HTML
-
Wild edible fungi (WEF) have been consumed by humans for thousands of years. The earliest evidence of consumption dates back to the Upper Paleolithic period around 18, 700 years ago (Power et al. 2015). They have also played a paramount role in the structure and functioning of natural ecosystems across the planet. As saprotrophs, mycorrhizal symbionts, or parasites, WEF have contributed in shaping all life on the planet. Since the Carboniferous period, saprotrophic mushrooms, mainly those belonging to Basidiomycetes, have played a vital role in nutrient cycling, primarily through digesting lignocellulosic materials previously resistant to decomposition. Ectomycorrhizal mushrooms function as a type of communication network in the forests by establishing connections among plants through which nutrients, water and signal molecules are exchanged. Parasitic mushrooms regulate plant community structures in ecosystems. It has even been shown that mushroom spores (including those of WEF), the production of which is estimated to be around 50 million tonnes annually, can stimulate rainfall (Hassett et al. 2015). A metanalysis of the diversity of WEF around the world revealed that approximately half of global diversity is concentrated across only eight families: Agaricaceae, Amanitaceae, Boletaceae, Cantharellaceae, Cortinariaceae, Gomphaceae, Russulaceae and Tricholomataceae (Fig. 1). Six of these families contain typical ectomycorrhizal species, showing the enormous dominance of this ecological group. In contrast, in China and Mexico, the two main biocultural repositories of WEF in the world, just five fungal families comprise around half of all WEF species consumed: Agaricaceae, Boletaceae, Gomphaceae, Russulaceae and Tricholomataceae (Fig. 2A, B, respectively).
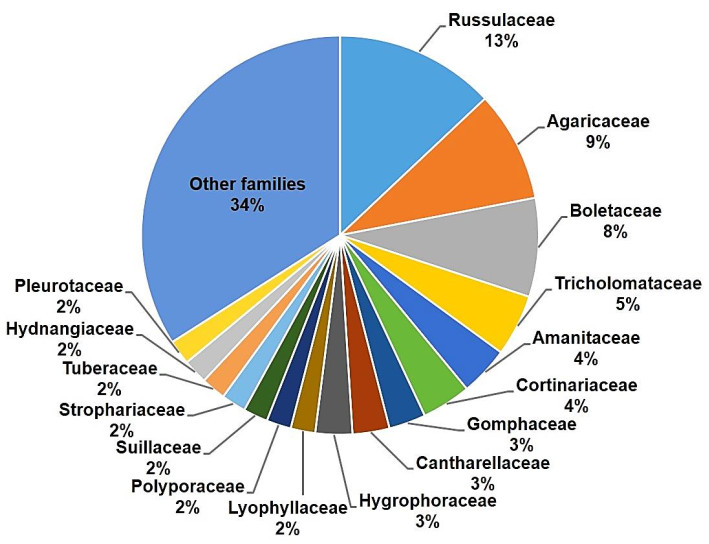
Figure 1. Distribution of families of 2, 100 species of wild edible fungi reported worldwide. Based on data from Boa (2004), Wu et al. (2019) and Pérez-Moreno et al. (2020d)
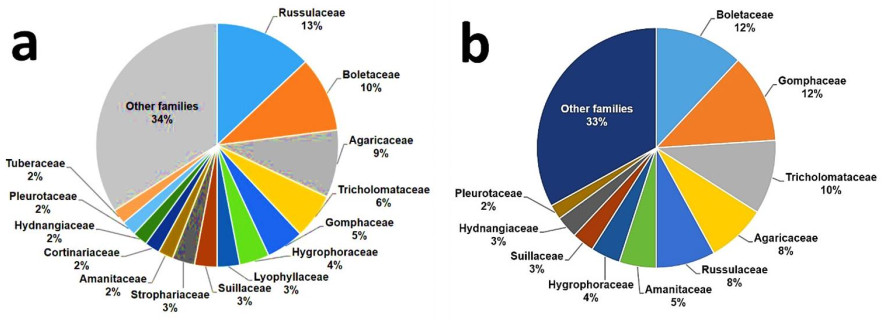
Figure 2. Distribution of families of WEF in the two main biocultural repositories of WEF in the world: 1, 020 species of WEF reported from China among 55 ethnic groups (a) and 450 species from Mexico among 68 ethnic groups (b). Based on data from Wu et al. (2019) and Pérez-Moreno et al. (2020d), respectively.
From an anthropocentric perspective, WEF and their multitudinous uses constitute an enormously important component of the traditional livelihood systems of numerous ancient cultures around the world (Pérez-Moreno et al. 2020a). It has been demonstrated that WEF are not only a nutritious food source, but that they also enhance the immune system and contain bioactive compounds with anticarcinogenic, antihypertensive, antioxidant, antibiotic and antiviral properties (Pérez-Moreno & Martinez-Reyes 2014). Therefore, the consumption of WEF constitutes not only a form of sustenance and economic activity for impoverished communities, but also a natural treatment for illnesses. The annual international trade of WEF has been estimated at 1.2 billion tonnes, with a 3-fold increase in the last two decades alone (de Frutos 2020). This trade has been estimated to be worth more than USD 5 billion. However, the use of WEF presently faces enormous challenges, including high rates of deforestation, biodiversity loss, rapid cultural erosion, unfair trade and climate change.
Fortunately, a promising future for WEF might yet be possible by implementing sustainable strategies such as adopting fair trade practices, successful reforestation efforts, application of innovations including mycotourism, sustainable harvesting techniques and widespread cultivation of mushrooms. Additionally, due to the fact that most edible mushrooms are quickly perishable, a suitable alternative is the technology transfer, to producers or gatherers, of methods to extend the WEF shelf life and at the same time to provide increasing revenues by providing and added value. These methods include for example drying, canning, preservation in vinegar or brine and production of jams, mushroom soup powder or bakery products. Successful commercialization of these added value products requires a quality control which should include information related to its nutritional data, date of packaging and due date. These strategies meet 11 out of the 17 Sustainable Development Goals of the United Nations Agenda for 2030 and have therefore attracted international interest (UN 2020). Accordingly, we present a review on the global ecological, sociocultural and economic relevance of WEF and analyze the challenges and perspectives towards WEF in the post-pandemic period.
-
The structural and functional diversity of WEF (Fig. 3) has played a crucial role in the development of life on the planet. Fungi are heterotrophic organisms, which mean that they are unable to generate their own food. For this reason, they have evolved to develop three trophic mechanisms. The first group are saprotrophic species, which feed on dead organic material of plants, animals or other organisms. The global ecological function of these fungi is essential for nutrient recycling in both natural and man-made ecosystems. Of particular importance are the Basidiomycetes fungi to which most WEF belong. Among the 2, 180 species of WEF reported worldwide by Li et al. (2021), 92.29% (2, 012 species) are Basidiomycetes, and the remaining 7.71% (168 species) are Ascomycetes (Fig. 4a). During the Carboniferous period 300 MYA ago, life on the planet as a whole was in a precarious state, largely because of the accumulation of plant debris, comprised mostly of cellulose and lignin. No organisms that were capable of degrading these compounds existed at the time, threatening a number of ecosystems across vast stretches of the planet. A mutation enabled an early lineage of Basidiomycetes fungi to produce enzymes belonging to the peroxidase family, which were able to degrade lignocellulosic compounds and recycle plant debris. As a consequence of this process, global ecosystem functioning was able to continue (Floudas et al. 2012). At the same time, the ancestors of Basidiomycetes experienced enormous structural and functional diversification that is still appreciable today. Basidiomycetous fungi are still excellent lignin decomposers in nature, demonstrating an "evolutionary footprint" which can be defined as a current characteristic present in living being's consequence of ancient genetic adaptations (Nagy et al. 2016, Ayuso-Fernandez et al. 2018).

Figure 3. Examples of the diverse range of morphological traits in wild edible fungi. A Amanita basii from pine forests in Central Mexico. B Auricularia auricula-judae from a cloudy subtropical forest in Machu Pichu, Peru. C Boletus reticuloceps in an Abies forest in China. D Terfezia arenaria from a sandy grassland in Morocco. E Tuber indicum from pine forest in Sichuan, China. F Cantharellus cibarius from a mixed broadleaf woodland in Scotland, United Kingdom.
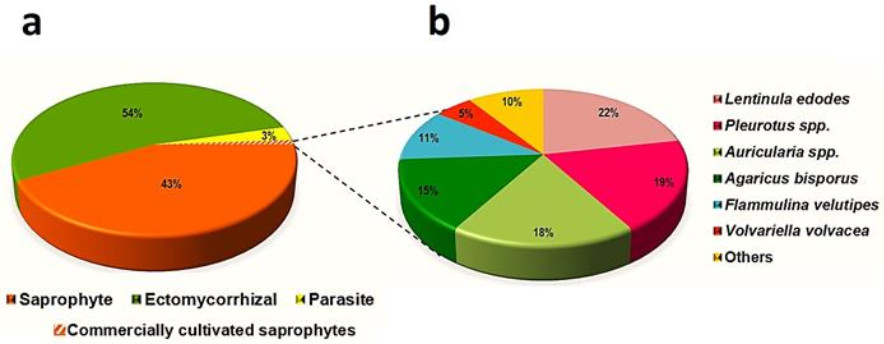
Figure 4. a Trophic groups (in percentages) of the 2, 180 known wild edible fungi. b the main cultivated saprophytic mushroom species in the world in relation as a percentage of the total global production (34 billion kg). Percentages were estimated from data presented by Li et al. (2021) and Royse et al. (2017).
A great variety of edible saprophytic fungi of enormous sociocultural and economic importance belong to Basidiomycetes, including numerous species belonging to the genera Auricularia, Agaricus, Flammulina, Lentinula, Pleurotus and Volvariella. However, there is also a small proportion of parasitic fungi that can be classified as WEF, such as members of Armillaria, Desarmillaria, Laetiporus and Ustilago. Parasitic fungi take their nutritional sources from plants, animals and microorganisms (including fungi themselves), causing damage, disease, death or simply living at the expense of the host organism. Paradoxically, epidemiological studies have shown that parasitic fungi regulate the structure of plant communities in natural ecosystems, maintaining biodiversity within an ecosystem by limiting the dominance of any particular species, thus fulfilling an ecological niche of enormous importance (Termorshuizen 2016).
The third trophic group consists of mycorrhizal fungi, which enter into mutualistic relationships with plants, significantly influencing forest structure and helping to facilitate the maintenance of all life on the planet. Mycorrhizal fungi feature a mutualistic feeding mechanism, wherein fungi receive carbon compounds from the plants with which they establish a relationship and in return they transfer water and macro- and micronutrients from the soil to their host plants. This symbiotic relationship is established among the roots of approximately 5, 000 plant species, including both gymnosperms and angiosperms, which dominate boreal, temperate and some subtropical as well as tropical biomes around the planet. The mycelium of these fungi penetrates the interstitial spaces between cortical cells of the host plant roots, from where they grow outward, forming fungal mantles that cover the host plant roots profusely and then later form mycelial networks that connect trees of different ages within the forest (Beiler et al. 2010). Thus, mycorrhizal networks constitute "… a kind of below ground internet which has been termed appropriately as the wood wide web…" (Rhodes 2017), since trees can utilize these complex assemblages to exchange water, carbon, nitrogen and other nutrients and minerals, conduct signaling between individuals, and send out defense compounds and allelochemicals. Moreover, because these networks facilitate the sharing of resources between trees of different species, including between angiosperms and gymnosperms, this discovery has shown that the functional traits of trees in forest ecosystems are more complex than previously believed (Tedersoo et al. 2020). The most economically lucrative WEFs in the world belong to this trophic group, which include truffles (Tuber spp.), Chantarelles (Cantharellus spp.), Porcini (Boletus spp.), Matsutake (Tricholoma matsutake and allied species) and Caesar's mushrooms (Amanita sect. caesarea). Li et al. (2021) reported 2, 180 species of WEF, among which 1, 181, 940 and 59 belonged to ectomycorrhizal, saprophytic and parasitic trophic groups, respectively (Fig. 4a). Despite this, only a miniscule number (100 species) are commercially cultivated, and only 10 of these are produced globally on an industrial scale: Auricularia auricula-judae, A. nigricans, Agaricus bisporus, Flammulina velutipes, Lentinula edodes, Pleurotus cornucopiae, P. eryngii, P. nebrodensis, P. ostreatus and Volvariella volvacea (Reis et al. 2012, Royse et al. 2017) (Fig. 4b).
Additionally, recent discoveries have revealed the enormous functional importance of wild fungi spores in their maintenance of local micro-climates by inducing rainfall. This includes WEF such as Lactarius hygrophoroides, Russula aeruginea, R. pulchra, R. variata and Suillus brevipes. The annual production of airborne fungal spores has been estimated to be around 50 million tonnes. It has been shown that due to their dispersal mechanisms, stimulated by the secretion of mannitol and other hygroscopic sugars, these spores constitute aerosols that have the potential to act as condensation nuclei for large water droplets, aiding in the formation of clouds and later in the generation of rain (Hassett et al. 2015).
-
The multitudinous uses of WEF constitute an enormously important component of subsistence livelihood systems developed over millennia. The first evidence of WEF consumption can be dated to 18, 700 years ago in the Upper Palaeolithic Period. An analysis of the dental plaque of the so-called "Red Lady" in northern Spain revealed the presence of various plant remains and fungal spores from an Agaric and a bolete (Power et al. 2015) related to Boletus edulis, a known WEF species from Europe. In other parts of the world, evidence of the consumption of WEF has also been documented: i) the Roman historian Pliny documented the consumption of wild edible mushrooms associated with oaks in his Natural History (Naturalis Historia) written between 77 and 79 AD, one of the most important encyclopaedic documents concerning ancient knowledge from the Roman Empire. In Chapter 11 of Book XVI, titled "The Natural History of the Forest Trees" he mentions: "… Such is the multiplicity of the products borne by the robur in addition to its acorns; and not only these, but mushrooms as well, of better or worse quality, the most recent stimulants that have been discovered for the appetite; these last are found growing about its roots. Those of the quercus are the most highly esteemed, while those of the robur, the cypress, and the pine are injurious…" (Bostock & Riley 1855). Furthermore, during the Song Dynasty in China from 960 to 1127 AD, Wu San Kwung discovered that by slashing the bark of forest logs where Lentinula edodes (shiitake) was naturally growing and vigorously hitting them, it was possible to increase the natural production of this mushroom. Due to this successful discovery, he is considered the father of shiitake cultivation. Even older documents record its use in ancient China, where it was called "ko-ko" or "hoang-mo" (Wasser 2005). Finally, the first known palaeographic evidence of WEF consumption in the Americas can be found in The Florentine Codex. This ancient book was written in Spanish and Nahuatl in Mexico between 1540 and 1577 by the friar Bernardino de Sahagún. Informed by traditional Aztec cultural practices, it contains a whole section in Chapter VII titled "Second paragraph: Of the mushrooms" (Parrapho Segundo: de las setas) that is dedicated to the WEF consumed in Mesoamerica, including those WEF with ceremonial uses (Fig. 5). This work reveals the wealth of WEF knowledge maintained generationally and transmitted by locals before the arrival of the conquistadors, including the common names of the WEF, cooking methods, and morphological and ecological characters of a number of species (Sahagún 2019). In a number of ancient cultures that are still living, there survives an incredible linguistic diversity used to describe WEF, which is evidence of prolific WEF knowledge widely disseminated across the five continents; for example, in Mexico, over 5500 native names are used to designate different kinds of WEF (Pérez-Moreno et al. 2020b).

Figure 5. The first paleographic evidence of wild edible mushroom consumption in the Americas can be found in the Florentine Codex (with pictures), written between 1540 and 1577 in Central Mexico by Aztec men and the friar Bernardino de Sahagún, currently held in the Laurentian Library of Florence, Italy.
Boa (2004) recorded over 2, 100 edible species of mushrooms and truffles across 85 countries. More recently, Li et al. (2021) identified 2, 189 edible species from 99 countries, among which 2, 006 can be consumed safely, and 183 species require a pre-treatment prior to safe consumption or are associated with allergic reactions by some people. Two of the most important biocultural centres in the world, where traditional knowledge that was generated over millennia still survives today, are China and Mexico. China has 1, 020 species of WEF (Wu et al. 2019) and 56 ethnic groups (55 of which are minority groups, with the Han people comprising the majority group); and Mexico has more than 450 species of WEF, 300 of which are commercialised, and 68 ethnic groups (Pérez-Moreno et al. 2020b, d). These ethnic groups are understudied, but recent surveys have shown them to be characterized by deep mycophilia. For example, in Mexico, only approximately 500 Tlahuica-Pjiekakjoo people are alive today, all of whom are over 60 years old. Members of this ethnic group, however, are capable of distinguishing more than 160 species of WEF (Pérez-Moreno et al. 2020b). In addition to this, there are important groups such as the Mixtec people (the third largest native group in Mexico, only after the Nahuatl and Maya groups) (Hernández-Santiago et al. 2016) or the Chinantec people (López-García et al. 2020), whose cultures have only recently been examined through an ethnomycological lens. Meanwhile, in Europe there are 87 distinct indigenous peoples, of which 33 form the ethnic majority population in at least one sovereign state, while the remaining 54 constitute ethnic minorities (Pan & Pfeil 2002). However, compared to Mexico, most European countries consume a relatively much smaller species number of WEF, e.gr. across 24 countries in Europe, the number of commercialized WEF species is as low as 268 (Peintner et al. 2014). Regions of Western Europe, however, such as the Mazovia region in Poland, where 72 species of WEF are consumed, feature extensive mycophilia as central parts of their cultures and economies (Kotowski et al. 2019). However, as mentioned above the largest centre of diversity of WEF with 1, 020 species (Wu et al. 2019), and 55 minority ethnic groups, is China. For example, very recently Zhang et al. (2021) collected and analysed 3, 585 samples of WEF from markets located in 35 counties across Yunnan Province and identified 159 WEF species. This is a representative example of the large diversity of WEF currently consumed in some Chinese areas, which should then be considered global hotspots. Recently, the opportunity to commercialize new WEF species in local markets dominated by ethnic groups such as the Yi people in southwest China has also gained attention (e.g., Wu et al. 2016, Wang et al. 2020).
Additionally, mycophilia is common in other countries around the world. In Europe, countries that have some mycophilic traditions include Finland, France, Italy, Spain, Sweden and Ukraine, as do Cameroon, Tanzania, Zimbabwe and Morocco in Africa, China, Japan, Russia and Mongolia in Asia, Mexico and Guatemala, with a growing interest in the USA and Canada, in North America, and finally New Zealand and Australia in Australasia (Pérez-Moreno et al. 2020a). Although the diametrically opposing concepts of mycophilia and mycophobia can be helpful to generalize viewpoints regarding WEF, we should be cautious about overutilizing this dualistic framework and understand that general attitudes toward mushrooms can be nuanced and change over time.
In general terms, there are three patterns of WEF consumption and trade in the world: i) self-consumption; ii) commercialization in domestic markets; and iii) commercialization in international markets. Each of these patterns faces its own challenges, weaknesses, and potential benefits. The most basic and oldest form of WEF consumption that can be widely found throughout the world is self-consumption. This category of consumption has been an important part of the subsistence and culture of low-income groups in Mesoamerica, Asia and Africa and continues to be an important part of the survival of millions of people in many cultures. Hundreds of WEF species are collected and consumed in these areas annually during their respective rainy seasons, and they constitute a fundamental element in the diet of numerous ethnic groups. Nutritionally, various studies have documented that, in general terms, WEF are an important source of protein, vitamins and minerals, and are low in fat, carbohydrates, cholesterol and triglyceride contents (Hall et al. 2003). Additionally, it has been documented that WEF species are an important source of bioactive compounds with medicinal properties, including compounds with anticarcinogenic, antidiabetic, antihypertensive, antioxidant, antimicrobial, antibacterial and antiviral properties as well as immune system enhancers (Pérez-Moreno & Martínez-Reyes 2014). For example, of the edible species consumed in China and Mexico, 30% and 15% have medicinal properties, respectively (Bautista-González & Moreno-Fuentes 2014, Wu et al. 2019). Of the WEF produced for self-consumption, some species that have a short shelf life or are produced in small quantities, such as members of the Suillus or Hygrophorus genera, are consumed by low-income groups where the value as a food is higher compared to that generated by their commercialization outside their communities. Additionally, species such as Neolentinus lepideus, which is the first WEF that appears during Mexico's mushroom season and has great biocultural importance for the Zapotec ethnic group, are not commercialized due to their high cultural and culinary value. In the last few decades, developed countries in North America and Europe have been increasingly organizing mycological field trips and forays in their local forests to collect species and later consume them. In the latter case, species that are either easily recognized based on their morphoanatomical characteristics or are comparatively abundant are usually collected and consumed, such as chanterelles (Cantharellus cibarius s.l.), boletes (Boletus edulis s.l.), morels (Morchellas pp.), "chicken of the woods" (Laetiporus sulphureus) and "Caesar's mushroom" (Amanita sect. caesarea). Currently, there is also a large and growing number of mycological fairs around the world, with a wide range of goals, including selling fresh and/or processed mushrooms, educating the public on how to distinguish between edible and toxic species, biocultural revaluation, conferences by specialists, mushroom identification workshops, commercialization of mushroom handicrafts, and the sale of artistic products created by local people.
Regulations have been established for the collection of WEF, whether for personal or commercial use, in various countries such as Austria, Argentina, Belarus, Bosnia and Herzegovina, Canada, Chile, Croatia, Finland, France, Italy, Macedonia, Montenegro, Poland, Rumania, Russia, Serbia, Spain, Slovakia, Spain, Sweden, Switzerland and USA (e.g., De Michelis & Rajchenberg 2006, Martínez-Peña et al. 2011, Peintnerpan2006bpeintner et al. 2013, BCG 2020, PFNMBN 2020, Wild Food UK 2020, VMS 2020). Regulations vary on a country-by-country basis, but in general they tend to limit which species are allowed for collection, the quantity allowed for collection (for personal or commercial consumption), and the frequency of collections in public areas (private areas or ecological reserves usually either require special permits or collection is outright forbidden). In addition, there are specific recommendations for sustainable harvesting of WEF, defined as the rate of extraction being equal to or less than the rate of production. Certain regulations designed to increase sustainable harvests include those meant to encourage collecting mushrooms using a knife and basket to promote the dissemination of spores in the gathering areas, cutting the mushroom stem above the ground in order to avoid damaging the mycelial masses, not collecting mushroom primordia, never picking more than half of the mushrooms that you find, or staying away from places of scientific interest, such as rare ecosystems.
Traditional knowledge of and culinary traditions associated with WEF constitutes an important part of the worldview of indigenous people, and is a valuable component of world cultural heritage (Fig. 6). Additionally, the collection and consumption processes of WEF have been used to strengthen social relationships in ethnic groups. This process begins with the gathering process, since it requires members of different generations to spend long periods of time together. It continues when members of the communities with more knowledge transmit their valuable expertise to younger generations, oftentimes communicating in their native languages. This is how traditional knowledge and its attendant vocabulary has been maintained across millennia in cultures around the world, and it continues even now in low income groups. After the collection stage, there are cultures that still use surplus gathered WEF as gifts to friends or family members of the community, or as barter for other foods or goods, further strengthening cultural ties. Finally, during the process of preparing food dishes, there is a sort of revalidation of cultural identity, since there is an enormous diversity of dishes that use local ingredients, and that constitute part of the myco-gastronomical characteristics of each of the different regions of the world where WEF are consumed. In fact, the astonishing diversity of local gastronomy is considered part of the biocultural heritage of humankind, and as such should not only be preserved, but also promoted as a strategy to maintain food security through the diversification of food sources. These perspectives and their global relationships to food security, biocultural conservation and forest sustainability have been recently reviewed in detail by Pérez-Moreno et al. (2021).

Figure 6. Sociocultural diversity of edible wild mushrooms in different cultures. A Nahua gatherer selling a diversity of mushrooms in Central Mexico. B Lyophyllum decastes marketing by Nahua women in Texcoco, Mexico. C Tricholoma matsutake sale in Ciba Market, Kunming, China. D Ramaria araiospora being sold by Otomi people in Jilotepec, Mexico. E Yi woman holding specimens of Tuber indicum in a truffle festival in Yongren, China. F Tajin with desert truffles (Terfezia arenaria and T. claveryi) in Morocco. G Berber woman collecting desert truffles associated with Helianthemum guttatum in a sandy grassland in Morocco, northern Africa.
WEF themselves are a genetic resource of enormous importance for global food security. There is an enormous number of species with the potential for cultivation, many of which could contribute to food self-sufficiency, creation of local jobs and poverty mitigation. In fact, the fungal germplasm of the 30 or so species of edible mushrooms cultivated globally at an industrial level were once wild mushrooms. This number dramatically contrast with the more than 2, 000 EWF which have been worldwide recorded (Li et al. 2021). However, only a small number of them (lower than 1.5%) has been industrially cultivated constituting therefore an untapped genetic pool. This despite the fact that EWF constitute a source of healthy food, which is rich in essential aminoacids, fibre, vitamins and minerals and low in cholesterol, and calories. More recently their importance as functional foods and their potential as health potentiators and elicitors of immune system has also been documented. As a consequence, rising consumption of unhealthy processed food items and increasing awareness among consumers related to the health benefits of mushrooms are important factors which are driving an increasing production of mushroom cultivation. For example, world production of cultivated edible mushrooms increased 30-fold since 1978 (from 1 billion Kg that year to 34 billion Kg in 2013). Meanwhile, world population increased only about 1.7-fold during the same period (from 4.2 billion to 7.1 billion), which represented an increase of per capita mushroom consumption, especially since 1997, accounting annually 1 kg per person that year versus 4.7 kg in 2013 (Bunyard 2021). Despite this fact, a global demand for healthy and nutritious food, including edible mushrooms continues to accelerate, and then a diversification boost of the cultivation of the known EWF genetic pool is currently an urgent need.
-
The economic importance of WEF exists on multiple levels. The most basic level is the commercialization in local communities in which the gatherers directly participate, the next level is the sale of WEF in regional markets carried out by 1–2 broker chains, followed by sale in national markets conducted by chains of up to 3 to 5 brokers. A crucial factor to consider is that in developing countries, mushroom pickers, regardless of gender, often belong to marginalized groups. Mushrooms are accordingly an important source of family income during harvest time, given the vulnerable socioeconomic status of mushroom collectors in parts of Africa, Mesoamerica and Eastern Asia. Little data exist regarding the commercialization of WEF in domestic markets around the world. However, there are some examples that are emblematic of what might exist globally. For example, in some Mexican communities, it has been documented that up to 100% of family income during the wet season comes from mushroom harvesting (Pérez-Moreno et al. 2010). In western North America, WEF collection constitutes an important source of income for immigrant groups coming from mycophilic cultures (Pilz & Molina 2002); and in China, there has been a radical change in the social position of previously marginalized groups that have traditionally collected and sold WEF such as matsutake (Arora 2008).
At the highest level, WEF are traded internationally for billions of US dollars annually (Table 1). Some species fetch exorbitant prices, placing some WEF among the world's most expensive foods. Some examples of these are as follows: i) In 2019 one fruiting body of the European white truffle (Tuber magnatum), weighing 1.005 kg, was sold for USD 145, 000 at an auction in the "89th International Alba White Truffle Fair" in Alba, Italy (AFP 2019); ii) Japanese matsutake (Tricholoma matsutake) can be worth up to USD 2, 000 per kg, and USD 1, 000 per kg during the beginning of the season in Tokyo is a common price (Japan Info 2020); iii) Perigord black truffle (Tuber melanosporum) prices ranged from USD 2, 811 to 3, 565 per kg from November 2019 to July 2020 (Truffle farm 2020); and iv) Annual world production of chanterelles (Cantharellus cibarius s.l.) ranges from 150, 000 to 200, 000 tonnes with an estimated value of USD 1.67 billion (Watling 1997). In addition to these examples, there are a number of WEF whose international commercial value is of relevance due to their exceptionally high level of production, e.g., Porcini (Boletus edulis s.l.), Caesar's mushroom (Amanita sect. Caesarea), Morels (Morchella spp.), Saffron Milk caps (Lactarius sect. Deliciousus) and Coral Fungi (Ramaria spp.). For example, a Finnish company harvested 1, 100 tons of WEF in a year, mainly porcini, with a revenue of USD 7.4 million (Cai et al. 2011).
Table 1. Highly prized wild edible fungi and estimated world trade value in US dollars
Common Name Scientific name Annual in-season retail market Wholesale price (per kilogram, grade one) Chanterelle Cantharellus cibarius s.l. $1.67 billion $\$$8 to $\$$22 Chinese black truffle Tuber indicum $\$$170milliona $\$$50 to $\$$200b Desert truffle Terfezia spp. and Tirmania spp. Unknownc $\$$27 to 334 Ganbajun Thelephora ganbajun Unknownd $\$$50 to $\$$250 Italian white truffle Tuber magnatum $\$$150 million $\$$1, 000 to $\$$7, 500b Matsutake Tricholoma matsutake s.l. $\$$500 million $\$$30 to $\$$1, 000 Périgord black truffle Tuber melanosporum $\$$150 million $\$$250 to $\$$3, 200b Porcini Boletus edulis s.l. $\$$250 million $\$$13 to $\$$198 Updated from Pérez-Moreno & Martinez-Reyes 2014; aestimation based on an average price of USD 170 provided by Truffle farm (2020) and a production of 1, 000 tonnes (Bin et al. 2021). bBased on retail prices in 2019 in Truffle farm (2020); cmostly sold in Arab countries with no official data available; dmostly sold in domestic markets in Southwestern China, without current accurate estimations of natural production. International trade of WEF has increased dramatically in recent decades. According to United Nations data, the volume of WEF trade has increased more than three-fold in the last 15 years (Fig. 7). The main variables that seem to drive this growing business are dynamic changes of trade relations between developed countries, income (gross domestic product) changes in some countries, and modification of consumption habits (including the increasing desire for healthy foods and diet diversification). The countries that have shown the greatest increase in trade of WEF between 2002 and 2017 in America are Canada, Mexico and Peru; in Europe they are Belgium, Bulgaria, Latvia, Macedonia and Romania; in Africa it is Belgian Congo; in Asia they are Armenia, Azerbaijan, Bhutan, India, Kyrgyzstan and Pakistan; and in Australasia it is New Zealand (de Frutos 2020). In addition to these figures, it should be considered that the domestic trade in many countries, such as Australia, China, France, Italy, Japan, Mexico, New Zealand and Spain, has yet to be holistically evaluated and likely accounts for millions of USD annually (Fig. 8).
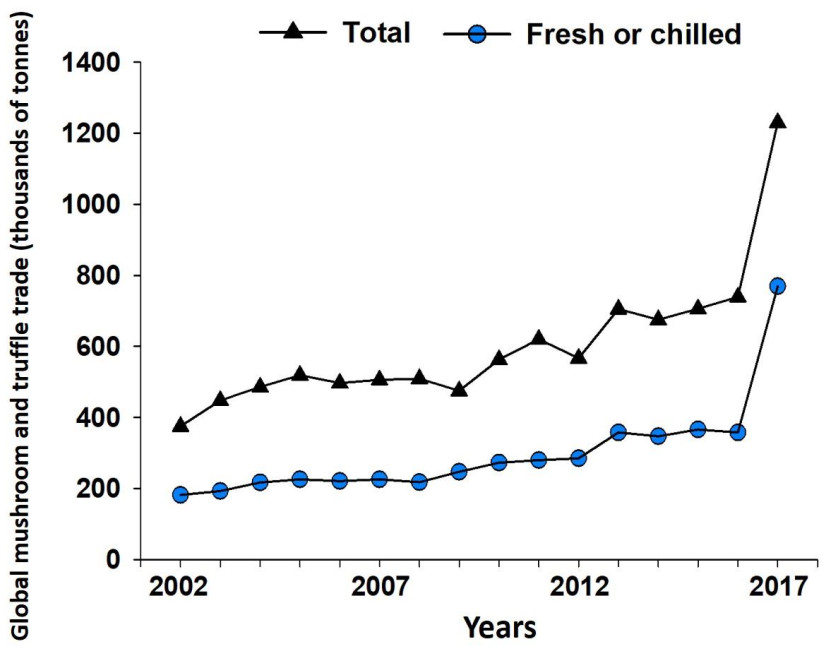
Figure 7. Annual global trade of edible wild mushrooms and truffles from 2002 to 2017. Graph created with data from the United Nations published by de Frutos (2020). "Total" includes the sum of international mushroom and truffle trade indexes labelled with the codes 070959 (truffles and mushrooms fresh or chilled), 071159 (mushrooms, provisionally preserved but unsuitable in that state for immediate consumption), 071239 (mushrooms and truffles, whole, cut sliced, broken or in powder but not further prepared, dried), and 200390 (mushrooms prepared or preserved otherwise than by vinegar or acetic acid). Meanwhile "Fresh or chilled" refers only to international trade labelled with the trade code 070959 (truffles and mushrooms fresh or chilled).

Figure 8. Economic relevance of edible mushrooms in different continents. A Mexican gourmet dish with the native "Blue milk cap" (Lactarius indigo). B Chinese gourmet desert with Asian truffles (Tuber indicum); C Vodka aged for 5 years with Cantharellus cibarius s.l. in Russia; D Complex factory of enoki mushrooms (Flammulina velutipes) in Nagano, Japan with an annual production of 300 thousand tonnes. E Specialized store selling Périgord black truffle (Tuber melanosporum) products in Cahors, France. F Hazel and oak tree plantation "Tewnion Truffiere" which produce high quality Périgord Black Truffles in Christchurch, New Zealand.
Currently cultivation of edible saprotrophic mushrooms constitutes a multibillion-dollar industry, with cutting edge technological applications. For example, in Suwa, Japan, there is an industrial complex dedicated exclusively to the cultivation of enokitake mushrooms (Flammulina velutipes), where more than 300, 000 tonnes are produced per year. Amazingly, as most of the processes are automated, the factory complex is run by only 200 workers. Furthermore, a novel stream of income for rural communities might exist in the future via the large-scale trade of edible ectomycorrhizal mushrooms used to produce bioinoculants useful for forest plant production, which are critical to ensuring the success of reforestation efforts (Pérez-Moreno et al. 2020c).
-
One of the main challenges faced by humankind today is figuring out how to balance nature conservation and human livelihoods. Nature conservation priorities include the maintenance of forest masses and natural ecosystems in order to mitigate climate change, slowing and reversing the accelerated extinction rate in order to preserve biodiversity, and preparing for the possibility of new pandemics (zoonoses) in the near future. At the same time, protecting human livelihoods should include the fair distribution of financial profits for people living in impoverished conditions, the promotion of rural development, food diversification, food sustainability and job creation, and reduction of environmental pollution and hunger. A comprehensive outline of the specific challenges facing humankind is the 17 sustainable development goals set by the 2030 United Nations agenda (UN 2020). To advance the strategic development of these goals, WEF constitute a genetic, biocultural and economic resource of enormous global import. In order to contribute to these 17 goals, the following actions must be promoted: i) the diversification of the cultivation of saprobic species at an industrial scale; ii) the improvement of techniques for the cultivation of ectomycorrhizal species; iii) the development of bioinoculant technologies based on edible ectomycorrhizal fungi that are useful for successful reforestation efforts; iv) the promotion of sustainable harvesting techniques to avoid overharvesting of WEF; v) the application of innovations such as mycotourism and the use of technologies to imbue added value to WEF, particularly in rural communities; vi) the dissemination of ecological, sociocultural and economic knowledge of mycological resources to children and young people; vii) the rescue and revaluation of local mycogastronomical practices; viii) the development of gourmet mycogastronomy; and finally, ix) promotion of the fair distribution of generated profits for local communities. Achieving these objectives requires the promotion of strategic alliances between four sectors: a) rural and urban communities; b) decision makers and policy makers; c) groups of scientists; and d) entrepreneurs, either as local cooperatives, small industries or big companies. It is important to consider that in the specific case of WEF, given the enormous cultural, social, economic, environmental and geographical variation that exists at a global level, a great amount of creativity will be required to design and implement context-specific strategies so as to suit the particular needs of an individual region, while at the same time working toward common objectives. An additional factor to consider is that in the short- and medium-term, the world will experience complex financial and sociocultural consequences of the so-called "great confinement" caused by COVID-19, which is already considered the largest economic recession in history (Snooks 2020). Therefore, immediate action to apply multiple strategies at the local, regional, national and global levels is urgently needed to counteract these negative effects. Promoting the sustainable management of WEF offers a potential pathway going forward for both vulnerable groups living in extreme poverty, as well as in urban and rural populations. The potential sustainable uses of WEF are countless; at the same time, many challenges must also be overcome to develop new innovations. The degree to which we meet these challenges will be consequential for our future.
-
Our coexistence with fungi dates back nearly 20, 000 years. Indeed, WEF have left an indelible mark on the evolution of all life forms on earth. Most of the 2, 200-known species of WEF establish ectomycorrhizal symbiosis with plant roots, accounting for 54% of total fungi, with the remaining 46% either saprotrophic (43%) or parasitic (3%). Surprisingly, only a small proportion of WEF are cultivated commercially or at an industrial scale (around 4.5%). These facts suggest that developing strategies to preserve WEF genetic resources for the purposes of food security is extremely relevant. Hundreds of WEF have traditionally been important biocultural resources critical to the livelihood strategies of poor rural communities in low or middle-income countries. On a larger scale, WEF global trade is worth billions of dollars; according to the United Nations, more than 1.2 billion tonnes were commercialized in 2017 alone. Furthermore, the volume of this trade has increased more than three-fold in the last 15 years. Therefore, WEF constitute an important natural resource which, if used sustainably, can contribute to food security, creation of jobs and hunger mitigation in the post-pandemic era to come.
- This study was funded by a grant (No. 31861143002) of NSFC-CGIAR. The first author would like to thank Dr. Faustino Hernández-Santiago and Dr. Magdalena Martínez-Reyes for his technical help to analyze the information presented in Figures 1, 2 and 4 and to the Mexican Council of Science and Technology (CONACYT) PRONACES FOP07-2021-03 Project 316198. Peter E Mortimer would like to thank the "High-End Foreign Experts" in the High-Level Talent Recruitment Plan of Yunnan Province, 2021. Samantha C. Karunarathna thanks the CAS President's International Fellowship Initiative (PIFI) young staff under the grant number: 2020FYC0002 and the National Science Foundation of China (NSFC) under the project code 31851110759.
- Conflict of interest
- No conflict of interest associated with this work.
- Copyright: © 2021 by the author(s). This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| J Pérez-Moreno, PE Mortimer, J Xu, SC Karunarathna, H Li. 2021. Global perspectives on the ecological, cultural and socioeconomic relevance of wild edible fungi. Studies in Fungi 6(1):408−424 doi: 10.5943/sif/6/1/31 |












