HTML
-
Maize (Zea mays) is a food crop that is consumed throughout the year in Kenya. It is mainly grown in the country in small holder farms as a basic source of food. Besides, the crop is also used as raw materials for many industrial processes. Majority of the communities in Kenya use maize to make a dish called 'Ugali' which is widely consumed in the country and therefore considered to be a staple food. Furthermore, maize is regarded as a source of income by farmers in Kenya (Birgen et al. 2020)
Majority of the families in Kenya consume maize meal at least once a day. Thus, to ensure constant availability of the product it is necessarily for farmers to store their maize after harvest. The preservation of the quality of the maize over the storage period depends on the status of the storage facilities. Storage facilities vary with the economic status of the producers. The poor resource producers are likely to have unsuitable storage facilities whose conditions predispose stored maize to post-harvest fungal infections, while more endowed producers may not. Post-harvest fungal infection on products leads to deterioration of both the quality and quantity of the products (Akwa et al. 2020). The most notable genera of the post-harvest fungi that infect maize and contaminate with mycotoxins are Aspergillus, Fusarium and Penicillium (Farrell & O'Keefe 2007). The most frequently encountered fungus in stored maize is A. flavus which produces aflatoxins that cause teratogenic, carcinogenic, mutagenic and hepatotoxic health problems (Birgen et al. 2020).
Fungicides has been used in the control of post-harvest fungi in stored food products. However, adverse effects on human health following consumption food products treated with fungicides has been observed (Dubey et al. 2007, Kumar et al. 2007). Some of these fungicides irritate the skin, tear glands; and disrupt neuron microtubules. This necessitates the search for safer alternatives for example the use of plant products.
Hence this publication addresses the use essential oils from Ocimum kilimandscharicum plants as an alternative to fungicides for the post-harvest control of Aspergillus flavus which is predominant in stored maize. Findings from this study will provide an alternative in the control of post-harvest fungi in maize rather than the use of fungicides.
-
The study took place in Murang'a County located in Kenya. Murang'a County has a population of about 942, 841 inhabitants spanning over an area of about 756 square kilometres (Mutahi 2010). Bordering this county to the North is Nyeri District, to the Southwest is Marang'a District, to the West is Nyandarua District and to the East is Kirinyaga District. This county experiences variations in rainfall. Long duration of rains is observed in the period March to May with rainfall ranging between 1400 mm to 1600 mm. The shorter duration being observed from October to November experiences average rainfall less than 900mm. Maximum agricultural productivity is observed during the long periods of rains. Temperatures vary in this part of Kenya with respect to altitude. At high altitudes, temperatures become as low as 6℃.
In Murang'a County, land is widely used for agricultural purposes with different crops being cultivated such as maize, coffee and tea. A proportion of about 70% of agricultural land is used for the cultivation of maize (Mutahi 2010).
Collection of Maize samples
-
Sampling of maize farms was performed in Kangema which is a sub county in Murang'a County. Agriculture is the major economic activity in Kangema, with most of the land being used for cultivation of food crops (Ndagi 2017). A total of eighty farms were sampled where maize had already been harvested and no synthetic chemicals had been initially dusted in the granaries, houses and other storage. One Kilogram of threshed maize sample were randomly collected from the stores in each of the eighty selected farms making a total of 80 kg that formed the bulk sample. The moisture contents of the sampled maize were reduced to 13% by sun drying. This moisture content was established with the help of an electronic moisture meter (Wile 55 type). The collected maize samples were then transported aseptically in polythene bags to the Kenyatta University Plant Science Laboratory for fungal isolation.
Isolation of Aspergillus flavus from maize samples
-
Initial surface sterilization (sterilization of maize kernel) was carried out on the maize samples using the Schulz et al. (1993) standard sterilization protocol. For the isolation of fungi from the maize, Potato Dextrose Agar (PDA) prepared following the protocol by Aryal et al. (2015) was used as the culture media. The sterilized maize grains were directly plated on the culture medium. The maize grains were arranged at equidistance 3.0 cm apart in the plates. Five grains were plated on each petri plate which was replicated four times. The plates were later sealed using parafilm and incubated at a temperature of 25℃ for a period of 7 days to ensure maximum growth of fungi (Samson et al. 2010).
Morphological identification of Aspergillus flavus from maize
-
Each of the fungal colonies which emerged from the plated maize grains after the period of incubation were individually sub- cultured in freshly prepared PDA media in petri dishes to obtain pure cultures. The subcultures were kept in an incubator maintained at 25℃ for 7days. Cultures which were uniformly growing with respect to color, texture and pigmentation were considered as pure colonies. Pure colonies of the fungi were identified following the methods outlined by Klich (2002) which comprised of fungal growth patterns, mycelia coloration and the appearance of the vegetative and reproductive structures.
Pathogenicity test of Aspergillus flavus on maize grains
-
To determine whether the A. flavus infects maize grains, pathogenicity test as outlined by Nikolić et al. (2016) of the identified fungi was performed in vitro. The pathogenicity test of Aspergillus flavus on maize was determined by inoculating clean/healthy maize grains with spore suspension of the fungus. The spore suspension was obtained from the pure cultures growing on PDA in petri dishes after the 7 days period of incubation. The spore suspension was obtained by adding 5 ml of sterile distilled water into the petri dishes containing the pure cultures. The petri dishes were gently agitated to bring the spores into the suspension. The spore suspension was then dispensed or poured into a universal bottle. The concentrations of the spores in the universal bottle were estimated by a haemocytometer count. The healthy maize was placed on moistened filter papers and inoculated with a drop of the spore suspension whose concentration was estimated to be 1x103 spores/ml. The plates were later sealed with parafilm and incubated at 27℃ for a period of 7 days to verify the fungal activity. Other maize samples were treated with sterile water only (no spore inoculation) and also incubated for the same period of time. These served as controls. After the duration of incubation, the developed symptoms on the spore inoculated maize samples were compared to the symptoms of the natural infected maize control to confirm the pathogenicity of the fungal isolates.
Ocimum kilimandscharium sample collection and oil extraction
-
Ruiru, Kiambu County was the site of collection of the Ocimum kilimandscharium plant (Fig. 1). The leaves of the plants were taken from their wild population. The taxonomic identification of the plant was performed in the plant sciences herbarium in which voucher specimens were kept.
Prior to oil extraction, the collected O. kilimandscharium leaves were air dried by placing them under a well-ventilated shade. This was done over a period of 5 days. Essential oil extraction was then performed on the plant, following the method of hydro distillation as documented by Erkan et al. (2012). This was then followed by the extraction of the distillate which was performed by passing over a solution anhydrous sodium sulphate. Solvent removal was carried out with the use of a rotary evaporator. The collected oil was then stored in a refrigerator using air tight bottles.
Antifungal assay of Ocimum kilimandscharicum on Aspergillus flavus
-
The antifungal activity of Ocimum kilimandscharicum was carried out using agar dilution method and disc diffusion technique following the procedure done by Bansod & Rai (2008).
Antifungal Assay by Agar Dilution Method
-
In the method involving agar dilution, amended PDA medium of different concentrations (3.33, 6.67, 13.33, 20 and 26.67) µl/ml were used. The different concentrations were prepared by incorporating separate volumes (400, 300, 200, 100 and 50) µl of the essential oil extracts of Ocimum kilimandscharicum plant into 15 ml of melted PDA. An aliquot of 20 ml of each amended medium was dispensed aseptically into sterile petri plates. The centre of each of the plates were then inoculated with 6mm mycelia disc taken at the periphery of Aspergillus flavus pure cultures. For each inoculated PDA plate four replications were made. The arrangements of the plates were performed in a completely randomized manner.
Control treatments comprised of plain PDA (not amended with oil extract) inoculated with A. flavus. The petri plates were then incubated at 27℃. The diameter of the colonies was recorded at 2 days intervals (1, 3, 5 and 7) spanning a period of 7 days. The growth inhibition was assessed by comparing the mean colony diameters of Aspergillus in the treatments to the controls.
Antifungal Assay by Disc Diffusion Technique
-
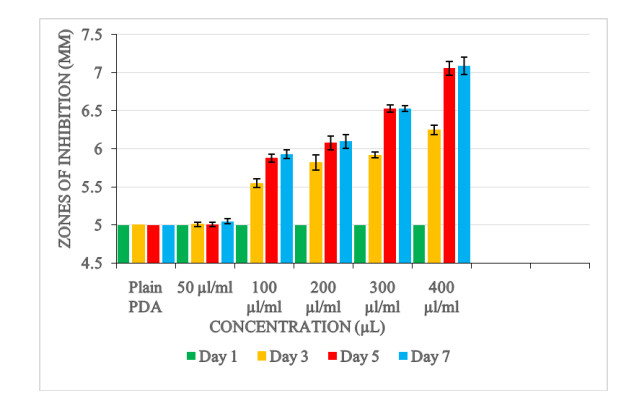
In the disc diffusion method, filter paper disc of diameter 5 mm was cut and sterilized in an autoclave. Amended Ocimum kilimandscharicum of different concentrations (400, 300, 200, 100 and 50) µl/ml were used. The different concentrations were prepared by diluting separate volumes (400, 300, 200, 100 and 50) µl of the essential oil extracts of into 1 ml of hexane. The sterilized paper disc was then immersed for 30 minutes in these concentrations. The paper disc was later removed from these concentrations, air dried and placed carefully on plates containing solidified PDA medium that had been amended with Aspergillus flavus spores suspension (1×106 spores). For each of the plates four replications were made. The arrangements of the plates were performed in a completely randomized manner. The plates were then incubated at 27℃. Control treatment comprised of sterile filter discs (not immersed in oil extracts) placed on PDA plates amended with A. flavus suspension. The size of the zone of inhibition were recorded at 2 days intervals (1, 3, 5 and 7) spanning a period of 7 days.
Data analyses
-
Data on radial growth inhibition were entered in a spread sheet, Microsoft Excel and normality determined. Transformation by logarithm, log10(x+1) was carried out followed by two-way ANOVA using SPSS Version 20 software. The means were separated using Tukey's HSD test at 0.05 level significance level. Tables and graphs were used in presenting the results.
Description of Study Site
-
Macroscopically, the Aspergillus flavus colonies isolated from the maize grains showed rapid growth and appeared dark on upper side (surface) of the plate and creamy on its lower side (reverse). The texture was cottony and granular in appearance. Microscopically, the conidiophores were long, colourless and contained phialides. At the end of the conidiophore was borne globose conidia (Fig. 2).
Pathogenicity test of Aspergillus flavus
-
Maize inoculated with spore suspension of Aspergillus flavus started to rot after 4 days of incubation. The inoculated grains were totally consumed with extensive mycelial growth that formed dark coloration over the testa of the grains (Fig. 3). Comparison of the symptoms that emerged from the inoculated kernels to those of the control (no spore inoculation) confirmed the pathogenicity of the A. flavus isolates on the grains.
Antifungal activity of Ocimum kilimandscharicum oil on Aspergillus flavus by Agar dilution method
-
Results showed that the Ocimum kilimandscharicum oil inhibited the radial growth of Aspergillus flavus. The magnitude of the growth inhibition of the oil varied with its concentrations. Radial growth inhibition was 100% (no further growth of A. flavus occurred) at high concentrations (26.67, 20, 13.33, and 6.67) μl/ml. In the control experiment (plain PDA), a rapid growth of Aspergillus flavus was observed (Fig. 4).

Figure 4. Amended PDA plates with varied concentration of Ocimum kilimandscharicum oil showing Aspergillus flavus colony.
Analysis of variance showed no significant difference of radial growth inhibition amongst the oil concentration level of 26.67, 20, 13.33, and 6.67 μl/ml. The oil concentration level of 3.33 μl/ml resulted in 43% radial growth inhibition. Analysis showed a significant difference (p < 0.0001) in the growth inhibitory effect between the 3.33 μl/ml concentration level and plain PDA (Table 1).
Table 1. Mean colony diameter (in millimetres) of A. flavus on PDA amended with different concentration of O. kilimandscharicum oil
Mean Colony diameter (mm) Concentration (μl/ml) Plain PDA (control) 1.36±0.05a 3.33 1.17±0.30b 6.67 0.79±0.001c 13.33 0.79±0.001c 20 0.79±0.001c 26.67 0.79±0.001c Days Day 1 0.79±0.001d Day 3 0.85±0.20c Day 5 0.91±0.50b Day 7 0.93±0.60a P-values Concentration 0.0001 Day 0.0001 Concentration*Day 0.0001 Values expressed a r e means ± SEM for four replicates. Means within respect columns accompanied by similar lower-case letters do not differ significantly at P < 0.0001 A significant difference (p < 0.0001) in colony diameter of Aspergillus flavus was also observed between the days (day 1, day 2, day 3, day 5 and day 7).
Furthermore, a significant interactive effect (p < 0.0001) was observed between concentrations of the oil and days of colony diameter (Fig. 5).
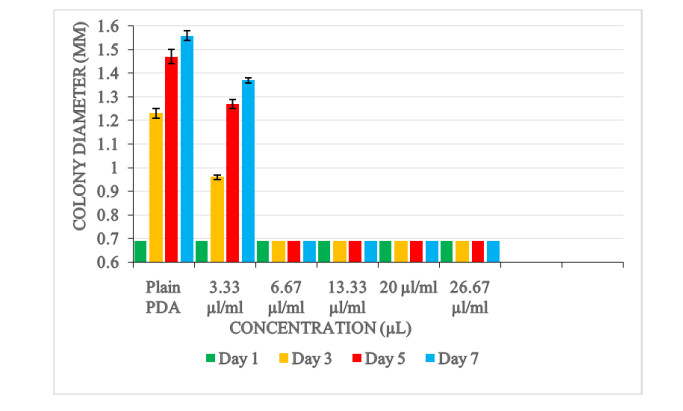
Figure 5. Interaction effects of concentration of Ocimum kilimandscharicum oil and days of incubation on colony diameter.
Antifungal activity of Ocimum kilimandscharicum oil on Aspergillus flavus by disc diffusion
-
Just as the agar disc diffusion method, similar radial inhibition pattern was observed using the disc diffusion method. Radial zones of inhibition recorded after 7 days for the different concentrations were as follows; 400 μl/ml (radial inhibition = 7.08 mm), 300 μl/ml (radial inhibition = 6.53 mm), 200 μl/ml (radial inhibition = 6.10 mm), 100 μl/ml (radial inhibition = 5.93 mm) and 50 μl/ml (radial inhibition = 5.05 mm).
Analysis of variance showed that a highest zone of inhibition (p < 0.0001) occurred at concentration level of 400 μl/ml. There was also a significant difference between the treatment concentrations and the control experiment (p < 0.0001). Furthermore, there was a significant difference in the zone of inhibitions between the days of incubation (day1, day 3, day 5 and day 7).
A significant interactive effect (p < 0.0001) was also observed between the concentrations of the oil and days on colony diameter (Fig. 6).
Table 2. Mean zone of inhibition (mm) of Aspergillus flavus culture grown on filter paper discs impregnated with Ocimum kilimandscharicum oil
Size of zone of inhibition Concentration (μl/ml) Plain PDA (control) 5.00±0.00f 50 5.06±0.01e 100 5.58±0.09d 200 5.76±0.12c 300 5.98±0.16b 400 6.34±0.22a Days Day 1 5.00±0.00c Day 3 5.74±0.09b Day 5 6.11±0.15a Day 7 6.14±0.16a P-values Concentration 0.0001 Day 0.0001 Concentration*Day 0.0001 Values expressed are means ± SEM for four replicates. Means within respect columns accompanied by similar lower-case letters do not differ significantly at P <0.0001 by Tukey's HSD test.
Identification of Aspergillus flavus from maize
-
The result indicated that Aspergillus flavus is capable of infecting maize grains as demonstrated by the colonization of the maize grains by the fungi, which eventually led to the complete rot of the grains. This observation therefore confirms that A. flavus is an important post-harvest rot agent of maize. Studies by Kinyungu et al. (2019) identified A. flavus as one of the major fungal contaminants and mycotoxin producer on post-harvest maize. The presence of this fungi contributes to the loss in quality and quantity of the maize after harvest (Birgen et al. 2020). The loss in quality of the maize is reflected by its change in color due to the presence of the pigmentation of the fungus. Pigmentation of fungi is a typical characteristic of its growth (Lopes et al. 2019).
The fact that there was no growth on the control maize sample indicates that the maize were truly healthy from the onset of the experiment and thus had not been infected by any fungi. It further confirms that when healthy maize comes in contact with A. flavus it gets infected.
Antifungal effect of Ocimum kilimandscharicum oil on growth of Aspergillus flavus using agar dilution method
-
Result showed that the oils extracted from Ocimum kilimandscharicum exhibited antifungal effects on Aspergillus flavus. Using the agar disc diffusion method, complete inhibition of A. flavus growth was observed with concentrations 26, 20, 13.33 and 6.66 μl/ml. Concentration level of 3.33 μl/ml showed a slight growth of A. flavus. In the control experiment (plain PDA), a rapid growth of A. flavus was observed. The fungal activity varied on the concentrations of the oil used. The antifungal effects were proportional to the concentrations of the oil extracts. Increase in level of concentration of the extracts enhanced the antifungal activity. The findings concur with similar reports done by Mares et al. (2004), Sharma & Tripath (2007) who showed that growth inhibition levels increase with increase concentration of antimicrobials used.
Antifungal effect of Ocimum kilimandscharicum oil on growth of Aspergillus flavus using disc diffusion method
-
Variations in the growth inhibition of the fungi at different concentrations using the disc diffusion method was similar to that obtained from the agar disc diffusion method. Higher concentrations resulted in higher zones of inhibition. The 400 μl/ml concentration level had a significant highest zone of inhibition while the 50 μl/ml concentration level had the significant lowest inhibition zone compared with the control experiment (plain PDA). This further proves that the antifungal activity is proportional to the concentrations of the oil extracts. Increase in level of concentration of the extracts enhanced the antifungal activity.
Several evidences have been brought forth showing the existence of active compounds in plant materials. Studies by Eliningaya et al. (2009) showed that aqueous extracts of leaves of Ocimum kilimandscharicum contains active components such as camphene, 4-terpeneol, limonene, α-terpineol, and linalool. These components have been shown to possess antimicrobial activities. Similar findings from Paschapur et al. (2009) also showed that leaves of O. kilimandscharicum contained biological active compounds such as triterpenoids saponins, tannins, sterols, flavonoids, carbohydrates and proteins. These compounds possess antimicrobial properties which protects the plants against infection by pathogens. Camphor has been shown to be one of the major components of O. kilimandscharicum oil (Sethi et al. 2012).
Other studies carried out by Gupta & Saxena (2012) has shown that the essential oils from O. kilimandscharicum possess significant antifungal properties. Some of these oil exhibits various mechanisms of action. Reports by Rasooli & Abyaneh (2004) showed that the use of thyme oil extracts brought about alterations in the morphology of species of Aspergillus parasiticus. These alterations included; hyphal distortion, sporulation failure, reduce conidiophore development, and loss of pigment.
Our present findings have shown inhibition of mycelia growth as one of the effects of O. kilimandscharicum oil on Aspergillus flavus. This inhibitory effect of the oil extracts may be as a result of natural bioactive chemicals found in the extracts (Rasooli & Abyaneh 2004).
Pathogenicity test of Aspergillus flavus on maize
-
From the results, it can be concluded that O. kilimandscharicum oil acts against the Aspergillus flavus, and hence a potential source for developing a product that controls this fungus. It is desirable to encourage the communities where the plant grows to domesticate it, and to extract oil, for local use and possibly for export. This would create additional source of income, thus improving their livelihood. In addition, it is a natural product, hence environmentally safe, and can be made available by domesticating it resulting in an additional benefit of conserving biodiversity. Furthermore, it is also recommended that, the efficacy of the oil be tested on other post-harvest fungi.
-
Many thanks go to the farmers of Murang'a County for the provision of maize used throughout the research.
-
The research does not involve any studies dealing with humans and animals
-
No conflict of interest is declared by the authors in the publication of these findings.
-
On behalf of the other authors, I (Corresponding Author) confirm that the manuscript has been read and is approved for submission
- Copyright: © 2021 by the author(s). This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| SM Kaguchia, JK Birgen, C Lang'at-Thoruwa, TE Akwa. 2021. Antifungal effects of Ocimum Kilimandscharicum oil on Aspergillus flavus Infecting Maize after Harvest. Studies in Fungi 6(1):288−298 doi: 10.5943/sif/6/1/19 |