-
With global warming, water scarcity is becoming increasingly serious, and arid and semiarid areas are expanding. The screening, breeding, and popularization of new drought-tolerant plant germplasm have become an important research topic. Among many environmental factors, water is particularly significant in limiting plant growth and development. Drought stress affects plant growth at various levels, such as morphological structure, photosynthetic growth, physiology, and biochemical reactions[1,2]. Drought stress can induce various adverse reactions in plant cells, such as increased osmotic pressure, accumulation of harmful substances including reactive oxygen species (ROS), and inhibition of photosynthesis[3,4]. Therefore, plants must quickly initiate responses to these adverse reactions for their survival.
The effects of drought stress on plant photosynthesis are more complex. Stomatal closure aimed at reducing water transpiration, limits CO2 fixation, and reduces NADP+ regeneration by the Calvin Cycle. This inhibition affects the initial light energy conversion, electron transport, photophosphorylation, and dark and light reactions, ultimately leading to a decline in photosynthesis and ROS accumulation[5,6]. These ROS can attack lipids, leading to lipid peroxidation, protein denaturation, and DNA mutation[7,8].
Plants possess several antioxidant enzyme systems that protect cells from the negative effects of ROS. Many reports suggested that the extent of oxidative cellular damage in plants exposed to abiotic stress is controlled by the capacity of their antioxidant systems, the relationship between enhanced or constitutive antioxidant enzyme activities, and increased resistance to drought stress[9,10].
The leaf is the main site of photosynthesis and water loss. Moreover, it is also the most sensitive organ in response to environmental changes. By studying the drought tolerance of leaves, we can more directly understand the adaptability of plants to drought, as well as the physiological and biochemical changes of plants under drought conditions. In addition, among the organs, leaves are also the first line of defense organs for plants to cope with drought stress and their response to drought stress is faster than roots[11,12]. Plants can adapt to different environments by adjusting the morphological characteristics of their leaves to improve their survival ability. Environmental changes often result in morphological and anatomical changes in leaf size and thickness, stomata, epidermal cells and appendages, palisade tissue, spongy tissue, thick horn tissue, and leaf veins. Previous studies have reported that leaf color, shape, leaf area, epidermal cell thickness, epidermal cell surface wax, stomatal density, stomatal size, and palisade tissue layer are related to the drought resistance of plants[13]. The thickening of palisade tissue in the mesophyll of xerophyte leaves can improve photosynthetic efficiency[14]. Furthermore, the epidermal structure of the leaves also exhibits characteristics of adaption to drought environments, e.g., thickened cuticle, small and sunken stomata, different forms of the surface fur, and other appendages[15]. Among rice varieties with varied drought tolerance, the leaf thickness and stomatal density in leaves are different[16]. The density of stomata increases with decreasing water in the environment, causing the stomata to become miniaturized, and some stomata collapse to form stomatal pits[17,18]. Therefore, studying the response of leaves to drought can more comprehensively evaluate the overall drought tolerance of plants.
Rosa rugosa is a deciduous shrub of the Rosaceae family. It is known for its strong drought tolerance and its presence in sandy land, saline-alkali flat land, and even desert steppe. It provides ecological benefits such as wind prevention and sand consolidation. Its petals can be used to extract essential oil; its fruits are edible, and its buds and fruits can be used as medicine. R. rugosa has many varieties. Drought resistance, plant appearance, physiology, and photosynthetic characteristics of wild R. rugosa and some varieties have been compared. Numerous studies have reported that under drought stress, R. rugosa from different provenances consistently exhibit decrease relative water content (RWC) in leaves, increased plasma membrane permeability, increased in free proline and soluble sugar contents, and decreased and then increased soluble protein content[19−21].
However, the phenotype and physiological response of R. rugosa from different provenances differ under varying durations or degrees of drought stress, indicating varied drought resistance among R. rugosa from different provenances. Leaves are important organs for photosynthesis and transpiration in plants. The net photosynthetic rate, transpiration rate, and stomatal conductance of four R. rugosa varieties were reported to be affected by drought stress[21]. However, the relationship between leaf structure and drought resistance of R. rugosa has not been reported. Wild R. rugose in the Shandong coastal area (China) is a precious breeding germplasm resource with strong environmental adaptability. And R. sertata × R. rugose, which is native to Gansu Province (China), exhibits strong drought, salt, and cold resistance, and is an important germplasm resource for drought resistance breeding. Moreover, R. rugosa has ornamental, health, edible, medicinal, and other useful values, with great product development potential and economic benefits. R. sertata × R. rugose, R. rugosa 'Fenghua', R. rugosa 'Zizhi', R. rugosa 'Plena', and R. rugosa 'Ziduan' are widely cultivated in northern China and exhibit strong drought resistance, cold resistance, and high economic value. The objective of this study was to assess the drought resistance of six genotypes by examining their photosynthetic and physiological characteristics under polyethylene glycol-6000 (PEG-6000) treatments and to explore their drought tolerance mechanism by observing their leaf structure.
-
Various genotypes used in this study were wild R. rugose (W), R. rugosa 'Fenghua' (F), R. rugosa 'Zizhi' (ZZ), R. rugosa 'Plena' (P), R. sertata × R. rugose (S), and R. rugosa 'Ziduan' (ZD). Wild R. rugosa were obtained from the seaside of Rongcheng (Weihai, China, 37°13' N, 122°34' E), and five R. rugosa varieties in this study were obtained from Pingyin County (Jinan, China, 36°15'55' N, 116°25' E).
One-year-cuttings of six genotypes were planted at the campus of Qingdao Agricultural University (Qingdao, China, 120°12'E, 36°20'N) for the analysis of leaf structure characteristics. For photosynthetic and physiological characterization, the 1-year-cuttings were cultivated in the intelligent greenhouse of Qingdao Agricultural University for 2 months. The test pot is 30 cm in diameter and 20 cm in height. Each pot was filled with 5 kg of potting medium (soil:sand = 1:3), supplemented by 1/2 Hoagland nutrient solution. The daytime temperature was controlled at 25 ± 3 °C for 16 h, and night temperature was maintained at 20 ± 3 °C for 8 h. The light intensity was 1,000 μmoL·m−2·s−1. Air humidity was controlled at 60%.
The cuttings were randomly divided and daily treated with 100 mL of 1/2 Hoagland nutrient solution containing different concentrations of PEG-6000 [CK (0%), C1 (5%), C2 (10%), and C3 (15%)] to simulate the drought stress for 7 d. Each treatment had five replicates.
To assess the soil water content after PEG treatment, soil water potential after irrigation with different concentrations of PEG in 1/2 Hoagland nutrient solution (0%, 5%, 10%, and 15%) and soil water potential at different soil field capacities (20%, 40%, 60%, 80%, and 100%) were measured using a dewpoint water potential instrument (Psypro, WESCOR, USA). Based on the relationship between field water holding capacity and water potential, the water potential after PEG treatment was substituted into the fitting formula to calculate the field water holding capacity.
Photosynthetic characteristics and chlorophyll content determination
Photosynthetic characteristics
-
Plants with the same growth characteristics were selected, and mature and healthy leaves in the middle of the outermost main branch was randomly selected. The photosynthetic transpiration rate (Tr), net photosynthetic rate (Pn), and stomatal conductance (Gs) of R. rugosa leaves were measured by LI-6400XT photosynthesometer (LI-6400XT, LI-COR, USA). The experiment was performed on each genotype.
Chlorophyll content
-
Leaf samples (0.1 g) were cut and soaked in 10 mL of 95% ethanol solution in the dark for 12 h to extract chlorophyll. The content of chlorophyll was determined according to the method previously described by Wintermans & Mots[22].
Physiological characteristics
Relative water content (RWC) in leaf
-
After drought treatment, 10 mature and healthy leaves in the middle of the branch was weighted using an analytical balance to calculate the fresh weight (FW). Further, the leaves were heated at 105 °C for 10 min, followed by heating at 80 °C till constant weight was obtained, which was considered as the dry weight (FD). The RWC in leaf was calculated as follows:
$\rm Leaf\; RWC\; ({\text{%}}) = (FW - FD)/FW \times 100{\text{%}} $ Superoxide anion detection (O2−)
-
First, 0.1% (w/v) Nitrotetrazolium blue chloride (NBT) staining solution was prepared in 0.05 M phosphate buffer (pH 6.4). The leaves were immersed in NBT dyeing solution and exposed to light for 6 h until a dark blue color appeared in the positive samples. The leaves were rinsed with distilled water and immersed in 95% ethanol at 40 °C for 3−16 h to remove chlorophyll.
Observation of leaf structures
Leaf surface characteristics
-
A total of five leaves were collected from each sample. Leaf areas of 1 cm2 were cut between the middle part of the leaf main vein and the leaf margin, then fixed with 2.5% glutaraldehyde for 6 h; and dried using 70%, 85%, 95%, and 100% alcohol gradient dehydration. After freeze-drying, the samples were fixed on the sample cup with the double-sided tape, and gold-plated with sputter coater. The images were taken using a JEOL7500F scanning electron microscope (7500F, JEOL, Japan) at a high voltage of 4 kV. The adaxial and abaxial planes were photographed. Five replicates were used for each genotype.
Stomatal characteristics
-
Ten mature leaves were selected for each genotype, and transparent nail polish was applied on the abaxial surface of the leaves to collect stoma marks. After the nail polish dried, it was carefully torn off using tweezers. The stomatal density, stomatal length, and stomatal width of leaves were observed using a microscope and photographed.
Histochemical and histological analyses
-
To observe the anatomical differences between the leaves of the six genotypes, the 0.5 cm × 0.5 cm area from the middle part of the mature leaf was cut and fixed in formaldehyde–acetic acid solution [formaldehyde:glacial acetic acid:70% ethanol (1:1:18)] for 24 h. Further, it was dehydrated using graded concentrations of ethanol and embedded in tertiary butanol[23]. The samples were sectioned at a thickness of 8 μm and stained with safranin and fast green. Thickness of blade, palisade tissue, stratum corneum, upper epidermal cell, lower epidermal cell, leaf vein, and lower epidermal cell; area of leaf vein, and area of vascular tissue was measured using ImageJ software.
Statistical analysis
-
SPSS 11.0 statistical software was used to conduct one-way analysis of and multiple comparison statistical analysis of the data (p < 0.05). The significance of differences was compared using Turkey's test.
-
Based on the relationship between field water holding capacity and soil water potential, the following formula was obtained:
ψ = 56.418θ − 76.724 (R2 = 0.9783) (Supplemental Fig. S1).
ψ, soil water potential, kPa; θ, field water holding capacity, %.
The water potential after PEG treatment was substituted into the formula to calculate the field water holding capacity (Table 1).
Table 1. The field water holding capacity after PEG treatment.
PEG-6000 concentration Soil water potential (kPa) Field water holding capacity 0% –21.51 97.86% 5% –39.75 65.54% 10% –46.25 54.05% 15% –59.862 29.89% After 7 d of PEG treatment with different concentrations, the leaves of six genotypes showed different degrees of wilting (Fig. 1a). After 15% PEG treatment, the leaf margin of W was slightly rolled back without scorching, whereas the leaf margins of ZZ, S, and P were both rolled and scorched. Additionally, the leaf margins of ZD and F were reverse-rolled and scorched after 10% PEG treatment.

Figure 1.
The leaf phenotypes and relative water content (RWC) of six R. rugosa genotypes under drought stress. (a) Leaf phenotypes of six R. rugosa genotypes. (b) RWC of six R. rugosa genotypes. W, Wild Rosa rugosa; F, R. rugosa 'Fenghua'; ZZ, R. rugosa 'Zizhi'; P, R. rugosa 'Plena'; S, Rosa sertata × R. rugosa; ZD, R. rugosa 'Ziduan'. Error bars represent standard error for three replicates.
Drought stress significantly decreased the relative water content (RWC) of leaves in the six genotypes of R. rugosa (p < 0.05) (Fig. 1b). Under 5%, 10%, and 15% PEG treatments, the RWC of leaves in W and ZZ was smaller than that of other four varieties (S, P, F, and ZD). Under 15% PEG treatment, the leaf RWC of F, ZD, P, S, ZZ, and W decreased by 28.34%, 22.66%, 22.24%, 20.25%, 10.82%, and 7.87%, respectively. These results indicated that the water loss in the leaves of W and ZZ under drought stress was the least.
Photosynthetic characteristics of R. rugosa leaves under drought stress
-
The genotype's Tr, Pn, and Gs of all genotypes decreased under drought stress. Under normal growth conditions, the Tr followed the order of S > ZZ > F > W > P > ZD and the Tr of all genotypes decreased with the increase in PEG concentration (Fig. 2a). Under 5% PEG treatment, the Tr in W was decreased by 29.42% and in other varieties by 57.63%–74.54%. The Pn followed the order of W > ZZ > S > P > ZD > F (Fig. 2b). Under CK and 5% PEG treatments, the Pn of W was significantly higher than that of other genotypes, and the Pn of W, ZZ, and S was significantly higher than that of the other three genotypes. The chlorophyll content in the leaves was significantly higher in W than in P, ZD, and F, which also indicated that the leaves of W had strong photosynthetic capacity (Supplemental Fig. S2).
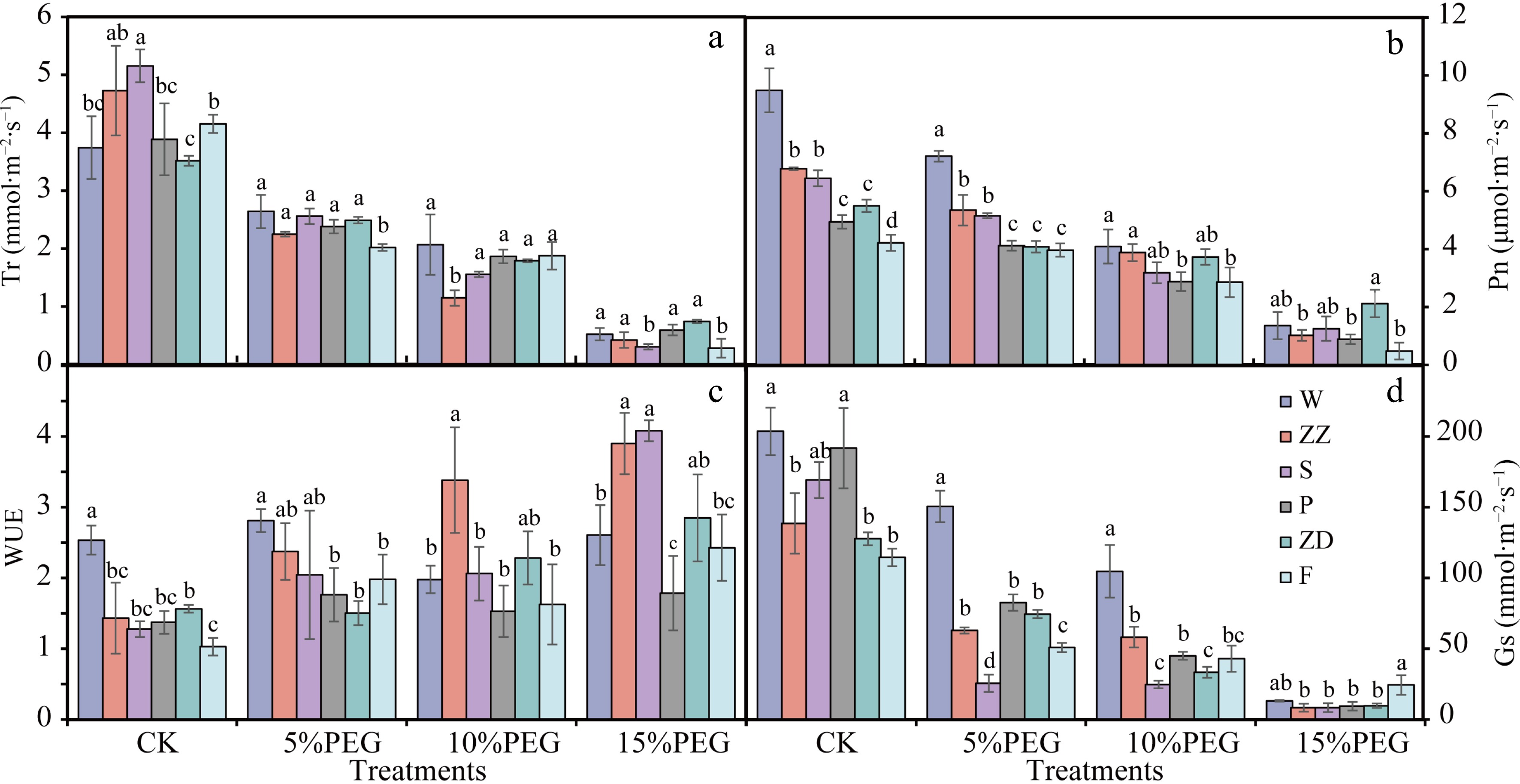
Figure 2.
Photosynthetic characteristics of the leaves of six R. rugosa genotypes under drought stress. (a) Transpiration rate (Tr). (b) Net photosynthetic rate (Pn). (c) Water use efficiency (WUE). (d) Stomatal conductance (Gs). W, Wild R. rugosa; F, R. rugosa 'Fenghua'; ZZ, R. rugosa 'Zizhi'; P, R. rugosa 'Plena'; S, R. sertata × R. rugosa; ZD, R. rugosa 'Ziduan'. Error bars represent standard error for 10 replicates. Different lower case letters in the same column indicate significant difference at p < 0.05.
Water use efficiency (WUE), which is the amount of biomass a plant produces per molar unit of water lost through transpiration, is a trait closely related to drought resistance in plants. Compared with the other five varieties, the WUE of W under CK and 5% PEG treatments were the highest (Fig. 2c). These results indicated that the leaves of W maintained a higher photosynthetic rate and WUE than other genotypes under low drought stress.
Under 5% and 10% PEG treatments, the Gs of W were significantly higher than that of other varieties, which may be one of the reasons why W could maintain higher Pn and Tr under mild drought stress (Fig. 2d). Under 5% and 10% PEG treatments, the Gs of W was significantly higher than that of other varieties, which may be one of the reasons why wild R. rugosa could maintain a higher photosynthetic rate and transpiration rate under mild drought stress (Fig. 2d).
Determination of O2–, MDA and soluble protein content
-
NBT staining can detect the accumulation of O2− in plants after drought stress. NBT staining results revealed that there were only a small number of blue spots in the leaves of W, ZZ, S, and P after 5% PEG treatment, whereas more blue spots were observed in the leaves of ZD and F. This indicated that ZD and F accumulated more O2− under mild drought stress. Compared with S and P, the number of blue spots in W and ZZ leaves after 10% PEG treatment was less (Fig. 3a). After 15% PEG treatment, the blue coloration in the leaves of all genotypes was deep, indicating damage due to drought stress.
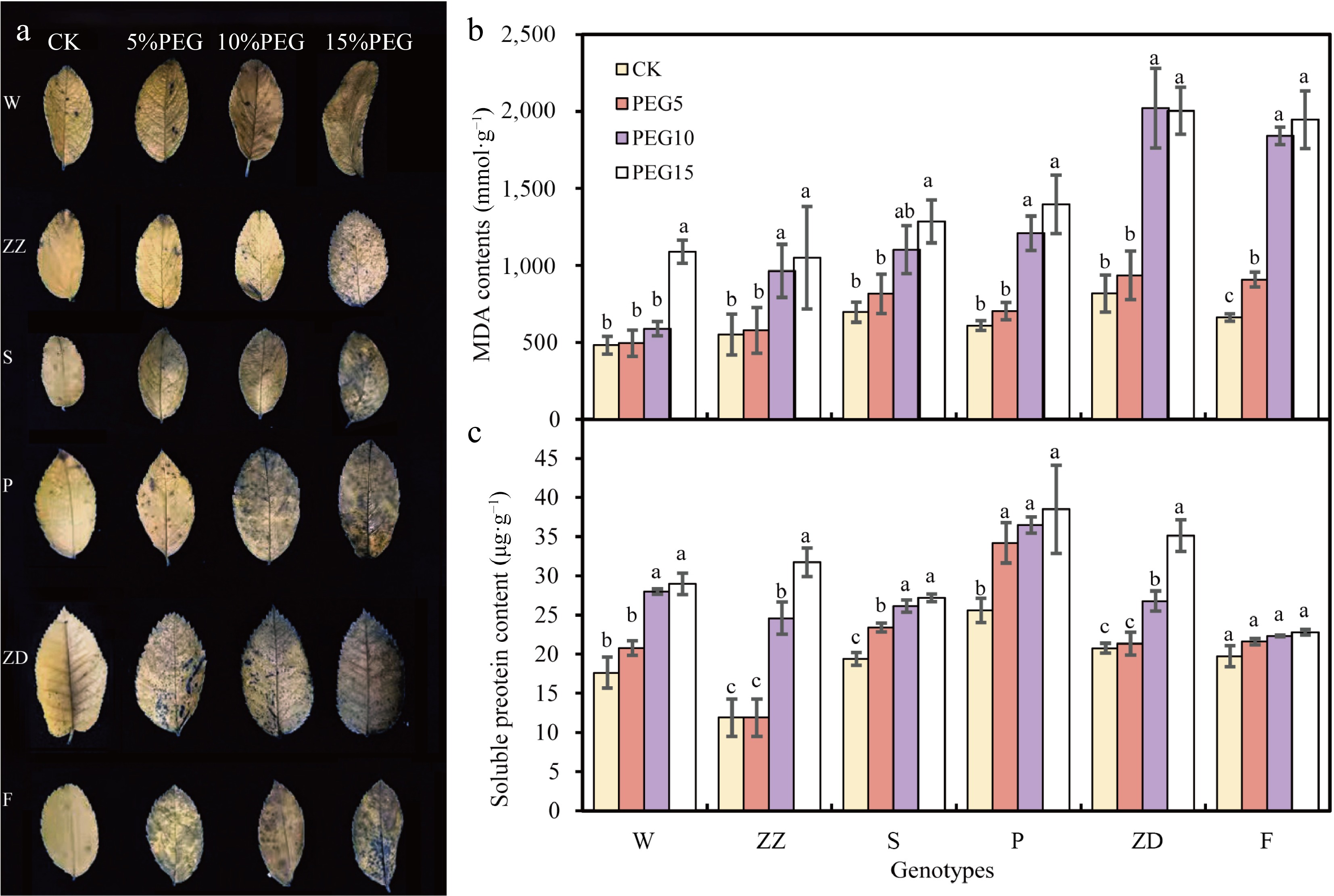
Figure 3.
Contents of superoxide anions (O2−), malondialdehyde (MDA), and soluble proteins in the leaves of six R. rugosa varieties under drought stress. (a) O2− content determination using 0.1% Nitrotetrazolium blue chloride (NBT) staining. (b) MDA contents. (c) Soluble protein content. W, Wild R. rugosa; F, R. rugosa 'Fenghua'; ZZ, R. rugosa 'Zizhi'; P, R. rugosa 'Plena'; S, R. sertata × R. rugosa; ZD, R. rugosa 'Ziduan'. Error bars represent standard error for three replicates. Different lowercase letters in the same column indicate significant difference at p < 0.05.
Similarly, the MDA contents in the leaves of six genotypes exhibited increasing trends with the increase of PEG concentration (Fig. 3b). Among them, the MDA content of F was significantly increased under 5% PEG treatment; that of ZZ, S, P, and ZD was significantly increased under 10% PEG treatment; and that of W was significantly increased under 15% PEG treatment. These results suggested that the membrane permeability of F was damaged even under mild drought stress and that of W was damaged after 15% PEG treatment.
With the increase in PEG concentration, the soluble protein contents in all genotypes showed an increasing trend, except for F. This indicated that increasing the concentration of soluble protein in R. rugosa leaf cells is a key mechanism to prevent water loss under drought stress (Fig. 3c). Under 5% PEG treatment, the soluble protein contents in W, ZZ, and ZD exhibited no significant change; however, those in S and P increased.
The drought resistance of six R. rugosa genotypes were different according to the changes in leaf RWC; photosynthetic water utilization rate; and O2−, MDA, and soluble protein contents under PEG stress. The drought resistance of wild R. rugosa was better than that of other varieties.
Blade surface characteristics
-
To explore the leaf microstructure and surface structure of different varieties of R. rugosa, paraffin sectioning, and scanning electron microscopy were used. All the leaves of the six R. rugosa genotypes were both heteromorphic leaves with stomata distributed only on the distal axial surface of the leaves (Figs 4−6). Their upper and lower epidermis were composed of single layer cells and the upper epidermis cells were covered with developed cuticle and rich wax.
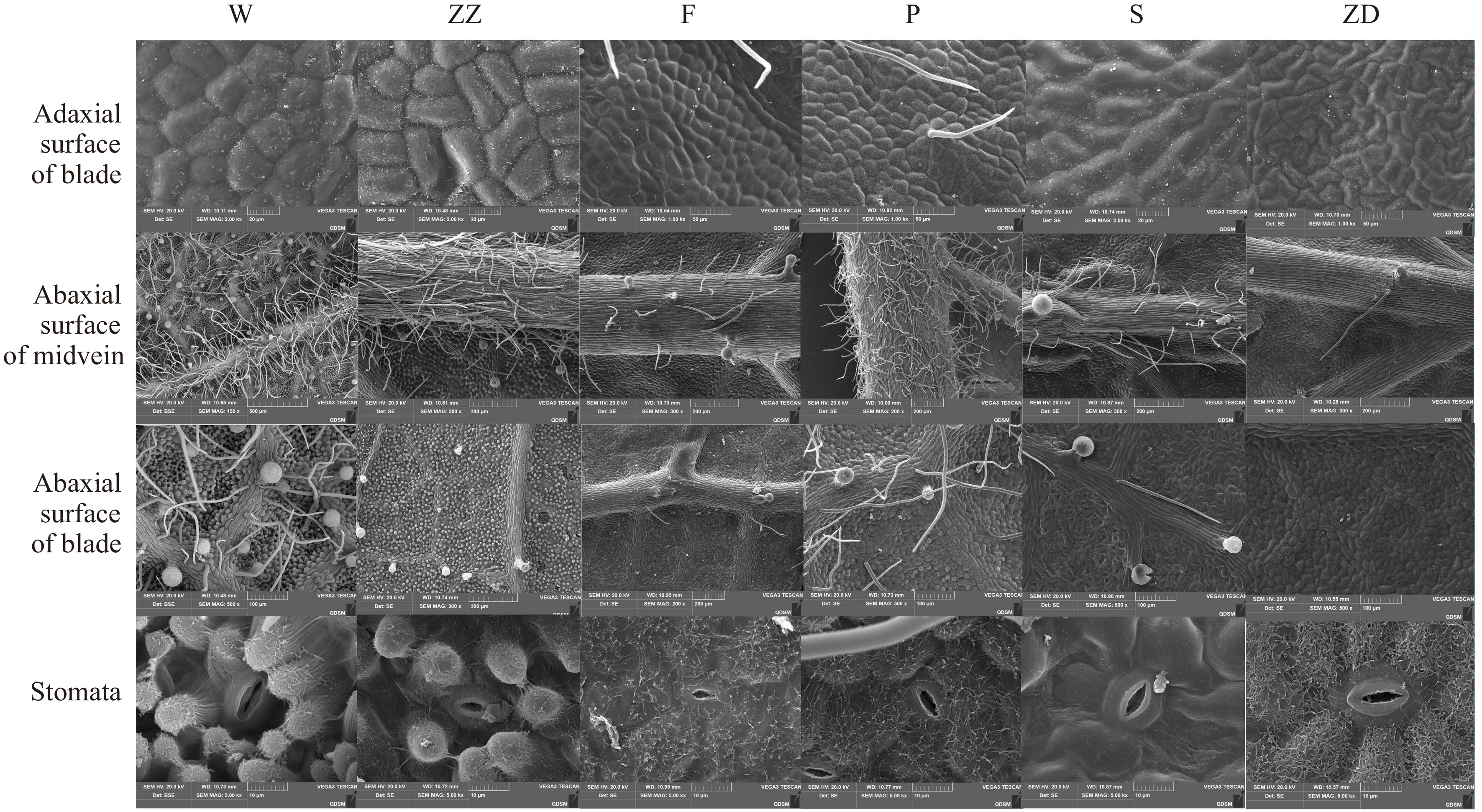
Figure 4.
Observation of leaf surface structure of six R. rugosa genotypes. W, Wild R. rugosa; ZZ, R. rugosa 'Zizhi'; F, R. rugosa 'Fenghua'; P, R. rugosa 'Plena'; S, R. sertata × R. rugosa; ZD, R. rugosa 'Ziduan'.
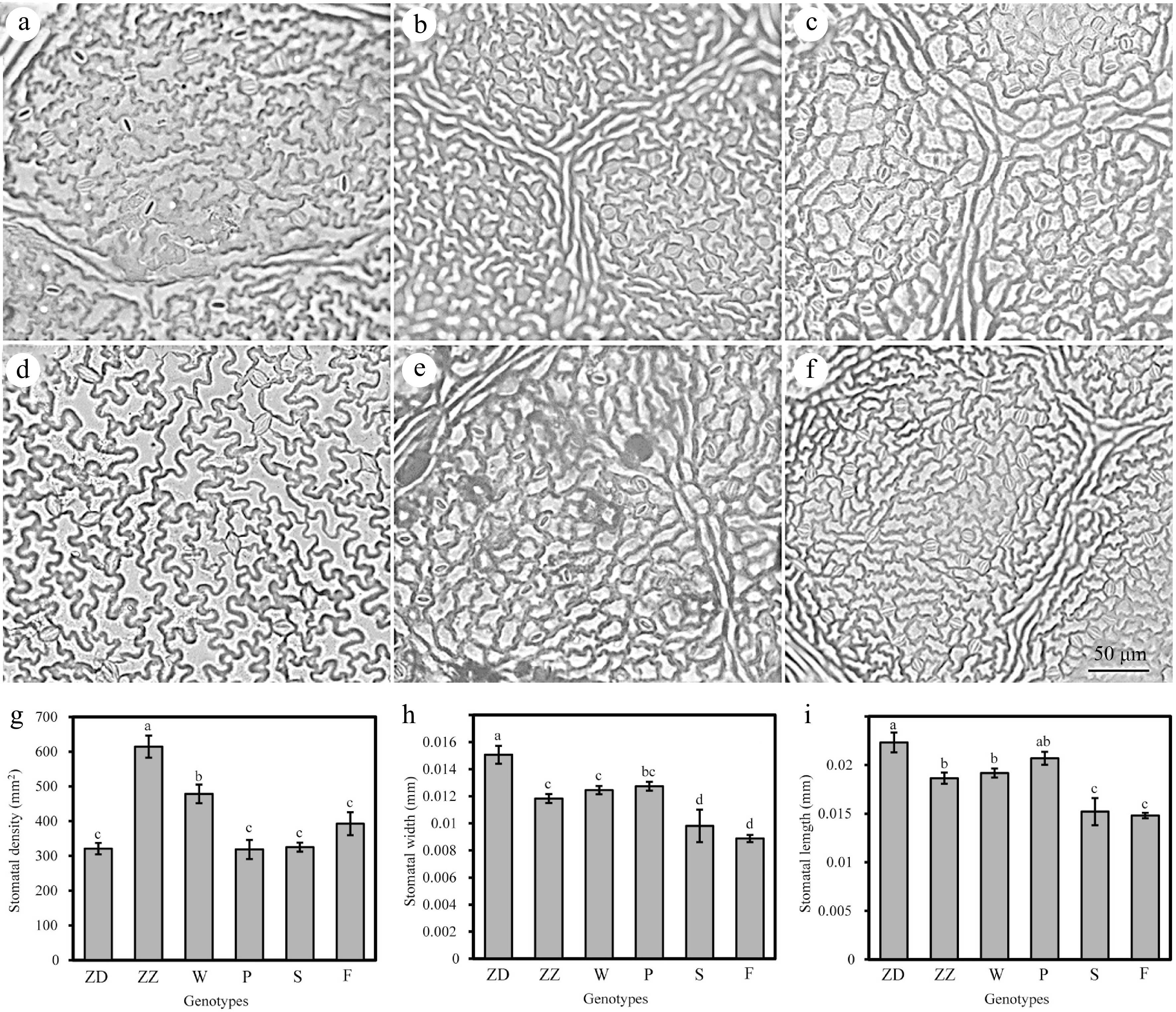
Figure 5.
Observation of lower epidermal cells and stomata in six genotypes of R. rugosa leaves. (a) Wild R. rugosa. (b) R. rugosa 'Zizhi'. (c) R. rugosa 'Fenghua'. (d) R. rugosa 'Plena'. (e) R. sertata × R. rugosa. (f) R. rugosa 'Ziduan'. (g) Stomatal density. (h) Stomatal width. (i) Stomatal length. Error bars represent standard error for 10 replicates. Different lowercase letters in the same column indicate significant difference at p < 0.05.
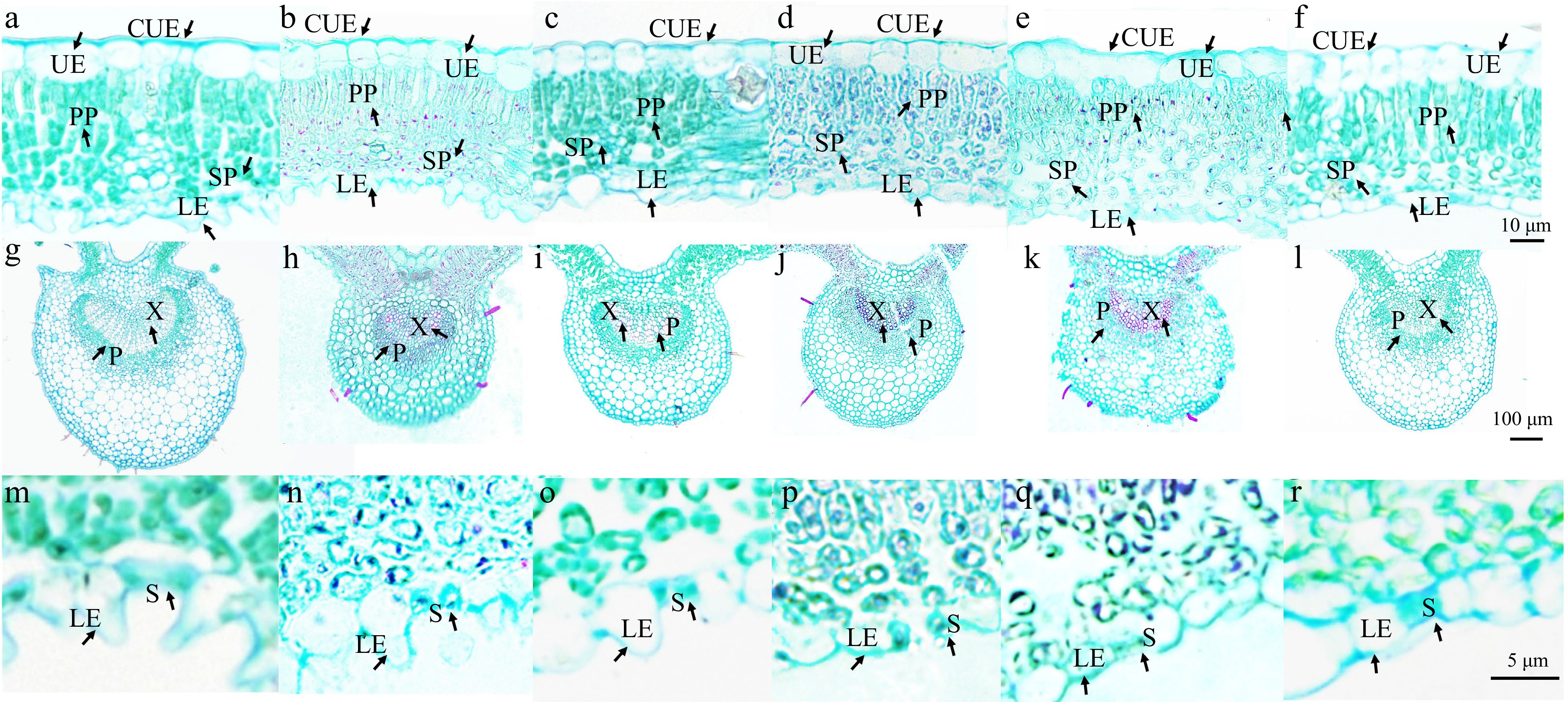
Figure 6.
Microstructure of the leaves of six R. rugosa genotypes. Leaf mesophyll tissue of: (a) wild R. rugosa; (b) R. rugosa 'Zizhi'; (c) R. sertata × R. rugosa; (d) R. rugosa 'Plena'; (e) R. rugosa 'Ziduan'; (f) R. rugosa 'Fenghua'. Leaf vein structure of: (g) wild R. rugosa; (h) R. rugosa 'Zizhi'; (i) R. sertata × R. rugosa; (j) R. rugosa 'Plena'; (k) R. rugosa 'Ziduan'; (l) R. rugosa 'Fenghua'. Lower epidermal cells and stomata of: (m) wild R. rugosa; (n) R. rugosa 'Zizhi'; (o) R. sertata × R. rugosa; (p) R. rugosa 'Plena'; (q) R. rugosa 'Ziduan'; (r) R. rugosa 'Fenghua'. CUE: Cuticle of upper epidermis; UE: Upper epidermis; LE: Lower epidermis; PP: Palisade; SP: Spongy; X: Xylem; P: Phloem; S: Stomatal apparatus.
It was observed that stomatal density and size could regulate the plant drought resistance by affecting the water transpiration rate of leaves. The lower epidermal cells of W and ZZ exhibited obvious tuberculous protrusion in the plain wall, causing the stomatal apparatus to sink into the lower epidermal cells. The outer walls of the other four genotypes of lower epidermal cells were microwave-like, and the stomatal organs and epidermal cells were almost in the same plane. However, the stomatal densities of ZZ and W (614.36 and 478.16 per mm2, respectively) were significantly higher than those of other genotypes. ZD had significantly larger stomatal length and width than other genotypes, whereas F had the smallest stomatal length and width. The stomatal apparatus type of all genotypes was cyclocytic; that is, the guard cell was surrounded by 6–9 cells in a ring (Fig. 4). The lower epidermal cells of the F and S were polygonal, whereas those of the other four genotypes were irregular.
Moreover, the lower epidermal cells of six R. rugosa varieties exhibited tuberculous protrusion in the plain wall, particularly in W and ZZ, causing the stomatal apparatus to sink into the lower epidermal cells (Figs 4, 6m & n). The ratio of the thickness and width of the lower epidermal cells can reflect the degree of the protrusion of the lower epidermal cells. It was significantly higher in W and ZZ than in other genotypes (Fig. 7h).
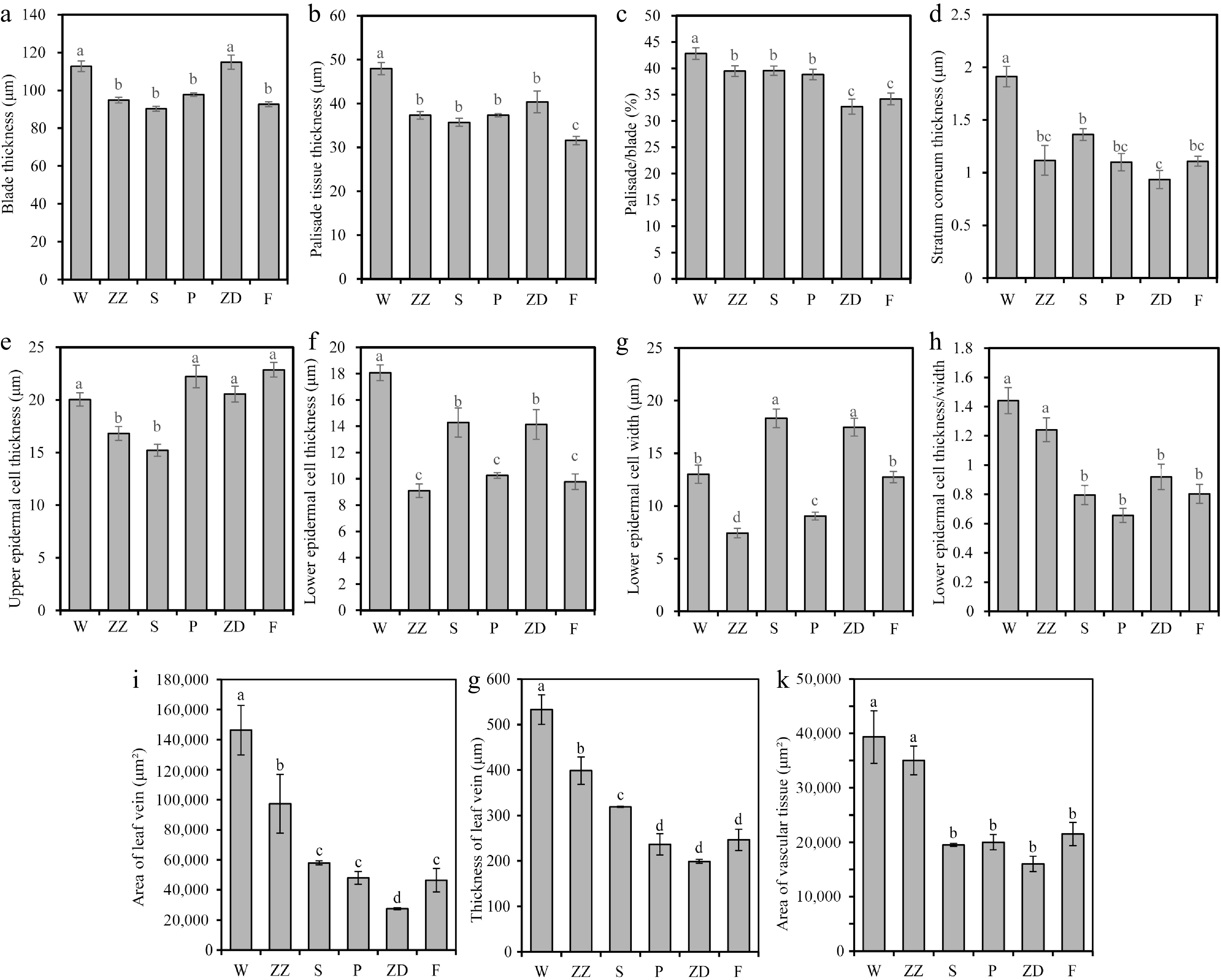
Figure 7.
Characteristics of leaf microstructure of six R. rugosa genotypes. (a) Blade thickness. (b) Palisade tissue thickness. (c) Palisade/blade. (d) Stratum corneum thickness. (e) Upper epidermal cell thickness. (f) Lower epidermal cell thickness. (g) Lower epidermal cell thickness. (h) Lower epidermal cell thickness/width. (i) Area of leaf vein. (j) Thickness of leaf vein. (k) Area of vascular tissue. W, Wild R. rugosa; ZZ, R. rugosa 'Zizhi'; F, R. rugosa 'Fenghua'; P, R. rugosa 'Plena'; S, R. sertata × R. rugosa; ZD, R. rugosa 'Ziduan'. Error bars represent standard error for 10 replicates. Different lowercase letters in the same column indicate significant difference at p < 0.05.
The leaf surface features, such as the shape of epidermal cells, the size of stomata, and the cuticle of epidermal cells, were significantly different among the six genotypes. All six R. rugosa genotypes had non-glandular and glandular hair in the lower epidermis. However, W had a lot of non-glandular hair and glandular hair in the leaf veins and leaves; ZZ only had glandular hair in the leaf veins and blade, and F, S, and ZD had non-glandular hair and glandular hair in the leaf veins but not in leaves. In addition, F and P had a small amount of non-glandular hair in the upper epidermal cells. The surface of the lower epidermal cells of all genotypes were rich in wax, except in S in which the surface of lower epidermal cells was smooth with less wax. These results indicated that the leaves of W and ZZ with strong drought resistance have more fur and wax in epidermal cells.
Anatomical structure of leaf vein and mesophyll tissue
-
The leaf thickness of W and ZD was significantly greater than that of other genotypes (Figs 6 & 7a). The palisade tissue of W was composed of 2–3 layers of cells, while that of ZZ, S, P, and F was composed of two layers of cells, and that of ZD was composed of 1–2 layers of cells (Fig. 6). The thickness and proportion of palisade tissue, thickness of the stratum corneum, and the thickness of lower epidermal cells in W were also significantly greater than those of other genotypes (Figs 6, 7b–d & f). The palisade tissue thickness in F was the lowest, and the proportion of palisade tissue in the leaves of F and ZD was significantly lower than that in the leaves of other genotypes (Fig. 7b & c).
Leaf veins are responsible for material transport, including water transport. The vein area, vascular bundle area, and vein thickness of W and ZZ were significantly larger than those of other genotypes, reflecting their stronger water transport capacity (Figs 6, 7i–k).
-
Plant plasma membrane plays key roles in regulating the flow of water, ions, and other substances as well as regulating drought signal transduction in the cell, to protect plants from drought stress. Under water stress, plants not only need to continue to absorb water from the medium with low water potential to maintain water balance in the body, but also need to keep cell turgor pressure unchanged to ensure the normal operation of physiological and biochemical processes in the body. Osmotic regulation is an important way to actively reduce osmotic potential. Under drought stress, organic (such as proline, soluble proteins, soluble sugars, free amino acids, etc.) and inorganic substances (such as K+, Ca2+, Mg2+, etc.) will accumulate in large quantities in plants[24,25]. The soluble protein as an osmoregulatory substance can reduce the osmotic potential and maintain the growth of cells. Except in F with poor drought resistance, the soluble protein contents of the five genotypes began to increase after 5% or 10% PEG treatments (Fig. 3c). Previous studies indicated that under PEG water stress, the addition of 50 mmol·L–1 NaCl could significantly increase the Na+ content in the leaves of Atriplex halimus, significantly reducing the leaf's osmotic potential, and significantly enhancing the osmotic regulation ability[26].
The stable structure and function of the plasma membrane are closely related to the drought tolerance of plants. Drought stress can cause peroxidation of plant cell membranes, resulting in the reduction or loss of selective permeability of cell membranes. MDA, as a product of membrane lipid peroxidation, can indicate the damage degree to the cell membrane[27]. Moreover, excessive accumulation of O2– can also damage plant cell membrane system and inhibit plant growth[28,29]. Under suitable environmental conditions, various antioxidant enzymes in the body can remove excessive O2– in the cell in time to maintain the normal metabolic activities of plant cells. The cell membrane of six genotypes was damaged under drought stress, as reflected by increased MDA and O2– contents in the leaves, which is consistent with the results of similar studies on many plants[30−32]. These results suggested that drought stress alters the equilibrium between free radical production and enzymatic defense reactions in R. rugosa. The contents of MDA and O2– can reflect the degree of damage to plant cells. However, the MDA and O2– contents were relatively lower in wild R. rugosa until 15% PEG treatment. This suggested that the wild R. rugosa is more resistant to drought than cultivated varieties.
Leaf RWC in R. rugosa in response to drought stress
-
The RWC of leaves can directly reflect the water loss from leaves under environmental stress. Under drought stress, the accumulation of soluble protein content in R. rugosa leaves cells can increase the concentration of cell fluid, reduce osmotic potential, and reduce water loss. The RWC of most R. rugosa genotypes sharply decreased, and soluble protein contents sharply increased under 10% PEG treatment. The transpiration rate of all genotypes decreased to less than 1 mmol·m−2·s−1, and the transpiration rate decreased to less than 1.5 µmol·m−2·s−1 under 15% PEG stress. Those results indicated that the leaves of most genotypes were more seriously damaged under 10% PEG stress. Compared with other genotypes, the RWC in W decreased slowly as the PEG concentration increased, indicating less water loss from the leaves.
Pn in R. rugosa in response to drought stress
-
Photosynthesis is a unique physiological function of green plants and is the basis for maintaining their growth and development. Drought stress can not only damage plant leaves but also affect plant growth by inhibiting photosynthesis. The Tr, RWC, and stomatal conductance were significantly higher in wild R. rugosa than in other varieties under 5% and 10% PEG treatments, which were the possible reasons why the Pn of W was higher than in other varieties. These results indicated that the leaves of W were less damaged under drought stress, and W was more drought-tolerant than other genotypes. Under 10% and 15% PEG treatments, RWC of F and ZD was the lowest, and their MDA and O2− contents were the highest, indicating poor drought tolerance. Considering all indicators, the drought resistance of wild R. rugosa was the strongest, followed by that of ZZ, S, and P. The drought resistance of ZD and F was the poorest.
Leaf structure affects drought resistance in R. rugosa
-
Leaf is an organ that is sensitive to environmental changes and has great plasticity during plant evolution. R. rugosa is an important component of sandy shrubland on temperate coasts. In this study, wild R. rugosa was introduced from the seaside in Weihai, Shandong Province, China, and grown in a dry, windy, and cold winter environment. The soil of the wild R. rugosa community was tested and was found to be alkaline with a salt content of 0.28% (Supplemental Table S1).
To adapt to the drought environment, wild R. rugosa leaves employ various strategies. First, compared with cultivars, wild R. rugosa has the most fur on the back of its leaves, including glandular hair and non-glandular hair. In addition, their epidermal cells are covered with a thick cuticle. The cuticle and epidermis both reflect sunlight and prevent water loss[33,34]. Second, wild R. rugosa has multiple layers of palisade tissue, and palisade cells are smaller and more closely arranged than those of cultivated varieties. Studies in Camellia plants have reported that palisade tissue thickness and palisade/spongy tissue were significantly positively correlated with Pn[35,36]. The stomata of the leaves of the six genotypes were all distributed in the lower epidermis of the leaves, indicating that they had a certain tolerance to drought. Among them, the periclinal wall of epidermal cell of W and ZZ caused stomatal depression, whereas the other four cultivars had no obvious protrusion in epidermal cells and stomatal depression (Figs 6, 7). In addition, the leaf stomatal densities of W and ZZ were significantly higher than those of other varieties; however, the Tr in CK was lower, and the WUE was also significantly higher than that in of other genotypes. Unlike the wild R. rugosa from Huichun (Jilin, China), R. rugosa 'ZiZhi', and R. sertata × R. rugosa, and the wild R. rugosa from Muping City (Shandong, China) had no photosynthetic siesta, which indicated that the wild R. rugosa from Shandong had strong photosynthetic capacity under high temperatures[37]. It was speculated that the developed palisade tissue and sunken stomata can reduce the transpiration intensity and increase the photosynthetic efficiency, resisting the drought and windy environment of the seaside.
-
In this study, the photosynthetic characteristics, physiological response, and blade structure characteristics of six R. rugosa genotypes were assessed under PEG treatment. Physiological analysis revealed that drought stress significantly decreased the leaf RWC of six R. rugosa genotypes. Under PEG treatment, the increase in MDA content was ranked as follows: F > ZD > P > S > ZZ > W. Additionally, with the increase in PEG concentration, the soluble protein contents in all varieties exhibited an increasing trend, except for F. In terms of photosynthetic characteristics, the Tr, Pn, and Gs of all genotypes decreased under drought stress. However, the Pn and Gs of W were significantly higher than those of other genotypes under 5% PEG treatment. To explore the adaptation characteristics of R. rugosa leaves to drought environment, the leaf surface and microstructure of six genotypes were observed. The stomatal densities of ZZ and W were significantly higher than those of other genotypes. The lower epidermal cells of W, ZZ, and S exhibited obvious tuberculous protrusion in the plain wall, causing the stomatal apparatus to sink into the lower epidermal cells. In addition, the palisade tissue proportion, cuticle thickness, and lower epidermal cell thickness of wild R. rugosa were significantly greater than those of other genotypes. The current study elucidated the difference in drought resistance in six R. rugosa genotypes, revealed the regulation mechanism of drought tolerance, and provided insights into the improved breeding efficiency of R. rugosa. Compared with cultivated varieties, wild R. rugosa have stronger drought resistance, utilized and is a precious germplasm resource for drought-resistant breeding.
-
The authors confirm contribution to the paper as follows: study conception and design: Li L; data collection: Li W, Ju Y, Lu Y; analysis and interpretation of results: Lv Z, Zhu H, Qian C; draft manuscript preparation: Li L, Li W. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This work was supported by the Subject of Key R & D Plan of Shandong Province (Major Scientific and Technological Innovation Project) 'Mining and Accurate Identification of Forest Tree Germplasm Resources' (2021LZGC02303), 'Innovation Team for Conservation and Utilization of Precious Tree Species Germplasm' project of the Department of Natural Resources of Shandong Province (LZYZZ202398).
-
The authors declare that they have no conflict of interest.
- Supplemental Table S1 Soil index of wild R. rugosa population in Weihai, Shandong, China.
- Supplemental Fig. S1 The relationship between field water holding capacity and soil water potential.
- Supplemental Fig. S2 Leaf chlorophyll contents in the leaves of six R. rugosa genotypes.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Li L, Zhu H, Ju Y, Lv Z, Qian C, et al. 2024. Comparison of microstructure and physiological response of the leaves of six Rosa rugosa genotypes under drought stress. Ornamental Plant Research 4: e016 doi: 10.48130/opr-0024-0014
Comparison of microstructure and physiological response of the leaves of six Rosa rugosa genotypes under drought stress
- Received: 19 December 2023
- Revised: 03 April 2024
- Accepted: 09 April 2024
- Published online: 03 June 2024
Abstract: Rosa rugosa is a deciduous shrub of the rosa family having ecological, economical, and ornamental values. In this study, the cutting seedlings of wild R. rugosa and five cultivated varieties were treated with polyethylene glycol-6000 (PEG-6000) solution to mimic drought stress. The leaf transpiration rate (Tr), photosynthetic rate (Pn), stomatal conductance (Gs), and the relative water content (RWC) of all genotypes decreased under drought stress. Importantly, the Tr in wild R. rugosa (W) was decreased by 29.42% under 5% PEG treatment, whereas that in other varieties was decreased by 57.63%–74.54%. Similarly, the Pn and Gs of genotype W were significantly higher in W than in other genotypes under 5% PEG treatment. The malondialdehyde (MDA) contents in five cultivated varieties were significantly increased under 5%–10% PEG treatments, and that in W was increased under 15% PEG treatment. In summary, the drought resistance of wild R. rugosa was the strongest, followed by that of ZZ, S, and P, and the drought resistance of ZD and F was the poorest. To explore the adaptation characteristics of R. rugosa leaves to drought stress, the leaf surface and microstructure of six genotypes were observed. The characteristics of the wild leaves such as sunken stomata, developed palisade tissue, and low stomatal density may reduce the water loss, resulting in significantly higher drought tolerance than the other five cultivated varieties. This study elucidated the difference of drought resistance among the six R. rugosa genotypes, and enhanced the understanding of the regulatory mechanisms of drought tolerance.
-
Key words:
- Drought stress /
- Physiological response /
- Blade structure /
- Rosa rugosa













