-
Wheat (Triticum aestivum L.) and maize (Zea mays L.) are the most consumed cereals in the world[1]. Because both species are naturally sensitive to aluminum (Al) toxicity[2], genetic improvement programs have been trying to develop Al-tolerant cultivars. Al3+ is present in toxic concentrations in 40% of the agricultural soils across the globe, limiting the yield of Al-sensitive cultivars such as wheat and maize[3]. The highly weathered soils of tropical and subtropical regions generally have high acidity, toxic levels of Al, and low reserves of phosphorus (P), calcium (Ca), magnesium (Mg), and potassium (K)[4,5]. In Brazil, acid soils predominate in the North and Midwest regions, followed by the Southeast and South regions[6].
When grown in acid soils, Al-sensitive plants respond with morphological, physiological, and biochemical changes[7]. An irreversible consequence of the toxic effects of Al is the cessation of root growth. At the root apex, cell division is blocked, which alters root elongation, increases cell wall rigidity, and consequently interferes with water and nutrient uptake efficiency in plants and impairs grain production[8−14]. A reduction in root length of maize plants with increased exposure time to Al due to the inhibition of both cell division and elongation was observed by Batista et al.[15]. Thus, plant height and dry mass of both roots and shoots were also affected. According to Clark[16], morphological changes caused by Al can lead to nutritional disorders with reduced levels of P, Ca, Mg, and K in plant tissues.
The reduction in Mg content in the maize root because of Al toxicity was more pronounced than that of other nutrients[16]. Since Mg has a positive effect on root growth, low Mg content can be an important response in plants sensitive to Al. Al induces the deposition of callose in the plasmodesmata channels, physically inhibiting symplastic transport between cells and promoting nutritional deficiencies[17]. Several studies suggest that the main toxic effect of Al in plants is due to an Al-induced Mg deficiency caused by a blockage in Mg transport[18−20].
Mg deficiency exerts a significant influence on the partitioning of dry matter and carbohydrates between the aboveground and belowground parts. Stress due to Mg deficiency causes a sharp increase in the aboveground-to-root dry mass ratio, which is associated with massive accumulation of carbohydrates in the leaves, especially sucrose and starch[21]. These effects are indicative of severe impairment in the export of photoassimilates by the leaves caused by Al toxicity.
In plant cells, Mg2+ ions play a specific role in the activation of enzymes involved in respiration, photosynthesis, and nucleic acid (DNA and RNA) synthesis. They are required by many enzymes in phosphate transfer, including the enzymes ATPases, ribulose-1,5-diphosphate (RuDP) carboxylase, RNA polymerase, and protein kinases. In addition, Mg2+ ions play an important role as a central constituent of the chlorophyll molecule[22,23].
Considering that Mg has an important role in reducing Al phytotoxicity[2,24,25], the objective of this study was to evaluate the root growth of maize and wheat genotypes with different sensitivities to Al in a minimal solution containing different combinations of Al and Mg. It was hypothesized that (i) the minimal solution method with 0.15 mmolc L−1 of Al is efficient to classify Al-tolerant maize and wheat genotypes; (ii) Al-sensitive wheat and maize genotypes show a greater reduction in root length compared to Al-tolerant genotypes when subjected to a solution containing a toxic level of Al; (iii) the presence of Mg in the solution, especially at higher concentrations, alleviates the toxic effects of Al on root growth of wheat and maize genotypes; and (iv) the alleviation of Al toxicity by the addition of Mg is more pronounced in Al-sensitive than Al-tolerant wheat and maize genotypes.
-
The study was conducted at the Plant Breeding Laboratory of the State University of Ponta Grossa, Paraná, Brazil. Two experiments were carried out, one with wheat and another with maize. A randomized complete block design in a split-plot arrangement was used, and the treatments were replicated three times in both experiments. Four combinations of Al and Mg in minimum solution were used in the main-plot treatments. The subplot treatments consisted of four wheat genotypes in the first experiment and four maize genotypes in the second experiment. Both experiments included Al-sensitive and Al-tolerant genotypes.
All plots received 2 mmolc L−1 of Ca. The main-plot treatments were: (i) control (without Al and Mg); (ii) with 0.15 mmolc L−1 of Al; (iii) with 0.15 mmolc L−1 of Al plus 2 mmolc L−1 of Mg; and (iv) with 0.15 mmolc L−1 of Al plus 10 mmolc L−1 of Mg. The Al and Mg concentrations were based on the study by Silva et al.[26].
The wheat genotypes used in the subplot treatments of the first experiment were: cultivar BH 1146 (tolerant), cultivar Anahuac (sensitive), line L01, and line L14. The cultivars BH 1146 and Anahuac were provided by the seed bank of the Agronomic Institute of Campinas (IAC). The lines L01 and L14 were provided by the seed bank of the Plant Breeding Program of the State University of Ponta Grossa. The L14 line is more sensitive to Al than the L01 line[27].
The maize genotypes used in the subplot treatments of the second experiment were: line Al 237 (tolerant), line Al 53 (sensitive), and two hybrids with unknown Al-tolerance (AG 9025 PRO3 and K 9606 VIP3). The two inbred maize lines (tolerant and sensitive to Al) were provided by the seed bank of the Brazilian Agricultural Research Corporation (EMBRAPA).
Experiment management and evaluation
-
The minimum solutions were prepared in rectangular polyethylene pots with a capacity of 8 L of solution. The Ca, Al, and Mg sources used to prepare the solutions were CaCl2.H2O, AlCl3.6H2O, and MgCl2.6H2O, respectively. The seeds of different wheat and maize genotypes were placed to germinate on paper rolls Germitest® in a germination chamber for 64 h at 23 °C and 72 h at 25 °C, respectively, with 100% relative humidity and a 24-h photoperiod.
The germinated seeds of wheat and maize were removed from the Germitest® paper and selected for similar size of the primary root. During the selection process, the seedlings were sprayed with water to prevent root dehydration. The initial measurement (IM, in cm) of the primary root length was taken using a ruler, considering the value from the seed to the apex of the primary root. The selected seedlings were transferred to expanded polystyrene trays with 96 cells (12 × 8 cells), each with a hole in the bottom to allow contact of the primary root with the treatment solution.
The seedlings were distributed in two rows of 12 cells for each genotype so that each subplot was composed of 24 seedlings. All trays in each block were placed in their respective solutions at the same time and remained immersed for 48 h. Aeration was uniform and constant throughout the experimental period, assisted by an oxygenation pump. The pH of all solutions was measured during the immersion period and, when necessary, adjusted to a value of 4.3. The adjustment was made using NaOH solutions (to raise pH) or HCl (to lower pH). After the 48-h-exposure period, the trays of minimal solutions were removed and the measurement of the length of the main root of all seedlings was again taken with a ruler, which was considered the final measurement (FM, in cm). The difference in root length (DIF, in cm) was calculated by subtracting the FM from the IM, based on the method proposed by Mazzocato et al.[28].
Statistical analysis
-
The results were subjected to analysis of variance (ANOVA), and the effects of treatments were compared by the LSD test at p < 0.01. When there was a significant interaction effect of treatments with Al and Mg solutions (main plots) and wheat or maize genotypes (subplots), the necessary unfolding was performed. The statistical analyses were carried out using the SISVAR software[29].
-
ANOVA showed a significant interaction effect of the treatments involving solution containing Al and Mg (S) and plant genotypes (G) on DIF (Table 1).
Table 1. ANOVA F-test probabilities for the effects of solution containing Al and Mg, wheat and maize genotypes and their interactions effects on difference in root length (DIF).
Crop Source F-probability Wheat Solution (S) < 0.001 Genotype (G) < 0.001 S × G < 0.001 Maize Solution (S) 0.001 Genotype (G) < 0.001 S × G < 0.001 Overall, all four wheat (Fig. 1) and maize (Fig. 2) genotypes showed a reduction in DIF with the addition of 0.15 mmolc L−1 of Al in the solution.
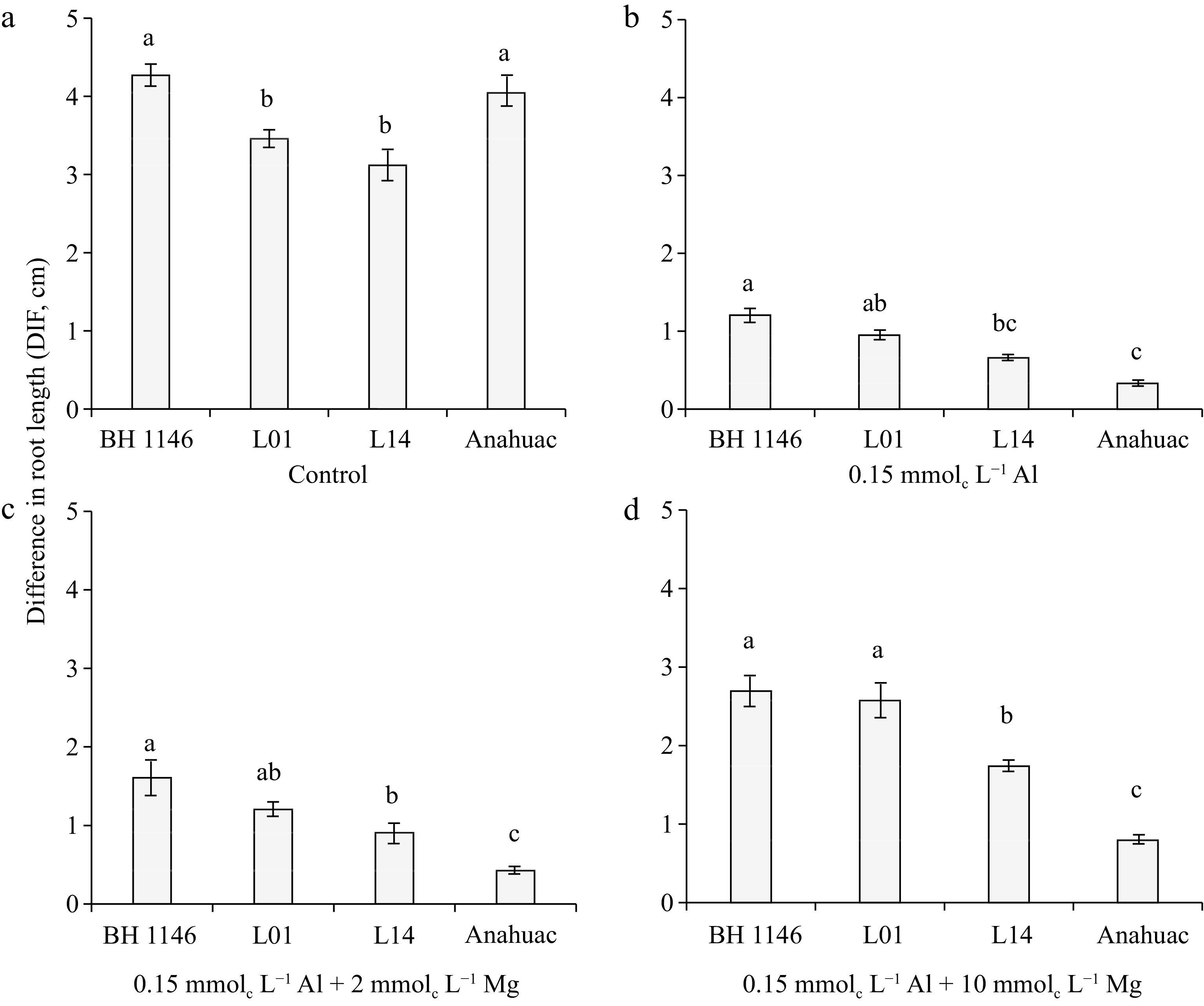
Figure 1.
Differential behavior of wheat genotypes within each minimal solution treatment: (a) control, (b) 0.15 mmolc L−1 of Al, (c) 0.15 mmolc L−1 of Al + 2 mmolc L−1 of Mg, and (d) 0.15 mmolc L−1 of Al + 10 mmolc L−1 of Mg, for the variable DIF after 48 h of exposure. Means followed by the same letters do not differ significantly by the LSD test at p < 0.01. Error bars express the standard error of the mean (n = 3).
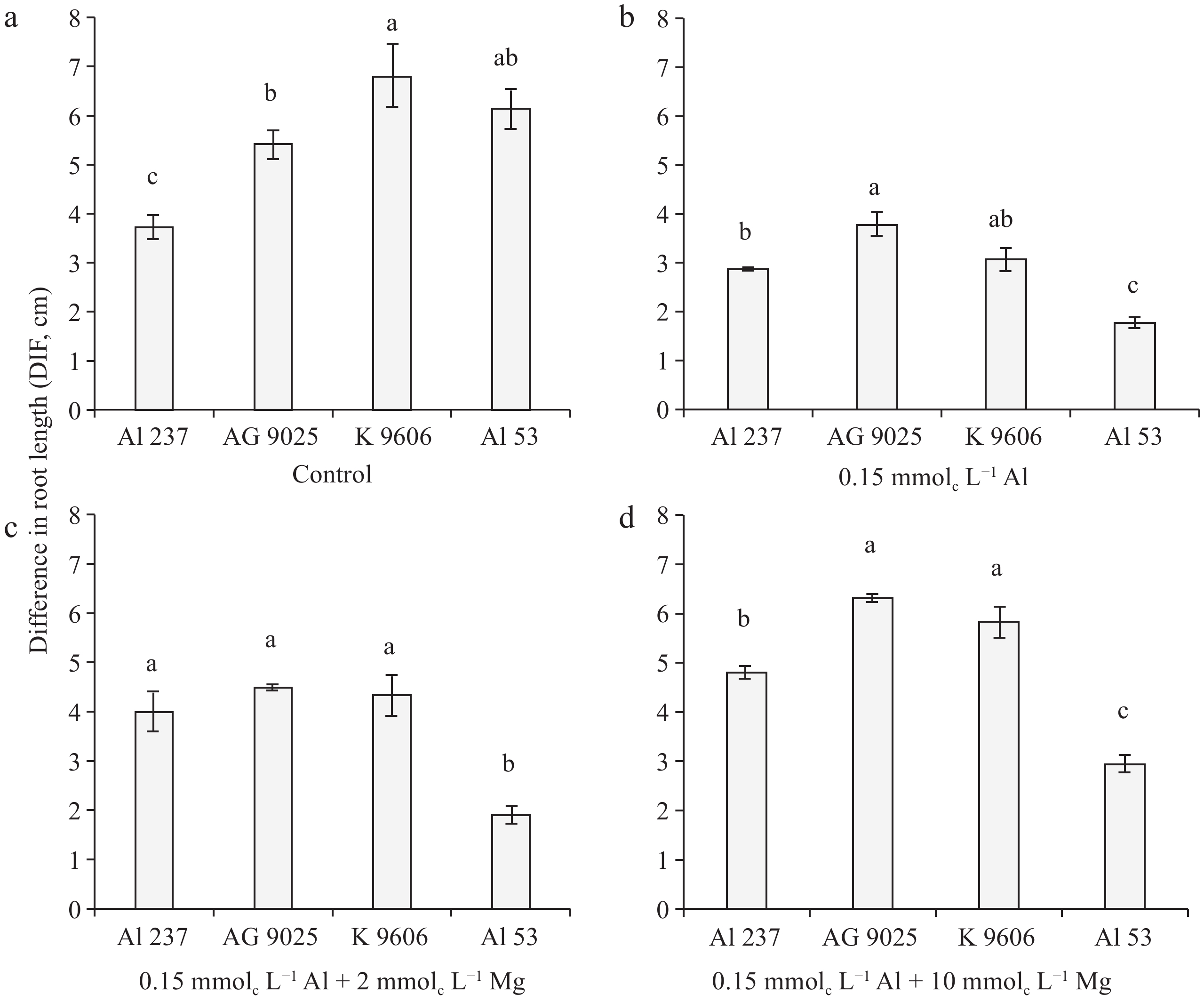
Figure 2.
Differential behavior of maize genotypes within each treatment in minimum solution: (a) control, (b) 0.15 mmolc L−1 of Al, (c) 0.15 mmolc L−1 of Al + 2 mmolc L−1 of Mg, and (d) 0.15 mmolc L−1 of Al + 10 mmolc L−1 of Mg, for the DIF variable after 48 h of exposure. Means followed by the same letters do not differ significantly by the LSD test at p < 0.01. Error bars express the standard error of the mean (n = 3).
In the control treatment without Al, wheat cultivars BH 1146 and Anahuac showed significantly higher DIF values than the L01 and L14 lines (Fig. 1a). When 0.15 mmolc L−1 of Al was added to the solution, the BH 1146 cultivar showed the highest DIF value, the Anahuac cultivar showed the lowest DIF value, and the L01 and L14 lines showed intermediate DIF values compared to the BH 1146 and Anahuac cultivars (Fig. 1b). In this treatment with Al, the L01 line was significantly similar to the BH 1146 cultivar, while the L14 line was similar to the Anahuac cultivar. In the presence of 0.15 mmolc L−1 of Al along with 2 or 10 mmolc L−1 of Mg, the lines L01 and L14 demonstrated higher DIF values than the sensitive cultivar Anahuac (Fig. 1c & d). However, the DIF of L01 was like that of the tolerant cultivar (BH 1146) and greater than that of the L14 line with the addition of 10 mmolc L−1 of Mg (Fig. 1d).
The maize genotypes AG 9025, K 9606, and Al 53 showed higher DIF values than the Al 237 in the control treatment without Al (Fig. 2a). Considering maize hybrids, K 9606 showed a higher DIF than AG 9025 in this control treatment (Fig. 2a). With the addition of 0.15 mmolc L−1 of Al in the solution, there was a significant difference in DIF between the Al 237 and Al 53 maize lines (Fig. 2b). In a solution containing 0.15 mmolc L−1 of Al plus 2 mmolc L−1 of Mg, genotypes Al 237, AG 9025, and K 9606 showed a higher DIF than the sensitive line (Al 53) (Fig. 2c). In the treatment containing 0.15 mmolc L−1 of Al and 10 mmolc L−1 of Mg, hybrids AG 9025 and K 9606 revealed a DIF value higher than the sensitive line (Al 53) and the tolerant line (Al 237) (Fig. 2d).
The presence of Al in the minimal solution visibly affected the length growth of wheat (Fig. 3) and maize (Fig. 4), depending on the tolerance of the genotypes to Al and on the concentration of Mg in the solution.
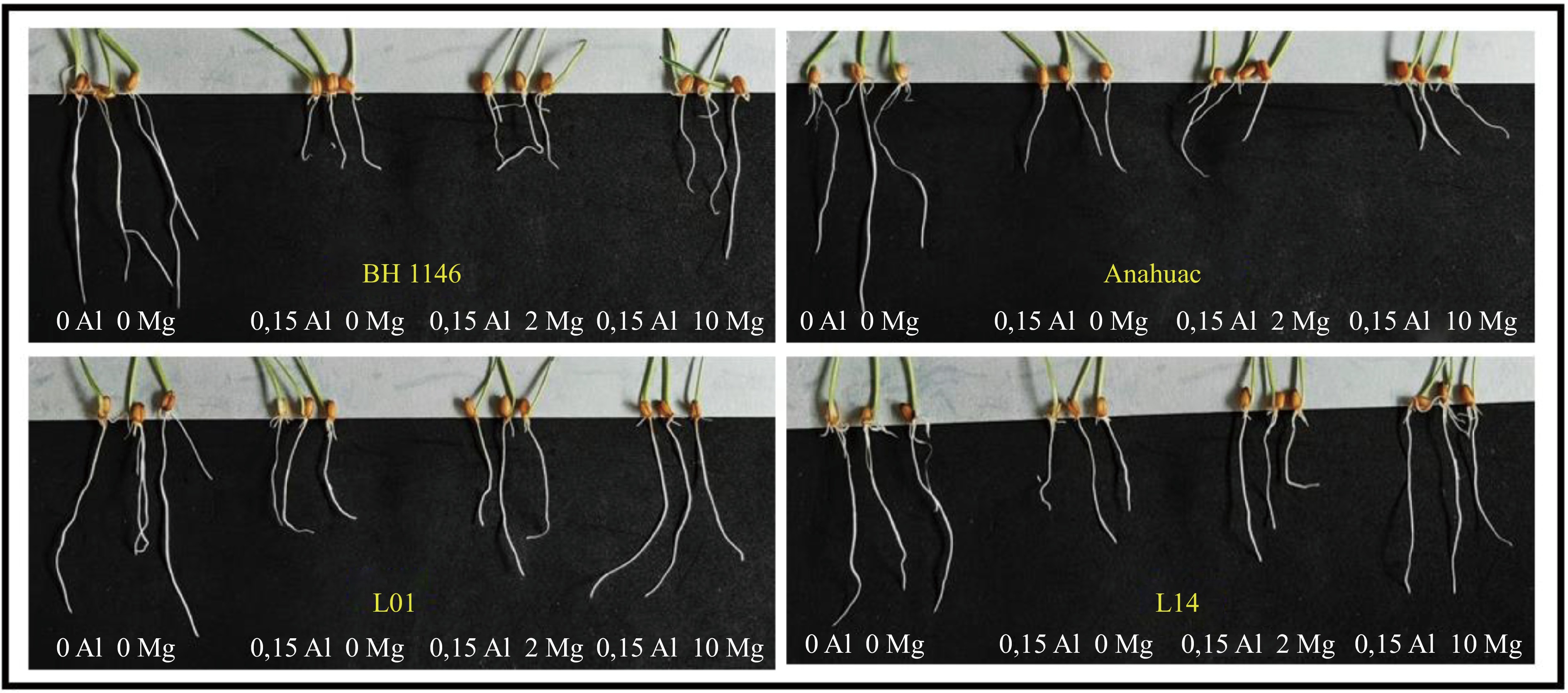
Figure 3.
Effects of Al and Mg concentrations on two wheat cultivars (BH 1146 and Anahuac) and two wheat lines (L01 and L14), after 48 h of exposure.
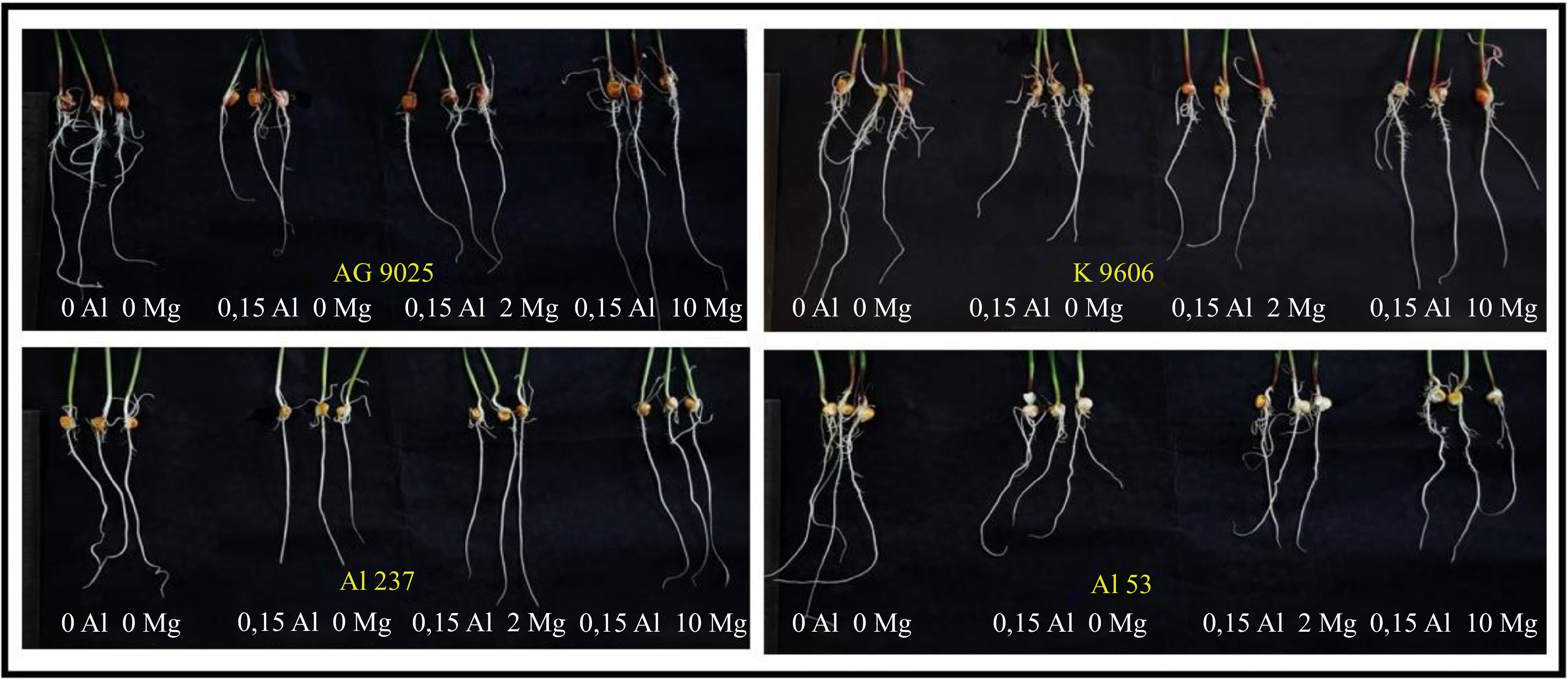
Figure 4.
Effects of Al and Mg concentrations on two maize hybrids (AG 9025 and K 9606) and two maize inbred lines (Al 237 and Al 53), after 48 h of exposure.
For the four wheat genotypes (BH1146, Anahuac, L01, and L14), there was no significant difference in DIF between the treatment containing only 0.15 mmolc L−1 of Al and the treatment containing Al along with 2 mmolc L−1 of Mg (Fig. 5). However, all wheat genotypes showed higher DIF in the treatment with 0.15 mmolc L−1 of Al + 10 mmolc L−1 of Mg compared to the treatment containing only 0.15 mmolc L−1 of Al.
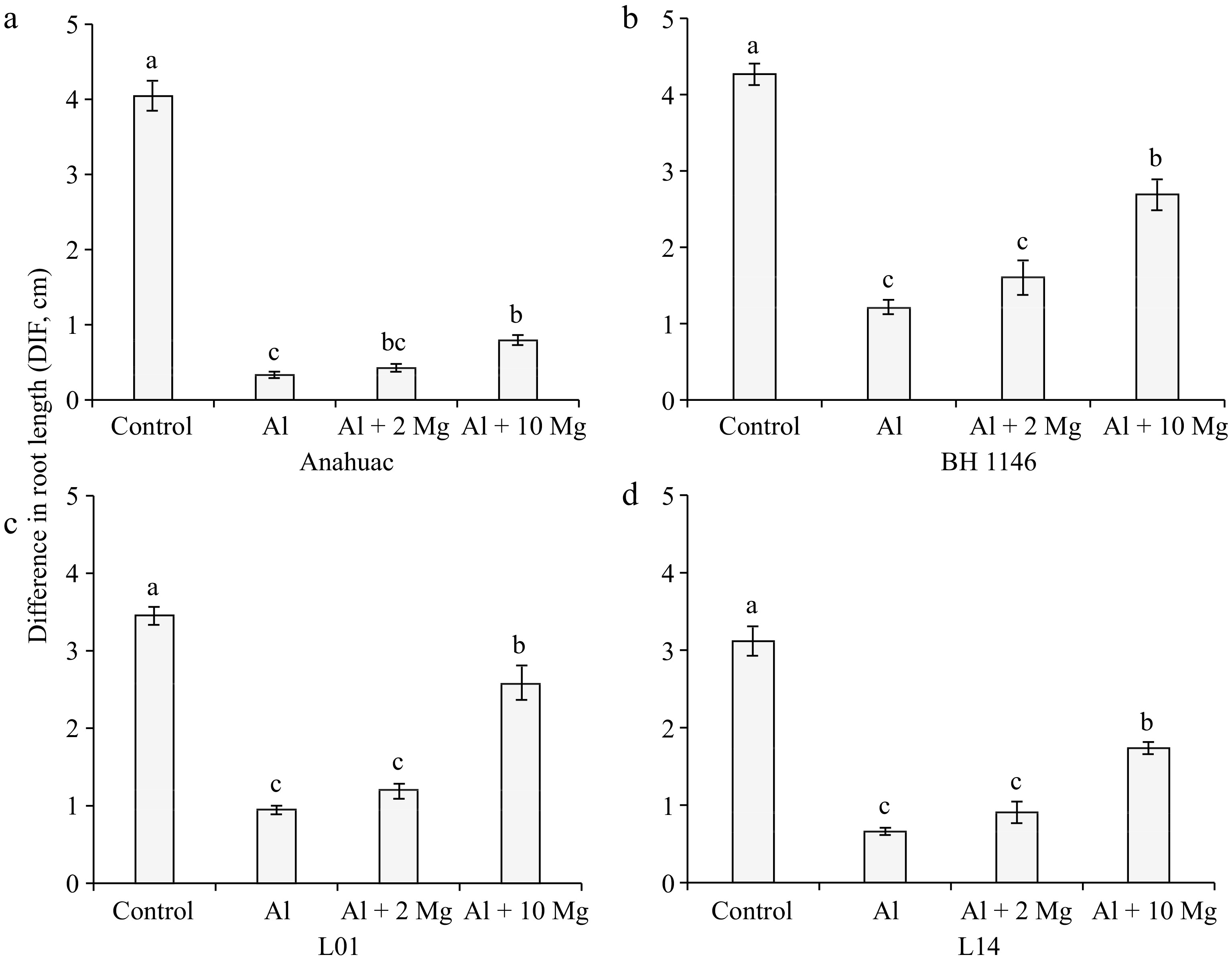
Figure 5.
Effect of solutions containing Al (0.15 mmolc L−1), without and with Mg (2 or 10 mmolc L−1), on the variable DIF after 48 h of exposure of wheat genotypes (a) Anahuac, (b) BH 1146, (c) L01, and (d) L14. Means followed by the same letters do not differ significantly by the LSD test at p < 0.01. Error bars express the standard error of the mean (n = 3).
For maize genotypes Al 53 (Fig. 6a) and AG 9025 (Fig. 6c) there was no significant difference in DIF between treatments containing only 0.15 mmolc L−1 of Al and 0.15 mmolc L−1 of Al + 2 mmolc L−1 of Mg. For the genotypes Al 237 (Fig. 6b) and K 9606 (Fig. 6d), the DIF was higher in the treatment with 0.15 mmolc L−1 of Al + 2 mmolc L−1 of Mg compared to the treatment containing only 0.15 mmolc L−1 of Al. In the treatment containing 0.15 mmolc L−1 of Al + 10 mmolc L−1 of Mg, the DIF was higher for the four maize genotypes compared to the treatment with only Al (0.15 mmolc L−1).
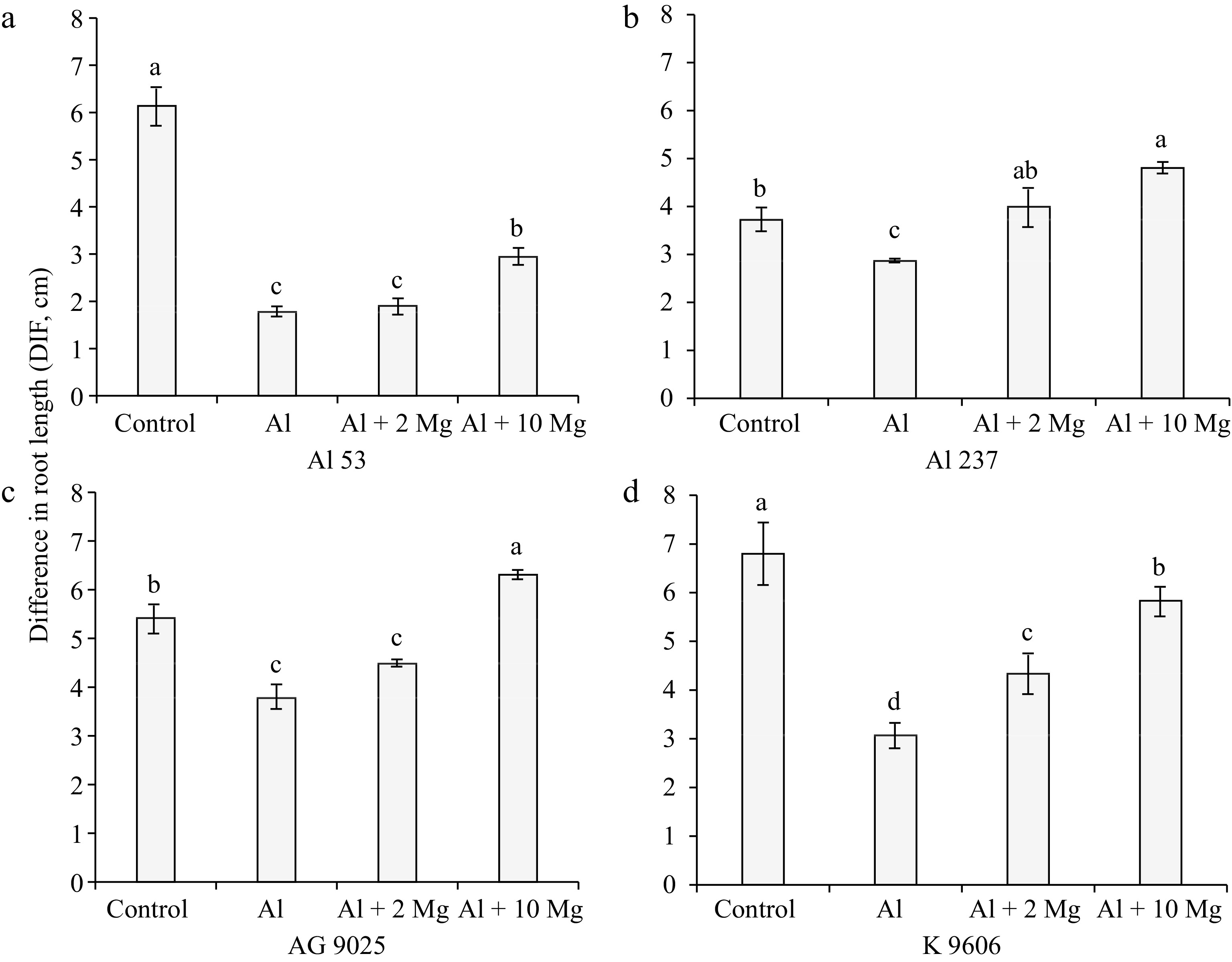
Figure 6.
Effect of solutions containing Al (0.15 mmolc L−1), without and with Mg (2 or 10 mmolc L−1), on the variable DIF after 48 h of exposure of maize genotypes (a) Al 53, (b) Al 237, (c) AG 9025, and (d) K 9606. Means followed by the same letters do not differ significantly by the LSD test at p < 0.01. Error bars express the standard error of the mean (n = 3).
-
The wheat cultivars BH 1146 and Anahuac were found to be tolerant and sensitive to Al, respectively, consistent with the classification of these genotypes described in several studies[30−35]. Also, L01 can be classified as moderately tolerant and L14 as moderately sensitive to Al. In the study by Ferraz et al.[27], it was also found that the L01 line showed higher tolerance to Al while the L14 line showed higher sensitivity to Al than the other wheat genotypes studied.
The inbred lines of maize Al 237 and Al 53 showed tolerance and sensitivity to Al, respectively, in agreement with the classification of these genotypes by EMBRAPA[36,37]. The sensitivity to Al of the two maize hybrids used in our study (AG 9025 and K 9606) was unknown. Based on treatment with Al in the solution, the hybrids AG 9025 and K 9606 behaved as tolerant to Al.
The impairment of wheat (Fig. 3) and maize (Fig. 4) root growth due to Al toxicity was visible. Rout et al.[13] reported that Al decreases cell division in the tips and sides of roots, increases the stiffness of the cell wall by cross-linking pectins, reduces replication by increasing the stiffness of the DNA double helix, makes P less available to plants, decreases root respiration, interferes with enzymatic activity that governs phosphorylation and deposition of polysaccharides in the cell wall and hinders the absorption of water and various nutrients by plants. In other studies conducted with wheat and maize in nutrient solution, a reduction in root length was also observed when the seedling was subjected to a solution containing Al[33,38,39]. The interaction of Al3+, the main toxic form of Al, with oxygen-donating ligands (proteins, nucleic acids, and polysaccharides) results in the inhibition of cell division, extension, and transport[40]. The plasma membrane of root cells, particularly at the root apex, seems to be one of the main targets of Al toxicity[40]. The toxic effect of Al appears to be related to a Mg deficiency induced by Al due to a blockage in Mg transport[18−20].
Alleviative the effect of Mg on Al toxicity in wheat and maize
-
The present data revealed that adding 10 mmolc L−1 of Mg to the solution increased wheat root growth by minimizing the toxic effect of Al. The data also showed an attenuation of the toxic effect of Al and a better response of the maize root growth when 10 mmolc L−1 of Mg was added in a solution containing 0.15 mmolc L−1 Al. Therefore, Mg showed a protective action against the toxic effect of Al.
The effect of Mg supply alleviating Al phytotoxicity and increasing root elongation has been demonstrated in several studies[2,25,41]. Assessing the alleviation of Al toxicity by cations (Ca2+, Mg2+, and Na+) in the wheat crop, Kinraide & Parker[42] found that competition between the cation and Al for external binding sites could be responsible for most of the decrease in Al toxicity. In another study that evaluated the ability and interaction of Ca and Mg to decrease Al toxicity in Al-sensitive wheat plants, Keltjens & Dijkstra[24] found that Mg was more effective than Ca in protecting roots against adsorbed/precipitated Al and in excluding Al from roots. Under Al stress conditions, the reduction in root growth is mainly due to Mg deficiency induced by Al[18−20]. Both Mg and Ca could exclude Al from active absorption sites. However, Mg is more effective than Ca[20,24]. Chemically, Al is more like Mg than Ca[43] and competitively inhibits Mg uptake by plant roots[18].
Several studies have investigated the interaction among Al, Mg, and Ca in different agricultural species. Keltjens & Tan[44] observed that Mg was more effective than Ca in alleviating Al toxicity in monocotyledonous plants, while the opposite occurred in dicotyledonous plants. However, an interaction between Mg and Al on soybean root growth was found by Silva et al.[26], who obtained an increase in primary and lateral roots when 2 or 10 mmolc L−1 of Mg were added. In another study[20], it was found that Mg alleviated Al toxicity and had a higher protective effect than Ca on the crops of rice, bean, maize, wheat, and soybean.
Increased concentrations of Ca2+ and Mg2+ in solution appear to protect plants against Al3+ toxicity by improving the uptake of Ca2+ and Mg2+ and alleviating the toxic effect of Al3+ on root growth[44]. Although the positive effect of Mg in reducing Al toxicity is not fully understood, it could be based on a physiological mechanism in the apoplast that appears to go beyond alleviating a Mg deficiency through competitive inhibition of Mg uptake by Al[20]. In roots of Populus subjected to treatments with and without both Al and Mg, it was found that Mg reduced the concentration of Al and increased the concentration of Mg in the root when both Al and Mg were added[25]. In addition, in the study by Zhang et al.[25] it was also observed that Mg promoted the transport and distribution of polar auxins, which led to a regulation in the increase of the root surface pH and alleviation in Al toxicity. Silva et al.[20] reported that the positive effect of Mg in reducing Al phytotoxicity is maximized when it is provided to plants in a pre-treatment solution (before the addition of Al), along with Al (time zero '0'), or within 6 h after root exposure to Al. After 6 h of root exposure to Al in the absence of Mg, the phytotoxic effects of Al becomes irreversible and the subsequent addition of Mg cannot completely restore root elongation. The results obtained from the present study showed that the use of Al-tolerant genotypes and the maintenance of adequate Mg levels in the soil solution are important practices in reducing Al toxicity for wheat and maize plants. Further research on assessing Al toxicity and tolerance in plants with overexpressed or antisense Mg transporters[45] could help to better clarify the effect of the Al-Mg interaction.
-
The minimum solution method with 0.15 mmolc L−1 of Al was efficient for classifying the Al tolerance of wheat and maize genotypes. The maize hybrids AG 9025 IPRO and K 9606 VIP3 behaved as tolerant to Al. Al-sensitive wheat and maize genotypes showed a greater reduction in root growth compared to Al-tolerant genotypes when subjected to the Al-containing solution. The presence of Mg in the solution, especially at higher concentration (10 mmolc L−1 of Mg), alleviated Al toxicity, and increased root growth of wheat and maize genotypes. The use of Al-tolerant wheat and maize genotypes and the maintenance of adequate Mg levels in the soil solution are important strategies to alleviate Al toxicity in plants.
-
The authors confirm contribution to the paper as follows: study conception and design: Caires EF, Matiello RR, Duart VM; data collection: Duart VM, de Oliveira ÉC; analysis and interpretation of results: Caires EF, Duart VM, Matiello RR; draft manuscript preparation: Duart VM, Caires EF; review & editing: Caires EF. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This work was supported by the CAPES and CNPq. The authors would like to thank the Agronomic Institute of Campinas (IAC) for providing Al sensitive and tolerant wheat cultivars, and the Brazilian Agricultural Research Corporation (EMBRAPA) for providing maize inbred lines both sensitive and tolerant to Al.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Duart VM, Caires EF, de Oliveira ÉC, Matiello RR. 2024. Magnesium alleviates aluminum toxicity in wheat and maize seedlings. Technology in Agronomy 4: e025 doi: 10.48130/tia-0024-0022
Magnesium alleviates aluminum toxicity in wheat and maize seedlings
- Received: 13 June 2024
- Revised: 03 July 2024
- Accepted: 11 July 2024
- Published online: 02 September 2024
Abstract: Aluminum (Al) toxicity inhibits root growth, affecting the ability of plants to absorb water and nutrients. Although magnesium (Mg) can decrease Al phytotoxicity, its effect on plant root growth in minimal solution containing calcium (Ca) and Al is not yet known. We evaluated the root length of wheat and maize genotypes with different sensitivities to Al in minimal solution containing different combinations of Al and Mg: (i) control (without Al and Mg); (ii) with 0.15 mmolc L−1 of Al; (iii) with 0.15 mmolc L−1 of Al plus 2 mmolc L−1 of Mg; and (iv) with 0.15 mmolc L−1 Al plus 10 mmolc L−1 of Mg. By measuring the difference in root length, we found that the minimum solution method with 0.15 mmolc L−1 of Al was efficient to classify the wheat and maize genotypes tolerance to Al. The Al-sensitive wheat and maize genotypes showed a higher reduction in root length compared to the Al-tolerant genotypes when subjected to a solution containing Al. The addition of Mg in the solution, especially at higher concentration (10 mmolc L−1 of Mg), effectively alleviated Al toxicity and favored root growth of wheat and maize genotypes. Our results suggest that Mg alleviates the toxic effects of Al on root growth of wheat and maize genotypes in minimal solution containing Ca and Al and that both the use of Al-tolerant wheat and maize genotypes and the maintenance of adequate levels of Mg in the soil solution are important strategies to alleviate Al toxicity to plants.












