-
Powdery mildew (PM) is one of the three most susceptible diseases in cucumber production, manifesting in both facility cultivation and open-field planting. Two pathogens, Podosphaera xanthii and Golovinomyces cichoracearum, have been reported as the most common fungi causing PM in cucumber, with P. xanthii being predominant in China[1]. Upon infection, cucumber leaves are covered by irregular white powdery spots of varying sizes, the leaves turn yellow and wither, leading to light suppression in cucumber plants, damage to the photosynthetic system reduced dry matter accumulation, and restricted growth of the roots and the above-ground parts[2,3]. Infection during the flowering period can reduce cucumber yield by 20%−40%[4]. Therefore, researching cucumber powdery mildew is of crucial significance for enhancing cucumber yield and quality.
Currently, planting disease-resistant varieties is the most economical, effective, environmentally friendly, and sustainable measure to control cucumber powdery mildew. The selection of powdery mildew-resistant cucumber varieties is the fundamental approach to control the disease. The premise for screening and cultivating cucumber materials resistant to powdery mildew is a scientific, convenient, and accurate resistance identification technology system[5]. In existing reports, research on cucumber powdery mildew inoculation mainly focuses on inoculation concentration and method. Xu et al.[6] mentioned that when inoculating cucumber powdery mildew, the concentration of spore suspension needs to be set at 1 × 106 spores per mL, and cucumber leaves at the two-true-leaf stage are used as inoculation materials. Zhang et al.[7] adopted a similar protocol as Xu et al.[6] and enhanced the adsorption capacity of powdery mildew spores on leaves by adding Tween-20 to the spore suspension. When identifying the powdery mildew resistance of different cucumber materials, Ma et al.[8] diluted the spore suspension with a 10 μg/mL SDS solution to a concentration of 5 × 105 conidia per milliliter and inoculated the cucumber seedlings at the three-true-leaf stage with powdery mildew using the spray method. Jia et al.[9] compared the effects of rubbing and spraying asexual spore suspension on the young leaves of cucumber seedlings, and concluded that the rubbing method had a higher incidence rate, but the specific parameters were not detailed. Wang et al.[10] mentioned in a report on controlling cucumber powdery mildew in high-temperature greenhouses that when preparing a spore suspension, the powdery mildew spores can be kept at 20−24 °C for 24 h to improve the activity of asexual spores, and then prepared into a suspension with 10−15 spores per field of view (10 × 10 microscope) for inoculation. Although the inoculation method for cucumber powdery mildew is relatively mature, there has been no reports on a systematic identification method for cucumber powdery mildew at the seedling stage.
Therefore, based on previous research, this experiment, by artificially inoculating the cucumber variety Xintai mici with powdery mildew and adopting the four-factor three-level (L9(3)4) orthogonal test design, which includes factors such as inoculation seedling stage, inoculation concentration, inoculation method, and different moisturizing times after inoculation, screened out the best indoor seedling stage resistance identification method for cucumber powdery mildew. The experiment also explored the impact of different identification methods on cucumber growth and photosynthesis, with the aim of providing a scientific basis and theoretical foundation for the identification of cucumber powdery mildew, resistance research, and the breeding of resistant varieties.
-
The cucumber inbred line Xintai mici was used for all experiments. The PM pathogen (Podosphaera xanthii) used in this test was isolated from diseased cucumber leaves from the greenhouse at Zhejiang University of Agriculture and Forestry (Zhejiang, China) and maintained by infection of susceptible cucumber cultivar plants.
Orthogonal test for indoor seedling stage resistance identification method
-
Cucumber plants were grown in soil (nursery substrate : garden soil = 2:1, V:V) under a long-day photoperiod (16-h light/8-h dark at 26 °C, 20,000 lx) up to one-true-leaf stage~three-true-leaf stage[11]. For the disease resistance test, a four-factor three-level (L9(3)4) orthogonal experimental design was employed, with factors including seedling age (a), spore suspension concentration (b), inoculation method (c), and moisturizing time (d). The detailed factors and levels are shown in Tables 1 & 2. Severely diseased cucumber plants from the greenhouse were selected, and fresh powdery mildew spores were collected and re-suspended in a water solution containing 0.01% Tween-20. The spore suspension was adjusted to the desired concentration (104, 105, or 106) spores per mL, and the spores were inoculated onto leaves at different seedling ages (one-true-leaf stage, two-true-leaf stage, or three-true-leaf stage) using different inoculation methods (spraying: spray a certain concentration of spore suspension onto flattened cucumber leaves with a spray bottle; rubbing: mix a specific concentration of spore suspension with quartz sand, and use gauze to evenly spread the mixture onto cucumber leaves; leaf brushing: use a specially made soft-bristled brush to evenly apply a certain concentration of spore suspension onto the leaves of cucumber plants). The inoculated plants were then placed in a growth chamber under controlled lighting conditions and subjected to 12, 24, and 36 h of moisturizing treatment. Furthermore, infection of leaf was recorded every 3 d after inoculation, and the incidence and disease index were investigated 15 d later. The experiment was set up with two biological replicates, each replicate experiment contains 90 samples (for each orthogonal scheme, 10 samples were prepared).
Table 1. Factors and levels of cucumber powdery mildew seedling resistance identification test.
Factor Level 1 Level 2 Level 3 a Seedling age one-true-leaf two-true-leaf three-true-leaf b Spore suspension concentration: spores/mL 104 105 106 c Inoculation method spraying rubbing leaf brushing d Moisturizing time 12 h 24 h 36 h Table 2. Cucumber powdery mildew seedling resistance identification implementation programme.
Test
no.Factor Scheme of orthogonal design a b c d T1 1 1 2 2 a1b1c2d2 one-true-leaf 104 rubbing 24 h T2 2 1 3 3 a2b2c3d3 two-true-leaf 104 leaf brushing 36 h T3 3 3 3 1 a3b3c1d1 three-true-leaf 106 leaf brushing 12 h T4 1 1 1 1 a1b1c1d3 one-true-leaf 104 spraying 12 h T5 2 2 2 1 a2b2c2d1 two-true-leaf 105 rubbing 12 h T6 3 2 1 3 a3b3c3d2 three-true-leaf 105 spraying 36 h T7 1 2 3 2 a1b1c3d1 one-true-leaf 105 leaf brushing 24 h T8 2 3 1 2 a2b2c1d2 two-true-leaf 106 spraying 24 h T9 3 3 2 3 a3b3c2d3 three-true-leaf 106 rubbing 36 h The calculation of disease index (DI) followed the method described by Nie et al.[12]. For details, refer to Table 3. The formula for calculating the disease index (DI) is:
$ \text{DI =}\ \dfrac{\sum_{ }^{ }\left({S}_{{i}}\cdot {n}_{{i}}\right)}{{4}{N}}\times100 $ (1) Where Si represents the disease grade, ni represents the number of plants in the corresponding disease grade, i represents each level of disease severity, and N represents the total number of plants investigated.
Table 3. Disease grading criteria for indoor seedling resistance identification.
Grade Symptom 0 no symptom 1 < 5% of the surface area of the leaf 2 6%−25% of the leaf infected 3 26%−50% of the leaf infected 4 > 50% of the leaf infected Identification of disease resistance of cucumber germplasm resources during the indoor seedling stage
-
In this study, four cucumber varieties with distinct resistance profiles, as determined through preliminary experiments, were chosen as test subjects. Among them, R1641 was designated as a highly resistant variety, BK2 as disease-resistant, 9930 as moderately resistant, and H136 as susceptible. At the stage when the cucumber plants developed three true leaves, the leaves were cut off and laid flat in sterilized plastic Petri dishes (with a moist filter paper placed at the bottom of the dish). The optimal indoor seedling disease resistance identification method, which had been screened, was utilized to inoculate powdery mildew on the leaves of the four germplasm resources. Details regarding the investigation of the incidence rate refer to Table 3. Furthermore, the four cucumber germplasms were graded based on their resistance levels: 0 ≤ DI < 15 was classified as highly resistant, 15 ≤ DI < 35 as resistant, 35 ≤ DI < 55 as moderately resistant, 55 ≤ DI < 75 as susceptible, and DI ≥ 75 as highly susceptible. Three plants were cultivated for each material, and three leaves were cut from each plant.
Observation of the germination process of powdery mildew conidia on cucumber leaves under different treatments
-
At 72 h after inoculation with powdery mildew, samples were taken from the leaves of the nine schemes of orthogonal design using a puncher, and the leaf discs were placed in 10 mL centrifuge tubes. Three plants were randomly selected for each treatment, and one leaf was taken from each plant. Three leaf discs were taken from each leaf, resulting in a total of nine replicates. Before observing the conidia, the leaf discs were placed in a decolorizing solution (acetic acid : ethanol = 1:3) for 3 h. They were then rinsed with deionized water two to three times and stained with cotton blue in lactophenol for 1−2 min. Subsequently, the leaf discs were clamped onto a glass slide with forceps, covered with a cover glass, and observed under a 10 × 40 magnification microscope to observe the germination status of powdery mildew conidia on cucumber leaves under different treatment combinations. Twenty conidia were observed per leaf disc.
Measurement of chlorophyll (Chl) contents
-
Twenty days after inoculation with powdery mildew, the chlorophyll content of cucumber in each orthogonal design scheme was measured. The third leaf from the top of the cucumber plants was selected for measurement. Chl contents of cucumber leaves were determined using a SPAD-502 PLUS chlorophyll meter. The experiment was set up with two biological replicates, and in each replicate, five strains were randomly selected for measurement.
Measurement of photosynthetic rate
-
Twenty days after inoculation with powdery mildew, the Pn, Ci and Gs of the third leaf from the top of the cucumber in each orthogonal design scheme was measured by a portable photosynthetic instrument LI-6800 (LI-COR, Lincoln, USA). The experiment was set up with two biological replicates, and in each replicate, five strains were randomly selected for measurement.
Biomass measurement
-
Twenty-one days after inoculation with powdery mildew, the fresh weight and dry weight of cucumber plants were measured for each orthogonal design scheme as well as the control group (sprayed with water). After being killed at 105 °C for 15 min, the samples were dried at 80 °C until reaching a constant weight, and the dry weight of all cucumber plants in each treatment combination was determined. The experiment was set up with two biological replicates, and in each replicate, five strains were randomly selected for measurement.
Statistical analysis
-
All data were expressed as the mean ± standard error of the mean (SEM). Software of statistical product and service solutions (SPSS) 18.0 was used for the evaluation of the statistical experimental design.
-
At 15 d post-inoculation, the cucumber Xintai mici demonstrated full disease onset. Initially, an individual factor analysis was conducted. As depicted in Fig. 1a, with the increase in cucumber seedling age, both the disease incidence and disease index exhibited a gradual ascension, with no significant differences in disease incidence among different treatments. The disease index for inoculation at the three-true-leaf stage was the highest at 82.40, significantly higher than at the one-true-leaf stage, but bore no significant difference with the two-true-leaf stage inoculation. As illustrated in Fig. 1b, an upward trend in both disease incidence and index is observed by an increase in the concentration of inoculating spore suspension. At an inoculation concentration of 106 per mL, the disease index stands at 86.78, remarkably outstripping those of the two treatments with inoculation concentrations of 105 per mL (65.70) and 104 per mL (58.83). Conversely, there are no notable disparities in either the disease index or the incidence among treatments differing in inoculation method and post-inoculation hydration time (Fig. 1c & d).
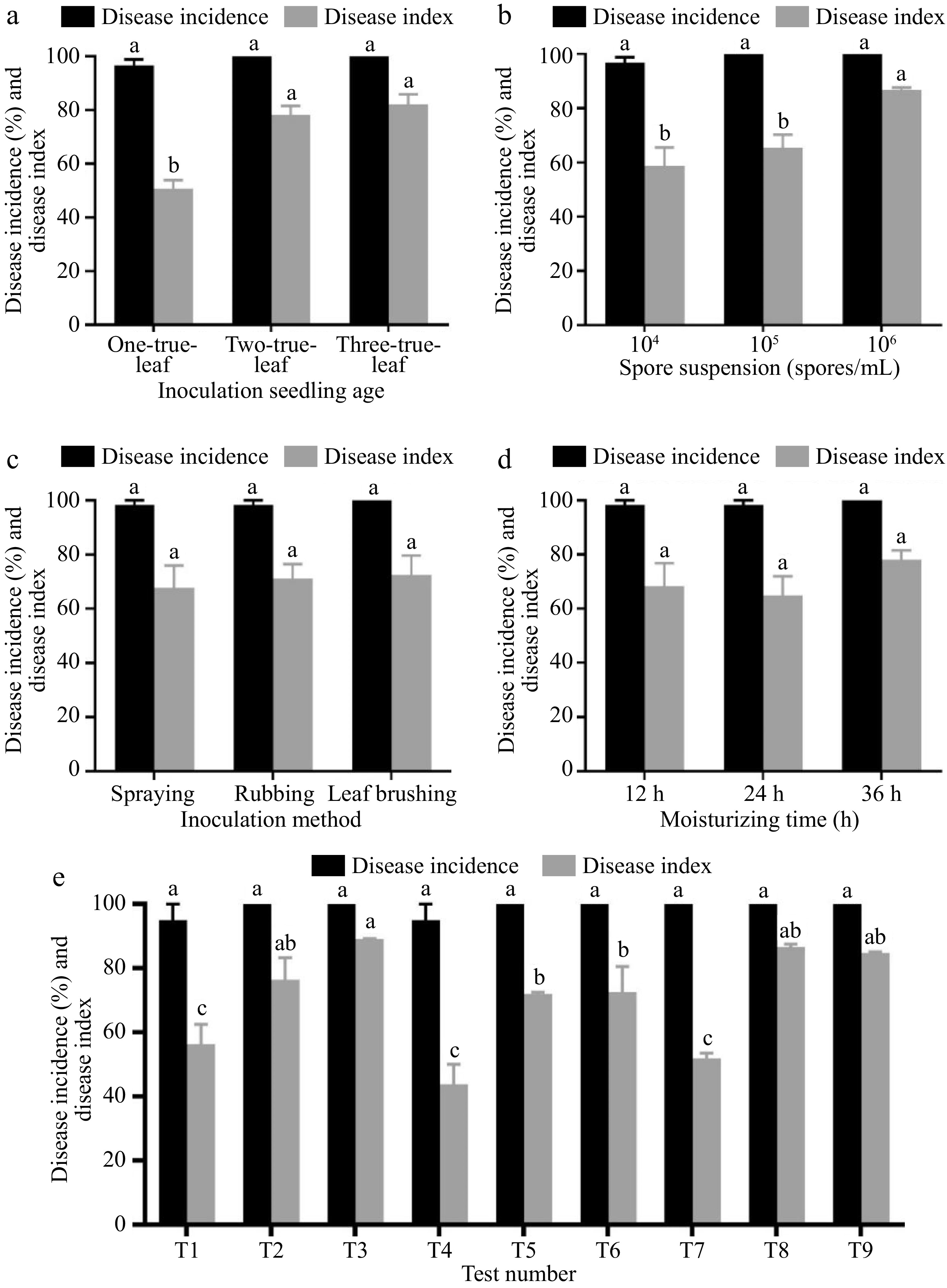
Figure 1.
Resistance identification results of combinations of indoor seedling resistance identification methods (15 d). Effects of (a) different seedling ages, (b) spore suspension concentrations, (c) inoculation methods, and (d) moisturizing time on the incidence rate and disease index of cucumber seedlings. (e) The influence of different inoculation schemes on the incidence rate and disease index of cucumber seedlings. Values are means of minimum two replicates, with each containing 9 to 10 seedlings. Data are expressed as mean ± Standard error of mean, and significances were examined by one-way ANOVA, followed by Duncan's multiple comparisons test and different letters represent significant differences (p < 0.05).
Upon analyzing the disease incidences and indices of nine treatment combinations, it is discerned that there are no significant differences in disease incidence among the treatments, all being above 96.67%. The disease progression of treatment T3 is the swiftest, with a disease incidence of 100%, and the symptoms are distinct. Fifteen days after inoculation with powdery mildew, the disease index of T3 reaches 89.03, significantly surpassing most treatments. Consequently, this treatment is regarded as the optimal method for early-stage powdery mildew resistance determination in cucumber seedlings, namely, inoculating at the three-true-leaf stage, with a spore suspension concentration of 106 per mL, using the leaf-brushing method, and maintaining hydration for 12 h post-inoculation (Fig. 1e).
Disease resistance identification of four cucumber germplasm resources
-
The indoor seedling disease resistance identification method was adopted to evaluate the powdery mildew resistance of four cucumber germplasm resources. The results indicated that R1461 exhibited high resistance, BK2 demonstrated resistance, while both 9930 and H136 showed susceptibility to the disease (Fig. 2). Except for 9930, the resistance types of the other three materials were consistent with those determined in previous experiments. These findings suggest that the indoor seedling disease resistance identification method screened in this study is reliable.

Figure 2.
Identification of disease resistance in different cucumber germplasm. The onset of disease in (a) R1461, (b) BK2, (c) 9930, and (d) H136 after 15 d of inoculation with powdery mildew.
Growth of powdery mildew spores under different treatment combinations
-
At 72 h after inoculation of powdery mildew on cucumber leaves, the spores in all treatment combinations began to germinate (Fig. 3). The spore growth on treatment combinations T1, T4, T5, and T7 were slow, with few germ tube branches, and some germ tubes had not yet begun to elongate. In contrast, the germ tubes of the spores on treatment combinations T2 and T6 had extended, branched, and started to spread on the leaf surface, forming hyphae. Meanwhile, the spores on treatment combinations T3, T8, and T9 also began to form hyphae, but the growth process was slightly faster than treatments T2 and T6.
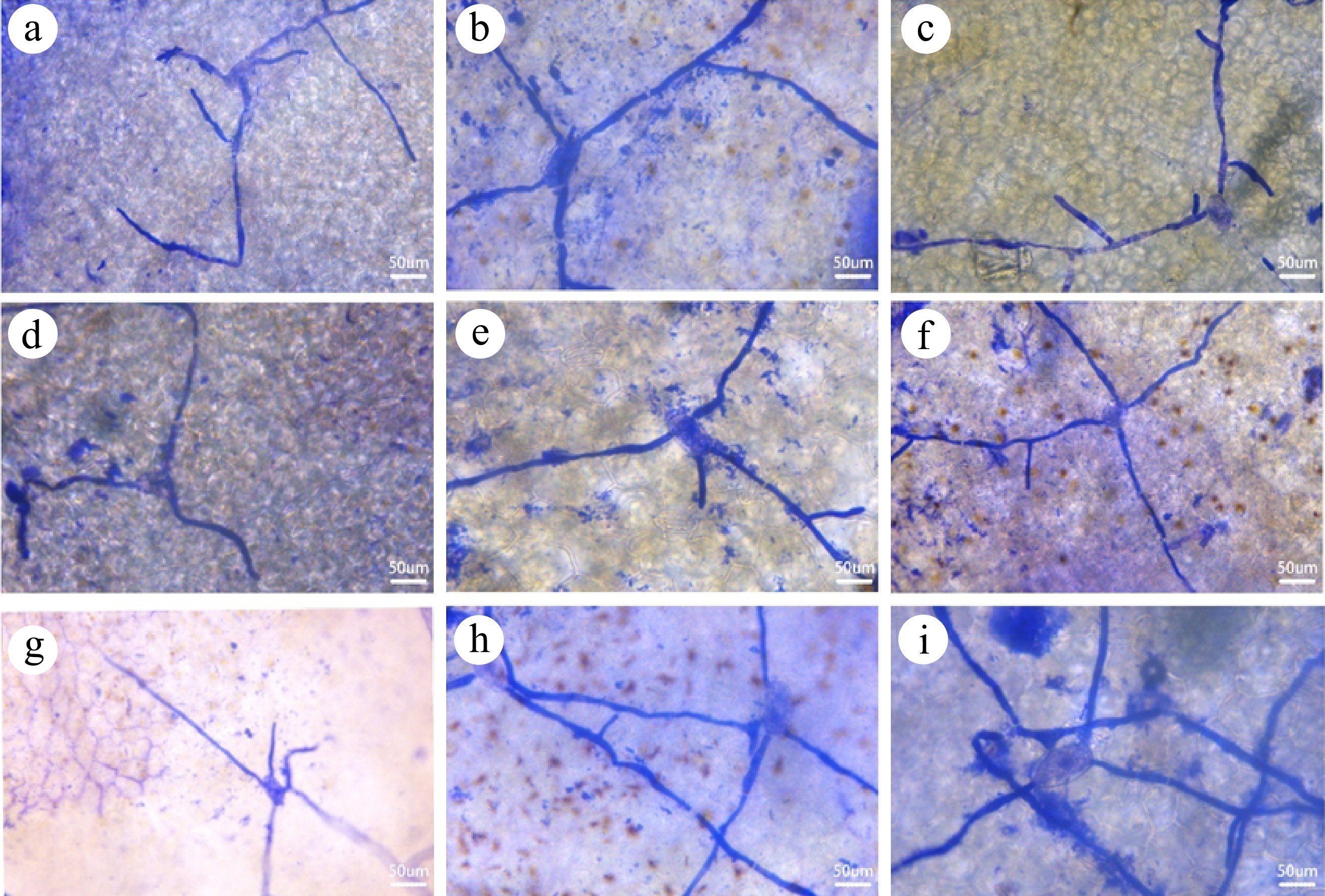
Figure 3.
Growth and development of powdery mildew fungus spores under different treatment combinations. (a)−(i) Correspond to the spore germination of treatment combinations T1−T9 after 72 h of inoculation.
Effect of powdery mildew on the growth of cucumber seedlings
The impact of powdery mildew on cucumber biomass accumulation
-
As shown in Fig. 4, compared to the control group, the fresh weight and dry weight of cucumber plants inoculated with powdery mildew fungi decreased significantly. In addition, it is observable that the impact of powdery mildew on the dry weight and fresh weight of plants diminishes as the disease index decreases. Compared to other treatments inoculated with powdery mildew at the one-true-leaf stage, the fresh and dry weights of cucumber seedlings in treatment T4 were the highest, but there was no significant difference between treatments T1 and T7. Among the treatments inoculated with powdery mildew at the two-true-leaf stage, the fresh weight under treatment T8 significantly decreased by 14.05% and 13.74% compared to T2 and T5, respectively, and the dry weight significantly decreased by 38.89% and 37.74%. When inoculated with powdery mildew at the three-true-leaf stage, compared to treatments T6 and T9, the fresh weight of cucumber plants under treatment T3 decreased by 34.01% and 13.83% respectively, and the dry weight decreased by 31.28% and 3.12%. It can be seen that the infection of powdery mildew inhibits the accumulation of dry matter in cucumber seedlings, and this inhibitory effect gradually intensifies with the worsening of the disease.
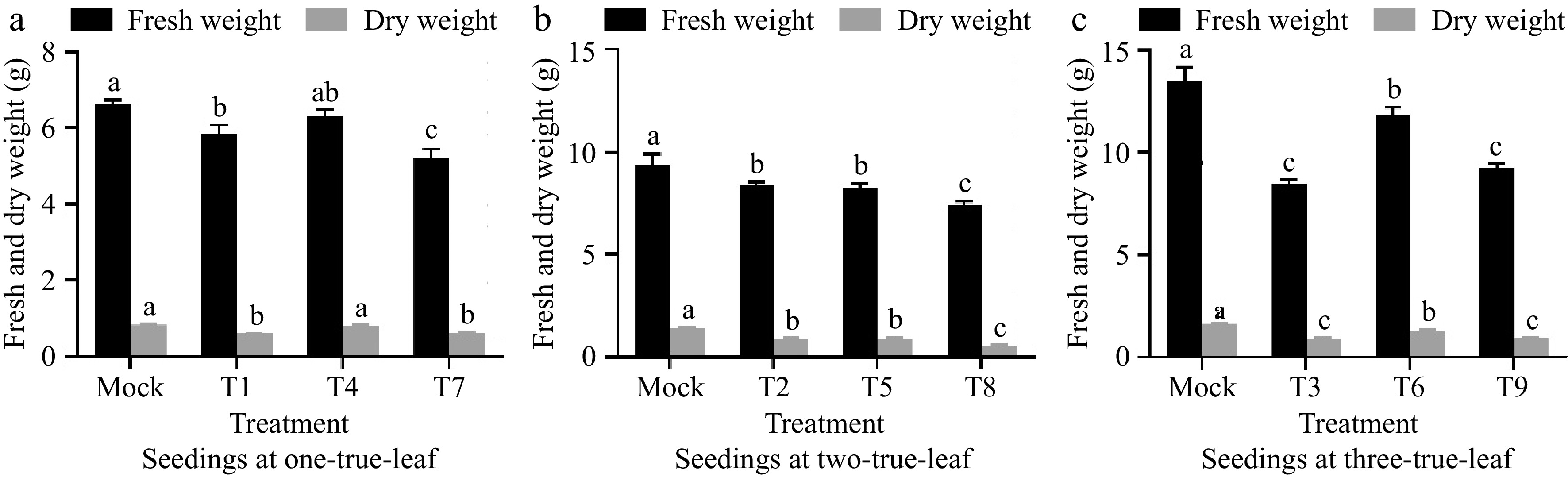
Figure 4.
Effect of powdery mildew on dry and fresh weight of plants at different seedling ages. (a) The impact of inoculating powdery mildew at the one-true-leaf stage on the growth of cucumber seedlings. (b) The impact of inoculating powdery mildew at the two-true-leaf stage on the growth of cucumber seedlings. (c) The impact of inoculating powdery mildew at the three-true-leaf stage on the growth of cucumber seedlings. Values are means of minimum two replicates, with each containing five seedlings. Data are expressed as mean ± Standard error of mean, and significances were examined by one-way ANOVA, followed by Duncan's multiple comparisons test and different letters represent significant differences (p < 0.05).
The impact of powdery mildew on photosynthesis of cucumber seedlings
-
As shown in Table 4, after inoculation with powdery mildew, cucumber leaves with different degrees of disease showed significant differences in Pn values. Compared with other treatments, T3, T8, and T9 had the greatest decline in Pn, which were only 2.42, 2.98, and 1.92, respectively. Similarly to Pn, the transpiration rate and stomatal conductance under treatments T3, T8, and T9 also significantly decreased compared to other treatments, with a decrease of more than 40%. Among the nine treatments, there was no significant difference in Ci values of most of the leaf treatments, but the Ci values of treatments T3, T8, and T9 were significantly lower than other treatments, indicating that powdery mildew inhibits the photosynthetic rate of cucumber leaves. When the disease index is low, the reduction in Pn is mainly due to non-stomatal limitations, and when the disease is serious, the reduction in Pn is mainly due to stomatal limitations.
Table 4. Comparison of photosynthetic parameters of diseased plants in different treatments.
Treatments Pn
(μmol/m2/s)Ci
(μmol/mol)E
(mmol/m2/s)Gs
(mol/m2/s)T1 3.29 bc 341.17 a 1.78 ab 0.13 ab T2 3.08 bc 325.84 a 1.41 b 0.09 b T3 2.42 de 229.12 b 0.49 cd 0.03 c T4 3.91 a 321.45 a 1.56 b 0.10 b T5 3.84 ab 343.73 a 2.18 a 0.15 a T6 3.71 ab 332.47 a 1.83 ab 0.12 ab T7 4.01 a 335.53 a 1.98 a 0.14 a T8 2.98 cd 220.68 b 0.81 c 0.05 c T9 1.92 e 208.43 b 0.29 d 0.02 c Different lowercase letters indicate significant differences between treatments (p < 0.05). The impact of powdery mildew on chlorophyll content in cucumber seedling leaves
-
Analysis of Fig. 5 shows that among the treatments of powdery mildew inoculation at the one-true-leaf stage, the SPAD value of the functional leaves in treatment T4 was the largest, but there was no significant difference with T1 and T7. At the two-true-leaf stage, compared to treatments T5 and T8, the SPAD value under treatment T2 significantly increased by 14.25% and 29.95%, respectively. Among the treatments of powdery mildew inoculation at the three-true-leaf stage, the SPAD value of treatment T6 was the largest, significantly higher than T3 and T9 by 16.37% and 13.86%. It can be seen that the infection of powdery mildew will reduce the chlorophyll content in cucumber leaves, thereby affecting the photosynthesis of cucumbers.
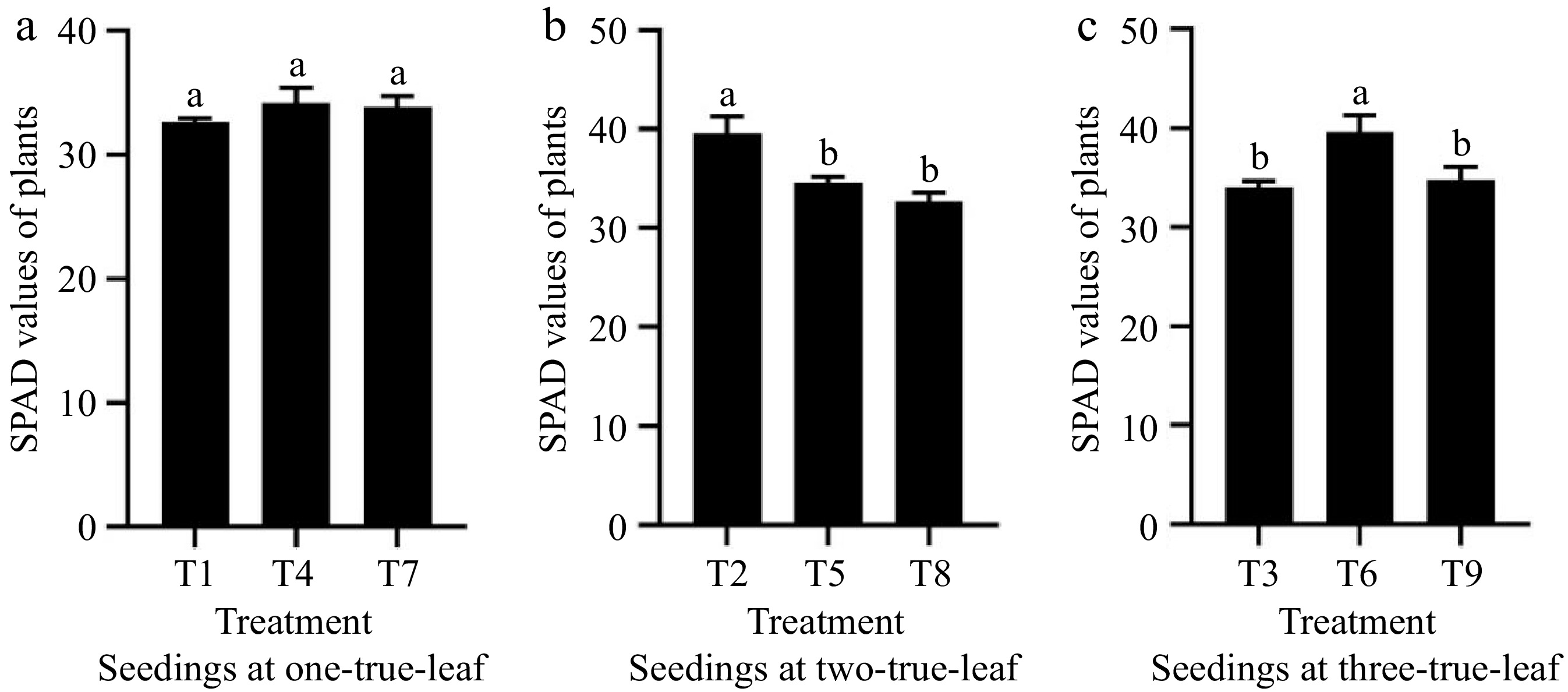
Figure 5.
Statistics of SPAD values of plants at different seedling ages under powdery mildew stress. (a) The effect of treatments T1, T4, and T7 on chlorophyll content in cucumber. (b) The effect of treatments T2, T5, and T8 on chlorophyll content in cucumber. (c) The effect of treatments T2, T5, and T8 on chlorophyll content in cucumber. Values are means of minimum two replicates, with each containing five seedlings. Data are expressed as mean ± Standard error of mean, and significances were examined by one-way ANOVA, followed by Duncan's multiple comparisons test and different letters represent significant differences (p < 0.05).
-
To screen a simple and accurate method for determining the resistance to powdery mildew during the seedling stage of cucumber, this experiment employed a four-factor three-level (L9(3)4) orthogonal experimental design. The inoculation method, inoculation timing, inoculation concentration, and hydration time involved in resistance determination were explored. Moreover, the best inoculation scheme for powdery mildew during the cucumber seedling stage were screened: inoculating with a powdery mildew spore suspension concentration of 106 per mL using the leaf brushing method at the three-true-leaf stage, followed by 12 h of hydration. This aligns with the research findings of Wang et al.[13] on the inoculation method for melon powdery mildew. It is also consistent with the conclusion of Kang et al.[14] that the optimal inoculum concentration for melon is 106 per mL. In contrast, Wang et al.[15] conducted similar research on the resistance identification scheme of Ningxia melon during the seedling stage using a four-factor three-level orthogonal experimental design. They demonstrated that the optimal identification scheme was to inoculate powdery mildew spore suspension concentration of 105 per mL using the rubbing method at the four-true-leaf stage, followed by 36 h of hydration. While the inoculation conditions were somewhat similar, differences were also present, which might be attributed to the inconsistent resistance of the two cucurbits or the results of interaction among different treatment factors. Additionally, both studies did not identify the physiological races of powdery mildew, so it is speculated that the differences in pathogenicity might be due to the dominant physiological races in Ningxia and Hangzhou being disparate[16,17].
The infection process of powdery mildew directly determines the disease rate and plant disease index[18]. After the powdery mildew spores fall on the host surface, they begin to germinate under suitable conditions, forming two to four germ tubes. Subsequently, these germ tubes begin to extend, differentiate to produce separate hyphae, form septa at the base of the hyphae, swell at the top to form attachment cells, and form infection pegs to pierce the host cuticle and epidermal cells. The infection pegs swell at the top to form haustoria that absorb nutrients to support hyphal growth. Finally, conidiophores are formed[19−22]. In both wheat and cucumber, powdery mildew can complete its growth cycle within five days[18,20]. In this experiment, Xintai mici, a medium-resistant variety to powdery mildew, was used as the test material. The growth of powdery mildew 72 h after inoculation with nine treatment combinations were compared. It was found that the spores on treatments T1, T4, T5, and T7 grew at a slightly slower rate, and some germ tubes had not yet begun to extend. The spores on treatment combinations T2 and T6 formed hyphae and began to extend. The spores on treatment combinations T3, T8, and T9 also formed hyphae, and their extension speed was faster than treatments T2 and T6. This indicates that the inoculation scheme will affect the growth process of spores on cucumber leaves. This is also one of the reasons why the disease indices of treatments T3, T8, and T9 are significantly higher than other treatments. In addition, research has shown that the inoculation density of powdery mildew is positively correlated with the disease rate. In their exploration of the occurrence and prevalence of grape powdery mildew, Gadoury et al.[23] discerned that the rate of disease incidence escalates with the increase in the number of asexual spores deposited per square millimeter, a finding that resonates with the results of the present study. With the augmentation of the concentration of spore suspension, there is a gradual increase in the rate of disease incidence and disease index in cucumber powdery mildew, reaching its zenith at 106 particles per mL. Gadoury et al.[23] postulated that this phenomenon may be attributable to the sporulation of powdery mildew colonies being regulated by quorum sensing (a cell-cell communication system that exchanges a wide range of information by generating and sensing small molecule chemical signals). In cases of insufficient density of asexual spores in the colony, this sporulation signal is not triggered, resulting in the loss of sporulation capacity; however, with time progression or enhancement of the initial density of the colony, this quorum-sensing signal is triggered and rapidly propagates within the colony, stimulating the entire group's sporulation.
The physiological state of plants may provoke different disease resistance mechanisms. Ben-Naim & Cohen[24] , in their research on watermelon powdery mildew resistance, found that the resistance of certain materials inoculated with powdery mildew in the cotyledon stage is controlled by a pair of dominant genes, whereas, during the four-true-leaf stage, the plant's resistance is governed by three pairs of dominant genes. These findings indicate a close correlation between the seedling age of the plant and its disease resistance. In the current experiment, cucumber seedlings inoculated with pathogens at the one-true-leaf stage exhibited the lowest disease index, demonstrating significant disease resistance, while those at the two-true-leaf and three-true-leaf stages were severely afflicted, showing a significant increase in disease index. Therefore, it's hypothesized that cucumber, another cucurbitaceous crop like watermelon, may possess a similar resistance mechanism, wherein different genes mediate plant resistance to powdery mildew at different developmental stages, resulting in differing resistance in cucumber seedlings at different stages.
Chlorophyll is a pivotal indicator of plant photosynthetic capacity and the SPAD value closely correlates with the plant chlorophyll content[25]. It was observed in this study that, at the same seedling stage, with the aggravation of the disease index, the SPAD value of cucumber materials in each treatment group significantly diminished, indicating that the powdery mildew treatment impeded the synthesis of chlorophyll in cucumber leaves, a conclusion congruent with the findings of Khan & Rizvi[3], on five kinds of cucurbitaceous crops infected with powdery mildew. Chlorophyll degradation is partly due to the disruption of chloroplast structure and may also be a result of chlorophyll's participation in the plant's immune response to pathogens[26,27]. Research conducted by Zhang et al.[27] indicates that the ethylene-mediated signaling pathway plays a role in cucumber resistance to powdery mildew, with its key regulatory factor, CsEIN, promoting chlorophyll degradation and ROS synthesis by upregulating chlorophyll-degrading enzyme genes (including CsNYE1, CsPPH, CsRCCR) and ROS synthesis enzyme gene (CsRBOHs), thereby promoting leaf senescence to enhance cucumber resistance to powdery mildew.
Zhang et al.[28] pointed out that powdery mildew can inhibit the activity of key enzymes in the Calvin cycle, thereby reducing the production of photosynthetic products and subsequently weakening the plant's photosynthesis and affecting its growth and development. The present data support the results obtained by Zhang et al.[28], after inoculation with powdery mildew, with the increase in disease severity, the photosynthetic capacity of cucumber leaves decreased, resulting in a reduction of cucumber fresh weight and dry weight. Regarding the comparative research on inoculation methods, different scholars have drawn divergent conclusions. Wang et al.[13] inferred from their research that using the brush method for leaf inoculation can reduce the surface tension of the bacterial fluid, accelerate the disease onset, and hence is the optimal method for disease inoculation. Zhang et al.[29] however, opined that the spray method yields the highest infection rate and is simple to execute. This experiment, based on a single-factor analysis, found no significant differences among different inoculation methods. Therefore, the selection of the inoculation method should be based on experimental requirements. The spray method is the most convenient and is suitable for experiments with large quantities, reducing the operational difficulty, while brushing allows the powdery mildew fungus to be evenly spread on the leaf surface, enhancing the infection rate. Zhang et al.[30] found in rice that compared to the commonly used whole-plant pathogen inoculation method, the detached leaf inoculation method significantly enhances stability of the detection results. Deng et al.[31] also found in melon research that during detached inoculation, environmental conditions have relatively less influence and a single planting of melon seedlings can be used multiple times, yielding accurate and reliable resistance identification results. In cucumber, there is a dearth of research on the detached leaf inoculation method. This experiment also adopted the traditional whole-plant inoculation method to screen for resistance identification methods. However, during the experiment, it was found challenging to precisely control the environmental conditions after inoculating with powdery mildew, especially with increased sample size, substantial differences in temperature and humidity at different locations can lead to uneven disease occurrence in plants, impacting the accuracy of resistance identification. Therefore, future research can focus on the detached leaf inoculation method in cucumber to optimize the resistance identification system in cucumber.
-
In the current study, the most optimal indoor method for early-stage resistance determination against powdery mildew of cucumber was explored using a four-factor, three-level orthogonal experimental design and T3 (inoculating a spore suspension of powdery mildew at a concentration of 106 per mL on one-true-leaf stage using the leaf-brushing method and maintaining hydration for 12 h post-inoculation) proved to be the most favorable for the development of powdery mildew disease in cucumber at the seedling stage. Seedling age and spore suspension concentration had a greater influence on powdery mildew development in cucumber than the inoculation method and wetting time. The disease index of cucumber leaves inoculation with powdery mildew at the three-leaf stage increased by 62.34% compared to the one-leaf stage. Disease index of cucumber plants inoculated with 106 particles/mL spore suspension was 47.51% and 32.61% higher than those inoculated with 105 and 104 particles/mL spore suspensions. In addition, it was found that powdery mildew inhibits photosynthesis and reduces the chlorophyll content of cucumber leaves, which ultimately leads to a reduction in plant dry matter accumulation. This study lays a solid foundation for further breeding for powdery mildew resistance.
-
The authors confirm contribution to the paper as follows: Study conception and design: Miao L, Wang H; data collection: Tan J, Zhong L; analysis and interpretation of results: Fan S, Cheng S, Gao Y; draft manuscript preparation: Tan J, Zhang P. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
This study was supported by the National Natural Science Foundation of China (32202511), the Taishan Program of Shandong Province (Grant tsqn202306246), the Major Science and Technology Project of Plant Breeding in Zhejiang Province (2021C02065-2), the 'Pioneer' and 'Leading Goose' R&D Program of Zhejiang (2022C02051, 2023C02027), the Natural Science Foundation of Zhejiang Province (LY24C150002), College Student's Science and Technology Innovation Activity Plan of Zhejiang Province (Xinmiao Talent Project) (2022R412B047, 2023R412051).
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Jihong Tan, Liping Zhong
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Tan J, Zhong L, Fan S, Cheng S, Gao Y, et al. 2024. Methods for seedling identification of cucumber resistance to powdery mildew and its effect on the growth of cucumber seedlings. Vegetable Research 4: e030 doi: 10.48130/vegres-0024-0028
Methods for seedling identification of cucumber resistance to powdery mildew and its effect on the growth of cucumber seedlings
- Received: 24 March 2024
- Revised: 01 June 2024
- Accepted: 24 June 2024
- Published online: 08 October 2024
Abstract: The severity of powdery mildew in cucumbers gravely impinges upon both yield and quality. In order to probe efficacious methods for early-stage resistance determination against powdery mildew in cucumbers, we utilized Xintai mici as an experimental subject and adopted a four-factor, three-level (L9(3)4) orthogonal experimental design. This design encompassed four factors: inoculation seedling age, inoculation concentration, inoculation method, and different hydration periods post-inoculation. Through this, we sifted and discerned the most optimal indoor method for early-stage resistance determination against powdery mildew in cucumbers. Concurrently, we studied the influence of different determination methods on the accumulation of cucumber plant biomass, chlorophyll content, and leaf photosynthetic parameters to delineate the impact patterns of powdery mildew on cucumber leaf photosynthetic traits. The results underscored that disease incidence amongst different treatments were minute, all exceeding 96.67%. In contrast to other treatments, T3 (inoculating a spore suspension of powdery mildew at a concentration of 106 per mL on one-true-leaf stage using the leaf-brushing method and maintaining hydration for 12 h post-inoculation) exhibited a rapid onset of disease, with a disease index reaching 89.03, thereby proving to be the most superior method for early-stage powdery mildew determination. Various determination methods wielded a significant influence on dry and fresh weight, chlorophyll content, and leaf photosynthetic activity of cucumber seedlings. Relative to other treatments, the net photosynthetic rate (Pn) of T3, T8, and T9 was significantly suppressed post-inoculation with powdery mildew, all lingering below 3 μmol/m2/s, while the transpiration rate (E), intercellular CO2 concentration (Ci), as well as the stomatal conductance (Gs), drastically declined, the decrease fell between 25%−80%. The relative chlorophyll content maintained at a lower level, ultimately leading to a significant reduction in the accumulation of dry matter. This demonstrates that powdery mildew can significantly stifle the growth of cucumber seedlings.
-
Key words:
- Cucumbers /
- Methods /
- Seedlings /
- Identification /
- Resistance /
- Powdery mildew












