-
Tobacco (Nicotiana tabacum L.) as an industrial crop, is widely grown in Yunnan Province, China. Cold stress is a major factor affecting tobacco production[1]. When the temperature is lower than 10 to 13 °C, the plant growth will be seriously restricted in tobacco[2]. Especially, the poor seed germination and seedling establishment usually occur under cold stress in tobacco. Thus, understanding the mechanisms of seed germination under cold stress and developing the techniques to improve seed germination are important for tobacco production.
It has been reported that several genes regulate seed germination under cold stress in plants. For instance, heat shock protein 70 (HSP70) negatively regulates seed germination under cold stress by influencing abscisic acid (ABA) efflux from endosperm to embryo in Arabidopsis[3]. The ω-3 fatty acid desaturase gene Glycine max (GmFAD3A) of soybean positively regulates seed germination under cold stress by increasing proline content and antioxidant enzymes activities[4]. Cucumber qLTG1.1 candidate gene CsGAI regulates seed germination under cold stress involving gibberellic acid (GA) and ABA signaling pathways[5]. Overexpression of maize late embryogenesis abundant gene ZmLEA5C confers to transgenic tobacco cold tolerance[6]. However, the regulators of seed germination under cold stress are poorly understood in tobacco.
Isopropylmalate synthase (IPMS) catalyzes the committed step of leucine (Leu) biosynthesis[7]. It has been reported that OsIPMS1 regulates seed germination by influencing amino acid levels under normal and stress conditions in rice[8]. In tobacco, the expression of NtIPMS is positively correlated with the degree of seed ageing[9]. Amino acids play important roles on seed germination[10] and cold tolerance involving reactive oxygen species (ROS) homoeostasis[10−13]. Whether IPMS is involved in seed germination under cold stress through amino acid and ROS pathways is largely unknown in tobacco.
In this study, we revealed that NtIPMS regulates seed germination under cold stress possibly by influencing amino acid and ROS levels in tobacco. Our findings provide new insight into the mechanisms of NtIPMS regulating tobacco seed germination under cold stress. Further, our results also provide the clues to improve seed germination under cold stress by the exogenous applications of amino acids and H2O2 in tobacco.
-
The overexpression lines of NtIPMS were generated in tobacco cultivar Yunyan 87 background using an Agrobacterium tumefaciens mediated plant transformation approach[14]. Mature seeds were harvested for the following experiments.
Seed germination
-
Thirty seeds per replicate were used for seed germination. Briefly, seeds were germinated at 15 °C for 10 d, and 25 °C was used as the control. Meanwhile, exogenous applications of amino acids (100 μM Asp + Cys + Pro; 500 μM Asp + Cys + Pro, and 1 mM Asp + Cys + Pro) and H2O2 (0.05%) were conducted using Yunyan 87 (WT) and NtIPMS-OE-1 lines under 15 °C condition for 10 days. The germinated seeds were considered when the normal radicle protruded through seed coat, and the established seedlings were considered when the cotyledon unfurled. Three biological replications were conducted.
Quantitative real-time PCR assay
-
RNA extraction was conducted using the TransZol Plant kit (Transgen, www.transgen.com). The first-strand cDNA was synthesized using the HiScriptII Reverse Transcriptase system (Vazyme Biotech Co., Ltd.). Quantitative real-time PCR (qRT-PCR) assay was conducted under the following conditions: 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s. The NtEF1a gene was used as internal control (Supplemental Table S1). The comparative 2−ΔΔCᴛ method was used to calculate the normalized transcript levels[15]. Three biological replications were conducted.
Amino acid assay
-
Amino acid assay was conducted according to He et al.[16]. Briefly, 0.2 g seeds after 10 d germination under normal (25 °C) and cold stress (15 °C) conditions were used for amino acid assay. Amino acids were detected using the AAA L-8900 auto-amino acid analyzer (Hitachi Ltd., Japan). The content of amino acids was expressed as μg/g FW (fresh weight). Three biological replications were conducted.
Evaluation of reactive oxygen species
-
The O2·− and H2O2 content was evaluated using the nitroblue tetrazolium (NBT) staining[17] and 3,3'-diaminobenzidine (DAB) staining method, respectively[18]. The levels of O2•− and H2O2 were detected according to the manufacturer protocol (Beijing Solarbio Science & Technology Co., Ltd, China). Three biological replications were conducted.
Enzyme activity assay
-
The activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) were detected according to the manufacturer protocol (Beijing Solarbio Science & Technology Co., Ltd, China). Briefly, 0.1 g seeds after 10 d germination under normal (25 °C) and cold stress (15 °C) conditions were used for enzyme activity assay. The absorbance was measured using 96-well microplate reader (K LAB Co., Ltd, Korean). The enzyme activity was expressed as U/g FW (fresh weight). Three biological replications were conducted.
Data analysis
-
Two-tailed Student's t-test or one-way ANOVA were used to determine the significance of samples.
-
Seed germination of Yunyan 87 wild-type (WT) and overexpression NtIPMS lines (NtIPMS-OE1 and NtIPMS-OE2) (Supplemental Fig. S1) was conducted under normal (25 °C) and cold stress (15 °C) conditions. We observed that seed germination was not significantly different between WT and overexpression NtIPMS lines under normal condition (Fig. 1a). However, the overexpression of NtIPMS resulted in poor seed germination under cold stress; the germination percentage and percentage of seedling establishment in the overexpression NtIPMS lines were significantly reduced compared with WT plants (Fig. 1b). This suggested that NtIPMS involves in tobacco seed germination under cold stress.
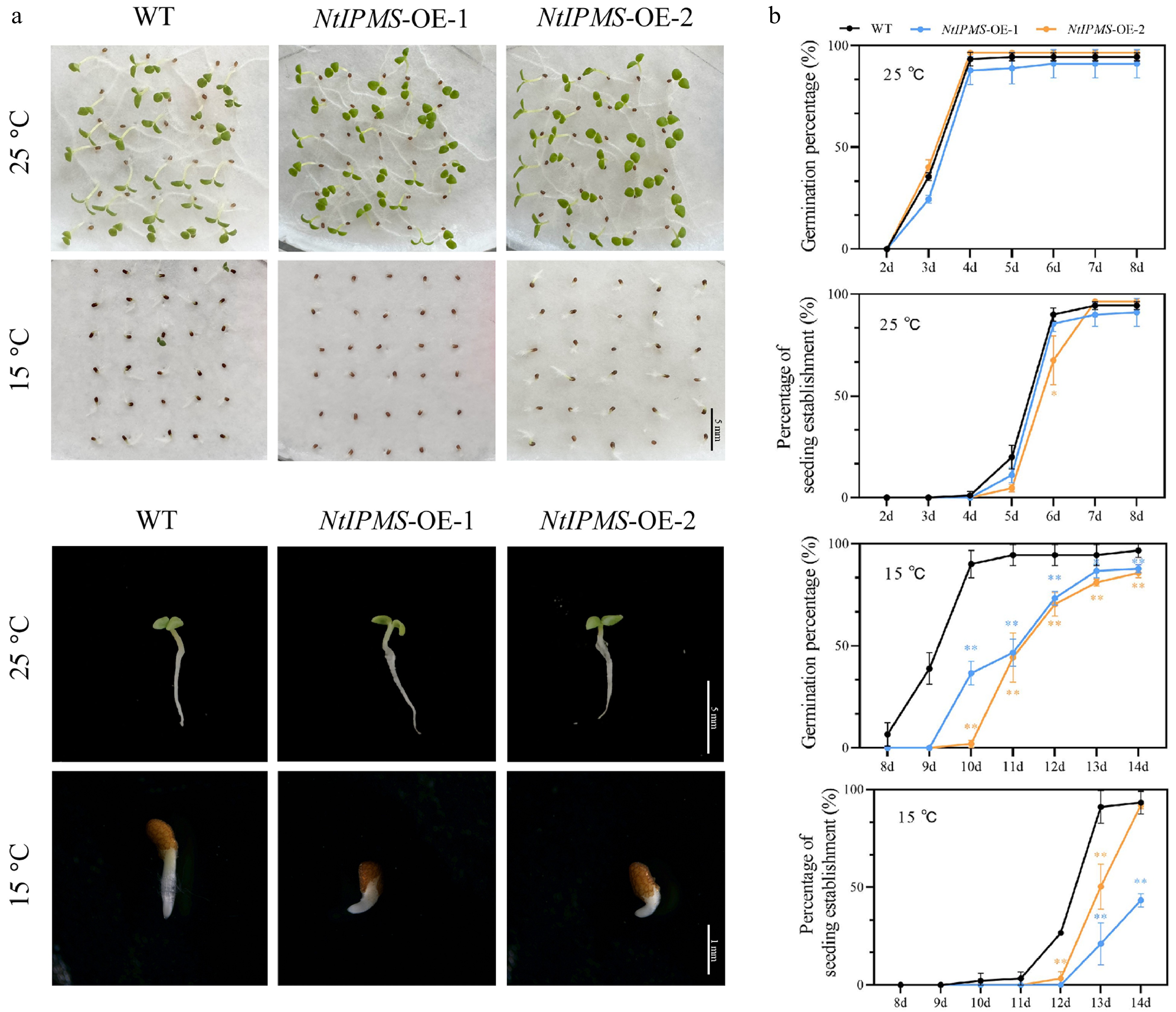
Figure 1.
Overexpression of NtIPMS reduces seed germination under cold stress in tobacco. (a) Representative images of seed germination under normal (25 °C) and cold stress (15 °C) conditions. Scale bars = 1 or 5 mm. (b) Comparison of germination percentage and percentage of seedling establishment in Yunyan 87 (WT) and NtIPMS-OE lines. Each point represents the mean ± standard deviation. n = 3. * and ** denote significant differences determined using Student's t-test at p < 0.05 and p < 0.01, respectively.
Expression pattern of NtIPMS during seed germination
-
qRT-PCR analysis showed that the expression of NtIPMS was gradually decreased during the early (0 to 72 h) seed gemination under normal (25 °C) and cold stress (15 °C) conditions (Fig. 2a). However, the expression of NtIPMS was gradually increased and then followed by the gradually decrease during the late (2 to 10 d) germination stage under normal (25 °C) and cold stress (15 °C) conditions (Fig. 2b). By comparison, the expression of NtIPMS was decreased during seed germination under cold stress compared with that of normal conditions.
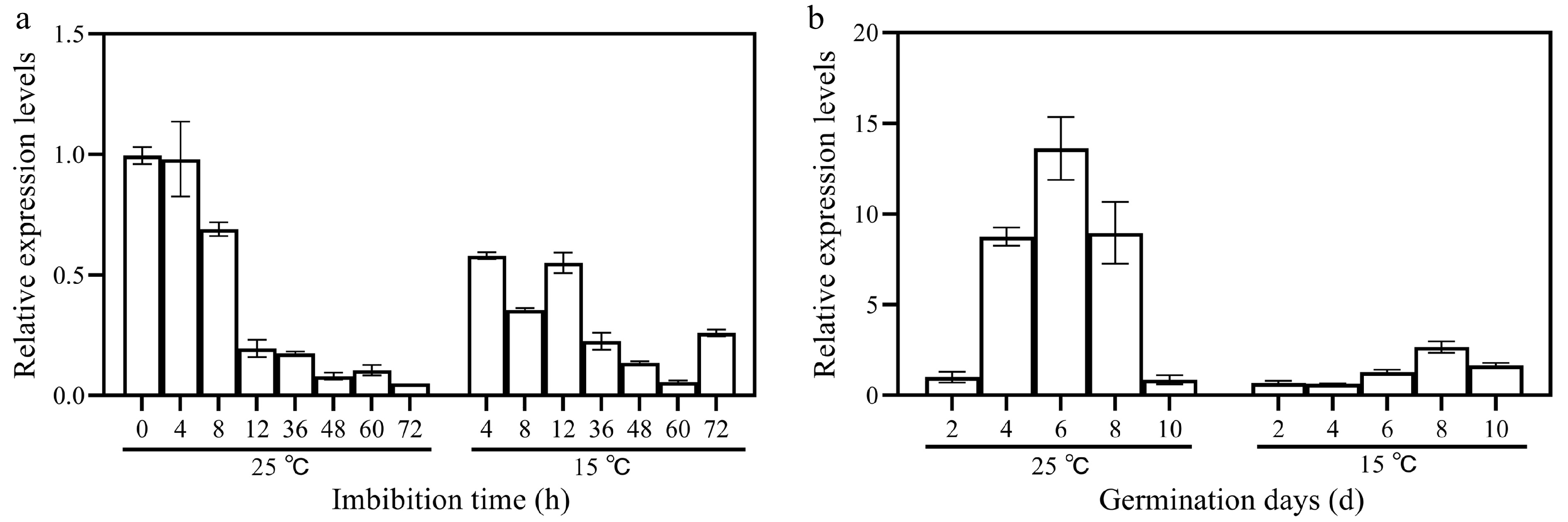
Figure 2.
Expression patterns of NtIPMS during seed germination under normal (25 °C) and cold stress (15 °C) conditions in tobacco. (a) Relative expression level of NtIPMS in Yunyan 87 tobacco during (a) the early germination stage and (b) the late germination stage determined via quantitative RT-PCR. NtEF1a gene was used as the internal control. Each column represents the mean ± standard deviation n = 3.
NtIPMS regulated seed germination involving amino acids under cold stress
-
It has been reported that IPMS involves in the regulation of amino acids in plants[7,8]. We observed that the levels of most amino acids were not significantly different between NtIPMS-OE-1 and WT line after 10 d germination under normal (25 °C) condition (Fig. 3). However, the levels of most amino acids were significantly increased in NtIPMS-OE-1 under cold (15 °C) stress, and the stress-associated amino acids, including asparatic acid (Asp), cysteine (Cys), and proline (Pro), were significantly decreased. It suggested that the reduction of stress-associated amino acids in NtIPMS-OE-1 may result in poor seed germination under cold stress. To confirm this hypothesis, the effects of amino acids (including Asp, Cys, and Pro) treatment on seed germination of WT and NtIPMS-OE-1 lines were determined. Expectedly, seedling growth of NtIPMS-OE-1 was nearly rescued by exogenous application of amino acids compared to that in WT plants (Fig. 4). It suggested that NtIPMS regulates seed germination involving amino acid pathway under cold stress.
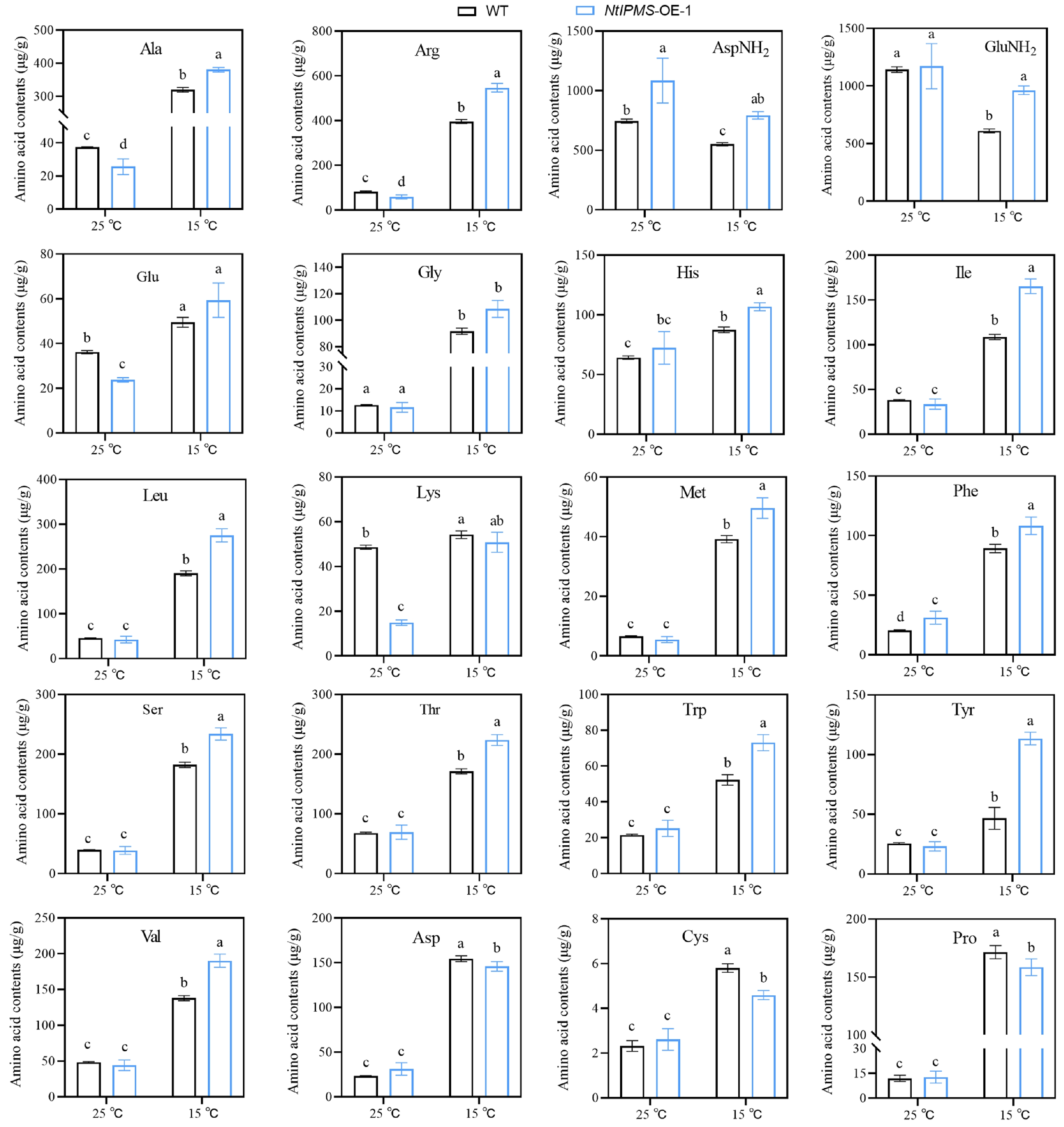
Figure 3.
Comparison of amino acids between Yunyan 87 (WT) and NtIPMS-OE-1 lines after 10 d germination under normal (25 °C) and cold stress (15 °C) conditions in tobacco. Each column represents the mean ± standard deviation. n = 3. Different letters indicate significant differences determined using Duncan's multiple range test: p < 0.05.
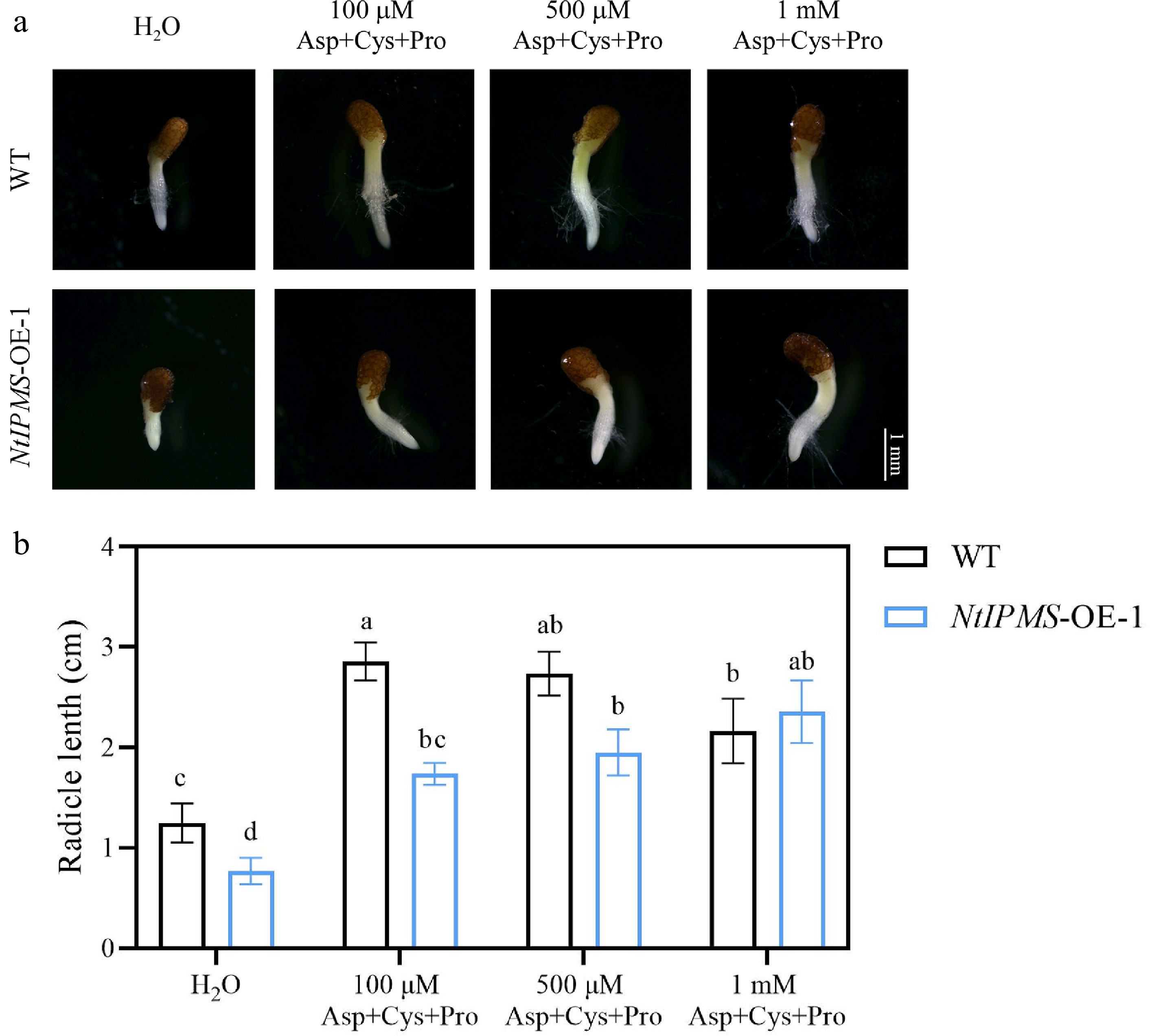
Figure 4.
NtIPMS regulates seed germination involving amino acid pathway under cold stress (15 °C) in tobacco. (a) Representative images of seed germination by exogenous amino acid (Asp, Cys, and Pro) treatment for 10 d in Yunyan 87 (WT) and NtIPMS-OE-1 lines under cold stress. Scale bars = 1 mm. (b) Comparison of radicle length between WT and NtIPMS-OE-1 lines under cold stress after amino acids treatment. Each column represents the mean ± standard deviation. n = 3. Different letters indicate significant differences determined using Duncan's multiple range test: p < 0.05.
NtIPMS regulated seed germination involving ROS pathway under cold stress
-
Amino acids have been reported involving antioxidative metabolism in plants[19,20]. By comparison, no significant differences of NBT and DAB staining color were observed between NtIPMS-OE-1 and WT lines under normal (25 °C) condition (Fig. 5a). However, a thin NBT and DAB staining color was observed in NtIPMS-OE-1 compared with that of WT line under cold stress (15 °C) (Fig. 5a). Expectedly, the significantly lower accumulations of O2•− and H2O2 were observed in NtIPMS-OE-1 line compared with that of WT under cold stress (Fig. 5b). It suggested that the reduction of ROS in NtIPMS-OE-1 may result in its poor seed germination under cold stress. To further confirm this, the effects of exogenous H2O2 upon seed germination of WT and NtIPMS-OE-1 lines were determined. Expectedly, seedling growth of NtIPMS-OE-1 was nearly rescued by exogenous application of H2O2 compared to that in WT plants (Fig. 5c). Moreover, the significantly higher activities of SOD, CAT, and POD were observed in NtIPMS-OE-1 compared with those of WT under cold stress (Fig. 6). We thus assumed that NtIPMS regulates seed germination involving ROS pathway under cold stress.
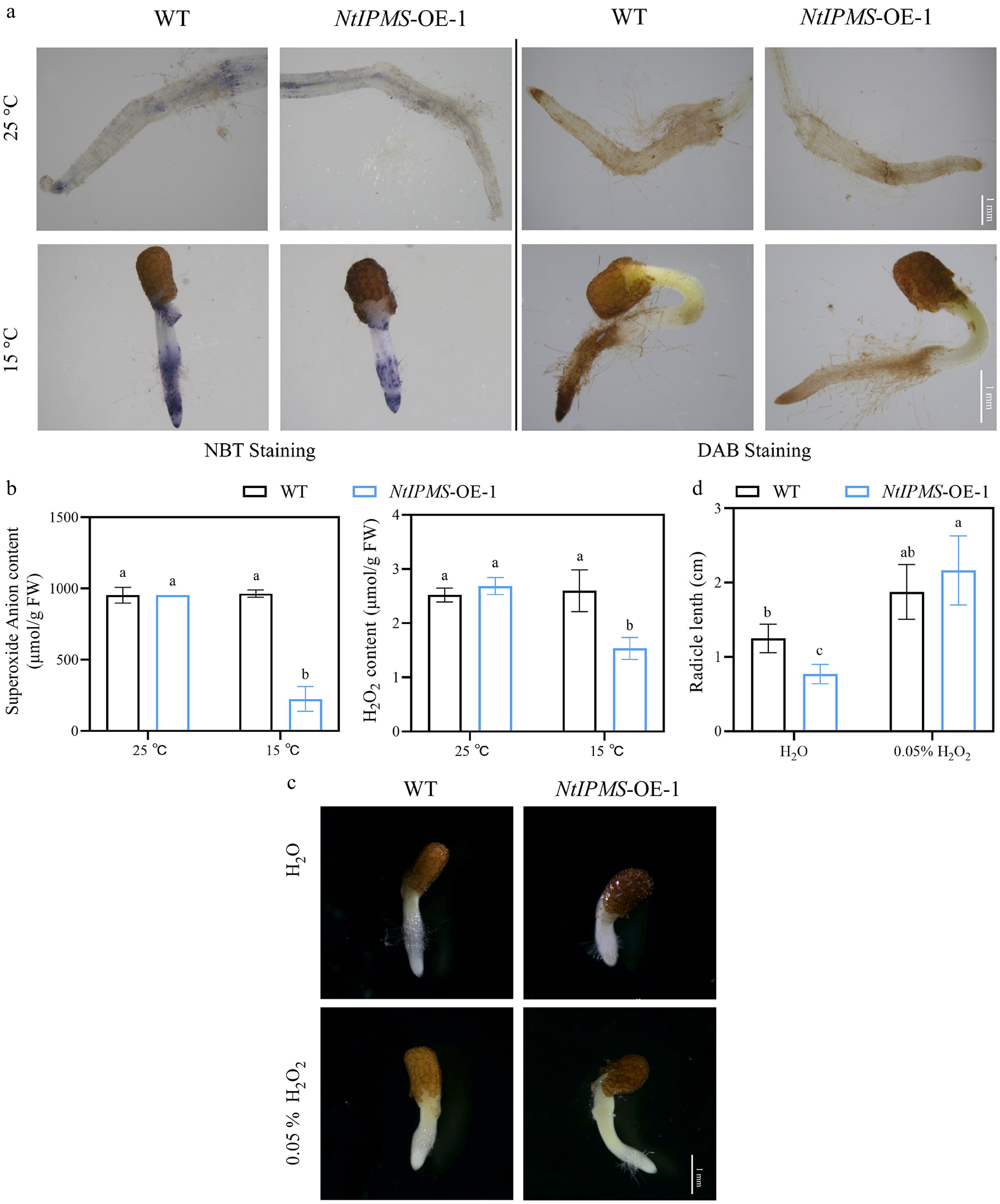
Figure 5.
NtIPMS regulates seed germination involving ROS pathway under cold stress (15 °C) in tobacco. (a) Representative images of NBT staining for O2•− content and DAB staining for H2O2 content. Scale bars = 1 mm. (b) Comparison of O2•− and H2O2 content between Yunyan 87 (WT) and NtIPMS-OE-1 line under normal (25 °C) and cold stress (15 °C) conditions. (c) Representative images of seed germination by exogenous H2O2 treatment for 10 d in Yunyan 87 (WT) and NtIPMS-OE-1 line under cold stress. Scale bars = 1 mm. (d) Comparison of radicle length between WT and NtIPMS-OE-1 lines under cold stress after H2O2 treatment. Each column represents the mean ± standard deviation, n = 3. Different letters indicate significant differences determined using Duncan's multiple range test: p < 0.05.
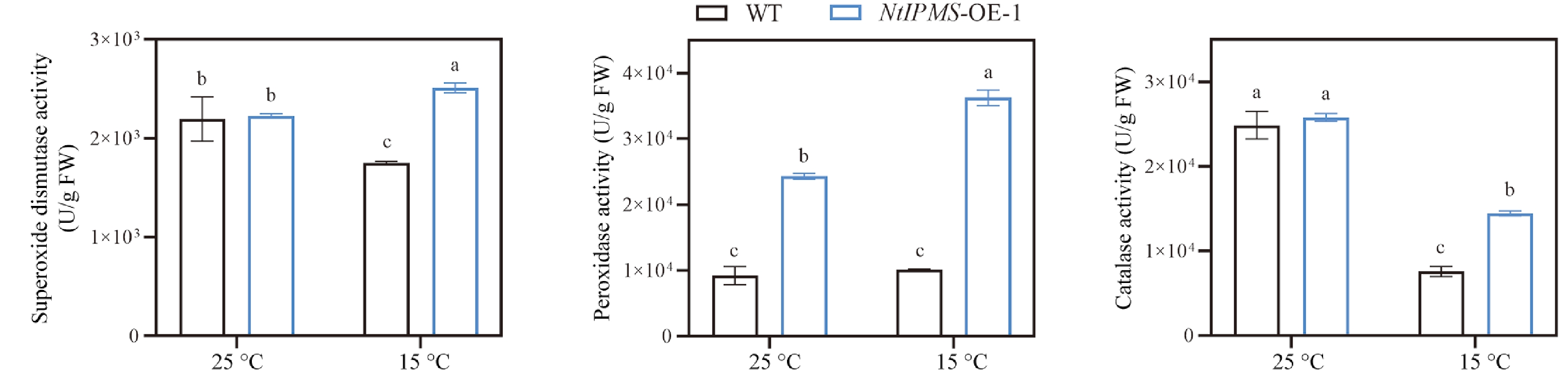
Figure 6.
Comparison of the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) between Yunyan 87 (WT) and NtIPMS-OE-1 lines under normal (25 °C) and cold stress (15 °C) conditions. Each column represents the mean ± standard deviation, n = 3. Different letters indicate significant differences determined using Duncan's multiple range test: p < 0.05.
-
Cold stress is one of the primary constraints to tobacco production during the whole growing season in the Yunnan Province of China[1]. Especially, seed germination and seedling establishment is seriously reduced under cold stress in tobacco. Similar with the previous studies[21], we observed that seed germination and seedling establishment of tobacco were significantly decreased under cold stress in this study. However, the regulatory mechanisms of seed germination under cold stress are limitedly understood in tobacco. In this study, one candidate gene NtIPMS was successfully identified that negatively regulates tobacco seed germination under cold stress. Our data showed that NtIPMS regulates tobacco seed germination under cold stress involving in amino acid and ROS pathways. These results will provide the theoretical and technical supports for the improvement of tobacco cold tolerance in the future.
The analysis of gene expression allows us to reveal the gene roles on seed germination. In this study, we observed that the expression of NtIPMS was gradually decreased during the early (0 to 72 h) gemination stage. We speculate that the transcripts of NtIPMS may be accumulated during seed development for the early seed germination. However, the expression of NtIPMS was gradually increased during the late (4 to 8 d) gemination stage. It suggests that the expression of NtIPMS may be important for the establishment of seedling during the late germination stage. These assumes need to be further investigated in the future. By comparison, the significantly lower expression of NtIPMS was observed in the germinating seeds under cold stress than that of normal condition. The reason might be that the delayed germination process was occurred under cold stress in tobacco; the high expression of NtIPMS is existed at the late germination stage when the seedling is established.
IPMS has been reported involving the regulation of amino acids in plants[7,8]. Similarly, we observed that the levels of most amino acids were significantly increased in overexpression NtIPMS line compared with WT under cold stress. However, three amino acids, including Asp, Cys, and Pro, that associated with stress tolerance were significantly reduced in NtIPMS overexpression line. The accumulation of amino acids such as Pro is effective way to enhance seed germination under stress conditions[22]. We assume that the low accumulation of Asp, Cys, and Pro in overexpression NtIPMS line may cause its poor seed germination under cold stress. It was further confirmed by the exogenous application of Asp, Cys, and Pro that could rescue the phenotype of NtIPMS overexpression line under cold stress. We thus speculate that NtIPMS regulates seed germination involving in amino acids under cold stress. However, the mechanisms of amino acids on seed germination under cold stress need to be further investigated.
Several researchers have revealed that amino acids are involved in antioxidative metabolism in plants. For example, amino acids, such as Cys, Pro, and gamma-aminobutyric acid (GABA), enhance seed germination or stress tolerance by influencing the activities of antioxidant enzymes and the ROS contents in plants[19,20]. In this study, we observed that the antioxidant enzymes, such as SOD, CAT, and POD, were activated in NtIPMS overexpression line, which may cause its low O2•− and H2O2 levels under cold stress. Usually, the excessive ROS will damage seed germination[23,24], while the low ROS level will promote seed germination[13,25] under normal conditions. Under cold stress conditions, the accumulation of ROS will activate plant defenses in plants[26]. We therefore assume that a relatively high accumulation of ROS will contribute seed germination under cold stress in tobacco. How to ensure a suitable ROS level by NtIPMS during tobacco seed germination under cold stress remains unclear in this study.
-
In conclusion, we revealed that tobacco NtIPMS regulates seed germination under cold stress involving amino acid and ROS pathways. Our results also provide the clues that the exogenous applications of amino acids and H2O2 contribute tobacco seed germination under cold stress. However, the regulatory mechanisms of amino acids and ROS levels by NtIPMS during seed germination under cold stress require further examination in tobacco.
-
The authors confirm contribution to the paper as follows: study conception and design: Wang Z, Zheng Y; data collection: Niu Y, Wang C, Wu Z, Wang D; draft manuscript preparation: Wang Z, Wang C. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article and its supplementary information files.
This research was funded by the Science and Technology Department of China National Tobacco Corporation Yunnan Company (No. 2021530000242032; 2023530000241007).
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Yongzhi Niu, Chengjing Wang
- Supplemental Table S1 The primer pairs used in this study.
- Supplemental Fig. S1 The relative expression of NtIPMS in overexpression lines. Relative expression level of NtIPMS in Yunyan 87 (WT) and NtIPMS-OE lines determined via quantitative RT-PCR. NtEF1a gene was used as the internal control. Each column represents the mean ± standard deviation. n = 3. * and ** denote significant differences determined using Student′s t-test at P<0.05 and P<0.01, respectively.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Hainan Yazhou Bay Seed Laboratory. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Niu Y, Wang C, Wu Z, Wang D, Suo W, et al. 2024. Overexpression of NtIPMS reduces tobacco seed germination under cold stress by influencing amino acids and reactive oxygen species. Seed Biology 3: e005 doi: 10.48130/seedbio-0024-0004
Overexpression of NtIPMS reduces tobacco seed germination under cold stress by influencing amino acids and reactive oxygen species
- Received: 30 January 2024
- Revised: 29 February 2024
- Accepted: 07 March 2024
- Published online: 15 April 2024
Abstract: Cold tolerance at the seed germination stage is a critical trait for tobacco production. However, the mechanism of seed germination under cold stress is largely unknown in tobacco. In this study, we revealed that tobacco isopropylmalate synthase gene NtIPMS negatively regulates seed germination under cold stress. Compared with wild-type (WT) of tobacco, the seed germination and seedling establishment were significantly reduced in overexpressing NtIPMS lines under cold stress. Physiological assays showed that NtIPMS regulates seed germination by influencing the levels of amino acids and reactive oxygen species (ROS) under cold stress. The lower accumulation of stress-associated amino acids and ROS were observed in overexpression NtIPMS line compared with WT under cold stress. The exogenous application of amino acids and hydrogen peroxide (H2O2) may be an effective way to enhance seed germination under cold stress in tobacco.
-
Key words:
- Amino acids /
- Isopropylmalate synthase /
- Reactive oxygen species /
- Cold stress /
- Seed germination /
- Tobacco














