-
T and B lymphocyte mediated immune tolerance disorder led to abnormal auto-antibodies invasion in thyroid glands, which was the main mechanism of Hashimoto thyroiditis (HT). The increase of HT continued[1], and gradually became the most common cause of thyroid hormone insufficiency. Besides, it was suggested a link to diseases including ischemic heart disease, osteoporosis, diabetes, cardiovascular disease and cancer[2−6]. Papillary thyroid carcinoma was the most common form of cancer associated with HT[7]. Even the health of children was threatened by HT with higher risk of dyslipidemia and cardiovascular disease[8]. Along with the progress of HT, the secretion of thyroid hormone was significantly insufficient, and patients had to replenish with Euthyroxin throughout their life, but still suffered from higher risk of HT related complications. Therefore, effective methods for HT prevention were considered to be urgent research.
Since Vitamin D receptors were shown to be present in the thyroid[9], Vitamin D was considered as HT prevention in resent studies. Krysiak et al.[10] suggested that Vitamin D combined with atorvastatin could improve thyroid autoimmunity. In addition, supplementation with cholecalciferol for HT patients was indicated to twist the balance of CD4+ T-cell subsets toward ameliorative composition[11]. Although the relationship between Vitamin D and HT seemed clear, it still needed further investigation[12], for there existed study reported they had no evident correlation[13]. These contradictory conclusions maybe due to the small sample size of the local population, but it was very difficult to conduct a high-quality epidemiological study with a large sample size. Therefore, to indicate whether vitamin D deficiency was really correlated to HT, which may affect clinical strategy whether we should use Vitamin D to prevent HT, evidence-based medical research tools such as systematic review and meta-analysis were necessary for a more reliable conclusion.
We conducted a systematic review and meta-analysis of the domestic and foreign studies that investigated the relationship between Vitamin D and HT. Compatible data were pooled into meta-analysis to provide rigorous evidence-based medical reference for the prevention of HT.
-
The present systematic review and meta-analysis were performed under PRISMA guidelines[14]. An online bibliographic search was performed in Pubmed (for English), and China National Knowledge Infrastructure (CNKI) and Wanfang Databases (both for Chinese) by two investigators with key words of 'Vitamin D' and 'Hashimoto thyroiditis'. Studies were considered if they were in English or Chinese, and updated to 27 September 2021. If the abstract displayed investigation about the relationship between Vitamin D and HT, the full text was read in detail by at least two authors (Fig. 1).
Inclusion criteria
-
Studies were finally included if they fulfilled the following criteria: (1) included the comparison of HT patient group with a healthy control group; (2) serum levels of 25(OH)D3 level and/or the quantity of patients with 25(OH)D3 insufficiency were reported; (3) written in English or Chinese; (4) with a quality score above or equal to 6 according to the coding manual for case-control studies[15] assessed by two authors respectively.
Data extraction
-
The following information was extracted from each study by two investigators independently: (1) the first author; (2) year of publication; (3) region; (4) sample size; (5) serum levels of 25(OH)D3; (6) cut-off of serum 25(OH)D3 insufficiency; (7) the quantity of patients with 25(OH)D3 insufficiency; and (8) quality score. After that, the extracted information was summarized and checked by another two authors.
Statistical analysis
-
RevMan 5.3 (the Cochrane Collaboration) was used to perform a meta-analysis on the data obtained. Firstly, Weighted mean differences (WMD) for continuous variable and Odds Ratios (OR) for binary variables were calculated. Subsequently, statistical heterogeneity was assessed with I2 test, and the main source of heterogeneity was revealed by subgroup analysis. Lastly, publication bias was evaluated by funnel plots. For statistical analysis above, 'P < 0.05' was considered a significant difference between groups.
-
Online search obtained 336 studies, in which 280 were excluded by abstract screening. Then, of the 56 remaining, 10 were excluded by full text in-detail evaluation; finally 46 studies with 15,336 individuals in total (6,138 HT patients and 9,198 healthy controls) were included into the present study for the systematic review[16−61]. Characteristics of included studies are summarized in Table 1.
Table 1. Characteristics of included studies.
Study
(published year)Region Sample size (HT:C) 25(OH)D3
Assay methodSerum 25(OH)D3 level
(HT vs C)
(ng/mL)Serum 25(OH)D3 insufficiency cut off (ng/mL) Number of 25(OH)D3
insufficiency (HT:C)Quality
scoreMaciejewski et al. 2015[23] Poland 62/32 ELISA 8.00 ± 5.06 vs
12.12 ± 7.80< 30 61/27 7 Ucan et al. 2016[27] Turkey 75/43 RIA 9.37 ± 0.69 vs
11.9 ± 1.01< 20 75/36 9 Bozkurt et al. 2013[12-17]] Turkey 360/180 CLS 12.2 ± 5.6 vs
15.4 ± 6.8< 10 150/37 8 Kim 2016[20] Korea 221/555 CLS 36.84 ± 22.96 vs
39.84 ± 21.48< 30 108/206 8 Sonmezgoz et al. 2016[25] Turkey 68/68 CLS 16.8 ± 9.2 vs
24.1 ± 9.4< 30 61/54 8 De Pergola et al. 2018[18] Italy 45/216 CLS − < 20 31/113 8 Botelho et al. 2018[16] Brazil 88/71 CLS 26.4 (7.6–48.2) vs
28.6 (13–51.2)< 30 61/39 7 Ma et al. 2015[22] China 70/70 ELISA 12.40 ± 4.46 vs
16.53 ± 5.79< 30 70/67 7 Yasmeh et al. 2016[29] America 97/88 CLS 24.5 ± 6.42 vs
20.6 ± 6.5< 30 66/74 7 Xu et al. 2018[28] China 194/200 CPBA 16.16 (13.72–18.76) vs
23.32 (20.84–25.92)− − 7 Kivity et al. 2011[21] Israel 28/98 CLS − < 10 22/30 8 Mansournia et al. 2014[24] Iran 41/45 SC 15.9 ± 1.21 vs
24.4 ± 1.73< 20 34/24 8 Tamer et al. 2011[26] Turkey 161/162 RIA 16.3 ± 10.4 vs
29.6 ± 2.55< 30 148/102 8 Chaudhary et al. 2018[32] India 35/50 HPLC 13.39 ± 6.8 vs
26.16 ± 12.28< 20 31/38 8 Evliyaoğlu et al. 2015[31] Turkey 90/79 HPLC 16.67 ± 11.65 vs
20.99 ± 9.86< 20 80/69 8 Unal et al. 2014[30] Turkey 254/124 CLS 17.05 (5.4−80) vs
19.9 (9−122.7)< 20 160/- 7 Ke et al. 2017[19] China 61/51 EBL 22.10 ± 1.52 vs
33.40 ± 1.56< 20 34/12 7 Camurdan et al. 2012[33] Turkey 78/74 HPLC 31.2 ± 11.5 vs
57.9 ± 19.7< 20 69/24 7 Dellal et al 2013[34] Turkey 51/27 RIA 17.3 ± 8.0 vs
21.8 ± 15.2− − 6 Siklar et al. 2016[35] Turkey 32/24 HPLC 16.02 ± 9.84 vs
21.91 ± 7.68< 20 22/10 7 Nalbant et al. 2017[36] Turkey 253/200 CLS 33 ± 29.6 vs
43.7 ± 26.2< 20 161/111 8 Giovinazzo et al. 2017[37] Italy 100/100 HPLC 21.2 ± 12.9 vs
35.7 ± 16.7< 20 70/18 7 Guleryuz et al. 2016[38] Turkey 136/50 HPLC 14.88 ± 8.23 vs
15.52 ± 1.34− − 6 Perga et al. 2018[39] Italy 55/59 CLS − < 20 37/42 Yavuzer et al. 2017 Turkey 49/34 ELISA 19.5 ± 15 vs
23.8 ± 19− − 6 Priya et al. 2016 India 25/27 ELISA 14.3 (12.65−17.90)
vs 26.2 (21.00−32.8)− − 6 Chao et al. 2020[42] China 373/4889 RIA 16.66 ± 6.51 vs
15.81 ± 6.42< 20 363/4738 9 Feng et al. 2020[44] China 36/30 ELISA 17.39 ± 8.49 vs
35.15 ± 14.16− − 6 Ahi et al. 2020[43] Iran 633/200 CLS 13.22 (8.1−24.27) vs
20.4 (11.2−29.6)− − 7 Liu and Zhang. 2012[46] China 30/20 RIA 16.48 ± 6.25 vs
24.31 ± 7.88− − 7 Xiang et al. 2017[47] China 41/106 CLS 19.71 ± 8.43 vs
20.56 ± 11.64< 30 38/90 6 Zhang et al. 2015[48] China 31/19 HPLC 17 ± 6 vs
24 ± 7− − 6 Chen et al. 2015[45] China 34/52 CLS 14.4 ± 5.6 vs
17.4 ± 5.6< 20 29/37 7 Li et al. 2015[49] China 50/56 − 21.19 (18.40−25.28) vs
24.06 (18.94−33.90)< 30 44/37 6 Cvek et al. 2021[50] Croatian 461/176 CLS 19.7 (14.4−25.2) vs
17.3 (13.2−22.7)< 20 127/65 7 Salem et al. 2021[51] Egypt 120/120 ELISA 7.6 ± 4.4 vs 20.6 ± 5.5 < 10 120/112 7 Hana et al. 2021[52] Egypt 112/48 HPLC 10.1 (8.7−11.7) vs 12.0 (9.3−15.6) < 30 101/40 6 Olszewska et al. 2020[53] Italy 30/20 − 17.9 ± 7.9 vs 18.5 ± 8.1 − − 6 Rezaee et al. 2017[40] Iran 51/45 CLS – − − 6 Ren et al. 2021[55] China 62/80 − 13.49 ± 4.32 vs 15.75 ± 5.85 < 30 60/76 6 Huang et al. 2018[56] China 61/50 CLS 16.27 ± 6.99 vs 29.01 ± 9.72 < 20 − 6 Chi et al. 2020[57] China 32/30 CLS 15.27 ± 5.98 vs 28.89 ± 9.58 − − 6 Yang et al. 2021[58] China 88/60 − 13.37 ± 3.49 vs 17.58 ± 5.63 − − 6 Ke et al. 2021[59] China 152/50 CLS 20.56 ± 1.4 vs 33.4 ± 6.5 < 20 90/6 7 Wang et al. 2015[64] China 31/30 ELISA 10.08 ± 0.44 vs 14.32 ± 3.74 − − 6 Fu et al. 2021[61] China 334/300 − 16.84 (11.81, 23.39) vs 16.66 (11.98, 22.13) < 30 214/209 7 H: hashimoto thyroiditis group; C: Healthy control group; ELISA: Enzyme Linked Immunosorbent Assay; RIA: Radioimmunoassay; CLS: Chemiluminesent lmmunoassay Assay; CPBA: competitive protein binding assay; SC: Solid Chromatography, HPLC: High Performance Liquid Chromatography, EBL: Euglobulin lysis method, −: Non reported. Evaluation of indexes
Serum 25(OH)D3 level
-
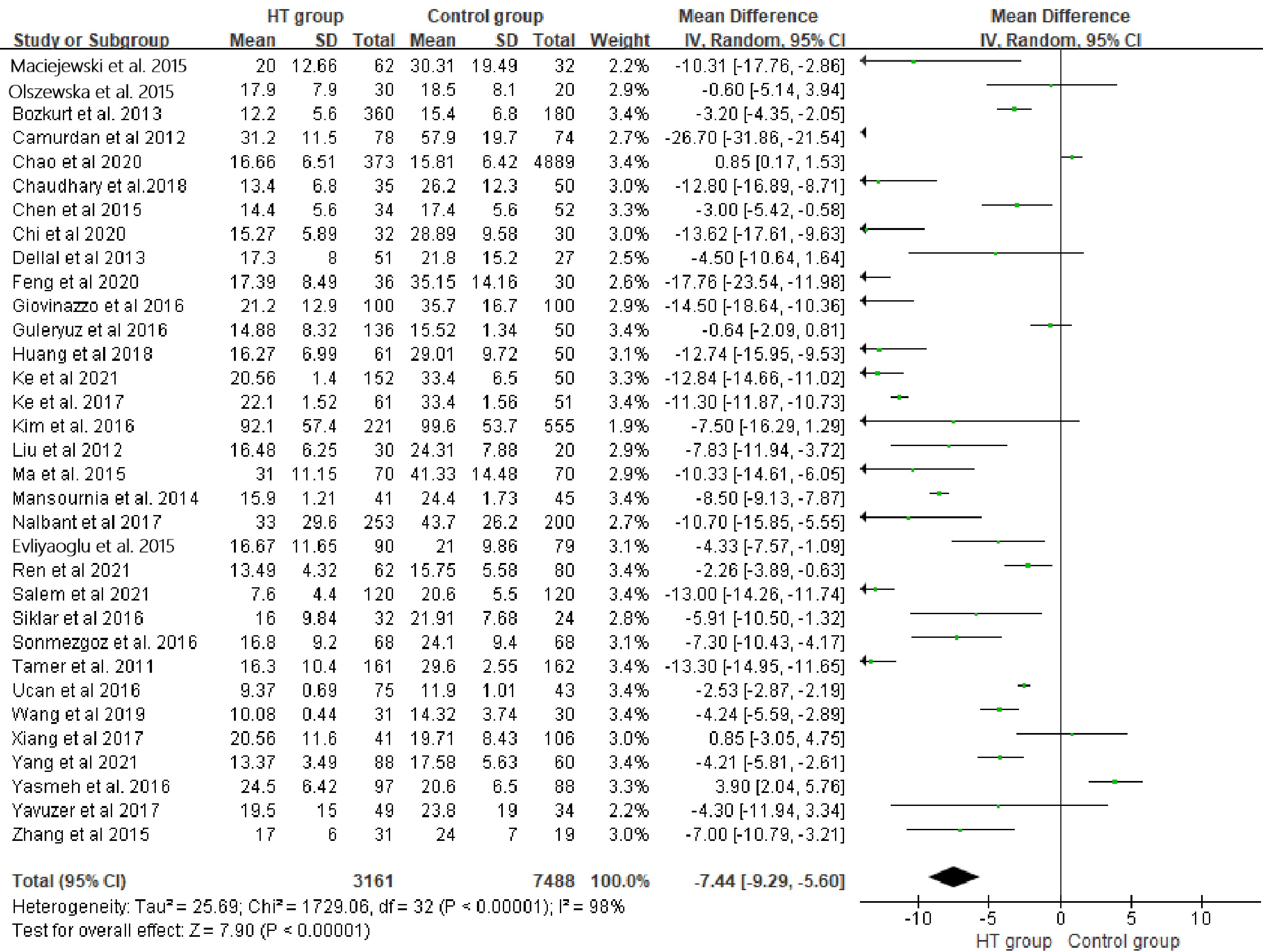
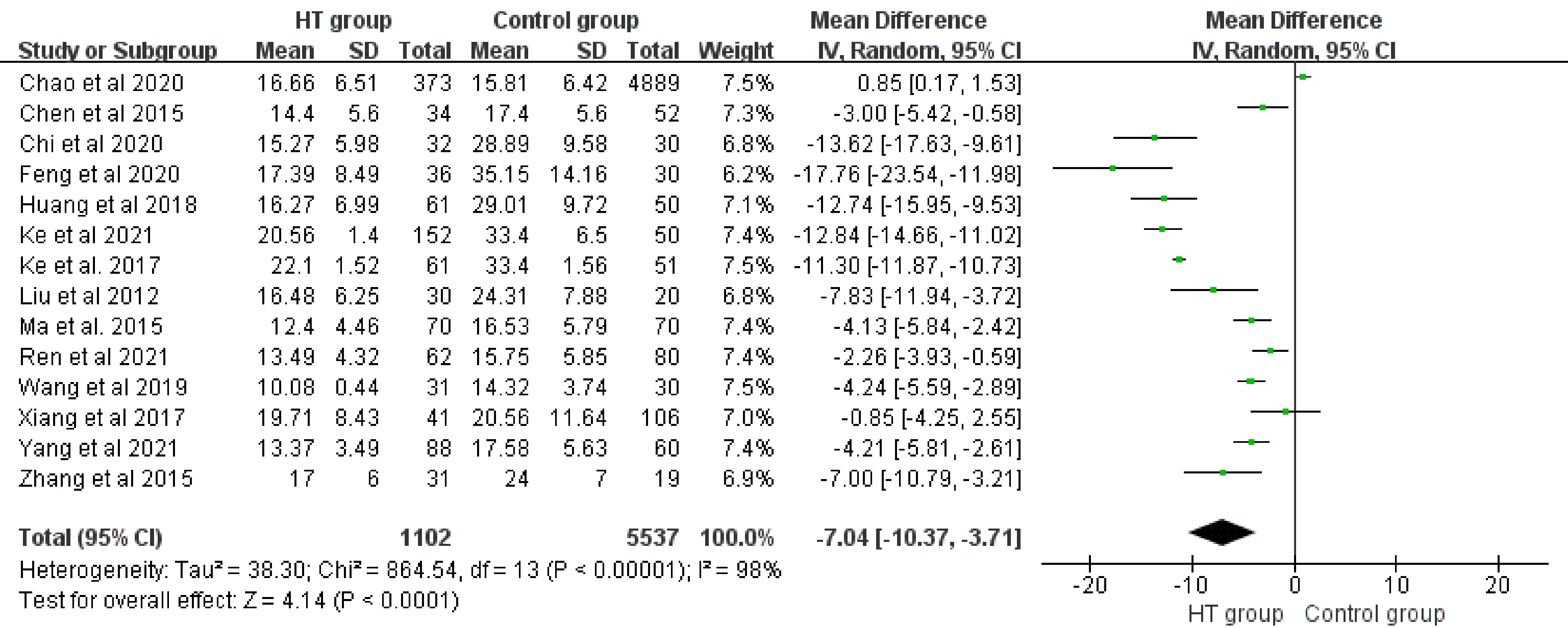
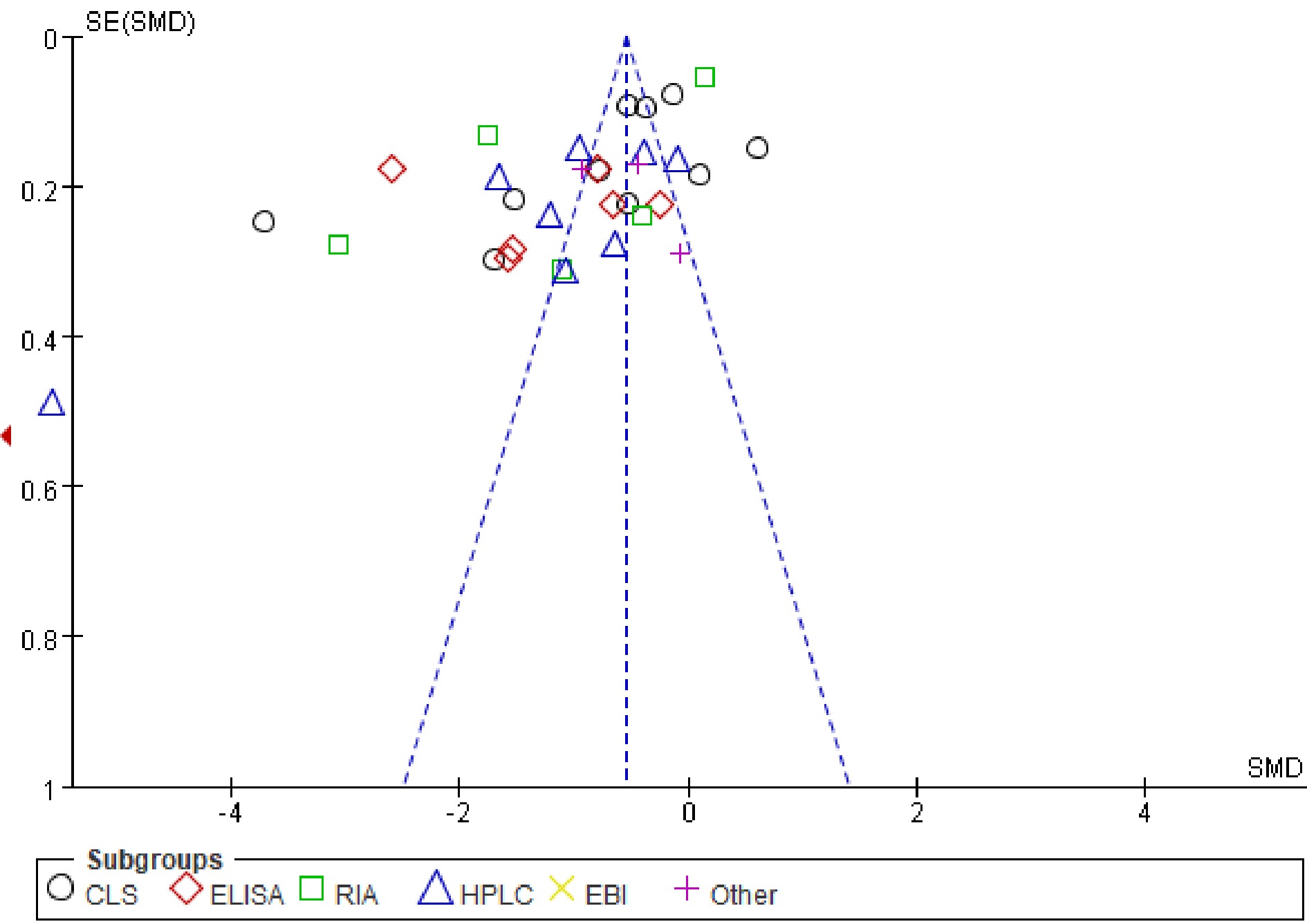
Meta-analysis included 33 studys with 3,161 patients in HT group and 7,488 healthy individuals in the control group for comparison. Random model indicated 25(OH)D3 levels of HT group were significantly lower than the control group (WMD: −7.44, 95%CI [−9.29, −5.60], P < 0.01). I2 test (98%) suggested significant heterogeneity in the meta-analysis (Fig. 1). The subgroup meta-analysis basing on 25(OH)D3 assays in a fixed model revealed similar results (WMDs: −0.55; 95%CI [−0.60, −0.49], P < 0.01), and its significant heterogeneity among subgroups represented by I2 = 98.3% suggested the difference of 25(OH)D3 assays was the main source of heterogeneity (Fig. 2,). Lastly, we separated the Chinese studies with 6,639 individuals (HT: 1,102 vs C: 5537) to perform another particle meta-analysis in random model. Result showed that, in the Chinese population, serum 25(OH)D3 level of HT patients was significantly lower than that of healthy individuals (WMD: −7.04, 95%CI [−10.37, −3.71], P < 0.01 ). Meanwhile, I2 = 98.0% also suggested a significant heterogeneity (Fig. 3).
Prevalence of 25(OH)D3 insufficiency
-
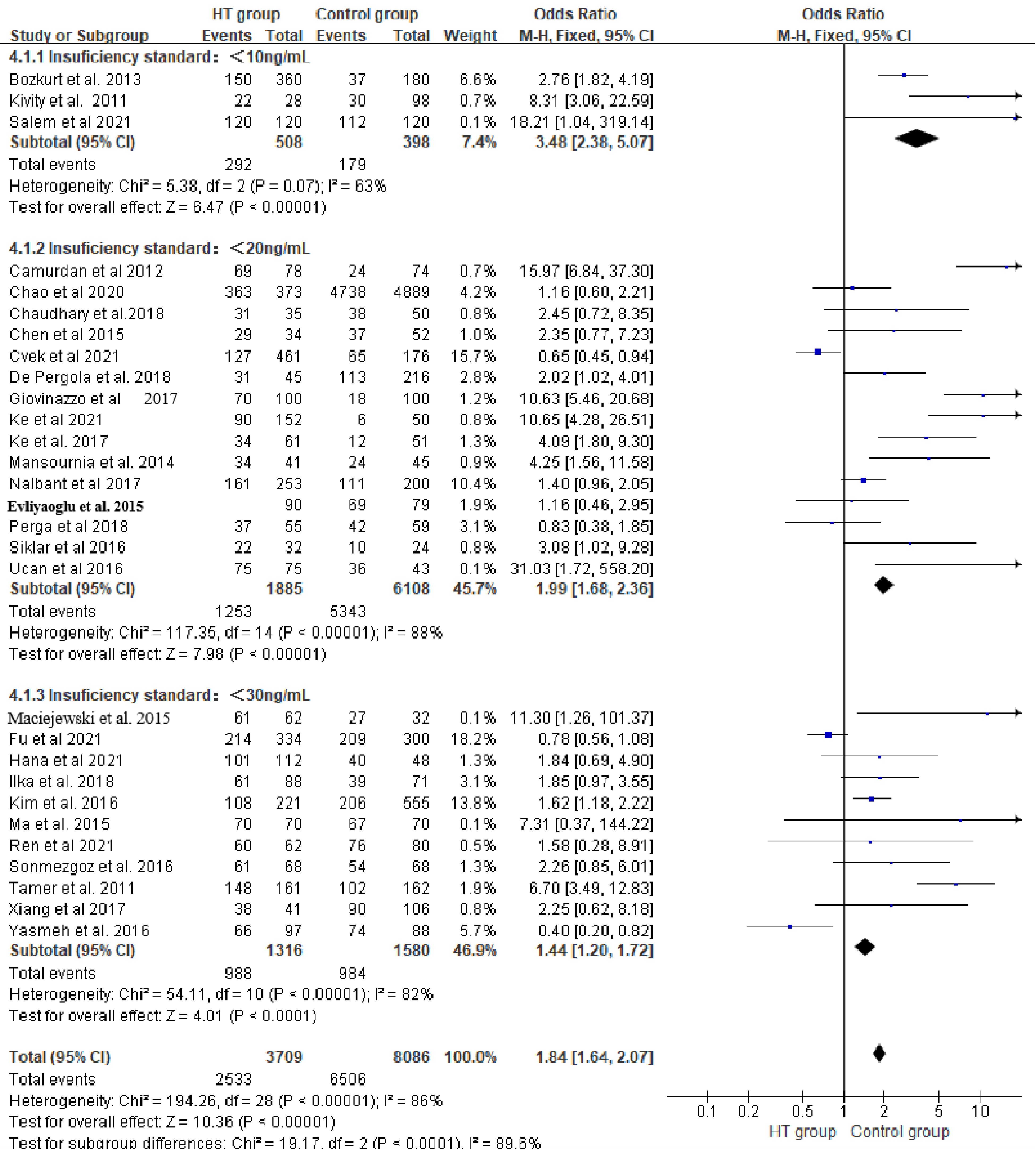
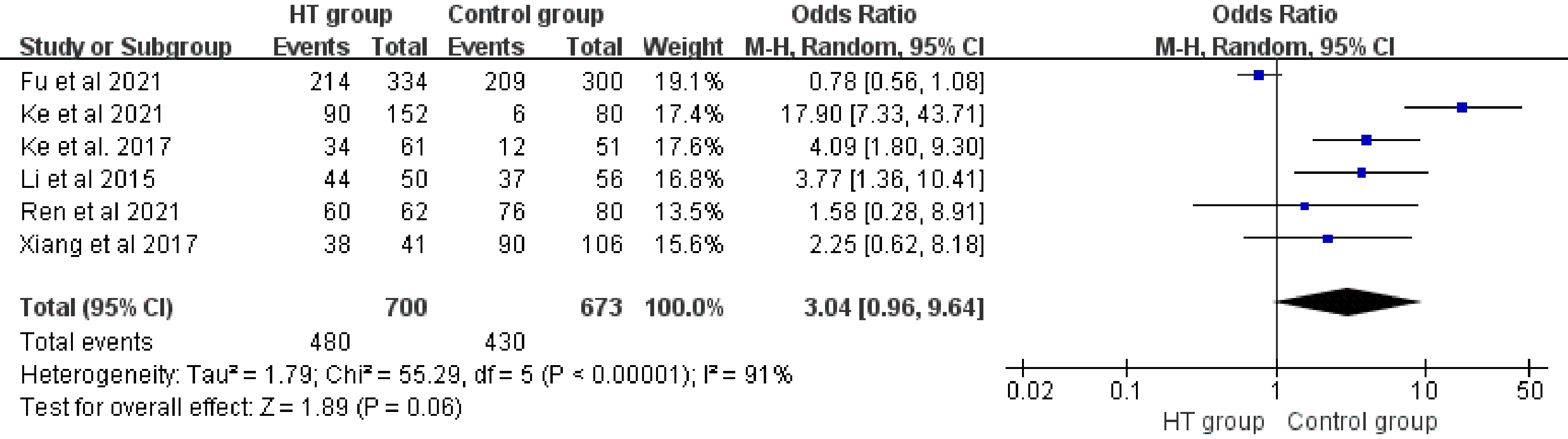
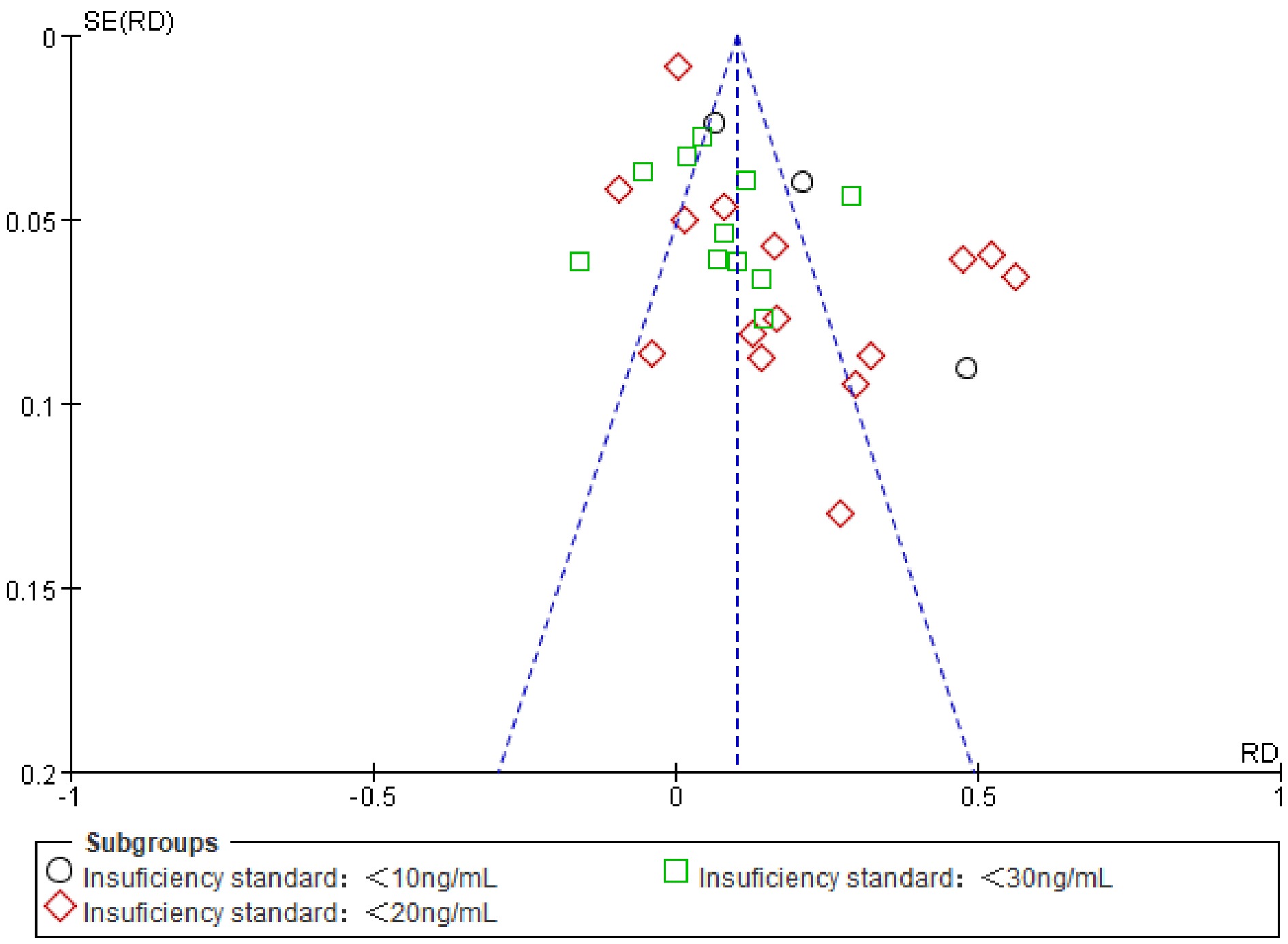
A total of 29 studies comprising 11,795 individuals (HT: 3,709 vs C: 8,086) were pooled for OR of 25(OH)D3 insufficiency. Random model indicated HT patients had higher prevalence of Vitamin D insufficiency compared to healthy individuals (OR: 2.54, 95%CI [1.77, 3.63], P < 0.01). I2 test (86%) suggested significant heterogeneity in meta-analysis (Fig. 4). Subgroup meta-analysis in a fixed model based on different 25(OH)D3 insufficiency cut-off also revealed similar results as above (OR: 1.84; 95%CI [1.64, 2.07], P < 0.01). Meanwhile, I2 equaled to 93% suggested the main source of heterogeneity was from the different cut-off of 25(OH)D3 insufficiency (Fig. 5). Chinese studies with 1,373 individuals (HT: 700 vs C: 673) were separated to perform another particle meta-analysis in random model. Results displayed a trend that the HT population had a higher prevalence of 25(OH)D3 insufficiency compared to healthy individuals, but it was not statistically significant (P > 0.05), and meanwhile significant heterogeneity was indicated by I2 equal to 91% (Fig. 6).
Publication bias
-
A funnel plot of serum 25(OH)D3 level in subgroup analysis exhibited that the included studies accumulated at the top of the funnel, which suggested that publication bias may exert little adverse effect on the confidence in the meta-analysis (Fig. 7). Similarly, results of the funnel plot suggested low risk of publication bias in prevalence of 25(OH)D3 insufficiency comparisons (Fig. 8).
-
The present study reinforced the close relationship between Vitamin D insufficiency and HT with methods of systematic review and meta-analysis. To our knowledge, the present systematic review summarized the most related studies to date; among them, Chinese studies, which may be ignored by other foreign researchers, were also included. Hence, we believe our conclusion produce more confident evidence for a relationship between Vitamin D insufficiency and HT.
Although a series of related factors of HT have been revealed, the real etiology has so far not been clearly understood[62]. Vitamin D has been proved to closely relate to HT, for it plays a vital role in regulating inflammatory response and maintaining immune balance[63]. Multiple epidemiological studies suggested a close relationship between Vitamin D and HT; however, differences in quality, region and population may affect the conclusions. Therefore, high quality systematic review or meta-analysis was still needed to acquire more reliable evidence. Wang et al.[64] published a meta-analysis in 2015 to indicate the relationship between Vitamin D insufficiency and HT. Štefanić et al.[65] subsequently included more recent studies for meta-analysis and drew a similar conclusion, but their results seemed to weaken the relationship of Vitamin D insufficiency and HT compared with Wang et al.[64]. However, these two studied did not include enough recent Chinese studies which should not be ignored. This may not only decrease the confidence of the conclusions, but also weaken the reliability for Chinese researchers. To fulfill this deficiency, we included Chinese studies into the present systematic review and meta-analysis. As anticipated, the general results including serum 25(OH)D3 level and quantity of individuals with Vitamin D insufficiency displayed similar results to Wang et al.[64] and Štefanić et al.[65], which meant the relationship of Vitamin D insufficiency and HT also existed in the Chinese population. We next separated the Chinese studies to perform a particle meta-analysis. The particle result of serum 25(OH)D3 levels of Chinese HT patients were significantly lower than healthy individuals generally, but its difference was less (−7.05 vs −7.44). However, concerning the prevalence of Vitamin D insufficiency, Chinese HT patients were not likely to have more Vitamin D insufficiency cases compared to healthy individuals, suggesting the relationship between Vitamin D insufficiency and HT in the Chinese population may not be as strong as in the global population. Note that, the Chinese population in the present study was only a small part of the total, the negative result maybe due to the small sample size. Further studies with larger sample sizes and high quality investigating the prevalence of Vitamin D insufficiency in the HT population are necessary in China in the future.
With regards to the studies included in the systematic review, most studies reported lower serum 25(OH)D3 levels and higher prevalence of Vitamin D insufficiency in HT patients compared to healthy individuals. The present study had drawn a similar conclusion, but the results contained significant heterogeneity. According to the systematic review, this heterogeneity may be due to the difference of 25(OH)D3 assays, thus we performed an analysis which separated studies with the same assay into several sub-groups. Results similarly indicated lower serum 25(OH)D3 levels in HT patients, and the most significant heterogeneity among sub-groups (I2 = 99.5%), which hinted that the heterogeneity was mainly caused by the difference in 25(OH)D3 assays. In parallel with lower serum 25(OH)D3 levels, HT patients were at higher risk of 25(OH)D3 insufficiency, indicated by our meta-analysis. Meanwhile, significant heterogeneity was indicated owing to the difference of serum 25(OH)D3 insufficiency criteria. With regard to the publication bias, we determined that heterogeneity would bring significant publication bias displayed by the funnel plot, but the results showed that the included studies accumulating at the top of the funnel; this suggested the publication bias exerted little influence on our results. However, our study also had limitations, as 25(OH)D3 level in a population could be affected by many other factors such as sunshine duration, season, area, economy, and education, which was not considered in our study. These factors may affect the conclusions of epidemiological research, and bring bias to the meta-analysis. Therefore, we can only indicate that vitamin D insufficiency was related to HT. Whether vitamin D insufficiency could lead to HT should be further investigated by biological research in the future.
Until recently, further investigations focussed on the HT mechanism in which vitamin D was involved. As is well understood, T lymphocytes including Th1, Th2 and Th17 cells infiltrate the thyroid gland due to immunological disorders in HT patients. Vitamin D can inhibit the differentiation of Th1 cells, and the production of inflammatory cytokines such as TNF-α, INF-γ. It could also suppress inflammatory Th1, but induce anti-inflammatory Th2 which produced anti-inflammatory cytokines such as IL-4 and IL-5[66]. Furthermore, the Th17 cells with their production of IL-17A could also be inhibited by Vitamin D at the transcriptional level[67]. On the other hand, vitamin D could increase the proportion of Treg cells to exert immune regulation[68]. Taken together, vitamin D may have the potential to prevent HT. However, clinical studies have shown contradictory results: Chahardoli et al.[69] reported activated vitamin D supplementation can decrease TSH and TG-Ab antibodies levels in HT patients, but another study showed that activated vitamin D supplementation had no effect on improving HT[70]. To our knowledge, the studies mentioned above may ignore the vitamin D receptors polymorphism. Vitamin D receptors in the thyroid gland have single nucleotide polymorphism and most typical Apal, Bsml, Fokl and Taql single nucleotide variations have been shown to be closely related to autoimmune diseases[71]. Therefore, more prospective studies are needed to confirm the preventive effect of vitamin D on HT.
-
In conclusion, the present systematic review and meta-analysis strengthened the relationship between vitamin D insufficiency and HT. HT patients potentially had higher propensity for having lower serum 25(OH)D3 levels compared to healthy individuals. Clinical staff may have to carefully consider the possibility of vitamin D insufficiency in HT patients.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Liu Z, Feng L, He Y, Yuan S, Xu C. 2022. The Association between Vitamin D and Hashimoto Thyroiditis: An Up-to-date Systematic Review and Meta-analysis. Food Materials Research 2:9 doi: 10.48130/FMR-2022-0009
The Association between Vitamin D and Hashimoto Thyroiditis: An Up-to-date Systematic Review and Meta-analysis
- Received: 27 May 2022
- Accepted: 31 May 2022
- Published online: 28 June 2022
Abstract: The objective of the surrent study was to summarize the up-to-date studies to investigate the relationship between vitamin D and Hashimoto thyroiditis (HT). An online search of English and Chinese databases was performed. The studies concerned the investigation of the relationship between vitamin D and HT including meta-analysis, meanwhile the heterogeneities were revealed by subgroup analysis. Fourty six elated studies containing 15,336 participants (HT: 6,138 versus control: 9,198) were included. HT patients had lower levels of 25(OH)D3 (standardised mean difference, −1.09; 95%CI: [−1.42, −0.75]; P < 0.01), and were more likely to be deficient in 25(OH)D3 (OR, 2.77; 95%CI, [1.88, 3.91]; P < 0.05). Obvious heterogeneities in the results of meta-analysis were down to the difference of detection methods and criteria of vitamin D insufficiency among studies. Vitamin D deficiency was colncluded to have a significant relation with HT.
-
Key words:
- Vitamin D /
- Hashimoto thyroiditis /
- Systematic review /
- Meta-analysis