-
Androdioecy, the coexistence of males and hermaphrodites in the same population, is an extremely rare sexual system in both plants and animals[1−5]. Currently, the sexual system of at least 15 plant species is reported as androdioecious. However, several species reported to be androdioecious are in fact functionally dioecious[6]. According to the phenotypic selection model, male functionally androdioecious species should have a siring success at least double that of hermaphrodites to remain in the population[7,8], leading to a male frequency of less than 0.5, with even lower male frequency in a metapopulation model[5]. Males should even have greater fecundity to remain in partially selfing populations than in outcrossing populations because fewer ovules are available for outcrossing[7−9]. Thus, functionally androdioecious species are expected to exhibit self-incompatibility or at least a low selfing rate, and a large male advantage[10,11].
Datisca glomerata (Datiscaceae) has completely satisfied theoretical predictions[12−15]. They have several common characteristics, such as wind pollination, relatively low (< 30%) male frequency in the populations, protogyny of hermaphrodite flowers, and larger pollen production in males than in hermaphrodites. Additionally, Fraxinus lanuginosa (Oleaceae), a wind- and insect-pollinated tree, has male frequency ranging from 10% to 50% in populations; the hermaphrodites in this species exhibit strong self-sterility, and the male advantage is 2.7-fold in terms of seed production[16].
However, the 1:1 ratio of males and hermaphrodites and self-fertility of hermaphrodites have been described in two Oleaceae species , Phillyrea angustifolia[17] and Fraxinus ornus[18,19]. P. angustifolia, a wind-pollinated shrub, can maintain high male frequency in natural populations because hermaphrodites belong exclusively to one of two self-incompatibility groups, and each group can fertilize only half of all pollen recipients[20]. F. ornus has the similar mechanism as that of P. angustifolia through the combined action of a Diallelic Selfincompatibility System (DSI) and male compatibility with both groups[21].
Since Lepart & Dommée[22] first reported the functional androdioecy of P. angustifolia in Oleaceae, androdioecy species were reported in at least three genera (Phillyrea, Fraxinus, and Osmanthus) in this family. Mock privet (P. latifolia) is morphologically androdioecious and functionally dioecious[23]. At least three species are functional androdioecious in the genus Fraxinus[18,24]. These species lack petals and are presumed wind-pollinated, whereas those species with petals are probably insect-pollinated. Another long-lived woody species, Osmanthus fragrans L., has also been reported as androdiecious[25]. The male frequency of O. fragrans in populations is near 0.5 (males versus all)[25,26]. O. fragrans grows in low to middle altitude on limestone mountains and naturally distributes from the Yangtze River Basin in South China to Southeast China[27].
Gender differences associated with reproduction in sexually dimorphic plants allow intersexual comparisons of energy allocation[28]. The intersexual comparison under androdioecy represents the most asymetrical situation (two extremes of the continuum)[19]. The hermaphrodite individuals of Fraxinus appear to produce small and non-functional anthers[4,24], and indicated that hermaphrodite individuals of Fraxinus already lost male function[26]. In F. ornus, males compensate the fitness advantage of hermaphrodites with greater reproductive, but not vegetative output[19]. In O. fragrans, the hermaphrodite flower has well-developed pistil and two stamens, and the male flower only has pistillode and two stamens. Pollen quantity per flower and their morphology between males and hermaphrodites are not significantly different. Moreover, both male and hermaphrodite individuals have high activity pollen and can germinate normally on stigmas[25] . It is necessary to qualify the pollen production of male and hermaphrodite individual and value the fitness of each sexual individuals.
For androdioecious species, high male frequency in populations is presumed to result high outcrossing rate and maintain high genetic diversity. A significant negative correlation exists between male frequency and inbreeding coefficient in Schizopepon bryoniaefolius (Cucurbitaceae), indicating that outcrossing rate was affected by the population sex ratio[29]. Moreover, outcrossing rate increased with increasing male frequency among sub-populations of F. lanuginose in north Japan[30]. Male individuals of Laguncularia racemosa (Combretaceae) in androdioecious populations had significantly more visitors than hermaphroditic plants, increasing the number of vectors carrying pollen from male plants. Further, many insects visited few flowers during foraging bouts, which should increase outcrossing frequency[31]. However, the strength of inbreeding depression does not increase with male frequency[32]. As functional androdioecious species, the inbreeding level and genetic structure of O. fragrans may offer a clue for us to understand the sexual system.
The aim of this paper is to study the sexual system of O. fragrans and understand the maintenance of the 1:1 sex ratio among populations. We investigated the sex ratios in three populations. Second, to determine floral trait differences between genders and among populations, floral traits in three natural populations were investigated and the differences between genders and among populations were analyzed. Finally, genetic variations (heterozygosity and genetic differentiation) among natural populations and between genders were estimated using AFLP methods.
-
The number of males, hermaphrodites and male frequency of O. fragrans within the five sampled populations are presented in Table 1. The sex ratio of the reproductive trees in the five populations, except the CT population (χ2 = 9.96273, p = 0.01), did not deviate significantly from 1:1. The male frequencies varied from 0.422 to 0.620 with a mean of 0.524.
Table 1. Locality, the number of males, hermaphrodites, non-flowering plants and male frequency within five populations of O. fragrans.
Population Locality Description Males Hermaph-rodites Male frequency * χ2 CT Guanfang, Changting, Fujian Isolated island of limestone mountains 100 61 0.620 9.96273 LY Guihuaxia, Zhouluo, Liuyang, Hunan Along a stream, mainly east-facing 35 33 0.515 ns 0.01470 QDH Guihua island, Thousand-isle Lake, Jiande, Zhejiang Isolated island of limestone mountains 26 21 0.553 ns 0.34042 LQ-GC Gongcun, Longquan, Zhejiang Along a stream, west-facing 35 48 0.422 ns 1.73494 LQ-MYC Maoyucun, Longquan, Zhejiang Along a stream, west-facing 19 13 0.594 ns 0.78125 Total 189 172 0.524 ns 0.89751 Sex ratio and male frequency of O. fragrans in different populations were firstly stated by Hao et al.[25]. Here, we cite the part of data and newly add the results of QDH population and make further statistics analysis in this paper. 'ns' means sex ratio does not significantly deviate from 1:1. Phenology of O. fragrans
-
In the same population, the blooming process of all same-sex plants, all inflorescences of the same plant, and all florets was almost consistent. The flowering period was maintained at 7 to 9 days (Table 2). The flowers of male individuals bloom 1 or 2 days earlier than hermaphrodites, whereas the corresponding blooming process between males and hermaphrodites was almost consistent. In the same hermaphrodite, the time of pistil maturation and the best stigma receptivity were 1 to 2 days earlier than the time of pollen dispersal. This condition resulted in the synchronization of male function of the male and female functions of hermaphrodites to some extent in the same population.
Table 2. Flower blooming process of males and hermaphrodites from CT, LY and LQ-GC population.
Stage Stage description Day Males Hermaphrodites 1 Bracts dropped and florets appeared gradually. 1 2 2 All florets appeared. 2 3 3 The pedicels of florets elongated gradually. 3 4 4 Florets opened gradually while the petals have not expanded completely. 4 5 5 Florets opened completely while anthers have not dehisced. (However, if the weather was sunny and temperature was high, anthers would dehisce at several hours after flowering.) 5 6−7 6 Anthers dehisced gradually and pollen grains dispersed. (If anthers have dehisced last day, they would brown gradually.) 6 7−8 7 Anthers browned gradually. 7 8−9 8 Florets dropped gradually. 8−9 − 9 The petals of florets browned and wilted gradually but not dropped. − 9−11 Floral morphology and correlation analysis
-
The flower structure was significantly different between males and hermaphrodites. The hermaphrodite flower has a well-developed gynoecium and the gynoecium is absent in male flowers (Fig. 1). Male individuals produce more flowers than hermaphrodite individuals. The number of flowering node and flower number per node of male individuals is higher than hermaphrodite individuals among three populations (Table 3). Meanwhile, male individuals also make lager anther. The anther width of male individuals is significantly larger than hermaphrodite individuals (Table 3).

Figure 1.
Males and hermaphrodites of O. fragrans. (a) Hermaphrodite flowers; (b) Male flowers; (c) Pistil of hermaphrodite flower; (d) Pistillode of male flower.
Table 3. Floral morphology variations of O. fragrans within three different populations (CT, LY and LQ-GC).
Populations Gender Sample size No. of
flowering nodeFlower number
per nodeFlower diameter Petal length Petal width Anther width CT M 34 3.73 ± 0.64A 15.47 ± 4.90a 7.00 ± 1.81A 4.05 ± 0.74A 2.49 ± 0.56a 1.28 ± 0.21A H 26 3.17 ± 1.18B 13.73 ± 4.69a 5.81 ± 1.01B 3.48 ± 0.48B 2.49 ± 0.42a 1.11 ± 0.11B LY M 31 4.00 ± 0.83A 15.93 ± 5.32A 6.13 ± 0.52B 3.01 ± 0.67a 2.05 ± 0.31a 1.19 ± 0.08A H 33 3.47 ± 1.07B 13.33 ± 3.62B 6.69 ± 0.61A 3.05 ± 0.54a 2.05 ± 0.28a 1.06 ± 0.10B LQ-GC M 33 4.30 ± 0.88A 18.77 ± 8.94A 6.52 ± 1.47a 3.48 ± 0.43B 2.60 ± 0.43A 1.38 ± 0.14A H 34 3.37 ± 0.81B 14.50 ± 5.97B 6.87 ± 1.86a 3.65 ± 0.63A 2.42 ± 0.43B 1.24 ± 0.19B Males* M 98 4.01 ± 0.81A 16.7 ± 6.73A 6.55 ± 1.41a 3.51 ± 0.76a 2.38 ± 0.50a 1.28 ± 0.17A Hermaphrodites H 93 3.33 ± 1.03B 13.9 ± 4.83B 6.46 ± 1.34a 3.40 ± 0.60b 2.32 ± 0.43a 1.14 ± 0.16B CT** 60 3.45 ± 0.98b 14.60 ± 4.83b 6.42 ± 1.57b 3.77 ± 0.68A 2.49 ± 0.49a 1.19 ± 0.19B LY 64 3.73 ± 0.99a 14.63 ± 4.70b 6.41 ± 0.63b 3.03 ± 0.60C 2.05 ± 0.30b 1.12 ± 0.11C LQ-GC 67 3.83 ± 0.96Aa 16.63 ± 7.84a 6.69 ± 1.67a 3.56 ± 0.54B 2.51 ± 0.44a 1.31 ± 0.18A M: male; H: hermaphrodite. Different upper case letters mean significant differences at p < 0.01 and different lowercase letters mean significant differences at p < 0.05. * Comparison between genders, populations pooled; ** comparison among populations, genders pooled. The pollen production of males and hermaphrodites was estimated using formula (1), based on the flowering node, flowers number per node and anther width. It seems that male individuals produce 1.7−2 times pollen of hermaphrodite individuals among three populations.
The flower size between male and hermaphrodite individuals is not significantly different in all three populations (Table 3). The pistil traits except for stigma length of hermaphrodites among three populations were not significant (Table 4).
Table 4. Pistil variations of O. fragrans in hermaphrodite genders among populations.
Populations Sample size Ovary length Ovary width Stigma length Stigma width CT 26 2.01 ± 0.17a 1.21 ± 0.11a 0.99 ± 0.10a 0.84 ± 0.17a LY 33 1.91 ± 0.26a 1.26 ± 0.11a 0.95 ± 0.10ab 0.85 ± 0.19a LQ-GC 34 1.99 ± 0.16a 1.27 ± 0.14a 0.94 ± 0.12b 0.78 ± 0.10a The different letters indicate significant differences at p < 0.05. Genetic diversity
-
A total of 1,367 polymorphic loci from eight primer combinations were scored in 72 wild individuals, and the number of polymorphic scorable loci per number varied from 157 to 184. The percentage of polymorphic loci (PPL) of CT, LY and LQ-GC populations was 67.62%, 72.75%, and 71.24%, respectively (Table 5). Between two genders, hermaphrodites present higher PPL (Percentage of Polymorphic Loci) compared with males.
Table 5. Percentage of Polymorphic Loci (PPL), band frequency, estimated allele frequency with number of different alleles (Na), number of effective alleles (Ne), Shannon's information index (I), expected and unbiased expected heterozygosity (He and UHe) and band richness (Br) among different populations.
Population No. individuals sampled PPL (%) Na Ne I He UHe Br CT 30 67.62 1.399 ± 0.023 1.287 ± 0.009 0.274 ± 0.007 0.175 ± 0.005 0.178 ± 0.005 1.293 LY 30 72.75 1.503 ± 0.022 1.266 ± 0.008 0.265 ± 0.006 0.166 ± 0.005 0.169 ± 0.005 1.290 LQ-GC 22 71.24 1.472 ± 0.022 1.289 ± 0.009 0.282 ± 0.007 0.179 ± 0.005 0.183 ± 0.005 1.313 Mean — 70.54 1.458 ± 0.013 1.280 ± 0.005 0.274 ± 0.004 0.174 ± 0.003 0.177 ± 0.003 — CTm 15 58.74 1.252 ± 0.024 1.273 ± 0.009 0.257 ± 0.007 0.166 ± 0.005 0.172 ± 0.005 1.290 CTh 15 56.83 1.206 ± 0.025 1.267 ± 0.009 0.250 ± 0.007 0.162 ± 0.005 0.168 ± 0.005 1.285 LYm 15 62.77 1.326 ± 0.024 1.264 ± 0.009 0.257 ± 0.007 0.164 ± 0.005 0.169 ± 0.005 1.296 LYh 15 57.31 1.219 ± 0.025 1.243 ± 0.008 0.236 ± 0.007 0.150 ± 0.005 0.156 ± 0.005 1.278 LQ-GCm 6 50.48 1.080 ± 0.025 1.265 ± 0.009 0.245 ± 0.007 0.160 ± 0.005 0.175 ± 0.005 1.336 LQ-GCh 16 64.55 1.368 ± 0.023 1.275 ± 0.009 0.265 ± 0.007 0.169 ± 0.005 0.174 ± 0.005 1.304 Mean — 58.45 1.242 ± 0.010 1.265 ± 0.004 0.252 ± 0.003 0.162 ± 0.002 0.169 ± 0.002 — M 36 78.83 1.606 ± 0.020 1.305 ± 0.009 0.300 ± 0.006 0.190 ± 0.005 0.192 ± 0.005 1.326 H 46 84.29 1.709 ± 0.018 1.301 ± 0.008 0.300 ± 0.006 0.188 ± 0.005 0.191 ± 0.005 1.327 Mean — 81.56 1.362 ± 0.012 1.288 ± 0.004 0.275 ± 0.003 0.177 ± 0.002 0.190 ± 0.003 — CTm and CTh represent male and hermaphrodite gender respectively in Changting population; LYm and LYh represent male and hermaphrodite gender respectively in Liuyang population; LQ-GCm and LQ-GCh represent male and hermaphrodite gender respectively in Longquan population; M and H represent male and hermaphrodite gender respectively from all three populations. Among three populations, Shannon's information index (I) and band richness (Br) resulted in similar estimates of diversity. The LQ-GC population had the highest (0.282, 1.313), and the LY had the lowest (0.265, 1.290) (Table 5). Shannon's information index (I) and band richness (Br) are similar between males and hermaphrodites. Slightly differences in the estimated allele frequency with the Na and Ne were observed among different populations and between male and hermaphrodite groups. Heterozygosity (He and UHe) in males was higher than that in hermaphrodites.
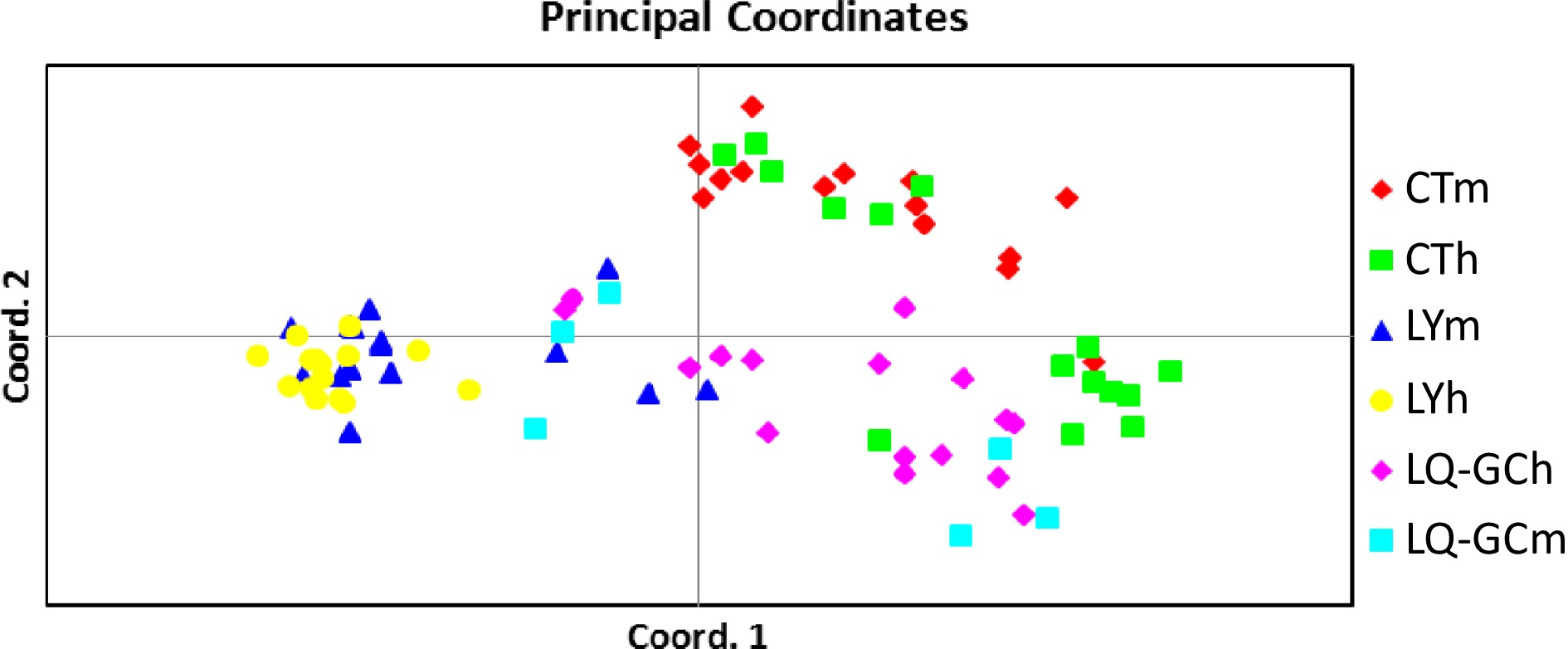
The PCA-based pairwise genetic difference showed three main groupings (Fig. 2). The individuals of the LY population were almost clustered together; however, the individuals of the CT and LQ-GC populations were relatively discrete. The total variation of the first three axes was 70.69%.
Genetic structure and genetic differentiation
-
In AMOVA, the total variation and differentiation (PhiPT) was 13% and 0.125 among populations, 2% and 0.023 between genders within population, and 85% and 0.145 within gender, respectively (Table 6). Among populations, the differentiation between CT and LY (0.166) were greater than CT and LQ-GC (0.087), and LY and LQ-GC (0.140) (Table 7). The differentiation between male and hermaphrodite groups was not significant from the Phi-value and the percentage of total variation. Among all genders, the greatest differentiation was present between CTh (the hermaphrodite group from CT population) and LYh (the hermaphrodite group from LY population) genders (0.211) and the least differentiation between LQ-GCh (the hermaphrodite group from LQ-GC population) and LQ-GCm (the male group from LQ-GC population) genders (0.009) (Table 8).
Table 6. Nested analysis of molecular variance (AMOVA) based on 1456 polymorphic loci among different populations.
Source df MS Est. Var. % Phi p-value AMOVA analysis 1 Among populations 2 759.002 20.962 13 0.125 0.001 Among genders/
populations3 186.594 3.354 2 0.023 0.006 Within gender 76 143.296 143.296 85 0.145 0.001 AMOVA analysis 2 Among sex groups 1 215.540 0.000 0 0.042 1.000 Among genders/sex groups 4 465.561 24.252 14 0.145 0.001 Within gender 76 143.296 143.296 86 0.109 0.001 p-value estimates are based on 999 permutations. df = degree of freedom and MS = mean squared deviations. Table 7. Pairwise Population PhiPT Values (below diagonal) and gene flow (Nm) (above diagonal).
Population CT LY LQ-GC CT — 1.256 2.624 LY 0.166 — 1.536 LQ-GC 0.087 0.140 — Table 8. Pairwise gender PhiPT Values (below diagonal) and gene flow (Nm) (above diagonal).
Sub-population CTm CTh LYm LYh LQ-GCh LQ-GCm CTm — 6.893 1.498 1.073 1.943 2.177 CTh 0.035 — 1.247 0.935 2.559 3.481 LYm 0.143 0.167 — 12.908 1.549 2.354 LYh 0.189 0.211 0.019 — 1.162 1.719 LQ-GCh 0.114 0.089 0.139 0.177 — 27.528 LQ-GCm 0.103 0.067 0.096 0.127 0.009 — -
High male biased sex ratio had been described in two species of Oleaceae, P. angustifolia[33] and F. ornus[18]. For O. fragrans, the 1:1 sex ratio was also observed in multiple populations (Table 1). Charlesworth[9] proposed that the 1:1 gender ratio observed in several suggested androdioecious species indicated that hermaphrodites preform female function in natural conditions, and that the populations functioned as a dioecious species such as F. ornus[18]. Pollen quantity and quality (normal pollen germination on the stigmas and pollen tube growth) of hermaphrodites in O. fragrans was not significantly different with that of males[25], and indicated that O. fragrans could be functional androdioecy. In P. angustifolia, Saumitou-Laprade et al.[20] reported the existence of a diallelic sporophytic self incompatibility (SSI) system that was controlled by two alleles. The SSI system can solve the paradox of high proportion of males in the populations. There are two groups of self-incompatible hermaphrodites and only the crosses performed between two different phenotypes of hermaphrodites and between males and hermaphrodites were compatible. Contributing to this, males can potentially sire twice as many ovules as hermaphrodites. This SSI system may exist in the wild, not only driving the diversification of the Oleaceae mating systems, but also other cases of distyly[20]. In O. fragrans, further work is necessary to clarify whether SSI systems exist or not.
Floral difference between males and hermaphrodites and male advantage
-
In androdioecious populations, males are expected to make more than twice the genetic contribution compared to hermaphrodites[8,9]. This contribution can be conducted either through a higher pollen dispersal, or through a higher siring success of dispersed pollen[4]. In F. ornus, the number of pollen gains per flower and the number of flowers per inflorescences were not significantly different between the genders, while males produced a significantly higher number of inflorescences than hermaphrodites[19]. In all three O. fragrans populations, males produce more flowers and larger anther. The pollen production of males is about 1.7−2 times that of hermaphrodites. This indicated that male individuals advance in male fitness compared with hermaphrodites.
The flowering phenology of males and hermaphrodites correspond to sexual function. In the population, males flower bloom 1 to 2 d earlier than hermaphrodites; for hermaphrodite pistil mature and stigma get receptive period 1 to 2 d earlier before pollen dispersal. Hermaphrodite perform female function earlier than male function and during the female function period of hermaphrodite, male individuals are offering pollen.
Significant difference between males and hermaphrodites was found not only in flowering phenology but also in some floral traits. In the flower blooming process, males flowered 1 to 2 d earlier than hermaphrodites did within the population. This synchronization provides a better chance of mating between male and hermaphrodite individuals and ensure the fitness of male individuals.
In summary, males have more male fitness advantage than hermaphrodites on producing more pollen and scheduled flower blooming process. That may explain the occurrence and maintainenance of male individuals in the population and the sexual separation of O. fragrans.
Genetic variation, mating system, and the maintenance of 1:1 sex ratio
-
The nested AMOVA analysis indicates that the population genetic structure of O. fragrans is genetically homogeneous at the species level. The largest percentage of genetic variation existed within the population and within gender not only among populations but also between males and hermaphrodites. The percentages of variation between male and hermaphrodite groups and between male and hermaphrodite genders in the same populations were both low (0% and 2%, respectively), and the percentage of variation among populations was higher (14%). This result indicates that the three populations experienced a relatively high gene flow and low level of genetic isolation. In general, outcrossing and long-lived seed plants maintain high genetic variation within populations. Whereas predominantly selfing, short-lived species harbor comparatively higher variation among populations[34]. The overall degree of genetic differentiation (Φpt = 0.143) among populations of O. fragrans was much lower than the average of 0.27 (genetic differentiation coefficient) for plants with a mixed breeding system, with an average of 0.25 for long-lived perennials and an average of 0.36 for dicotyledons[35,36]. Moreover, genetic differentiation was very low (Φpt = 0.003 and 0.020) between genders not only among populations but also in the same population. This genetic structure showed high levels of heterozygotes within populations especially between the two genders. This could be greatly attributed to the occurrence of a high level of xenogamy between males and hermaphrodites. Simultaneously, high male frequency (50%) and more male fitness advantage in males are the essential condition for high outcrossing between males and hermaphrodites. Males could not produce progenies directly and transmit genetic information relaying on the females (hermaphrodites) in the population. However, those have a similar degree of genetic diversity with hermaphrodites. It attributes to very high gene flow between males and hermaphrodites. And xenogamy between males and hermaphrodites is the principal mating pattern in population reproduction and regeneration in this species. The male function of hermaphrodites chiefly plays the role for reproductive assurance. Therefore, the 1:1 sex ratio in this species could be the result of the integrative effects of sexual system, mating system, and reproductive success.
Plants benefit from high levels of genetic diversity and gene flow to adapt to the variable environmental conditions. Although the three populations are far from each other, a certain degree of gene flow among populations through mediate flow was observed. Because O. fragrans is a prolific species, one of primary species of evergreen broad-leaved forest that is widely distributed in the south of the Yangtze River Basin, high gene flow among close populations occurs, and gene flow among populations with distance occurs through gene introgression. The current populations and their distribution patterns are widely affected by the destruction of a large number of populations, particularly during the recent two decades. Habitat fragmentation and rapid decrease of population size have no obvious effects on genetic diversity of the surviving individuals but have great influence on population proliferation, regeneration, and dispersal and genetic diversity of the next generations. This condition may cause numerous species becoming endangered in the immediate future. The conservation of existing populations and their habitats, particularly for some populations that have rich morphological variations, is important in maintaining the genetic structure of O. fragrans.
-
The androdioecious mating system of Osmanthus fragrans L. (Oleaceae) may be explained by three aspects of evidence: male frequency, the floral traits, and genetic variation. In three nature O. fragrans population, male individuals maintain at about 50% in the population. Males bear more flowering nodes, and more flowers per node, and larger anther in all three populations. Males produce almost twice pollen comparing to the hermaphrodites. Genetic differentiation was very low between genders not only among populations but also in the same population. This genetic variation could be attributed to the occurrence of high levels of xenogamy between genders. Therefore, high male frequency and more male fitness advantage in males are the essential conditions for this mating system, which plays an important role during population reproduction and regeneration. The 1:1 sex ratio could be the result of integrative effects of sexual system, mating system, and reproductive success.
-
The sex ratio of five natural populations from four locations of three provinces (Fujian, Hunan, and Zhejiang, China) and the floral morphology and blooming process of three larger populations including Guanfang, Changting (CT) (25°32.574' N,116°32.065' E, and 300 m altitude), Guihuaxia, Zhouluo, Liuyang (LY) (28°25.699' N, 113°40.217' E, and 370 m altitude) Gongcun ((LQ-GC)) and Maoyucun (LQ-MYC), Longquan (LQ) (28°11.545' N,119°15.007' E, and 220 m altitude), Guihua island (QDH), Thousand-isle Lake, Jiande (JD) (29°31.861' N, 119°8.341' E and 450 m altitude) were investigated (Table 1). In addition, individuals of CT, LY, and LQ-GC populations were sampled for amplified fragment length polymorphism (AFLP). Only the individuals with diameters more than 3 cm at breast height were sampled and investigated in every study site, covering the entire range of distribution. The sampled trees were at least 1 m far away from each other.
Sex ratio, flower blooming process, and floral trait data recording
-
The males and hermaphrodites in flowering individuals of O. fragrans from CT, LY and LQ-GC were tallied in September to October of 2008 and 2009. The blooming process of the plant, inflorescence, and single floret were recorded from at least 10 individuals (five male individuals and five hermaphrodite individuals), 15 inflorescences, and 20 florets every day during the flowering period in the same population. The floral traits, including the number of flowering nodes per twig, number of flowers per node, flower diameter, petal length, petal width, and anther width of at least 15 florets per individuals from at least 20 male and hermaphrodite individuals per population were measured using a Vernier caliper. The ovary length and width and stigma length and width of the hermaphrodite individuals were simultaneously measured at full-bloom stage.
The pollen production (Po) was estimated by flowering node (Fn), flowers number per node (F;) and anther width (Aw). Present as following:
$ {Po=Fn\times Fl\times \left(Aw\right)}^{2} $ (1) DNA extraction and AFLP analysis
-
Young flowers of O. fragrans were collected from September to October of 2009 and conserved in self-indicating silica gels for drying to extract genomic DNA. Total genomic DNA was extracted from dried materials using the modified CTAB method[37]. The fluorescence AFLP procedure was performed based on the protocol of Zhao et al.[38,39]. Eight pairs of polymorphism primers were selected from 64 primer pairs. A FISH-AFLP kit from Beijing Dingguo Biotechnology Company was used following the manufacturer’s guide. The main steps are shown as follows: (1) enzyme digestion and connection, (2) pre-amplification reaction, (3) selective amplification reaction, and (4) electrophoresis and data acquisition using a 377 DNA automatic sequencer (ABI PRISM 377 sequencer) to carry out 4% denaturing polyacrylamide gel electrophoresis for 2.4 h. The Run Module was used with the software GS Run 36F-2400 to acquire electrophoretograms automatically. The data of 0 (absence) or 1 (presence) were obtained from the electrophoresis patterns by using the program GeneScan 3.1 with hand-made correction.
Data analysis
-
The band patterns, band frequency, estimated allele frequency with number of different alleles (Na), number of effective alleles (Ne), Shannon's information index (I), and expected and unbiased expected heterozygosity (He and UHe, respectively) per population and per group were calculated using the GenAlEX version 6[40]. The pairwise Nei’s genetic identity and genetic distance, Nei’s unbiased genetic identity, and genetic distance between populations were also calculated using GenAlEX. Rarefaction was used to account for unequal sample sizes by using the program AFLPdiv by estimating band richness (Br)[41−43]. The range of Br was from 1 to 2, which could be interpreted as an allelic richness analogue[41,43,44].
Pairwise genetic differences between individuals were calculated using GenAlEX by counting the number of genetic differences between two individuals. This pairwise genetic difference was used in a principal coordinate analysis (PCA) in GenAlEX to validate and define naturally occurring genetic clusters. At last, a hierarchical AMOVA analysis with populations nested within regions was performed in GenAlEX to examine the distribution of variation and differential connectivity among populations, genders (males or hermaphrodites in a single population), and groups (males or hermaphrodites from all three populations). The pairwise phiPT (an analogue of Fst, i.e., genetic diversity among populations) and Nm (gene flow) was also estimated with GenALEX AMOVA analysis.
Statistics analysis
-
The significance of deviations from a sex ratio of 1:1 was tested by using the chi-square test ( χ2)[45]:
$ {\chi _{\left( {m - 1} \right)}}^2 = \sum\limits_{i = 1}^m {\frac{{{{(\left| {{O_i} - {T_i}} \right| - 0.5)}^2}}}{{{T_i}}}{\chi ^2}} $ (2) where O is the observed value, T is the expected value, and m is 2 on the basis of the Oleaceae sex (male and hermaphrodite). The genetic differences among populations and between males and hermaphrodites were analyzed with ANOVA by using SPSS 13.0 (2001, v. 13.0; SPSS Inc., USA) software package.
We gratefully thank Yangsong Li, Fangping Tang, Jianguo Feng, Xiaoxia Wang and Tao Sheng for their helps in collecting plant materials and field investigations. This research was supported by National Natural Science Foundation of China (Grant No. 32072615 and 31902057) and Zhejiang Science and Technology Major Program on Agricultural New Variety Breeding (2021C02071).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhou L, Yang L, Fang Q, Dong B, Wang Y, et al. 2022. Sex ratio, floral traits, and genetic variation of androdioecious Osmanthus fragrans L. (Oleaceae) and the implications for maintenance of high male frequency. Ornamental Plant Research 2:22 doi: 10.48130/OPR-2022-0022
Sex ratio, floral traits, and genetic variation of androdioecious Osmanthus fragrans L. (Oleaceae) and the implications for maintenance of high male frequency
- Received: 28 July 2022
- Accepted: 13 October 2022
- Published online: 28 December 2022
Abstract: Several examples of androdioecy appear to have evolved from dioecy and have low male frequency (< 0.5). However, the evolutionary pathway to androdioecy in Oleaceae may come from hermaphroditism. Osmanthus fragrans L. has a 1:1 sex ratio in nature populations. Significant differences are observed not only in flowering phenology but also in some floral traits between males and hermaphrodites. The protandry in the same population and the protogyny in the same plant may promote the xenogamy between genders. The majority of flower traits related with the pollen production are different between males and hermaphrodites. Males bear more flowering nodes, and more flowers per node, and larger anther in all three populations. This characteristic demonstrated that males have more male advantage than hermaphrodites. Population genetic structure of O. fragrans is genetically homogeneous at the species level, and most variations exist within a population. The percentage of variation among populations (13%) and between males and hermaphrodites (0%) is low. Moreover, genetic differentiation was very low between genders not only among populations but also in the same population. This genetic variation could be attributed to the occurrence of high levels of xenogamy between genders. Therefore, high male frequency and more male fitness advantage in males are the essential conditions for this mating system, which plays an important role during population reproduction and regeneration. The 1:1 sex ratio could be the result of integrative effects of sexual system, mating system, and reproductive success.
-
Key words:
- Androdioecy /
- Osmanthus fragrans /
- Sex ratio /
- Floral trait /
- Genetic variation /
- Mating system