-
Tomato chlorosis virus (ToCV) belongs to the Crinivirus genus of the Closteroviridae family. The virion of ToCV has a curved long linear shape, with a diameter of about 12 nm, a length of 800~850 nm, and a helical symmetrical structure with a pitch of 3.4~3.8 nm[1]. The ToCV genome is composed of two just single-stranded RNA, including a 8,594 nt RNA1 and a 8,242 nt RNA2, which is composed of 13 open reading frames (ORFs)[2]. ToCV is a phloem-restricted plant virus, which is transmitted by several whiteflies such as Bemisia tabaci in a semi-persistent transmission and seriously harms many dicotyledonous plants, especially tomato (Solanum lycopersicum). Tomato ToCV disease has spread throughout Asia, Europe, North America, South America, and Africa. Since the phenotype of ToCV-infected tomato at the early stage is similar to that of nutrient deficiency syndrome, this disease is easily ignored. However, when the symptoms are severe at the later stage, it can not be controlled and seriously affect the tomato plant growth, fruit yield, and quality, resulting in huge economic losses (usually more than 40%)[3]. So, more research on ToCV resistance mechanism of tomato and related breeding work needs to be carried out to ensure the stable development of the tomato industry.
For research on tomato-ToCV interaction and tomato breeding, it is necessary to establish an efficient and stable ToCV-inoculated method and evaluation criteria for tomato ToCV resistance. To date, there are no effective ToCV-inoculated methods and no evaluation criteria for tomato ToCV resistance. Though the natural incidence of tomato ToCV disease in the field is closer to the actual production, this method has great application limitations, and the incidence level is very uneven. In addition, the natural incidence of ToCV is transmitted by whiteflies, which always have a combination of ToCV and tomato yellow leaf curl virus (TYLCV) infection[4]. At present, the common methods of virus inoculation in plants are: mechanical friction, insect vector transmission, grafting transmission of virulent plants, transmission with virus-infectious clones, and so on[5−8]. ToCV can not be inoculated by mechanical friction. Insect vectors are difficult to control and carry a variety of viruses during transmission. At present, a method of transmitting ToCV by grafting has been reported in tomato plants[8]. However, the method of virus transmission by grafting ToCV-infected plants is extremely cumbersome. So, we want to build and use ToCV-infectious clones to achieve efficient inoculation.
The virus-infectious clones can be constructed by attaching their genomes to plant expression vectors, which stably maintain their genetic and biological characteristics and have been successfully used in virus inoculation[9,10]. In addition, since ToCV is a phloem-restricted virus, it is often difficult and time-consuming to vaccinate ToCV with leaves, resulting in a slow onset of disease symptoms even after successful inoculation[10]. Based on the above characteristics, we optimized the inoculation and disease evaluation methods based on Agrobacterium-mediated ToCV-infectious clones. The study of these methods is not only conducive to the study of tomato-ToCV interaction but also helpful to breeders to carry out resistance evaluation work, which has important reference value.
-
Total RNA was extracted from ToCV-infected tomato leaves using the TRIzol method (Invitrogen). RNA was reverse transcribed to cDNA using a SuperScript™ First-Strand Synthesis System for reverse transcription PCR (RT-PCR) (Invitrogen). The DNA fragments of ToCV RNA1 (NCBI: KC887998.1) and RNA2 (NCBI: KC887999.1) were amplified using these cDNA as templates. Referring to other successful cases of plant virus infectious clone construction[11,12], the binary vector pCass-RZ was also selected as the chassis of the ToCV-infectious clones. The DNA fragments of ToCV RNA1 was ligated into pCass-RZ using Stu I and BamH I restriction sites. DNA fragments of ToCV RNA2 was ligated into the pCass-RZ vector using the Stu I restriction site. Thus, the recombinant plasmids of pCassRz-ToCV-RNA1 and pCassRz-ToCV-RNA2 were constructed respectively. The constructed recombinant plasmids were transformed into Agrobacterium tumefaciens strain GV3101 to construct ToCV-infectious clones. The structure map of ToCV-infectious clones is shown in Fig. 1.
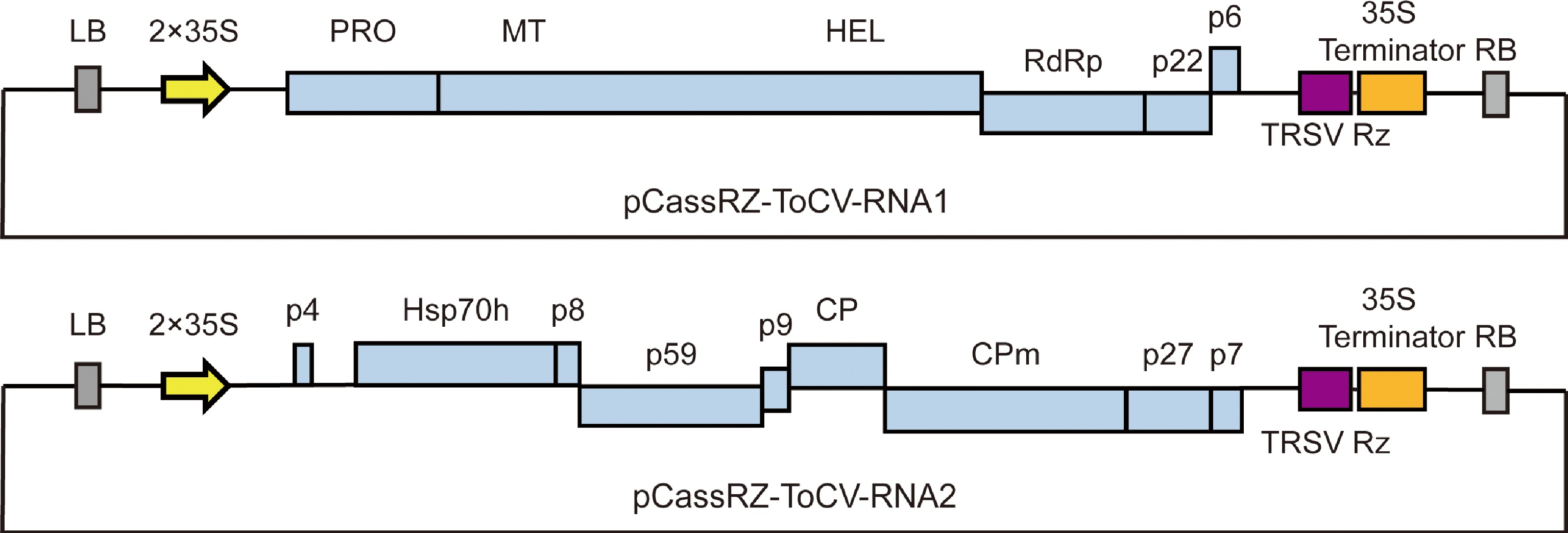
Figure 1.
Structure map of ToCV-infectious clones. LB: left border repeat of T-DNA; 2 × 35S: two tandem cauliflower mosaic virus (CaMV) 35S promoter; TRSV Rz: the cis-cleaving ribozyme sequence of tobacco ringspot virus; 35S Terminator: CaMV 35S terminator; RB: right border repeat of T-DNA. PRO: papain proteinase; MT: methyltransferase; HEL: helicase; RdRp: RNA dependent RNA polymerase; Hsp70h: heat shock protein 70 homolog; CP: coat protein; CPm: minor coat protein; p4, p6, p7, p8, p9, p22, p27, and p59 represent proteins of 4 kilodaltons (kDa), 6 kDa, 7 kDa, 8 kDa, 9 kDa, 22 kDa, 27 kDa and 59 kDa, respectively.
Tomato materials
-
Twenty-five tomato varieties (Table 1) that came from the Tomato Genetics Resource Center (TGRC), our laboratory, and commercial channels were used in this study. These tomato varieties come from different regions of the world and include different types of regular tomato, cherry tomato, and processed tomato.
Table 1. Information, disease index (DI), and disease resistance (DR) of tomato varieties.
Number Tomato varieties Source DI DR 1 Ailsa Craig TGRC (LA2838A) 96 HS 2 Fireball TGRC (LA3024) 62 S 3 Momor TGRC (LA2828) 45 MR 4 Red River TGRC (LA4350) 71 S 5 Nagcarlang TGRC (LA2661) 82 HS 6 New Yorker TGRC (LA2009) 44 MR 7 Moneymaker TGRC (LA2706) 75 S 8 Heinz 1706-BG TGRC (LA4345) 98 HS 9 M-82 TGRC (LA3475) 71 S 10 Shan Nong Tian Fen Yi Hao Our Lab. 26 R 11 Shan Nong Tian Fen Er Hao Our Lab. 82 HS 12 Lu Xiao Fan Yi Hao Our Lab. 20 R 13 Qing Lian Yi Hao Our Lab. 80 HS 14 Zhu Yu Our Lab. 58 S 15 Jin Peng Yi Hao Commercial 67 S 16 Bei Ying Commercial 85 HS 17 Tao Tai Lang Commercial 46 MR 18 Shang Hai Da Hong Commercial 70 S 19 Zhong Shu Si Hao Commercial 79 HS 20 Zhong Shu Wu Hao Commercial 71 S 21 Zhong Shu Liu Hao Commercial 62 S 22 Lu Fen Yi Hao Commercial 83 HS 23 Fu Shan 88 Commercial 28 R 24 Qian Xi Commercial 60 S 25 Bei Bei Commercial 58 S Tomato seed germination and seedling culture
-
Tomato seeds were disinfected with 10% sodium hypochlorite for 5−10 min and then rinsed with water five times. The sterilized seeds were placed in a petri dish containing three layers of wet filter paper and incubated at 25−28 °C in darkness. The germinated seeds were seeded in small pots containing a seedling substrate (peat : vermiculite = 2:1, v:v). Seedlings in these small pots were grown in a pest-free solar greenhouse (25−30 °C/15−20 °C, light/dark) or light incubator [16 h/8 h, 28 °C/18 °C; light (20,000 lx)/dark]. Water and nutrients were provided to the seedlings by daily watering of Hoagland nutrient solution [4 mM Ca(NO3)2, 6 mM KNO3, 2 mM MgSO4, 1 mM NH4H2PO4, 80 μM Fe-EDTA, 46.3 μM H3BO3, 9.5 μM MnSO4, 0.8 μM ZnSO4, 0.3 μM CuSO4, 0.02 μM (NH4)6Mo7O24]. Seedlings were used for ToCV inoculation when they were at the five-leaf stage.
Preparation of ToCV-inoculated buffer
-
Agrobacterium containing ToCV-infectious clones was cultured on solid Luria-Bertani (LB) medium (peptone 10 g·L−1, yeast extract 5 g·L−1, NaCl 5 g·L−1, 15 g·L−1 agar) with 50 mg·L−1 kanamycin and 50 mg·L−1 rifampin at 28 °C for 48 h. A single Agrobacterium colony was selected and cultivated in 5 mL LB liquid medium (without agar) with 50 mg·L−1 kanamycin and 50 mg·L−1 rifampin at 28 °C and 220 rpm for 24 h. After that, 15 mL of LB liquid medium was added to expand the culture for another 6 h. The Agrobacterium suspension was centrifuged at 6,000 rpm for 5 min and the bacterial precipitation was collected. Appropriate inoculation buffer [10 mM MgCl2, 10 mM 2-(N-morpholono) ethane sulfonic acid, 200 μM acetosyringone, and pH 5.6] was added to the bacteria precipitate and mixed by vortex. The OD600 of ToCV-inoculated buffer was adjusted to 0.5 ± 0.1, and then was allowed to stand in darkness for 3 h before inoculation.
Traditional leaf-inoculated method
-
The basal 2nd or 3rd fully extended leaves of seedlings (five-leaf stage) were selected for inoculation. A sterile needle was used to make a wound on the back of the leaf at the branch of the vein. After that, 0.5−1 mL of ToCV-inoculated buffer was drawn with a sterile syringe and slowly injected into the wound site until the inoculum infiltrated the entire leaf. After inoculation, the seedlings were placed in a dark environment for 24 h, and then transferred to the normal culture environment for further culture[13].
Improved stem-inoculated method
-
The stem tips of seedlings (five-leaf stage) were excised with a sterile scalpel blade, retaining two basal leaves (Fig. 2a, b). The incision of the stem segment is flat and phloem and xylem can be clearly distinguished (Fig 2b). About 0.5−1 mL of ToCV-inoculated buffer was absorbed with a sterile syringe. Then, a needle was inserted perpendicular to the stem section into the incision and a small amount of ToCV-inoculated buffer injected multiple times until the incision tissue is visibly infiltrated (Fig. 2c). The needle was slowly pulled out, and the ToCV-inoculated buffer will naturally form a liquid film at the incision (Fig. 2d, e). Tomato ToCV disease was detected 30 and 50 d post-inoculation (dpi).
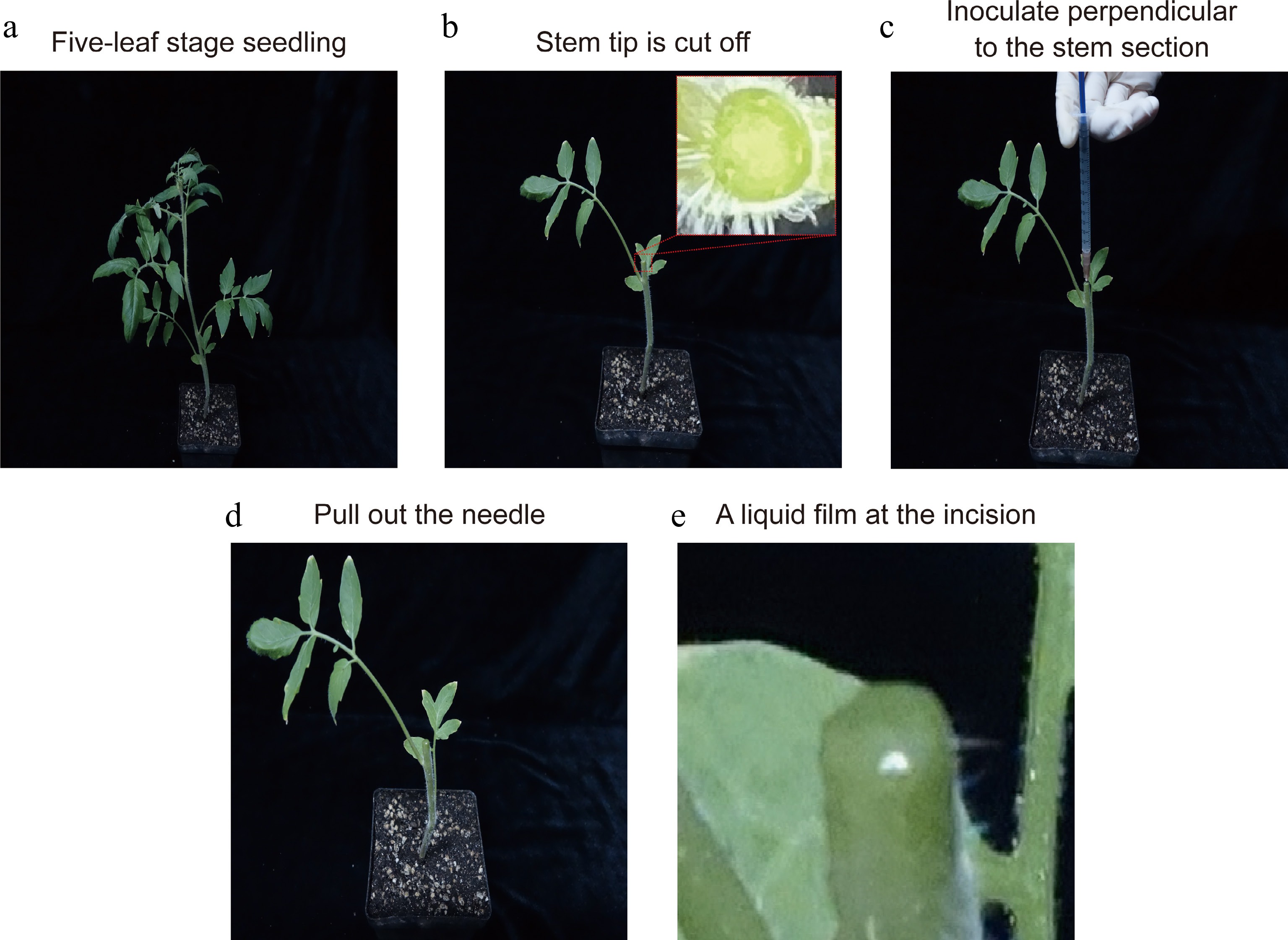
Figure 2.
The operation flow of the improved stem-inoculated method. (a) Preparing five-leaf-old tomato seedlings. (b) The stem tips of seedlings were excised with a sterile scalpel blade, retaining two basal leaves. The incision of stem segment is flat and can distinguish phloem and xylem clearly. (c) About 0.5−1 mL ToCV-inoculated buffer was absorbed with a sterile syringe. Then, a small amount of ToCV-inoculated buffer was injected multiple times until the incision tissue is visibly infiltrated. (d), (e) Slowly pull out the needle, and the ToCV-inoculated buffer will naturally form a liquid film at the incision.
Identification of tomato ToCV disease
-
The tomato ToCV disease was identified by the phenotype of interveinal chlorosis and the detection of the ToCV coat protein (ToCV CP) gene and its protein. At 30 dpi, the third leaf above the inoculated site was obtained for identification of tomato ToCV disease.
Gene identification of ToCV CP was performed with RT-PCR. Total RNA was extracted from leaves using the TRIzol method (Invitrogen). cDNA synthesis was performed according to standard procedures of a Revert Aid First Strand cDNA synthesis kit (Fermentas). Primers of ToCV CP (F: ATGGAGAACAGTGCTGTTGCAA, R: TTAGCAACCAGTTATCGATGCA) were used for PCR analysis.
Protein identification of ToCV CP was performed with a western blot. A total of 100 mg of leaves were ground into a frozen powder with liquid nitrogen. The frozen powder was suspended in 100 μL of 5 × SDS lysis buffer. The protein was denatured at 95 °C for 10 min, cooled to room temperature, and centrifuged at 12,000 g for 10 min at 4 °C. About 20 μL of protein extract was used for 10% SDS-PAGE. The proteins were detected using anti-CP (1:5,000) and the secondary antibody of Goat Anti-Mouse IgG (H&L)-HRP Conjugated (1:5,000). To verify the equal loading, the blot was stripped in strip buffer (1.5% glycine, 1% SDS, 1% Tween 20, pH 2.2) at room temperature for 30 min and then incubated with an anti-Actin (1:5,000) antibody. Quantitative analysis of protein was performed by ImageJ software.
Disease index analysis
-
Each tomato cultivar was inoculated with 20 plants. Another five uninoculated plants were used as a negative control. The experiment was repeated three times. The disease level (d) of all inoculated plants of each tomato cultivar was counted. The d of the inoculated plants and their corresponding symptom descriptions are shown in Table 2. The disease index (DI) per tomato cultivar is calculated as:
$ DI=\dfrac{\sum (d\times n)}{N\times D}\times 100 $ Table 2. Symptom description and classification of tomato ToCV disease.
Disease
level (d)Symptom description 0 No visible symptoms. 1 Several chlorotic yellow spots appear on local leaves of plant base. Plant is not dwarf. 2 Intervein yellowing and chlorosis appear on leaves of 1/3 base of the plant. Plant is not dwarf. 3 Half of the leaves of the plant are seriously yellowed. Leaves become brittle and hard. Plant is obviously dwarfed, the plant height is reduced by 10%−30% when compared to control. 4 Leaves of the whole plant are seriously yellowed and curled. Plant is obviously dwarfed, the plant height is reduced more than 50% when compared to control. No further planting value. Evaluation of tomato ToCV disease resistance
-
According to the DI of different tomato cultivars, the tomato ToCV disease resistance (DR) grade can be divided into highly resistant (HR, 0 ≤ DI < 15), resistant (R, 15 ≤ DI < 30), moderately resistant (MR, 30 ≤ DI < 55), susceptible (S, 55 ≤ DI < 77), and highly susceptible (HS, 77 ≤ DI ≤ 100).
Statistical analysis
-
Experiments were performed with three replicates. GRAPHPAD PRISM v.8.0 was used to draw all the charts and perform ANOVA analysis.
-
The ToCV-susceptible tomato variety Ailsa Craig (AC) and the improved stem-inoculated method with ToCV-infectious clones were used in this part. The phenotype of tomato ToCV disease were obtained at 30 and 50 dpi, separately. At 30 dpi, the ToCV-infected plants showed symptoms of interveinal chlorosis only in basal leaves (Fig. 3a). As time extends to 50 dpi, the symptoms of interveinal chlorosis spread from the basal leaves to the middle leaves (Fig. 3a). We randomly selected 12 AC plants inoculated with ToCV-infectious clones to monitor ToCV CP gene and its protein. The results showed that the presence of ToCV CP gene and its protein was detected in 10 of the 12 plants with obvious symptoms of interveinal chlorosis (Fig. 3b, c). In addition, there were two AC plants with mild disease, almost no difference from the uninoculated plants (mock treatment), and the presence of ToCV CP gene and its protein was not detected in these two plants (Fig. 3b, c). These results indicate that the phenotype and genetic test results of ToCV-infected plants are consistent.

Figure 3.
(a) The phenotype of tomato ToCV disease at 30 and 50 dpi. (b) ToCV CP gene identification of inoculated plants at 30 dpi. (c) ToCV CP protein identification of inoculated plants at 30 dpi.
Next, we compared the inoculation efficiency of the traditional leaf-inoculated method with that of the improved stem-inoculated method. We carried out three independent inoculation experiments with AC plants at different times and locations, each testing contains 50 plants per treatment. The phenotype of the tomato ToCV disease was used as a statistical basis. Figure 4 shows that the ToCV-inoculated efficiency of the traditional method is less than 40%, while the ToCV-inoculated efficiency of the newly invented method in this study is about 90%. This result indicates that the improved stem-inoculated method is more suitable in tomato plants.
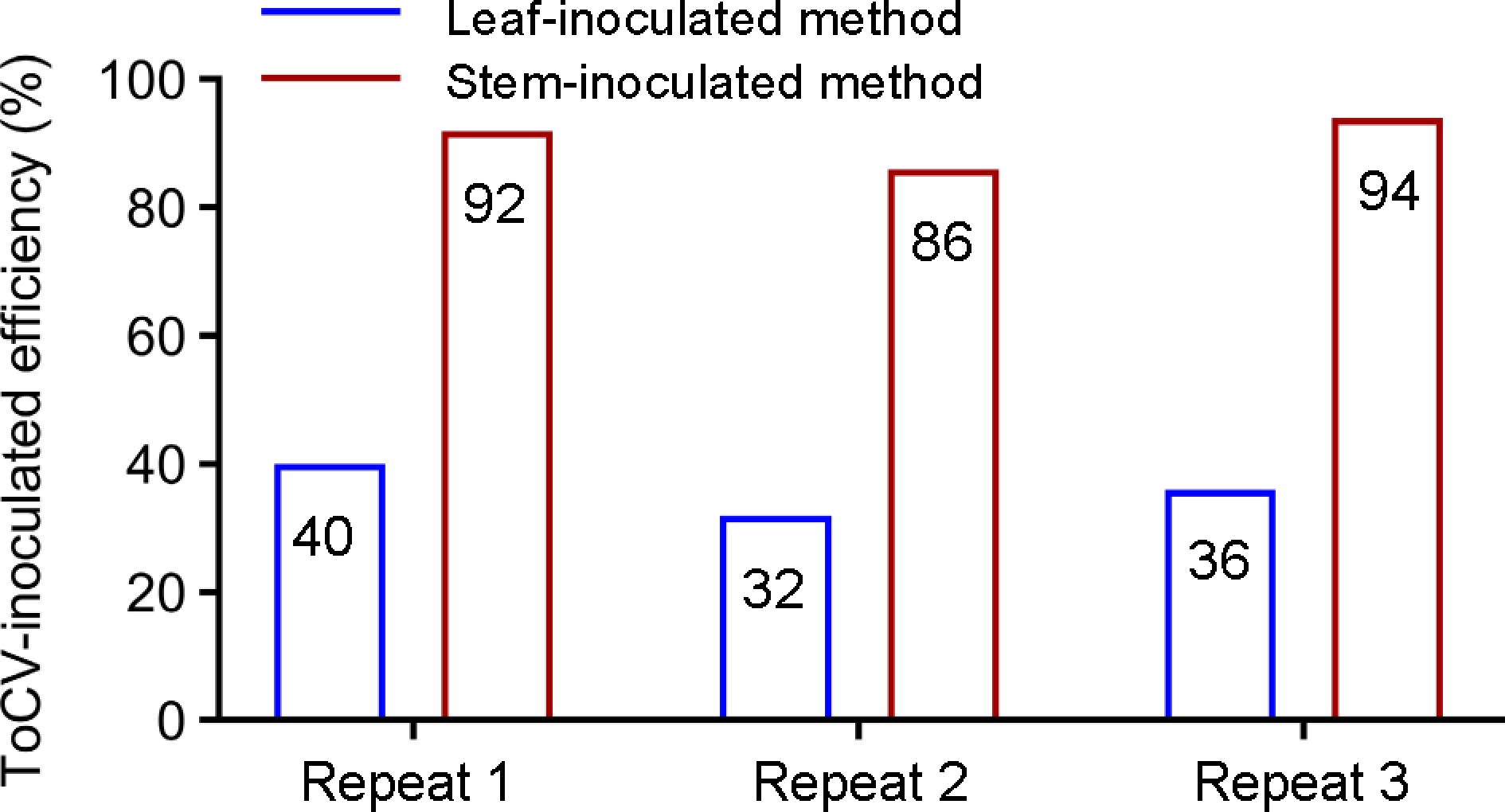
Figure 4.
Comparing the inoculation efficiency of traditional leaf-inoculated method and improved stem-inoculated method. Three independent inoculation experiments with AC plants were performed. Each testing contains 50 plants per treatment.
Finally, we want to explore the application of the improved stem-inoculated method in more tomato varieties and establish an effective tomato ToCV disease evaluation system. According to DI, these 25 tomato varieties can be divided into four DR grades, including three R varieties, three MR varieties, 11 S varieties, and eight HS varieties (Table 1). Though we did not identify the HR grade tomato varieties, this evaluation system can still effectively grade the ToCV-resistance of different tomato varieties.
The traditional leaf-inoculated method with ToCV-infectious clones is the most commonly used and most likely to be successful in model plants of Nicotiana benthamiana[14]. However, this study shows that this inoculation method is inefficient in tomato plants. This is because the tomato leaf is small, and the leaf pulse is dense, and the ToCV-inoculated buffer is not easy to penetrate the whole leaf. To increase the success rate of traditional leaf-inoculated method, we need to inject more times into the same leaf and inject more leaves. Therefore, the workload of the traditional leaf-inoculated method is very large. Although we have developed an efficient inoculation method and disease evaluation of ToCV in tomato, the operation still takes about 80 d from seeding to completion. How to shorten the experiment period is still a problem worth discussing. The removal of the main stem during stem-inoculated process was not suitable for tomato varieties with poor lateral bud germination ability. The whole evaluation process has high requirements for the control of other pests and diseases, especially vectors such as whiteflies that can transmit viruses. Despite these difficulties and shortcomings, this method is still the most efficient and feasible technical means known to us. In conclusion, an innovative modification was made to the ToCV-inoculated method, which significantly improved the inoculation efficiency. In addition, we first published the evaluation criteria of DI and the ToCV-resistance in tomato varieties. The publication of these new methods will strongly promote related research and breeding in the field of tomato and ToCV.
This work is supported by the Key R&D Program of Shandong Province, China (2022CXGC020710), Taishan Scholars Program (tsqn202306139), China Postdoctoral Science Foundation (2024M761852), and the 811 First-Class Disciplines Project of Shandong Agricultural University.
-
The authors confirm contribution to the paper as follows: study design, writing manuscript: Gong B, Zhu X; performing research, data analysis: Zhao D, Xia T, Zhao L; supplying guidance: Zhou T. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhao D, Xia T, Zhou T, Zhao L, Zhu X, et al. 2025. Inoculation method and disease evaluation of tomato chlorotic virus (ToCV) in Solanum lycopersicum. Vegetable Research 5: e006 doi: 10.48130/vegres-0025-0002
Inoculation method and disease evaluation of tomato chlorotic virus (ToCV) in Solanum lycopersicum
- Received: 02 December 2024
- Revised: 16 December 2024
- Accepted: 20 December 2024
- Published online: 14 February 2025
Abstract: Tomato chlorosis virus (ToCV) is a worldwide epidemic virus that seriously harms tomato production. However, there is no effective ToCV-inoculated method and relevant disease evaluation criteria in tomato plants. The lack of these basic techniques have severely limited the tomato-ToCV interaction research and ToCV-resistant tomato breeding. Here, we presented a method for constructing the ToCV-infectious clones using binary vector pCass-Rz and Agrobacterium-mediated transformation system. We further used ToCV-infectious clones to develope an improved stem-inoculated method, which can increase the ToCV-inoculated efficiency from less than 40% to more than 90% compared with the traditional leaf-inoculated method. According to characteristics of the tomato ToCV disease, we developed a detailed symptom description and grading plan, including the change degree of leaf color and texture, plant height, and planting value. The symptom description and grading plan was used to calculate the disease index (DI) and further to develop the tomato ToCV disease resistance (DR) of different tomato cultivars. The publication of these new methods will strongly promote related research and breeding in the field of tomato and ToCV.
-
Key words:
- Tomato /
- Tomato chlorosis virus /
- Inoculation method /
- Disease index /
- Disease resistance /
- Disease evaluation












