-
Insect pests present a formidable challenge to global agriculture, accounting for up to 35% of crop yield losses[1]. In response, synthetic chemical pesticides have been extensively used. However, their indiscriminate application resulted in significant environmental harm and adverse human health effects. These issues highlight the pressing need for eco-friendly and safe insecticides[2]. Notably, natural pyrethrin, extracted from the dried flower heads of Tanacetum cinerariifolium, along with neem oil and rotenone, plays a pivotal role in pest management[3]. Natural pyrethrin is especially valued for its minimal toxicity to mammals and its rapid, effective action against a broad spectrum of insects. However, the post-World War II advent of more affordable synthetic pyrethroids led to a decline in the use of natural pyrethrins in agriculture, significantly impacting their industry.
Recently, there has been a revival in the use of pyrethrins as insecticides. Their lower propensity to induce pest resistance, compared to synthetic pyrethroids, renders them effective against certain resistant pest species[4]. Pyrethrins comprise six distinct molecules with varying efficacies, resulting in lower toxicity but greater efficiency in pest control, including effective repellent properties, thus reducing the selection of resistant alleles[5]. Moreover, pyrethrins are biodegradable and decompose under UV light, minimizing their environmental persistence and impact on non-target organisms due to their short active period[6]. Used in safe concentrations and refined to eliminate sesquiterpene lactone residues, pyrethrins are deemed non-carcinogenic and safe for sensitive persons like pregnant women or infants[7,8]. These attributes have bolstered the resurgence of this traditional natural insecticide, particularly in household use and organic farming practices.
The global pyrethrum industry has witnessed substantial growth, driven by advancements in manufacturing processes, improved product quality, and effective marketing strategies. Overcoming its historical pattern of production fluctuations, the pyrethrum sector now represents the most commercially viable and promising natural source of environmentally protective insecticides. Pyrethrum flowers synthesize secondary metabolites, including pyrethrins and terpene volatiles, as defense mechanisms against insect predation[9,10]. Therefore, a thorough understanding of the biosynthetic pathways and regulatory mechanisms involved in the production of these compounds is crucial. Our focus extends to exploring the historical evolution of the pyrethrum production industry, particularly in terms of enhancing the natural pyrethrin content. Moreover, we investigate the potential of employing pyrethrum as a companion plant.
-
The insecticidal properties of some chrysanthemum-like plants, similar to those observed in modern pyrethrum, have been recognized since ancient times. Ancient Chinese texts, such as the 'Rites of the Zhou' during the Warring States period (475−221 BC), describe the use of ash, powder, or smoke from 'Muju' (translated as 'male chrysanthemum plants') during the Zhou Dynasty (1046−256 BC) to repel or eliminate noisy aquatic insects and frogs, thereby preventing their sounds from disturbing the nobility. This application exhibited toxicity akin to pyrethrins, particularly effective against aquatic insects. Zheng Xuan, a Han Dynasty (127−200 AD) scholar, noted the self-fertilization challenges of these plants due to their lack of ornate flowers, which affected pollination and seed quality. Zong Lin (499−563 AD) in his 'Jingchu Suishiji' (Festivals and Seasonal Customs of the Jing-Chu Region) also mentioned the use of chrysanthemum-like plant ashes or powder as insect repellents in the Jing and Chu regions. However, with the evolution of Chrysanthemum plants into symbols of purity and elegance and their use in ornamental, tea, and culinary applications, the insecticidal usage of these plants became less documented in later ancient Chinese literature. Therefore, despite some scholarly suggestions that these plants are akin to modern pyrethrum[11], there is no direct evidence to confirm that they are the same species.
In historical practices, the use of plant extracts and dried powders as insect repellents can be traced back to the Roman Empire. Around 400 BC, during Persian king Xerxes' reign, pyrethrum was used for its dried flower powder to delouse children[12]. Although records of this plant's insecticidal use are limited, it is believed to have been traded along the Silk Route to Europe, where its dried flowers became a popular means to deter insect bites in ancient times.
The widespread commercial cultivation of pyrethrum as an insecticidal agent commenced in the 19th century. The initially cultivated species, Tanacetum coccineum (commonly known as Persian pyrethrum), notable for its vivid, daisy-like red, pink, and white flowers[13], exhibited genetic variability in tetraploid (4n = 36) and diploid (2n = 18) forms. Originating from the Caucasus region, between the Black Sea and the Caspian Sea[14], the commercial cultivation of Persian pyrethrum began in Armenia in 1828. The product, known as 'Persian insect powder' derived from T. coccineum dried flowers, quickly became renowned for its efficacy in delousing children[12].
Around 1840, a significant shift in cultivation practices occurred with the introduction of T. cinerariifolium, or Dalmatian pyrethrum. This shift was driven by the discovery of higher pyrethrin content in Dalmatian pyrethrum flower heads compared to Persian pyrethrum. Native to the eastern Adriatic coast, Dalmatian pyrethrum thrives in areas like Croatia, Bosnia, Herzegovina, and Montenegro[15]. After the phylloxera outbreak in Dalmatian vineyards in 1910, the region pivoted to Dalmatian pyrethrum cultivation, leveraging its resistance to phylloxera and suitability for large-scale farming. Dalmatia soon became the leading global pyrethrum cultivation region, becoming the primary source of pyrethrins[16].
Global conflicts, notably World War I, disrupted the pyrethrum industry, hindering exports from Dalmatia and elevating Japan as a leading producer. World War II further complicated the supply chain, leading to the expansion of pyrethrum cultivation in Eastern African regions, including Kenya, Tanzania, and Rwanda. During this period, Kenya became the top pyrethrum producer, meeting the allied armies' needs. However, the mid-20th century saw a decline in the pyrethrin industry due to the rise of synthetic insecticides. By the late 20th century, as the demand for natural and environmentally friendly products grew, interest in natural pyrethrins resurged, driving growth in the Australian pyrethrum sector with efforts towards enhancing the industry's sustainability and efficiency[11].
Commercial cultivation of pyrethrum in China commenced in the 1930s in Jiangsu province. By 1935, the planted area in Jiangsu and Zhejiang provinces reached nearly 500 hectares. By 1952, the production of dry pyrethrum flowers in Jiangsu province was recorded at 350 metric tons. However, a notable decline in cultivation areas ensued due to an increased emphasis on cotton farming and the advent of synthetic pyrethroids, persisting until the onset of the new millennium. In the early 21st century, the focus of pyrethrum cultivation shifted to Yunnan province due to favorable growing conditions (Fig. 1). Since 2018, Yunnan's cultivation area has expanded to about 4,000 hectares, with Yuxi alone accounting for over 2,000 hectares, establishing Yunnan as a key player in the global market[17]. Today, Australia, China, and Kenya are the leading global pyrethrin producers.

Figure 1.
Cultivation of pyrethrum. (a) Blossoming pyrethrum fields in Yunnan, China. (b) Mechanical harvesting of pyrethrum in Yunnan, China.
Biological characteristics of pyrethrum
-
The Tanacetum genus, part of the Asteraceae family, comprises approximately 160 species of perennial plants, recognized for their daisy-like flowers, these species are characterized by their deeply incised leaves and flower heads, which exhibit a spectrum of colors from white to pink and yellow. Extracts from Tanacetum species display a range of biological activities, including antibacterial, anti-inflammatory, and anti-cancer properties[18]. Among the Tanacetum genus, only selected species like T. coccineum and T. cinerariifolium are renowned for their high insecticidal toxicity. T. cinerariifolium, in particular, is globally cultivated as a commercial pesticide plant. As an outcrossing diploid species (2n = 18), it displays high self-incompatibility, resulting in significant pyrethrin content variability within the same geographic region[19]. Dried flower heads of wild pyrethrum populations typically contain 0.36% to 1.3% pyrethrins, with individual levels ranging from 0.10% to 1.35%[20,21]. In comparison, plants grown in Yunnan province, China, show higher pyrethrin content, ranging from 0.45% to 2.38%[19].
Pyrethrum plants can flower throughout the year when vernalized, typically producing flowers for three consecutive years, extendable to 4−5 years under optimal conditions[22]. In China, farmers often replant pyrethrum annually after harvest, aiming to preserve its pyrethrin content and utilize the cleared fields for rotating high-value crops.
Pyrethrins mainly accumulate in well-developed seed embryos, protecting them from degradation at elevated temperatures[23]. The concentration of pyrethrins in pyrethrum leaves is approximately one-tenth of that found in the flower heads[24]. The industry typically discards flower stems and leaves, focusing on extracting essential oils solely from the flowers. Pyrethrum flower heads progress through eight developmental stages (S1 to S8), with harvest timing critical for optimal yield. Around 94% of pyrethrins are stored in the secretory ducts and achenes of mature flowers, with pyrethrin accumulation per flower closely related to the number of achenes, the yield per achene is notably higher in those with embryos compared to hollow ones[25]. Although the highest pyrethrin content is observed at stage 6 (S6), harvesting typically occurs during more uniform stages S4−S5 for mechanical efficiency.
Components and insecticidal activity
-
In terms of insecticidal activity, pyrethrum efficacy primarily stems from six structurally similar ester compounds found in its crude extract: pyrethrin I, cinerin I, jasmolin I (pyrethrin type I), and pyrethrin II, cinerin II, jasmolin II (pyrethrin type II)[26]. The total pyrethrins typically comprise 73% pyrethrin I and II, 19% cinerin I and II, and 8% jasmolin I and II[27]. Pyrethrin I and II demonstrate superior knockdown and insecticidal activity compared to cinerins and jasmolins[28], with pyrethrin I exhibiting greater lethality and pyrethrin II providing a quicker knockdown effect[29].
Pyrethrins target voltage-gated sodium channels in insects, altering nerve functions by prolonging channel opening[30]. Studies suggest that pyrethrum extract induces autophagy in insects non-nervous systems[31]. All six compounds inhibit fast-channel inactivation, with varying effects on channel deactivation, and pyrethrin II is the most potent[30]. A mere 0.001% (v/v) concentration of pyrethrum extract can cause 100% mortality in larvae of Corcyra cephalonica[32], and at 0.1%, it effectively controls the cherry weevil[33]. Beyond insect control, pyrethrins have applications in treating seborrheic dermatitis[34], and have been shown to induce oxidative DNA damage in SH-SY5Y cells and mediate autophagy through the AMPK/mTOR pathway[35].
-
The production of natural pyrethrin extract is a labor-intensive process, contributing to its higher cost than synthetic alternatives. However, the prospect of mass-producing natural pyrethrins through biotechnology, utilizing microorganisms or plant hosts offers a potential avenue for cost reduction[36]. A complete understanding of the pyrethrin biosynthesis pathway is imperative for this biotechnological approach to be feasible.
Pyrethrins, primarily biosynthesized in the flower heads of T. cinerariifolium, are esters formed from the esterification of a monoterpenoid acyl moiety (pyrethric or chrysanthemic acid) with an alcohol moiety (pyrethrolone, jasmololone, and cinerolone) (Fig. 2). The acid component originates from the methylerythritol-4-phosphate (MEP) pathway within plastids, part of the terpene biosynthetic network[37]. In contrast, the alcohol moiety derives from the jasmonic acid (JA) biosynthetic pathway, involving enzymes such as lipoxygenase (LOX), allene oxide synthase (AOS), allene oxide cyclase (AOC), oxo-phytodienoic acid reductase (OPR), and three β-oxidation stages[38−40]. Jasmolone hydroxylase (JMH), a P450 cytochrome oxidoreductase, is key in converting jasmone to jasmolone[41]. Pyrethrolone synthesis from jasmone involves PYS (CYP82Q3), a member of the CYP82 family[42].
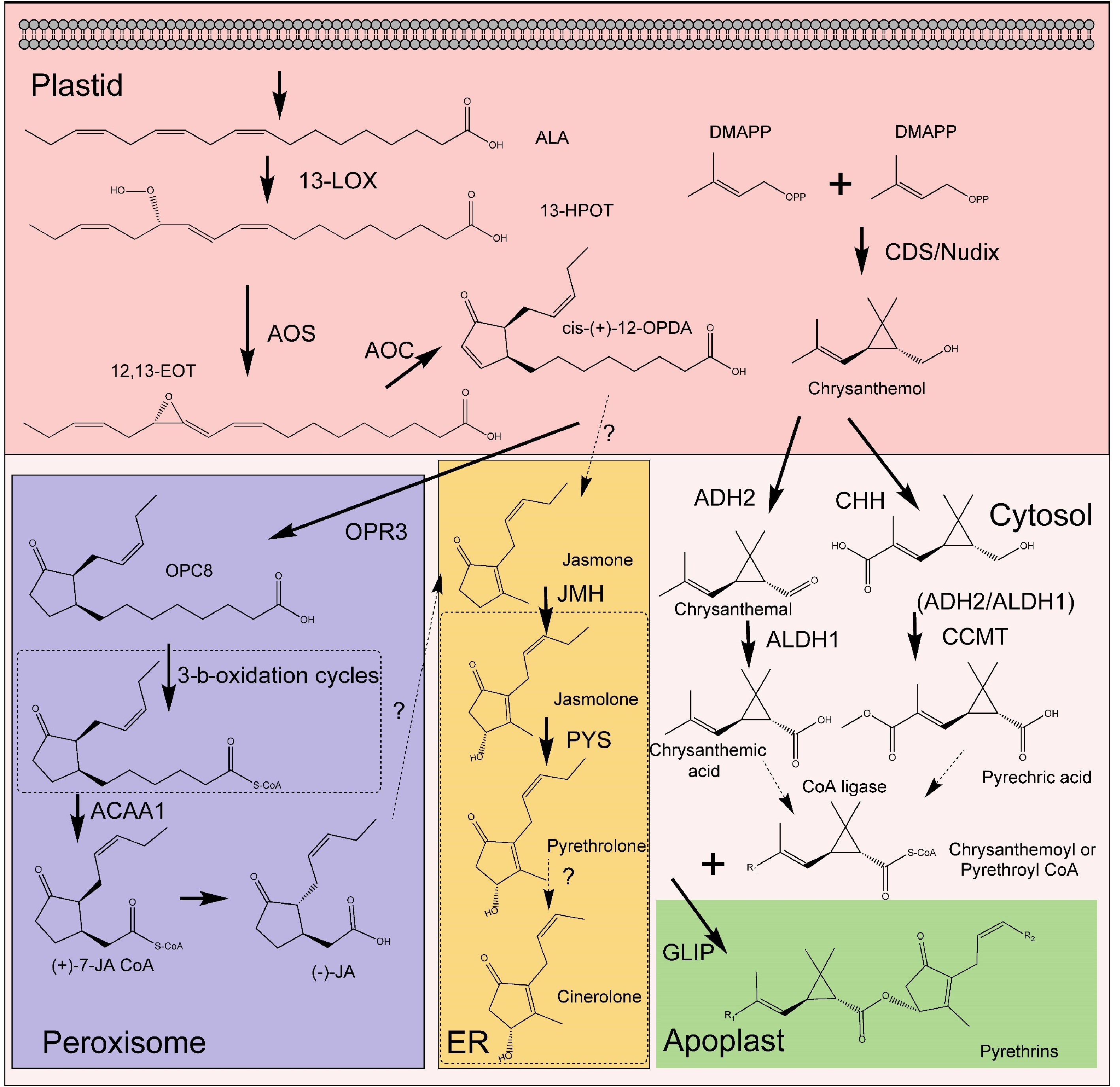
Figure 2.
The pathway of pyrethrins biosynthesis. Solid arrows represent the steps that have been elucidated in the pathway, while dashed arrows indicate the steps that remain to be clarified. 13-LOX (13-lipoxygenase), AOS (allene oxide synthase), AOC (allene oxide cyclase), OPR3 (OPDA reductase 3), CDS (chrysanthemyl diphosphate synthase), ADH2 (alcohol dehydrogenase 2), ALDH1 (aldehyde dehydrogenase 1), CHH (chrysanthemol 10-hydroxylase), GLIP (GDSL lipase-like protein), JMH (jasmolone hydroxylase), MT (10-carboxychrysanthemic acid 10-methyltransferase), PYS (pyrethrolone synthase).
The synthesis of trans-chrysanthemic acid begins with chrysanthemyl diphosphate synthase (CDS), which combines two dimethylallyl diphosphate (DMAPP) molecules to form chrysanthemyl diphosphate (CPP). The conversion of CPP to trans-chrysanthemol may be facilitated by a Nudix-like phosphatase enzyme[43,44]. Subsequent steps involve enzymes TcADH2 and TcALDH1, transforming trans-chrysanthemol into aldehyde and acid forms. The 10-carboxychrysanthemic acid 10-methyltransferase (CCMT) methylates the carboxyl group at C10 of trans-chrysanthemum acid, producing pyrethric acid. Lastly, a GDSL lipase-like protein (GLIP) links the acid and alcohol moieties to form the pyrethrin molecule[45−47]. This complete biosynthetic pathway for pyrethric acid has been reconstructed in Nicotiana benthamiana and Solanum lycopersicum[47,48].
Despite these advancements, gaps remain in our understanding, particularly in converting Jasmonates (JAs) to jasmone and jasmolone to cinerolone. Pyrethrolone in pyrethrin II likely originates from 12-oxo-phytodienoic acid (OPDA), iso-OPDA, and cis-jasmone, rather than methyl jasmonate (MeJA) or JA[49]. This suggests partial, but not complete, alignment with the JA biosynthetic pathway, raising questions about JA direct role in pyrethrin biosynthesis versus its regulatory function.
Recent discoveries have significantly enhanced our understanding of pyrethrin biosynthesis and its subcellular localization in pyrethrum plants. Initially believed to accumulate primarily in the trichomes of floral ovaries, advancements in isolation and extraction methods have revised this view. Contrary to earlier beliefs, the concentration of pyrethrins in trichomes is relatively low at about 0.55%. However, trichomes are reservoirs for nearly all pyrethrin precursors[50]. Enzymes critical for pyrethrin metabolism are predominantly expressed in trichomes covering the ovaries. Importantly, the final two enzymes in the pathway, CCMT and GLIP, are located within the ovary tissues[41,47]. This indicates that chrysanthemum acid is synthesized in trichomes and then transported to the pericarp of the achenes, where pyrethric acid and ultimately pyrethrins are synthesized.
-
Environmental factors play a pivotal role in the content and accumulation of pyrethrins in pyrethrum cultivation. Moderate drought conditions can expedite the flowering of pyrethrum, facilitating earlier harvesting. However, this may also limit the duration of pyrethrin accumulation and reduce the pyrethrin content at flower maturity[25]. Soil type and pH significantly impact pyrethrum yield. Typically, pyrethrum prefers well-drained, moderately fertile karst soils in its native regions. The optimal soils for pyrethrum production are volcanic and clay loams, which tend to be slightly alkaline[51]. Nevertheless, pyrethrum shows adaptability to various soil types; in Yunnan province, it is commonly cultivated on less fertile soils, including barren hillsides, yet still exhibits robust growth.
Temperature variations markedly influence yield. Field studies indicate that pyrethrum thrives in cool climates and can overwinter in the open fields of Wuhan. The ideal growth temperature range is 20−25 °C, with optimal flowering and pyrethrin content observed when plants experience cool nights and warm days. Vernalization at lower temperatures can enhance flower yield and stem elongation[52]. Conversely, high temperatures (above 30 °C) negatively affect pyrethrin accumulation[11]. In Yunnan's Yuexi, with an average annual temperature of 17.4–23.8 °C, pyrethrum flowers throughout the year and show optimal growth. In contrast, Wuhan's hot summers, occasionally exceeding 38 °C for around 20 d, can lead to dormancy or death of the above-ground parts of the plant. Moreover, high temperatures, particularly during the early flowering stage, can act as thermal stress, accelerating flower development and reducing pyrethrin content at maturity[53].
Pyrethrins and their regulatory mechanism
-
Pyrethrins, naturally present in pyrethrum leaves and flowers, are subject to hormonal regulation. Plant growth regulators such as gibberellins (especially GA3), IAA, and ABA have been shown to effectively enhance pyrethrum growth and increase pyrethrin accumulation[54,55]. Furthermore, salicylic acid treatment elevates the expression of pyrethrin synthesis genes and the accumulation of pyrethrin I[56]. Additionally, JAs play a particularly significant role, acting both as substrates and hormones in pyrethrin synthesis.
JAs are crucial to a range of plant processes, including seed germination, root hair development, stomatal regulation, flower and hypocotyl growth, leaf senescence, chlorophyll degradation, trichome production, and responses to biotic and abiotic stress[57,58]. Studies demonstrate a rapid increase in pyrethrin content in leaves following mechanical damage or insect feeding. However, leaf damage during the flowering stage does not similarly increase pyrethrin levels in flower heads[24]. In flower heads, pyrethrin composition is primarily regulated by endogenous JAs, with exogenous JAs unable to significantly boost pyrethrin content. The application of MeJA mimics the endogenous JA induction pattern, peaking in the expression of pyrethrin biosynthesis genes 6 to 12 h post-application[41]. Interestingly, in damaged pyrethrum plants, besides the rapid upregulation of JAs, the release of volatile organic compounds (VOCs) can increase pyrethrin accumulation in nearby undamaged pyrethrum plants[59,60], highlighting the complex relationship between pyrethrin synthesis and JAs.
Prolonged exposure to low concentrations of MeJA has been observed to increase gland density in T. cinerariifolium, indirectly leading to enhanced pyrethrin content[61]. This underscores the significant role of JAs in regulating pyrethrin synthesis. Treatment with JAs, particularly MeJA, activates a series of transcription factors crucially involved in pyrethrin biosynthesis. TcMYC2, a key transcription factor in the JAs signaling pathway, shows notable upregulation following MeJA treatment. Its protein binds to and promotes the expression of essential genes in pyrethrin synthesis, such as TcAOC, TcCHS, and TcGLIP, thus playing a central role in regulating pyrethrin production[61].
In addition to TcMYC2, TcWRKY75, localized in the glands, also contributes to the regulation of pyrethrin synthesis through this pathway[62]. The transcription factors, including TcMYB8, TcbZIP60, and TcbHLH14, influence genes involved in pyrethrin synthesis to varying degrees[63−65]. There is a general positive correlation between MeJA treatment and the expression of genes in both the acid and alcohol pathways of pyrethrin synthesis, including early alcohol pathway genes like TcLOX and TcAOC. However, for late synthesis genes such as TcJMH, TcPYS, and TcGLIP, there is a positive correlation at lower MeJA concentrations, but a rapid decrease in expression at higher concentrations[61].
In vitro studies focusing on TcMYB8 and TcbHLH14 genes have shown their strong affinity for binding to the TcGLIP promoter, initiating downstream gene expression. Nevertheless, in vivo studies indicate no significant increase in TcGLIP expression with the overexpression of TcMYB8 or TcbHLH14. This suggests a complex feedback regulatory mechanism involving JAs in T. cinerariifolium[64,65]. These insights into the transcriptional regulation of pyrethrin synthesis not only deepen our understanding of the plant's secondary metabolism but also open pathways for optimizing pyrethrin production through targeted genetic and hormonal interventions.
-
In addition to pyrethrins, pyrethrum flowers are a rich source of secondary metabolites. Pyrethrum extracts typically contain 70%−80% pyrethrins, along with a diverse range of plant-derived compounds such as terpenes, flavonoids, free fatty acids, and high-molecular-weight alkanes. The terpene fraction, complementing the pyrethrins from the MEP pathway, mainly consists of various sesquiterpene volatiles and sesquiterpene lactones (STLs) originating from the cytoplasmic mevalonate (MVA) pathway.
STLs, particularly pyrethrosin, are significant constituents of pyrethrum extracts. First identified in the 19th century, pyrethrosin often forms crystalline precipitates in refined pyrethrum extracts' isoparaffin solutions. Characterized by a reactive α-methylene and γ-lactone sub-structure, STLs exhibit a range of biological functions, including activity against herbivores and microorganisms and a role in interspecific competition[66]. In the STL biosynthetic pathway, germacrene A, synthesized from farnesyl diphosphate (FDP) by germacrene A synthase (GAS), acts as a precursor, followed by a series of oxidations and reductions leading to STL and other oxygenated germacratrien-12-oic acid derivatives. Genes implicated in STL biosynthesis, highly expressed in trichomes, exhibit a similar expression pattern to flower development[67].
Sesquiterpene volatiles
-
Pyrethrum flowers, compared to other Asteraceae species, exhibit lower volatility during flowering, with (E)-β-farnesene (EβF) being the predominant sesquiterpene volatile emitted[59,68]. However, in T. coccineum, known for its striking multicolored appearance, major volatile organic compounds (VOCs) include α-farnesene[69]. EβF, recognized as an aphid alarm pheromone, repels aphids, and attracts ladybird beetles, natural aphid predators, contributing to an indirect defense strategy[68]. As a common component in plant volatile blends, EβF likely plays a role in a broader defensive system, signaling a 'cry for help'[68]. In response to mechanical damage during the vegetative stage, pyrethrum emits specific VOC blends, mainly green leaf volatiles and EβF from wounded leaves[59,70,71]. These induced VOCs act as biological signals, triggering pyrethrin biosynthesis in intact leaves, suggesting a comprehensive role in plant defense mechanisms[71].
Pyrethrins, along with minor constituents like EβF, activate specific odor receptor neuron types in Egyptian mosquitoes. Pyrethrum achieves spatial repellency through a dual-target mechanism[72,73], and employs a defensive mimicry strategy based on the alarm pheromone EβF[68]. Furthermore, germacrene D, another major volatile compound in pyrethrum, may have regulatory effects on both defense and pollination[74].
-
The average pyrethrin content in global commercial pyrethrum populations ranges from 1.8% to 2.0%[75]. However, the cultivation of pyrethrum faces challenges due to inherent genetic factors like high self-incompatibility and heterozygosity, complicating efforts to stabilize and enhance pyrethrin content. As a result, developing a bioreactor-based pyrethrin synthesis process has garnered significant industrial and scientific interest.
Agrobacterium rhizogenes-mediated hairy roots have been explored as an alternative pyrethrin production source[76,77]. However, GCMS analysis reveals these hairy roots lack pyrethrins, predominantly containing high concentrations of EβF. Attempts to redirect the sesquiterpene biosynthetic pathway from the MVA pathway, synthesizing EβF, to the MEP pathway responsible for pyrethrin precursor synthesis, have been made using CRISPR-Cas9 technology to knock out the EβF synthesis gene. Despite successfully reducing EβF gene expression and content, there was no subsequent detection of pyrethrins or their precursors[61,62,78], raising concerns about the economic feasibility of producing pyrethrins via hairy root cultures.
Pyrethrum cultivation is notably labor-intensive, primarily due to the targeted harvesting of flowers, which contain the majority of the plant's pyrethrin content. Consequently, other plant parts, such as leaves, are often discarded, leading to resource wastage and potential environmental pollution from disposal practices like burning. Enhancing the pyrethrin content in leaves to commercially viable levels could significantly benefit the pyrethrum industry. Studies have shown that short-term treatments with exogenous MeJA at higher concentrations can induce an increase in pyrethrin content in leaves, although a subsequent decrease is observed. Conversely, prolonged exposure to low concentrations of MeJA can increase gland density in seedlings, thereby steadily enhancing pyrethrin levels in leaves[61]. In tobacco leaves, introducing pyrethrin synthesis genes enables the production of pyrethric acid, an essential precursor[47]. However, this production is challenged by the competition for substrates with carotenoids in plastids[79]. In transgenic T. cinerariifolium plants, overexpression of the TcCDS gene, driven by the rubisco promoter, elevated pyrethrin levels but resulted in significant chlorophyll reduction and slowed plant growth, indicating an adverse impact on overall plant health[80]. Overexpressing pyrethrin biosynthesis-related genes in the vegetative organs might substantially hinder plant growth and development, presenting a challenging dilemma between boosting pyrethrin synthesis and sustaining healthy plant growth.
An alternative and more promising method is the production of pyrethrins in plant fruits. This approach often hosts biosynthetic pathways for high-value metabolites without impacting overall plant growth or yield[81]. The successful synthesis of trans-chrysanthemum acid in tomato fruits[36], and the production of chrysanthemum acid in tomato-type VI glands[48], highlight this approach's potential.
Despite these advances, fully reconstructing the pyrethrin synthesis pathway in alternative plant species or biological reactors remains a complex challenge. To date, reports have only been made of chrysanthemum acid or pyrethric acid synthesis in heterologous plants, with no complete biosynthesis of pyrethrins reported in such systems. This underscores the complexity of reconstructing the complete pyrethrin biosynthesis pathway. The synthesis of pyrethrins is complicated by the final enzymatic step involving TcGLIP, which has shown a preferential affinity for pyrethrin I/II over other compounds, such as cinerin I/II and jasmonate I/II[9]. Emerging evidence suggests a potential non-competitive interaction between Coenzyme A (CoA) and specific compounds at the TcGLIP active site, particularly involving the adenosine portion of CoA. Although feedback inhibition of CoA in lipases has not been documented, it is hypothesized that CoA may play a crucial role in modulating TcGLIP reactions, possibly influenced by the hydrophobic nature of pyrethrins and the gene expression levels of TcGLIP[82].
While TcGLIP primarily functions as a transferase enzyme in synthesizing pyrethrin I, it also exhibits esterase activity, albeit less prominently[83]. The disparity between these activities presents an opportunity for genetic modification of the TcGLIP gene, potentially leading to more efficient and targeted pyrethrin production. Modifying TcGLIP to enhance its transferase activity while reducing its esterase function could significantly optimize pyrethrin synthesis.
Pyrethrum could be used as a companion plant in future agriculture
-
The indiscriminate use of pesticides has led to significant repercussions, including diminished quality and safety of agricultural produce, threats to human health, and environmental disruption. This situation is exacerbated by degrading beneficial organism populations, contributing to soil and groundwater contamination, and posing challenges to sustainable environmental and agricultural development. Although synthetic pyrethroids have encountered resistance issues in pest populations due to intensive use, natural pyrethrins derived from pyrethrum have proven effective against various herbivores, supporting their efficacy in pest control[84]. However, direct application of natural pyrethrins on organic farms can be harmful to insect-pollinated species like honey bees upon direct contact[85], and their short half-life in direct sunlight can potentially lead to increased application frequency and associated costs. Interplanting represents a viable alternative to direct application of pyrethrum extracts for pest management. This agricultural technique involves cultivating at least two different plant species within the same field. Interplanting with pyrethrum offers several advantages over direct application. Firstly, it eliminates the need for labor-intensive extraction processes, reducing costs and preventing the environmental harm associated with waste byproducts. Secondly, pyrethrins are concentrated in the plant's secretory vessels or cavities, where they are protected from photodegradation and isolated from pollen, thus posing no risk to pollinating insects. Additionally, pyrethrum plants produce a plethora of volatile secondary metabolites, such as (E)-β-farnesene, which repel aphids and attract beneficial predators like ladybirds, further contributing to pest control[68]. Moreover, pyrethrum has low nutritional and soil quality requirements and can bloom year-round in regions like Yunnan and Guizhou provinces. Even after the harvest of dry flowers, the plant can regenerate and bloom again, offering a sustainable option for perennial crop cultivation that can last 4−6 years. This long-term, low-input cultivation method not only provides an auxiliary source of income but also enhances the economic value of the main intercropped organic produce. Therefore, pyrethrum stands out as an intercropping plant with significant developmental potential and practical value in sustainable agriculture.
Historically, pyrethrum has been used in intercropping systems. Traditional agricultural practices along the Adriatic Sea coast have utilized pyrethrum as a cover crop in olive groves[86]. In Yunnan, China, its cultivation as an annual crop in mountainous regions makes it a popular choice for intercropping (Fig. 3). Pyrethrum is versatile, demonstrated by its integration with various crops and trees. It is commonly intercropped with vegetables like chili peppers (Capsicum annuum) and potatoes (S. tuberosum). Fruit trees such as citrus (Citrus spp.), Chinese jujube (Ziziphus jujuba), pear (Pyrus spp.), and peach (Amygdalus persica) are also integrated into pyrethrum intercropping systems, as well as with oil crops like olive (Olea europaea), ornamental plants including roses (Rosa chinensis) and Chrysanthemums, and field crops such as corn (Zea mays), rapeseed (Brassica spp.), and tobacco (N. tabacum). Intercropping pyrethrum with cabbage, for example, has been effective in controlling approximately 80% of aphids on cabbage, benefiting from the synchronization of pyrethrum flowering with pest occurrence[87].

Figure 3.
Pyrethrum intercropping systems. (a) Intercropping of pyrethrum with Chinese jujube. (b) Pyrethrum interplanted with olive trees. (c) Pyrethrum combined with citrus and cabbage in intercropping. (d) Pyrethrum intercropped with roses.
The agronomic benefits of pyrethrum intercropping are manifold. They include increased economic returns, optimized land use, effective weed control, soil and water conservation, mitigation of continuous cropping challenges, and achievement of ornamental and ecological layout objectives. Intercropping pyrethrum with forestry and fruit trees exploits temporal and spatial complementarity, creating layered agricultural spaces. For example, the intercropping of pyrethrum with Chinese jujube and olive forms a stratified structure, efficiently utilizing the land under fruit trees. The canopy of these trees provides shade for pyrethrum during the summer, enhancing its survival. Pyrethrum shallow-rooted perennial nature helps minimize ground-level weed growth, leading to cost savings in management. Additionally, its role in slope stabilization and soil conservation highlights its potential for widespread adoption in diverse agroecosystems.
Unraveling and transporting the complete pathway of pyrethrin synthesis
-
Although significant progress has been made, the biosynthetic pathway of pyrethrins still contains unresolved aspects, particularly concerning the origin of the alcohol portion. The transformation from cis-jasmone to jasmolone is now better understood[41]. But the precursor of cis-jasmone itself remains unclear. Current evidence suggests that the alcohol moiety in pyrethrin II likely originates from 12-oxo-phytodienoic acid (OPDA), iso-OPDA, and cis-jasmone, rather than MeJA or JA[49]. Studies in Mentha suavelons indicate that jasmonic acid and cis-jasmone biosynthesis are separate processes, especially from cis-OPDA to cis-jasmone[88]. Early stages of JA synthesis involving genes like LOX, AOC, and OPR, show co-expression patterns similar to those in the pyrethrin acid pathway[61], suggesting a potential synthesis of pyrethrins from OPDA. However, it remains a puzzle how trace amounts of OPDA contribute to substantial pyrethrin production.
The density of glandular trichomes on leaves significantly correlates with the content of monoterpene and sesquiterpene[89]. Glandular trichomes are widely present in Asteraceae plants[90], and tissue differentiation within these structures are essential for the production of specific compounds[91]. In pyrethrum, glands primarily contain sesquiterpenes such as EβF[74]. Interestingly, while precursors of pyrethrins are synthesized in the glands, the final enzyme, TcGLIP, operates extracellularly, meaning that pyrethrins are not stored within these structures[50]. It is intriguing that, despite the presence of established chloroplasts, no pyrethrins or their distinguishable precursors like chrysanthemol and chrysanthemic acid was detected in the hairy roots of pyrethrum. Instead, only high-purity EβF was observed, and notably, these hairy roots lacked glands[61]. For certain plants, tissue differentiation or specific structures are prerequisites for producing particular secondary metabolites[92]. Research in Artemisia annua suggests that the plant can generate artemisinin independently of glandular trichomes[93]. Therefore, the question arises: Are glands indispensable structures for the synthesis and accumulation of pyrethrins, and what is the biological significance of synthesizing precursors in the glands but not storing them there?
Pyrethrum leaves contain a variety of terpenoid compounds, some of which are chemically reactive and cytotoxic to plant cells. Understanding the transportation of these metabolites from their biosynthetic sites to storage locations is crucial. Terpenoid compounds typically undergo modifications like glycosylation or acylation and are stored in vacuoles or other subcellular compartments[94,95]. Considering 94% of pyrethrins accumulate in the flower heads and achenes of pyrethrum, it is challenging to conceive that high concentrations of pyrethrins are synthesized exclusively in the chloroplast-lacking cells of the flower heads. ATP-binding cassette (ABC) transport proteins, known to participate in terpenoid transport, may be involved in pyrethrin transportation due to their shared hydrophobic nature[96,97]. However, the specific role of transport proteins in pyrethrin transportation in pyrethrum remains to be discovered.
In summary, while there has been substantial progress in understanding pyrethrin biosynthesis, challenges remain in comprehending the intricacies of precursor synthesis, transportation mechanisms, and storage within the plant. Further research is vital to unravel the complex relationship between pyrethrin synthesis, transport, and storage, thereby enhancing our comprehension of these processes in pyrethrum.
-
Pyrethrum is a plant of unique significance, chiefly for its production of pyrethrins, a potent insecticidal compound. Research on pyrethrum presents numerous challenges, especially in decoding its expansive genome. Notably, the draft genomes for T. cinerariifolium and T. coccineum are notably large, exceeding 7.1 and 9.4 Gb[98,99]. This is in stark contrast to other Asteraceae genera, such as 1.74 Gb for Artemisia annua[100], and 2.53 Gb for Chrysanthemum indicum[101]. Even the hexaploid-cultivated Chrysanthemum × morifolium has a genome size of only 8.15 Gb[102]. Notably, pyrethrum synthesizes pyrethrins as a defense mechanism, a process intricately regulated by JAs. The complex relationship between JAs and pyrethrin biosynthesis poses a significant research challenge.
This review provides a comprehensive overview of pyrethrum cultivation history and its essential biological characteristics, particularly focusing on the enhancement of pyrethrin yield. It discusses the potential of pyrethrum as a companion plant, emphasizing its biological and ecological importance. Furthermore, the review delves into the complete biosynthesis pathway of pyrethrins, which may aid in replicating pyrethrin biosynthesis in other species. Future research is expected to further elucidate the regulatory mechanisms of pyrethrum's defense system and explore how these traits can be integrated into sustainable agricultural practices.
-
The authors confirm contribution to the paper as follows: study conception and design: Zeng T, Wang C; data analysis: Zhu L, Liu K, Bo J, Jiang Q; draft manuscript: Zeng T, Li Jinjin, Li Jiawen, Hu H; manuscript revision: Zeng T, Wang C. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this article.
This work was supported by the National Natural Science Foundation of China (grant no. 32160718), Natural Science Research Project of Guizhou (ZK[2022]301) and Guizhou Normal University QSXM[2022]19.
-
The authors declare that they have no conflict of interest. The authors Liu Kexin, Bai Jinxue, and Jiang Qijun were employed at Yunnan Nanbao Biotechnology Company. However, all the authors did not receive any funding or sponsorship from the company for the research or the publication of this article.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zeng T, Li J, Li J, Hu H, Zhu L, et al. 2024. Pyrethrins in Tanacetum cinerariifolium: biosynthesis, regulation, and agricultural application. Ornamental Plant Research 4: e015 doi: 10.48130/opr-0024-0013
Pyrethrins in Tanacetum cinerariifolium: biosynthesis, regulation, and agricultural application
- Received: 21 January 2024
- Revised: 13 April 2024
- Accepted: 22 April 2024
- Published online: 03 June 2024
Abstract: Pyrethrum (Tanacetum cinerariifolium), a perennial herb within the Tanacetum genus, stands as a crucial source of natural insecticides pyrethrins which have been utilized for centuries. This study delves into the historical utilization of pyrethrum and elucidates the biosynthetic pathways of pyrethrum, uncovering the majority of genes responsible for pyrethrin production. Moreover, pyrethrum flowers and stems are rich in sesquiterpene lactones, known for their antifungal attributes, and they release (E)-β-farnesene, an aphid alarm pheromone that lures predators such as ladybirds. These discoveries emphasize pyrethrum's multifaceted chemical defense against various biotic adversaries and its viability as a companion plant in agricultural settings. Farmers have recognized and begun utilizing pyrethrum in this capacity. The paper underscores the need for further research to thoroughly comprehend and exploit pyrethrum defense strategies for sustainable farming practices, underscoring its potential in ecological and agricultural spheres.
-
Key words:
- Pyrethrum /
- Pyrethrins /
- Flower defense /
- Biosynthetic pathway /
- Companion plant












