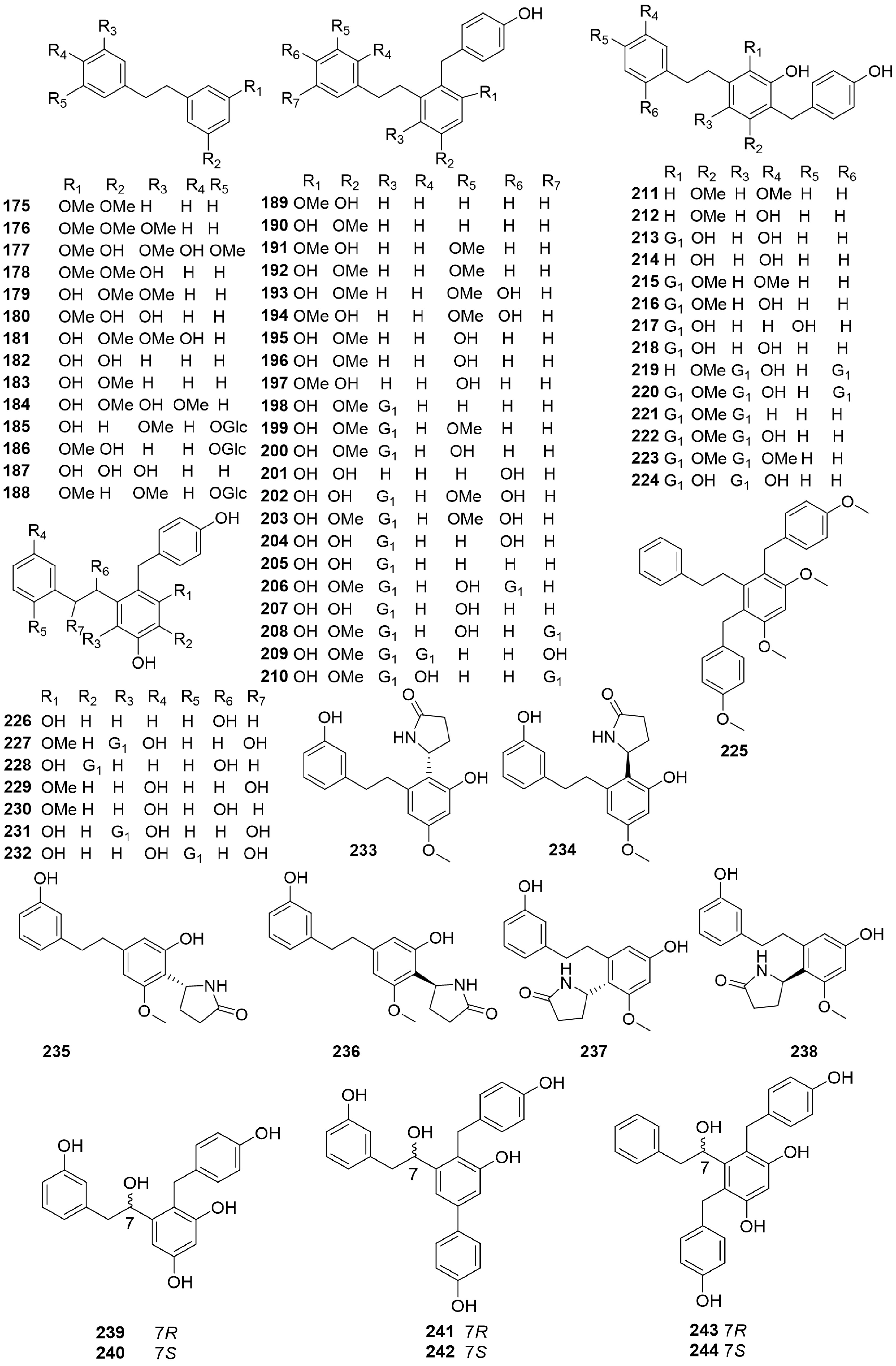
For example, depending on their structural characteristics, 70 bibenzyl compounds (Fig. 11, 175 to 244) can be grouped into three groups, simple bibenzyls (175–186, 233–238), complex bibenzyls (189–225) and chiral bibenzyls (226-232, 239-244)[58−60].
Flavonoids
-
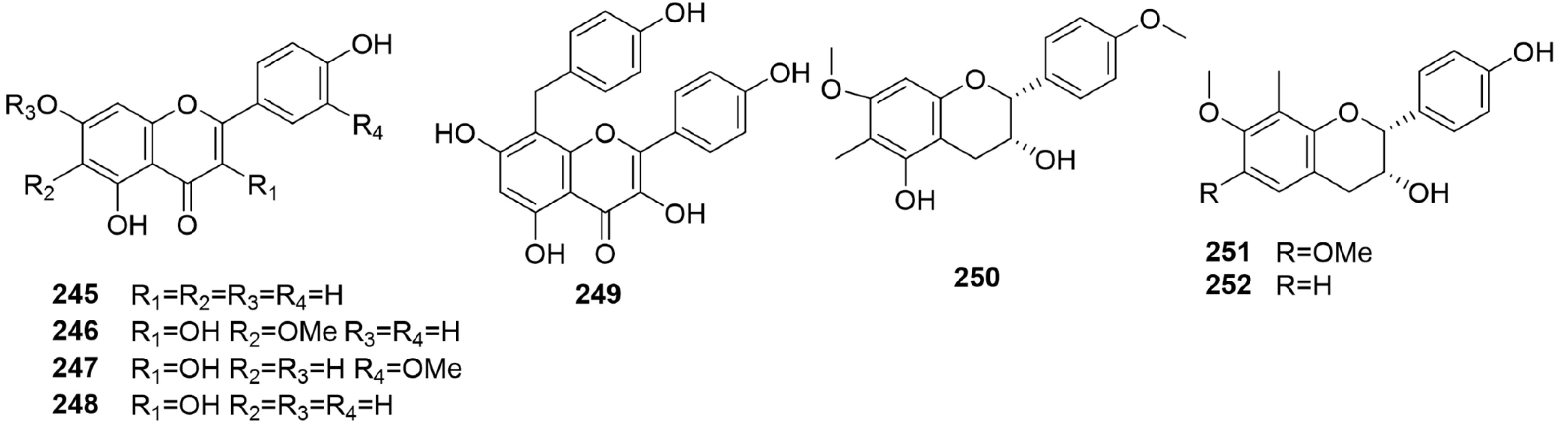
Flavonoids are among the most common plant pigments. Eight bibenzyls (Fig. 12, 245 to 252) have been isolated from B. formosana, B. ochracea, and B. striata. Apigenin (245) and 8-C-p-hydoxybenzylkaempferol (249) were isolated from the whole plant of B. formosana[45]. Bletillanol A (250), bletillanol B (251) and tupichinol A (252) were isolated from B. striata[61]. The names and chemical structures of the flavonoids reported from Bletilla are shown below (Fig. 12).
Triterpenoids and steroids
-
Twenty-four triterpenoids and steroids (Fig. 13, 253 to 276) have been reported from Bletilla (Fig. 13), such as, tetracyclic triterpenes (253–259) and pentacyclic triterpenes (189–225) and chiral bibenzyls (260)[62−64]. Steroids (261–276) isolated from the Bletilla and have shown some bioactivity. For example, bletilnoside A (272) was isolated from Bletilla species and displayed anti-tumor activity[65,66].
Other compounds
-
Thirteen other compounds (Fig. 14, 277 to 289) were isolated from B. formosana, B. ochracea, and B. striata. These compounds included amino acids, indoles and anthraquinones[67,68]. For example, syringaresinol (285) and pinoresinol (286) have been described in the methanol extract of the tubers of B. striata[61].
Based on the information about the chemical constituents of Bletilla species, it appears that there is a substantial body of research on these compounds. However, there are some areas that may warrant further investigation and research. At first, it would be valuable to investigate potential synergistic effects and interactions between the different classes of compounds within Bletilla species, as some of the compounds may work together. Besides, it is worth considering the improvement of compound yield. Optimizing extraction methods and finding the most efficient and environmentally friendly techniques are vital for both research purposes and potential commercial applications. It is also important to take into account the variability in chemical composition among different Bletilla species and even within the same species from different cultivars.
-
The rich and varied chemical components make the plants of Bletilla have a wide variety of pharmacological activities (Table 4). Many studies have shown that the plants of this genus have anti-inflammatory, antineoplastic, antiviral, antioxidant, hemostatic, antibacterial, and other biological activities, which help to support the traditional medicinal practice of Bletilla in folk medicine.
Table 4. Summary of the pharmacological activities of Bletilla species.
Pharmacological activity Tested substance/part Tested system/organ/cell Tested dose/dosing method Results Refs. Anti-inflammatory Ethanol extract of Bletilla striata RAW264.7 cells RAW264.7 cells were pre-treated with ethanol extract of B. striata for 1 h and then stimulated with LPS (200 ng/mL) for 12 h, 0.05% DMSO was applied as the parallel solvent control. The culture supernatant was collected for IL-6 and TNF-α detection. Ethanol extract of B striata significantly inhibited LPS-induced interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) expression at 2.5 µg/mL. [41] The ethyl acetate-soluble (EtOAc) extract of tubers of B. striata H2O2-induced PC12 cell injury model PC12 cells were seeded in 96-well multiplates at a density of 1.5 × 105 cells/mL. After overnight incubation at 37 °C with 5% CO2, 10 μM test samples and H2O2 (final concentration of 450 μM) were added into the wells and incubated for another 12 h. It protected the cells with the cell viabilities of 57.86 ± 2.08%, 64.82 ± 2.84%, and
64.11 ± 2.52%.[98] Ethanol extract of tubers of B. striata RAW264.7 cells Cells were treated with ethanol extracts (25 μM) dissolved in DMSO, in the presence of
1 μg/mL lipopolysacchride
(LPS) for 18 hThe anti-inflammatory activity with IC50 of 2.86 ± 0.17 μM. [54] PE extract of the tubers of B. striata LPS-stimulated BV2 cells Cells treated with extract
(0, 1, 10, 30, 100 μg/mL) and dihydropinosylvi (0, 1, 10, 30, 100 μM) in presence of LPS
(1 μg/mL)The anti-inflammatory activity with IC50 values of 96.0 μM. [96] Ethanol extract of the roots of B. striata Cox-1 and Cox-2 Treated with the ethanol extracts at various concentrations
(0, 1, 10, 100 μM)The compounds with sugar moieties displayed selective inhibition of Cox-2 (N90%). [38] B. striata polysaccharide (BSPb) Human mesangial cells (HMCs) HMCs were pre-treated with BSPb (5, 10, 20 μg/mL) BSPb efficiently mediated expression of NOX4 and TLR2, to attenuate generation of ROS and inflammatory cytokines. [12] Compounds extracted from the rhizomes of Bletilla ochracea RAW264.7 cells After 24 h preincubation,
cells were treated with serial dilutions in the presence of
1 μg/mL LPS for 18 h. Each compound was dissolved in DMSO and further diluted in medium to produce different concentrations. NO production in the supernatant was assessed by adding 100 μL of Griess regents.It showed the inhibitory effects with IC50 values in the range of 15.29–24.02 μM. [76] Compounds extracted from the rhizomes of B. ochracea Murine monocytic RAW264.7 cells After 24 h preinubation, RAW 264.7 cells were treated with compounds (25 μM) dissolved in DMSO, in the prenence of
1 μg/mL LPS for 18 h. NO production in each well was assessed by adding 100 μL of Giress regentIt showed the inhibitory effects with IC50 2.86 ± 0.17 μM. [86] Compounds extracted from the rhizomes of Bletilla formosana Elastase Release Assays Neutrophils (6 × 105 cells/mL) were equilibrated in MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide (100 μM) at 37 °C for 2 min and then incubated with a test compound or an equal volume of vehicle (0.1% DMSO, negative control) for 5 min. Most of the isolated compounds were evaluated for their anti-inflammatory activities. The results showed that IC50 values for the inhibition of superoxide anion generation and elastase release ranged from 0.2 to 6.5 μM and 0.3 to 5.7 μM, respectively. [49] Anti-tumor Two compounds from Bletilla striata A549 cells Compounds were tested for their ability to induce ROS generation in A549 cells at concentrations of 20 two compounds for 24 h, the cells were harvested to evaluate the ROS production. The two compounds exhibited antiproliferative effects using the MTT test; these effects may be due to cell cycle arrest and inducing ROS generation. [87] Stilbenoids from B. striata BCRP-transduced K562 (K562/BCRP) cells — It showed antimitotic activity and inhibited the polymerization of tubulin at IC50 10 μM. [78] Compounds extracted from the rhizomes of B. ochracea The human tumor cell lines HL-60 (acute leukemia), SMMC-7721 (hepatic cancer), A-549 (lung cancer), MCF-7 (breast cancer), and SW480 (colon cancer) 100 μL of adherent cells were seeded into each well of 96-well cell culture plates. After 12 h of incubation at 37 °C, the test compound was added. After incubated for 48 h, cells were subjected to the MTS assay. All isolated metabolites except 7 were evaluated for cytotoxic activity against five human cancer cell lines (HL-60, SMMC7721, A-549, MCF-7 and SW480). [76] Antiviral The tuber of B. striata Madin-Darby canine kidney model and embryonated eggs model As simultaneous treatment with 50% inhibition concentration (IC50) ranging from 14.6 ± 2.4 to 43.3 ± 5.3 μM. Phenanthrenes from B. striata had strong anti-influenza viral activity in both embryonated eggs and MDCK models. [107] The 95% ethanol
Extract of B. striataBALB/C mice — It has significant anti-influenza
virus effect in mice, which may be related to the increase of IL-2, INFα, INF-β and thus enhance the immune function of mice.[12] Antioxidant Compounds extracted from the rhizomes of B. formosana DPPH radical-scavenging assay Solutions containing 160 μL of various concentrations of sample extract, 160 μL of various concentrations of BHA, 160 μL of various concentrations of ascorbic acid, and the control (160 μL of 75% methanol) were mixed separately with 40 μL of 0.8 mM DPPH dissolved in 75% methanol. Each mixture was shaken vigorously and left to stand for 30 min at room temperature in the dark. Tthe seedlings grown by tissue culture of B. formosan collected in Yilan County had the best antioxidant capacity. In addition, B. formosana collected in Taitung County had the best scavenging capacity in the tubers, leaves and roots. [93] Fibrous roots of B. striata DPPH model and superoxide anion system The ABTS+ solution was prepared by reacting 7 Mm ABTS with 2.45 mM potassium persulfate (final concentrations both dissolved in phosphate buffer, 0.2 M, pH 7.4) at room temperature for 12–16 h in the dark. It removed free radicals and inhibit tyrosinase activity. [33] B. striata extracts (BM60) The murine macrophage cells NR8383, male SD mice (180~200 g) NR8383 were pretreated with extracts (1, 10 and 100 g/mL) for 4 h and then 65 stimulated with 1 g/mL of LPS for 24 h. Acute lung injury was induced in mice by nonhexposure intratracheal instillation of LPS (3.0 mg/kg). Administration of the BM60 extract of 35, 70, and 140 mg/kg (L, M, H) was performed by oral gavages. The BM60 treatment reduced the production of NO in NR8383 macrophages. Treatments with BM60 at the doses of 35, 70, 140 mg/kg significantly reduced macrophages and
neutrophils in the bronchoalveolar lavage fluid (BALF).[12] The crude
polysaccharides obtained from B. striataDPPH free radical scavenging activity Concentration
range of 2.5–5.0 mg/mLThe IC50 of BSPs-H was 6.532 mg/mL. [35] Hemostasis B. striata polysaccharide (BSP) Diabetes mellitus (DM) mouse models were induced by high fat-diet feeding combined with low-dose streptozocin injection DM mouse models were induced by high fat-diet feeding combined with low-dose streptozocin injection. The BSP solutions were applied on the surface of each wound at a volume of 50 μl. RD mice were assigned as normal controls and received saline treatment (n = 6). All mice were treated with vehicle or BSP once daily from the day of wounding (d0) until 12 days later (d12). BSP administration accelerated diabetic wound healing, suppressed macrophage infiltration, promoted angiogenesis, suppressed NLRP3 inflammasome activation, decreased IL-1β secretion, and improved insulin sensitivity in wound tissues in DM mice. [112] B. striata Micron Particles (BSMPs) Tail amputation model and healthy male Sprague-Dawley (SD) rats
(250 ± 20 g, 7 weeks of
age)Rats were divided into six groups of five treated with cotton gauze and BSMPs (350–250, 250–180, 180–125, 125–75, and < 75 μm), respectively. Compared to other BSMPs of different size ranges, BSMPs of 350–250 μm are most efficient in hemostasis. As powder sizes decrease, the degree of aggregation between particles and hemostatic BSMP effects declines. [109] Rhizoma Bletillae polysaccharide (RBp) Adult male SD rats weighing 220 ± 20 g After incubation for 1 min at 37 °C, 300 μL of PRP was dealt with different concentrations of RBp (50, 100, 150, and 200 mg/L) under continuous stirring, and the vehicle was used as the blank control. RBp significantly enhanced the platelet aggregations at concentrations of 50−200 mg/L in a concentration-dependent manner. [113] Antibacterial Bibenzyl derivatives from the tubers of Bletilla striata S. aureus ATCC 43300, Bacillus subtilis ATCC 6051, S. aureus ATCC 6538 and Escherichia coli ATCC 11775 Using a microbroth dilution method, bacteria were seeded at
1 × 106 cells per well (200 μL) in a
96-well plate containing Mueller-Hinton broth with different concentrations (from 1 to 420 μg/mL, 300 μg/mL and so on;
2-fold increments) of each test compound.It showed inhibitory activities with MIC of (3–28 μg/mL) against S. aureus ATCC6538 [116] The crude extract of B. striata S. album, A. capillaris, C. cassia They were seeded at 1 × 106 cells per well (200 μL) in a 96-well plate containing Mueller−Hinton broth (meat extracts 0.2%, acid digest of casein 1.75%, starch 0.15%) with different concentrations (from 1 to 128 μg/mL; 2-fold increments) of each test compound. It showed S. album (0.10%), A. capillaris (0.10%), and C. cassia (0.10%) to have the strongest antibacterial properties. [118] The ethyl acetate-soluble (EtOAc) extract of tubers of B. striata S. aureus ATCC 43300, S. aureus ATCC 6538, and Bacillus subtilis ATCC 6051) and Escherichia coli ATCC 11775) Bacteria were seeded at 1 × 106 cells per well (200 μL) in a 96-well plate containing Mueller Hinton broth with different concentrations (from 1 to 420 μg/ml; 2-fold increments) of each test compound. The extract was effective against three Gram-positive bacteria with minimum inhibitory concentrations (MICs) of 52–105 μg/ml. [98] The phenanthrene fraction (EF60) from the ethanol extract of fibrous roots of Bletilla striata pseudobulbs S. aureus ATCC 25923, S. aureus ATCC 29213, S. aureus ATCC 43300, E. coli ATCC 35218, and P. aeruginosa ATCC 27853, Bacillus subtilis 168 EF60 was active against all tested strains of Staphylococcus aureus, including clinical isolates and methicillin-resistant S. aureus (MRSA). The minimum inhibitory concentration (MIC) values of EF60 against these pathogens ranged from 8 to 64 μg/mL. EF60 could completely kill S. aureus ATCC 29213 at 2× the MIC within 3 h but could kill less than two logarithmic units of ATCC 43300, even at 4× the MIC within 24 h. The postantibiotic effects (PAE) of EF60 (4× MIC) against strains 29213 and 43300 were 2.0 and 0.38 h, respectively. [117] Anti-adhesive Bletilla striata extraction solution PPA was induced by cecal wall abrasion, and Bletilla striata was injected to observe its efect on adhesion in rats The rats in the sham operation group was not treated; the other rats of the three experimental groups were intraperitoneally injected with 8 ml of phosphate-buffered saline (Control group), 15% Bletilla striata extraction solution (BS group), and 0.2% hyaluronic acid solution (HA group), respectively. Bletilla striata decreased the development of abdominal adhesion in abrasion-induced model of rats and reduced the expression of the important substance which increased in PPAs. [120] Immunomodulatory B. striata polysaccharide (BSPF2) Mouse spleen cells To observe the immune activity of BSPF2, mouse spleen cells were stimulated with BSPF2 at 10–100 g/mL for 72 h. Immunological assay results demonstrated that BSPF2 significantly induced the spleen cell proliferation in a dose-dependent manner. [121] Anti-pulmonary fibrosis B. striata polysaccharide Clean grade male SD rats SD rats were randomly divided into 5 groups, sham operation group (equal volume of normal saline), model group (equal volume of normal saline), tetrandrine positive control (24 mg/kg) group and white and Polysaccharide low
(100 mg/kg) and high (400 mg/kg) dose groups.The Bletilla striata polysaccharide has remarkable regulation effect on anti-oxidation system and immune system, but cannot effectively prevent lung fibrosis. [127] Small molecule components of Bletilla striata Clean grade male SD rats SD rats were randomly divided into 5 groups, sham operation group (0.5 mL normal saline), model group (0.5 mL normal saline), and positive control group (tetrandrine 24 mg/kg) and low (20 mg/kg) and high (40 mg/kg) dosage groups of the small molecule pharmacological components of Bletilla, which were administered by gavage once a day for 2 consecutive months. The small molecule components of Bletilla striata can effectively prevent lung fibrosis though regulating the anti-oxidation system,immune system and cytokine level; SMCBS is one of the active components of Bletilla striata on silicosis therapy [124] —, not given. Anti-inflammatory
-
Many phytochemicals have been well characterized to lessen swelling or inflammation[89]. A series of phenolic acid and polysaccharide compounds isolated from Bletilla demonstrated anti-inflammatory bioactivity against BV-2 microglial, RAW 264.7, and PC12 cells[96,100−102]. For example, phochinenin K (106) exhibited growth inhibitory effects with an IC50 of 1.9 μM, and it is a possible candidate for development as neuroinflammation inhibitory agent[43]. Using the H2O2-induced PC12 cell injury model, (7S)-bletstrin E (242), (7R)-bletstrin F (243) and (7S)-bletstrin F (244) could clearly protect the cells with the cell viabilities of 57.86% ± 2.08%, 64.82% ± 2.84%, and 64.11% ± 2.52%, respectively[98]. With an IC50 of 2.86 ± 0.17 μM, 2,7-dihydroxy-4-methoxyphenanthrene (53) showed potential action against NO generation in RAW 264.7 macrophages[54]. The use of Bletilla in traditional skin care, it is said to function as an astringent, hemostatic and wound healing[33]. Modern medical pharmacology research has validated that this plant has antibacterial effects, which may may help to explain, in part, its traditional use in skin care[24].
Though it's mentioned that some of these compounds from Bletilla have demonstrated anti-inflammatory action, more extensive studies are needed to fully understand their mechanisms of action, potential therapeutic applications, and safety profiles. Conducting in vivo studies and clinical trials can provide more concrete evidence of their effectiveness.
Anti-tumor
-
There are important antineoplastic agents that have originated from plant natural products[103]. In recent years, several bibenzyl and flavonoid compounds have been discovered from Bletilla that have antineoplastic activity against A549 cells and other cells. For example, 7-hydroxy-2-methoxy-phenanthrene-3,4-dione (160) and 3′,7′,7-trihydroxy-2,2′,4′-trimethoxy-[1,8′-biphenanthrene]-3,4-dione (163) have shown strong antiproliferative effects and induced ROS production after 24 h in A549 cells[87]. The doxorubicin (Dox)/FA (folate)-BSP-SA (stearic acid) modified Bletilla striata polysaccharide micelles boosted the drug enrichment in tumors and improved the in vivo anticancer effects[104,105]. Micelles, nanoparticles, microspheres, and microneedles are examples of B. striata polysaccharide-based drug delivery systems that exhibit both drug delivery and anti-cancer functionality. These experiments confirmed that some of the compounds isolated from the Bletilla have potential activity for the treatment of cancer.
However, most of the evidence presented in the previous studies is based on in vitro experiments or cell culture studies. It is highly necessary to use animal models to study the in vivo anti-tumor effects of Bletilla extracts or compounds. These studies can help evaluate the safety and effectiveness of treatments based on Bletilla. Additionally, through such methods, researchers can further investigate the mechanisms of Bletilla's anti-tumor activities, exploring how Bletilla compounds interact with cancer cells, immune responses, and signaling pathways involved in tumor growth and metastasis.
Antiviral
-
Antiviral medications are essential for preventing the spread of illness, and are especially important nowadays with pandemics and drug-resistant viral strains[5, 6]. Therefore, it is vitally necessary to find novel, safe, and effective antiviral medications to treat or prevent viral infections[106]. B. striata plant contains compounds that have been recorded in ancient texts to cure cough, pneumonia, and skin rashes, and these may be related to potential antiviral constituents[23]. Some constituents of B. striata have antiviral activity, for example, phenanthrenes and diphenanthrenes from B. striata displayed potent anti-influenza viral in a Madin-Darby canine kidney model and embryonated eggs model, diphenanthrenes with parentally higher inhibitory activity than monophenanthrenes[107]. But more research is needed to further determine the antiviral activity of Bletilla, understand how Bletilla compounds interact with viral proteins or the host immune response, and conduct safety and toxicity studies, which are crucial for the development of related materials.
Antioxidant
-
Free radicals have the potential to exacerbate lipid peroxidation and harm cell membranes, which can lead to several prevalent human diseases, including cancer, cataracts, and coronary heart disease[108]. Research has shown that extracts from Bletilla possess strong antioxidant activity. However, this antioxidant activity can vary depending on the different growing environments of the plant. Additionally, the antioxidant capabilities of extracts from different parts of the Bletilla plant also vary[93]. Clinical studies have shown that traditional Chinese medicine formulas containing Bletilla can inhibit tyrosinase activity and possess antioxidant properties, thus resulting in skin-whitening effects[108]. Furthermore, some research reveals that the polysaccharides in the plant exhibit significant antioxidant activity, effectively scavenging free radicals and inhibiting tyrosinase activity[33]. This highlights the skin-whitening potential of the fibrous root of Bletilla striata, indicating promising prospects for the comprehensive utilization of the B. striata plant[33]. However, most studies on the pharmacological activities of Bletilla have focused solely on B. striata, neglecting other species within the genus. Different species may possess varying phytochemical compositions and antioxidant properties, which can lead to an incomplete understanding of the genus as a whole.
Hemostasis
-
Available hemostatic agents are expensive or raise safety concerns, and B. striata may serve as an inexpensive, natural, and promising alternative[109]. Polysaccharides of B. striata displayed hemostatic activity through inhibition of the NLRP3 inflammasome[110−112]. The ADP receptor signaling pathways of P2Y1, P2Y12, and PKC receptors may be activated as part of the hemostasis[113]. Alkaloids from Bletilla have hemostatic activities through platelet deformation, aggregation, and secretion. In addition, polysaccharides of Bletilla striata have potential wound-healing medicinal value[110]. Currently, Bletilla plants have been used in various traditional systems, such as traditional Chinese medicine and Ayurveda, to control bleeding.
Antibacterial
-
Previous studies revealed that Bletilla displayed antibacterial effects[114]. For example, bletistrin F, showed inhibitory activities with MIC of (3–28 μg/mL) against S. aureus ATCC 6538[115,116]. Antimicrobial screening of Bletilla showed S. album (0.10%), A. capillaris (0.10%), and C. cassia (0.10%) to have the strongest antibacterial properties[117,118]. In addition, phenanthrenes are worthy of further investigation as a potential phytotherapeutic agent for treating infections caused by S. aureus and MRSA[119]. However, further in vivo studies on the antibacterial activity of Bletilla are lacking, which is needed for clinical application. For example, the specific mechanism of antibacterial activity of Bletilla still needs to be elucidated. While research on the antibacterial activity of Bletilla plants is promising, it faces several shortcomings and challenges that need to be addressed for a more comprehensive understanding of their potential therapeutic applications. Further studies with standardized methodologies, mechanistic insights, clinical trials, and consideration of ecological and safety concerns are essential to advance this field.
Other
-
There are other pharmacological activities of Bletilla, like anti-fibrosis activity, anti-adhesive activity, and immunomodulatory activity. For example, B. striata has been studied as a new and cheaper antiadhesive substance which decreased the development of abdominal adhesion abrasion-induced model in rats[120]. However, the natural resources of Bletilla are also getting scarcer. To preserve the sustainable development of Bletilla species, proper farming practices are required, along with the protection and economical use of these resources. The immunomodulatory activity of the Bletilla species was assessed using the 3H-thymidine incorporation method test, and BSP-2 increased the pinocytic capacity and NO generation, which improved the immunomodulatory function[121,122].
B. striata extract was shown to have anti-pulmonary fibrosis effect[123]. B. striata polysaccharide can successfully prevent lung fibrosis through established by invasive intratracheal instillation method and evaluated by lung indexes[123,124]. Moreover, Bletilla species need further investigations to evaluate their long-term in vivo and in vitro activity before proceeding to the development of pharmaceutical formulation.
While there is currently a deep understanding of the pharmacological activity of plants in the Bletilla genus, there are still many gaps that need to be addressed. To overcome these shortcomings, future research on the pharmacological activity of Bletilla species should emphasize comprehensive, well-designed studies with a focus on species-specific effects, mechanistic insights, and rigorous clinical trials. Additionally, collaboration among researchers, standardization of methods, and transparent reporting of results can help advance our understanding of the therapeutic potential of Bletilla plants. Researchers should also consider safety aspects and explore potential herb-drug interactions to ensure the responsible use of Bletilla-based therapies.
-
There are several common clinical applications of Bletilla striata in TCM. The gum of B. striata has unique viscosity characteristics and can be used as thickener, lubricant, emulsifier and moisturizer in the petroleum, food, medicine, and cosmetics industries[125−130]. B. striata is used as a coupling agent, plasma substitute, preparation adjuvant, food preservative and daily chemical raw material[131−133]. In clinical practice, B. striata glue has also been proven to control the infections and is beneficial to the healing of burns and wounds[133−135].
In ethnic communities in Southwest China, the locals chew fresh Bletilla tubers directly or take them orally after soaking in honey to treat cough, pneumonia and other diseases[33, 34]. This traditional use is common in local communities in Southwest China, and suggests at the safety of Bletilla. However, current research shows it is still necessary to control the dosage when using Bletilla[136].
Zebrafish embryos and larvae respond to most drugs in a manner similar to humans[137]. Militarine, the main active ingredient of Bletilla, was tested in a zebrafish embryo development assay at concentrations of 0.025 g/L and 0.05 g/L, and with the increased concentration, the heart rate of zebrafish embryos is slowed. Mortality and malformation rates of zebrafish embryos gradually increased with time and militarine concentration[138]. Although Bletilla species are safe at therapeutic dose ranges, further research on their safety is required[136]. More in-depth studies should be carried out on Bletilla to extract effective ingredients and make better preparations for clinical use[139].
-
According to the traditional medicinal knowledge in ancient Chinese texts, Bletilla has been an important ingredient for skin care since ancient times. Many ethnic minority groups in China still retain the practice of using Bletilla for skin care, and the plant parts and preparation methods of use are consistent with the records in ancient texts. Almost 300 phytochemicals have been identified from Bletilla, and some of them possess important pharmacological activities, which support its traditional uses and suggest the important medicinal development potential of this genus. This review has demonstrated that Bletilla, as an important medicinal plant of Orchidaceae, still requires further research to fathom its medicinal potential.
For instance, it is necessary to enhance the quality control procedures based on the chemical components and pharmacological activity of Bletilla. The chemical composition and pharmacological properties of Bletilla are critical areas of current research. According to previous studies, the main bioactive components of Bletilla can vary greatly according to its origin, harvest time, distribution, storage, and adulteration. However, variation in bioactivities caused by the differences in Bletilla constituentshave not been explored extensively yet. To develop clinical applications of Bletilla, it is crucial to further explore the mechanism of action between its chemical composition variation and its pharmacological actions.
In addition, although the tuber has historically been the main medicinal part of Bletilla, research has shown that the chemical composition in other parts of Bletilla, such as stems, leaves, and flowers, also give these parts a variety of pharmacological activities. Further in-depth analysis of the chemical components and pharmacological activities of different parts of this genus is worthwhile, to explore the specific chemical basis of its pharmacological activities, develop related drugs, and promote clinical applications. For example, Bletilla polysaccharide has good hemostasis and astringent wound effects[110], so it may have the potential to be developed into a drug or related medical materials to stop bleeding and heal wounds.
Finally, as a cautionary note, many unrestrained collections and the destruction of habitats have made the resources of wild Bletilla rarer. In addition to protecting the wild populations of Bletilla, appropriate breeding techniques should be adopted to meet the commercial needs of this economically important genus, thereby allowing its sustainable use in commerce.
-
The authors confirm contribution to the paper as follows: study conception and design, funding acquirement: Long C; data analysis, draft manuscript preparation, literature review: Fan Y, Zhao J; manuscript revise and language editing: Wang M, Kennelly EJ, Long C. All authors reviewed the results and approved the final version of the manuscript.
-
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation, to any qualified researcher. Requests to access these datasets should be directed to Yanxiao Fan (fanyanxiao0510@163.com).
This research was funded by the Yunnan Provincial Science and Technology Talent and Platform Plan (202305AF150121), Assessment of Edible & Medicinal Plant Diversity and Associated Traditional Knowledge in Gaoligong Mountains (GBP-2022-01), the National Natural Science Foundation of China (32370407, 31761143001 & 31870316), China Scholarship Council (202206390021), and the Minzu University of China (2020MDJC03, 2022ZDPY10 & 2023GJAQ09).
-
The authors declare that they have no conflict of interest.
-
# These authors contributed equally: Yanxiao Fan, Jiaqi Zhao
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Fan Y, Zhao J, Wang M, Kennelly EJ, Long C. 2023. Ethnopharmacology of Bletilla orchid species: a comprehensive review on ethnobotany, phytochemistry and pharmacology. Medicinal Plant Biology 2:21 doi: 10.48130/MPB-2023-0021
Ethnopharmacology of Bletilla orchid species: a comprehensive review on ethnobotany, phytochemistry and pharmacology
- Received: 31 July 2023
- Accepted: 29 November 2023
- Published online: 29 December 2023
Abstract: Bletilla is an orchid genus with distribution in China, Japan, South Korea, and other Asian countries with many species that are overexploited and vulnerable medicinal plants. Some Bletilla have a long history of ethnobotanical application in Asia, especially China, and ethnic groups in Southwest China still use Bletilla as medicines to treat cough, dermatitis and pneumonia. About 289 chemical compounds have been isolated from Bletilla, mostly phenathrene and phenolic derivatives. These diverse chemical components are responsible for the anti-inflammatory, antineoplastic, antiviral, antioxidant, hemostatic, antibacterial, and other biological activities of Bletilla. Various pharmacological activities support the traditional medicinal efficacy of Bletilla, implying the medicinal potential of this genus. However, detailed information on the botanical characteristics, ethnobotanical uses, chemical components, pharmacological effects, clinical application, and safety evaluation is limited. To better understand the ethnobotany, phytochemistry, and bioactivity of Bletilla, this article assesses recent developments in the field.