-
Salinity is an important limiting factor in turfgrass management in many areas because of increasing use of low quality of water for irrigation and soil salinization. It was reported that more than 20% of cultivated land worldwide (~45 million hectares) is influenced by salt stress and the amount of the affected land is rising[1]. Salinity stress may manage plant cells by excess production of reactive oxygen species (ROS), imbalance of ions and hormones, suppressed antioxidant defense systems and photosynthesis[2−7].
Salt stress may inhibit gas exchange by reducing stomatal conductance[4]. The plants may experience oxidative injury because excess energy may be directed to oxygen (O2), generating various ROS including superoxide (O2–·), hydrogen peroxide (H2O2), hydroxyl radicals (OH·), and singlet oxygen (1O2)[2]. The ROS may damage important cell components and cause cell membrane electrolyte leakage (EL)[5−8] . Plants possess various antioxidant defense mechanisms to suppress ROS toxicity and reduce or prevent oxidative damage. Antioxidant enzymes, such as superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6), and ascorbate peroxidase (APX, EC 1.11.1.11), can protect plants against oxidative stress[8,9]. The SOD is considered as the first line of defense against ROS by dismutating the superoxide anion to H2O2, which is finely regulated by CAT, POD, and APX[1, 10] .
Plant hormones have been considered as metabolic signals in regulating plant adaptation to stress environments[5, 11−15]. Cytokinins (such as ZR and iPA) essentially regulate cell division and enlargement and chloroplast biogenesis, and delay leaf senescence[13]. Auxin such as IAA can enhance root initiation and also delay plant senescence[16]. Bioactive GAs such as GA4 regulate leaf expansion and stem elongation[13].
World population and potable water demand continue to increase. Therefore, shortage of fresh water for irrigation is an important issue facing crop production and turfgrass management[6, 16,17]. Turfgrasses are increasingly experienced salt stress due to the accelerated salinization of agricultural lands and increasing demand on use reclaimed or other secondary saline water for irrigation of turfgrass landscapes[8, 16,17] . Various chemical and agronomic approaches have been applied to improve turfgrass tolerance to abiotic stress including salt stress tolerance. Plant growth regulators have been used to improve turfgrass tolerance to salt stress tolerance[18−21]. The jasmonic acid and its derivatives such as methyl jasmonate (MJ), collectively referred to as jasmonates, are naturally occurring plant growth regulators that are found in the plant kingdom. They are reported to regulate plant development and improve plant tolerance to abiotic stresses[22−24]. Exogenous jasmonic acid (100 µM) improved vegetative and reproductive development in two cotton varieties under normal and water deficit conditions[24]. The MJ treatment along or with other plant growth regulators (ascorbic acid, alpha-tocopherol and brassinosteroids) improved photosynthesis and grain filling, and alleviated adverse effects of high temperature on rice plants[20,21]. Several studies have shown that MJ can reduce the inhibitory effect of NaCl on photosynthetic rate and enhance growth and development of plants[25,26]. The JA at 2 mM reduced MDA content and increased leaf SOD, CAT, and APX activity in wheat under salt stress[27]. The MJ at 500 mg L−1 increased salt stress tolerance in pepper by increasing chlorophyll, root length, and root biomass[28]. Exogenous MJ improved the black locust tree (Robinia pseudoacacia) tolerance to salt stress by increasing the activities of SOD and APX[29]. Lang et al. reported that MJ from 45 to 60 μM increased SOD, APX, and glutathione peroxidase (GPX) activities in salt-stressed G. uralensis seedlings, MJ from 15 to 30 μM promoted POD and CAT activities, and ascorbate peroxidase (AsA) and glutathione (GSH) contents, resulting in a reduction of ROS and MDA[30]. However, no report was on effects of MJ on hormone response in perennial ryegrass associated with salt stress tolerance. The mechanisms of MJ's impact on perennial ryegrass salt tolerance are not well understood.
Perennial ryegrass (Lolium perenne L.) is a turfgrass species widely used for home lawns, golf course, urban landscapes and other sports fields[2, 5]. Our hypothesis that exogenous MJ at proper rates may improve plant salt stress tolerance by enhancing antioxidant metabolism and hormonal balance in perennial ryegrass. Therefore, the objective of this study was to examine antioxidant and hormone responses to MJ treatments at various concentrations under salt stress conditions and investigate if foliar application of MJ could enhance selected antioxidant enzyme activity and regulate endogenous hormonal balance associated with salt stress tolerance in perennial ryegrass.
-
Salt stress negatively impacted on turf quality as measured at day 14 and 21. The MJ treatments alleviated turf quality decline as measured at day 14 and 21 (Fig. 1a). At day 21, MJ treatments at 50 µM and 100 µM increased turf quality rating by 20.6% and 26.5%, respectively, relative to salt stress alone.
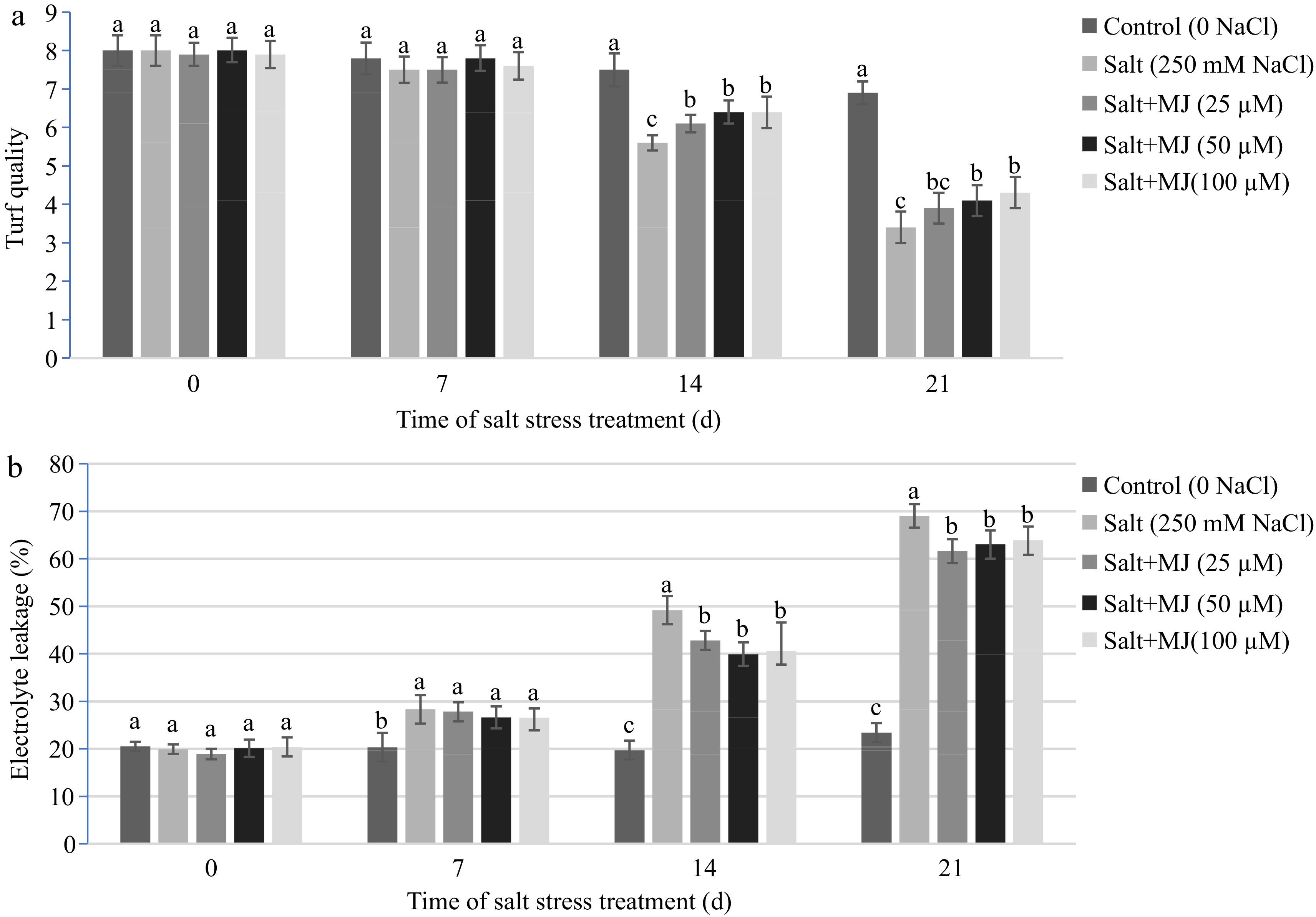
Figure 1.
Effects of methyl jasmonate (MJ) on (a) turf quality and (b) leaf electrolyte leakage (EL) in perennial ryegrass under salt stress. Treatments with same letters for each sampling date are not significantly different at P = 0.05.
Effects of MJ and salt stress on leaf electrolyte leakage (EL)
-
Salt stress caused an increase in EL as measured on day 7, 14, and 21 (Fig. 1b). The MJ application at all three rates decreased EL as measured at day 14, and 21. At day 21 of salt stress, MJ at 25, 50, and 100 µM reduced EL by 10.7%, 8.7%, and 7.4%, respectively, relative to the salt treatment alone.
Effects of MJ and salt stress on leaf malondialdehyde (MDA) content
-
Salt stress caused an increase in MDA content when compared to the control (no salt stress) as measured on day 7, 14, and 21 (Fig. 2a). The MJ application at 50 and 100 µM reduced MDA relative to salt stress treatment as measured at day 14 and 21. On day 21 of salt stress, EBR at 50 and 100 µM reduced EL by 11.3% and 15.9%, respectively, relative to the salt treatment alone.
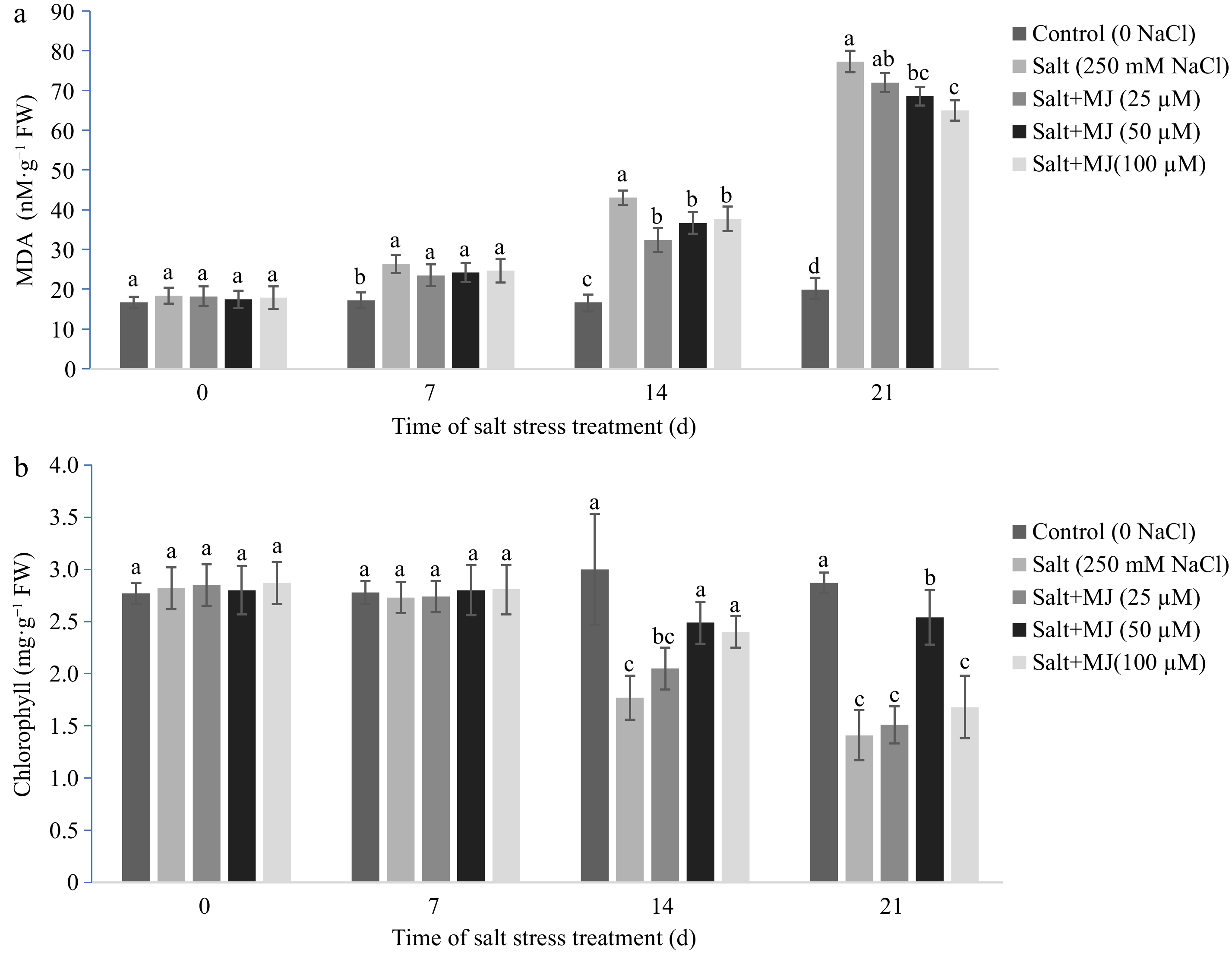
Figure 2.
Effects of methyl jasmonate (MJ) on (a) leaf malondialdehyde (MDA) content and (b) chlorophyll content in perennial ryegrass under salt stress. Treatments with same letters for each sampling date are not significantly different at P = 0.05.
Effects of MJ and salt stress on leaf chl content
-
Salt stress decreased chl content at day 14 and 21. The MJ treatments at 50 µM alleviated chl decline at day 14 and 21 (Fig. 2b). At day 21, MJ treatments at 50 µM increased chl content by 80.1% relative to salt stress alone.
Effects of MJ and salt stress on leaf superoxide dismutase (SOD) catalase (CAT), and ascorbate peroxide (APX) activity
-
Salt stress decreased SOD activity as observed at day 14 and 21 (Fig. 3a). The MJ treatments alleviated SOD decline as measured at day 14 and 21. Salt stress decreased CAT activity as observed at day 14 and 21 (Fig. 3b). The MJ treatment did not impact CAT activity when compared to the salt stress alone except for MJ at 100 µM which increased CT activity relative to salt stress along at day 14.
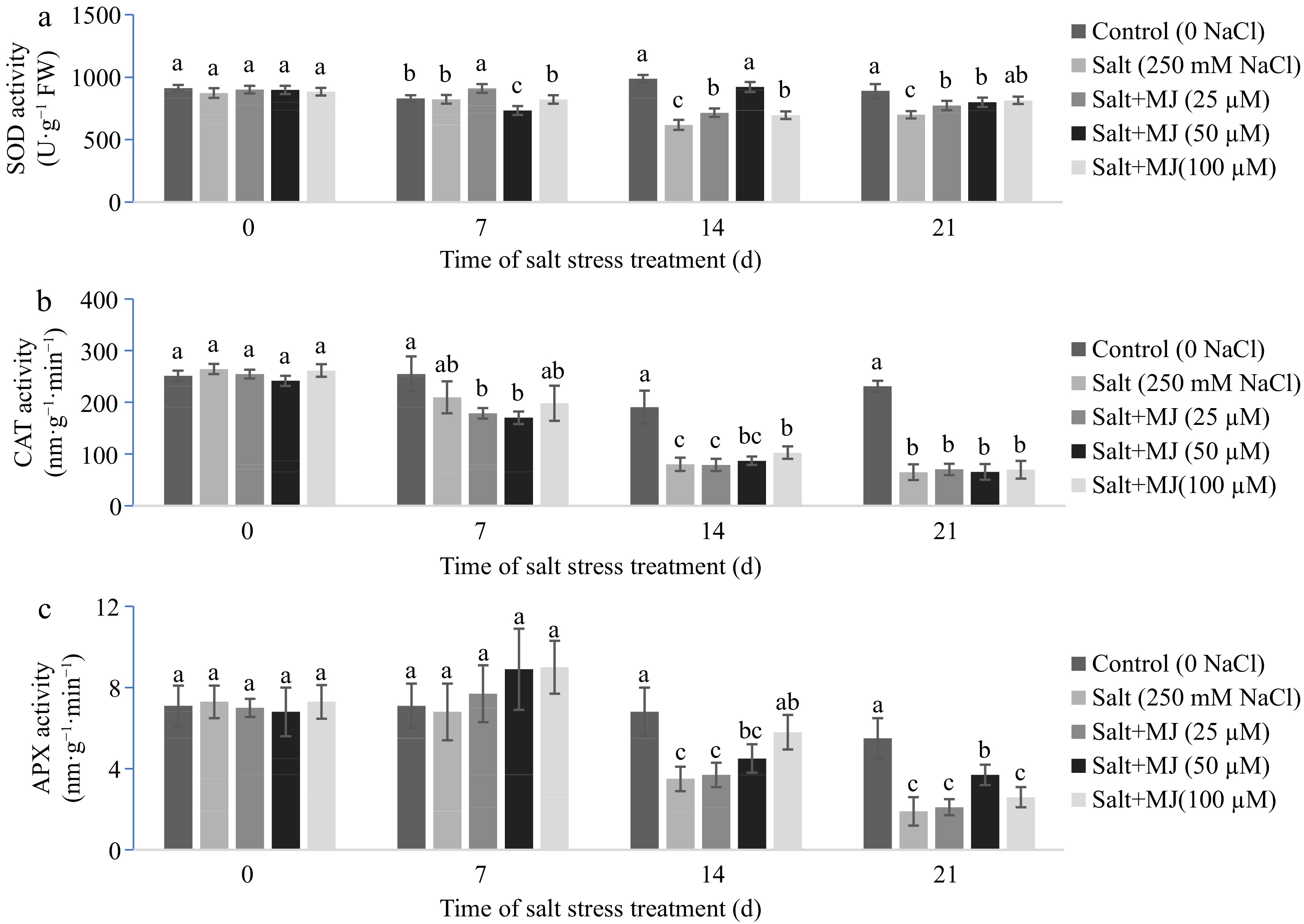
Figure 3.
Effects of methyl jasmonate (MJ) on (a) leaf superoxide dismutase (SOD), (b) catalase (CAT) and (c) ascorbate peroxidase (APX) activity of perennial ryegrass under salt stress. Treatments with same letters for each sampling date are not significantly different at P = 0.05.
Salt stress caused a reduction in APX activity as observed at day 14 and 21 (Fig. 3c). The MJ treatment at 100 and 50 µM alleviated APX reduction as found at day 14 and 21, respectively, relative to the salt stress alone.
Effects of MJ and salt stress on leaf indole-3-acetic acid (IAA) and gibberellin A4 (GA4)
-
Salt stress caused a reduction in leaf IAA content as observed at day 7, 14, and 21 (Fig. 4a). The MJ at treatment at all three rates did not impact leaf IAA when compared to the salt stress alone.
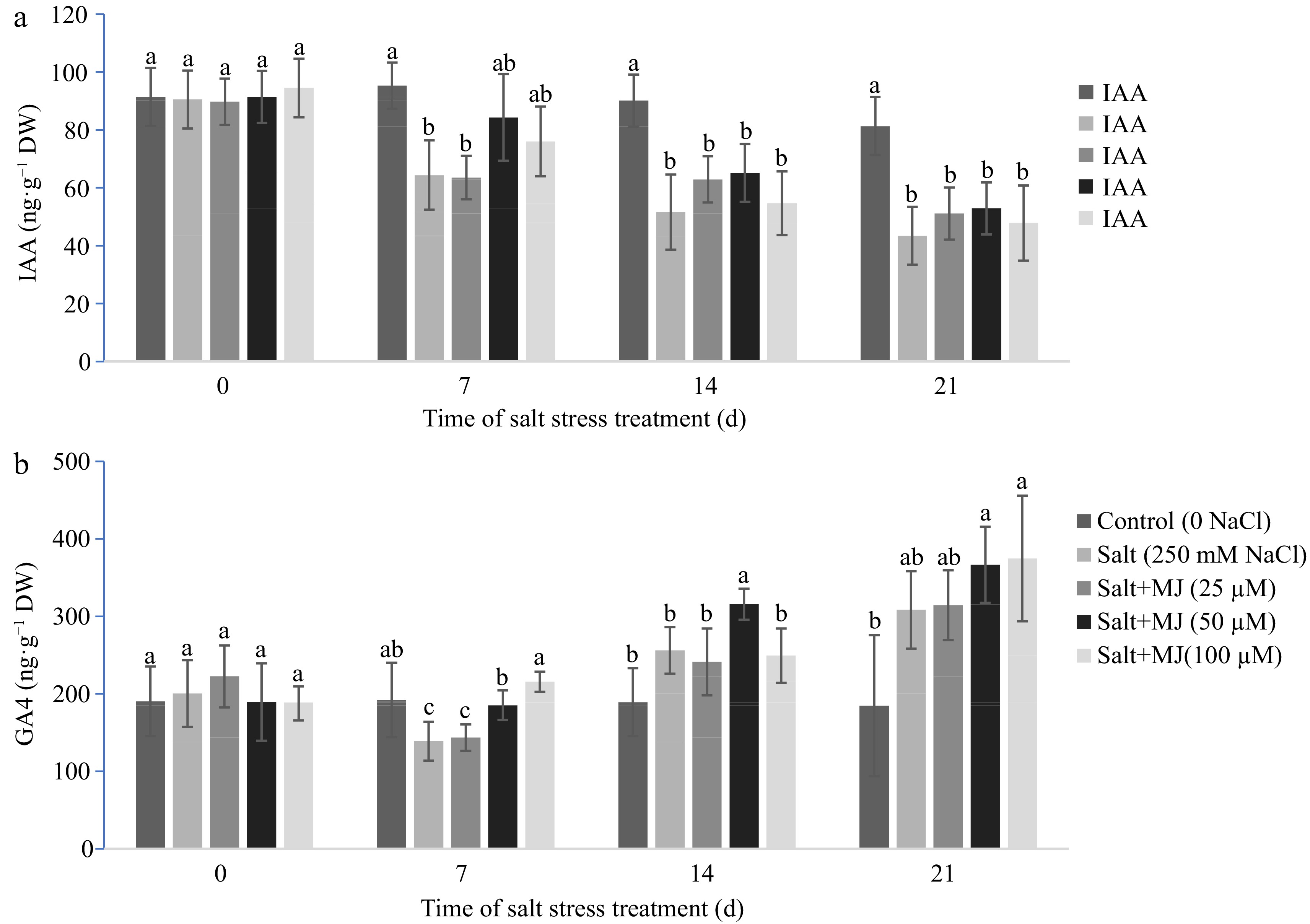
Figure 4.
Effects of methyl jasmonate (MJ) on (a) leaf indol-3-acetic acid (IAA), and (b) gibberellin A4 (GA4) of perennial ryegrass under salt stress. Treatments with same letters for each sampling date are not significantly different at P = 0.05.
Increases in GA4 content were found in responses to salt stress at day 7 and 21 (Fig. 4b). The MJ application at 50 µM promoted GA4 content relative to the sat stress treatment alone at day 7 and 14.
Effects of MJ and salt stress on leaf zeatin riboside (ZR) and isopentenyl adenosine (iPA)
-
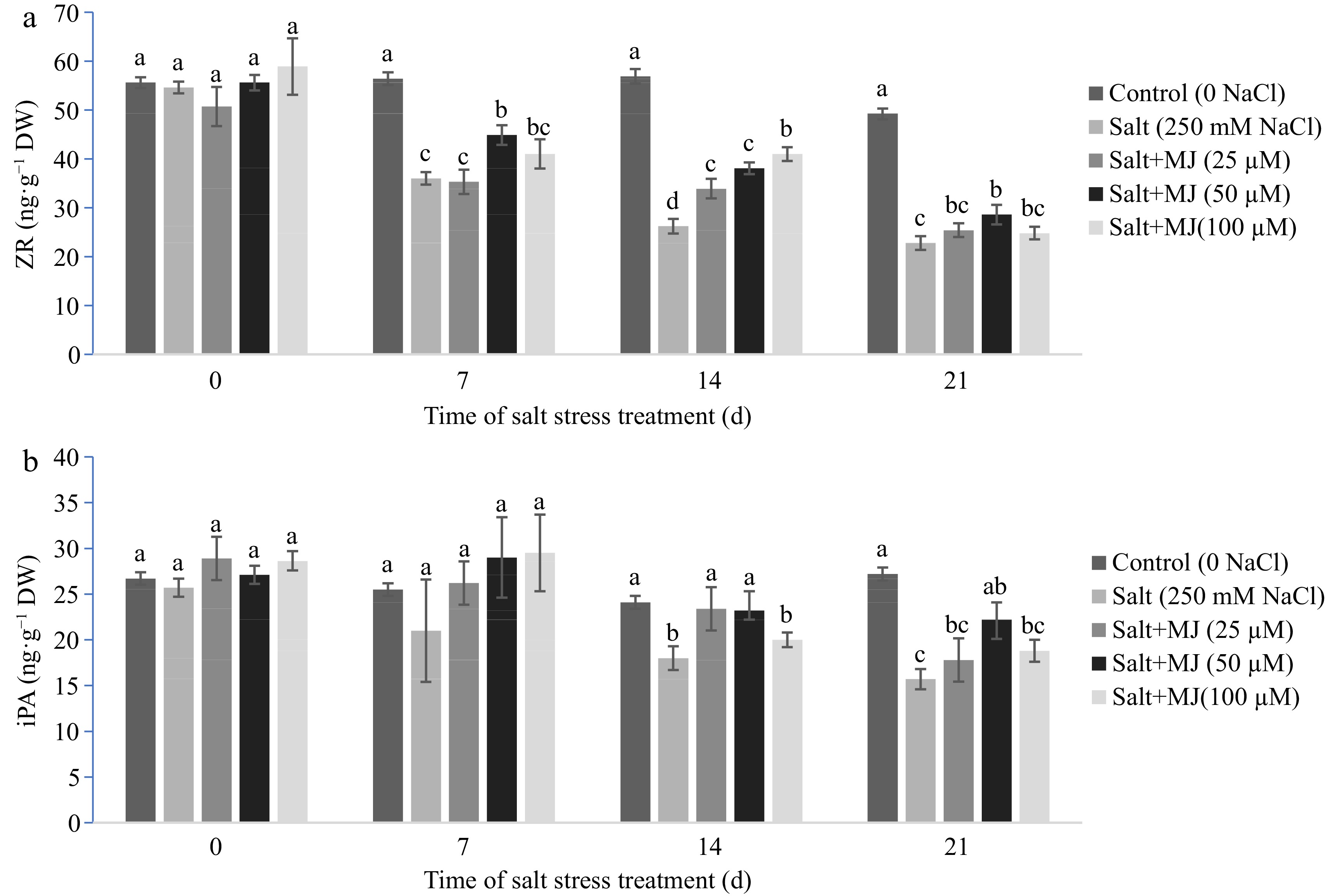
Salt stress caused a decline in leaf ZR content at day 7, 14, and 21 (Fig. 5a), and also decreased iPA content at day 14 and 21 (Fig. 5b). The MJ applied at 50 µM promoted ZR content relative to salt stress treatment alone as observed at day 7, 14, and 21. At day 21, MJ at 50 µM increased ZR by 25.4%. Similarly, MJ treatment at 50 µM promoted iPA content by 41.4% at day 21. In addition, MJ at 25 µM also increased ZR and iPA content relative to the salt stress treatment alone at day 14.
-
The results of this study indicated that salt stress damaged perennial ryegrass as determined by lipid peroxidation (higher MDA), cell membrane leakage (higher EL), and chlorophyll decline. The MJ treatments at 50 and 100 µM alleviated salt-induced damage of cell membrane and decline in leaf chlorophyll content. This is consistent with previous studies by Lang et al.[30] with Glycyrrhiza uralensis seedlings, Ahmadi et al.[31] with Brassica napus plants, and Jiang et al.[29]. Hu et al.[32] indicated that salt stress damaged perennial ryegrass by reducing stomatal limitation-induced accumulation of oxygen, resulting excess ROS accumulation and oxidative injury to cell membrane components. The results of this study showed that MJ treatments reduced EL and protected cell membrane integrity.
Previous studies indicated that plants possess antioxidant defense mechanisms to suppress excess ROS induced by salt stress, and the responses and capacity of antioxidant systems are closely associated with plant salt stress tolerance[1, 5]. The results of this study indicated that salt stress caused a reduction of SOD, CAT, and APX activity (Fig. 3). Application of MJ at 50, and 100 µM improved CAT and APX activity and MJ at 50 enhanced SOD activity under salt stress. This is consistent with previous studies[24, 30, 31]. Several studies have shown that application of MJ had a positive effect on antioxidant enzymes to protect plant under abiotic stress conditions[23, 33]. Lang et al.[30] applied MJ at 15, 30, 45, and 60 µM and found MJ at 45 and 60 enhanced SOD and APX activity and MJ at 15 and 30 µM increased CAT and peroxidase (POD) activity in salt stress-Glycyrrhiza uralensis seedlings. Qiu et al.[27] found that JA at 2 mM increased the activities of SOD, CAT, APX, and POD in wheat under salt stress when compared to salt treatment alone. Our data suggest that exogenous MJ-induced improvement of salt stress tolerance may be closely related to its up-regulation of antioxidant defense systems.
Hormonal balance and adjustment plays an important role in plant adaptation to abiotic stress including salt stress[13]. However, few studies have reported on hormonal responses to salt stress and exogenous MJ treatments in perennial ryegrass. The results of this study indicated that salt stress enhanced GA4 level but decreased levels of IAA, ZR, and iPA. Application of MJ alleviated decline of IAA and cytokinins (ZR and iPA) (Figs 4 & 5). Recent study has showed that IAA and cytokinins are closely associated with salt stress tolerance in Kentucky bluegrass[34]. Maggio et al.[35] found that exogenous GA3 improved water availability at low salt stress conditions. Our results suggest that MJ treatments-induced changes in endogenous hormones, especially cytokinins and auxin, may contribute to the improvement of salt stress tolerance in perennial ryegrass.
In summary, salt stress (250 mM NaCl) increased lipid peroxidation, and damaged cell membrane, and chlorophyll, and suppressed anti-senescence hormones (ZR, iPA, and IAA), and negatively impacted visual quality in perennial ryegrass. Application of MJ, especially at 50 µM, improved cell membrane integrity and chlorophyll content. The MJ treatments also increased antioxidant enzyme activity, and the selected hormones (IAA, ZR, and iPA) content (Figs 4 & 5). The shift of hormonal balance by MJ may signal an upregulation of antioxidant defense, delay leaf senescence and protect photosynthetic apparatus under salt stress. The results of this study suggest that MJ treatment may improve salt stress tolerance by enhancing plant antioxidant defense and regulating hormonal balance, protecting cell membrane and photosynthetic apparatus in perennial ryegrass exposed to salt stress.
-
This study was conducted from April to June 2020 in the growth chamber facility at Virginia Tech, Blacksburg, VA, USA. Seeds of perennial ryegrass 'Manhatan-5' were obtained from Turf Merchants (Albany, OR, USA) were planted in plastic pot (16 cm diameter and 15 cm deep) filled with a fine sand containing 10% peat at a rate of 30 g·m−2 pure live seeds on 17 April, 2020. The plants were grown in a growth chamber at temperatures (mean ± SD) at 22 ± 0.8/16 ± 0.6 oC (day/night), 65% ± 8% relative humidity, 14-h photoperiod and photosynthetically active radiation at 400 ± 10 µmol·m−2·s−1. The grass was fertilized at 1.5 g·m−2 nitrogen from 28−8−18 complete fertilizer with micronutrients biweekly and trimmed to 6 cm weekly, and irrigated by hand until water drained from bottom of the pots, three times per week.
Treatments and sampling
-
Twenty days after emergence, the grass was subjected to four treatments: (1) Control: normal water; (2) Salt Stress: 250 mM NaCl; (3) Salt stress (250 mM NaCl) plus 25 µM MJ (Sigma-Aldrich company, St. Louis, MO, USA); (4) Salt stress (250 mM NaCl) plus 50 µM MJ, and (5) Salt stress (250 mM NaCl) plus 100 µM MJ. The MJ solution was applied to foliage by using a hand-held sprayer at 10 mL per pot. After about 12 h of MJ treatment, the salt solution was added in gradually increasing concentrations in aliquots of 50 mM every 12 h until the concentration of 250 mM was attained within 48 h after initiation[5,36,37]. The NaCl concentration (250 mM) selected based on our preliminary experiment and the previous studies[2, 30] was suitable for perennial ryegrass. Then the pots were placed in plastic trays (20 cm diameter, 6.5 cm deep) filled with either salt treatment solution or distilled water only. The grass receiving the same volume of distilled water was considered as control. The lower 1/5 portions of the pots were submerged in the solutions all the time.
Leaf samples were collected at 0, 7, 14, and 21 d after the initiation of salt treatment and a part of each sample was stored at −80 °C for analysis of antioxidant enzyme, MDA, and various hormones. A small amount of fresh leaf tissues was collected for EL analysis.
Measurements
Turfgrass quality
-
Turfgrass quality was rated based on a visual scale of 1 to 9 based on leaf color, uniformity, and density, with 1 indicating complete death or brown leaves, and 9 indicating turgid and dark green leaves, with optimum canopy uniformity and density[9].
Leaf electrolyte leakage (EL)
-
Fresh leaf (50 mg) were placed in closed test tube containing 10 mL deionized water and EL was determined according to Wu et al.[5].
Leaf malondialdehyde (MDA)
-
Leaf cell membrane lipid peroxidation was measured based on MDA content. The MDA was determined following the procedure as described by Hodges et al.[38] with modifications[5].
Leaf chlorophyll (Chl) content
-
Leaf samples were extracted for chl in 100% acetone. Leaf chl content was determined by using a spectrophotometer as described by Zhang et al.[39].
Leaf antioxidant enzyme activity
-
Frozen leaf samples (100 mg) were ground into powder in liquid N2 and extracted in 1.8 ml of ice-cold 50 mmol sodium phosphate buffer (pH 7.0) containing 0.2 mM EDTA and 1% polyvinylpyrrolidone (PVP) in an ice-water bath. The homogenate was centrifuged at 12,000 gn for 20 min at 4 ºC. Supernatant was used for antioxidant enzyme activity.
Superoxide dismutase activity (SOD) was determined by measuring its ability to inhibit the photochemical reduction of nitro blue tetrazolium (NBT) according to the method of Giannopolitis & Ries[40] with minor modifications[5].
Activity of catalase (CAT) was determined by using the method of Chance & Maehly[41] with modifications[5]. The activity of ascorbate peroxidase (APX) was detected using the method of Zhang et al.[9].
Leaf hormone extraction and purification
-
Leaf hormones (IAA, ZR, iPA, and GA4) were extracted according to Edlund et al.[42], Zhang et al.[18] with some modifications[5].
Hormone analysis by LC-MS/MS
-
The hormones were analyzed by LC-MS/MS as described by Wu et al.[5]. An Agilent tandem LC-MS/MS system with an ESI sample introduction interface (Agilent, Santa Clara, CA, USA), consisting of 1290 UPLC and 6490 QQQ, was used for analyzing the hormone extracts. The selected hormones (ZR, iPA, IAA, GA4) were determined based on retention times and ion products and standards of each compound[5,42].
Experimental design and statistical analysis
-
A completely randomized block design was used with four replications. The data were analyzed using one-way analysis of variance using SAS software (v. 9.3 for Window). The mean separations were performed using the Fisher's protected least significant difference test at the 5% probability level.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhang X, Goatley M, Wang K, Conner J, Brown I, et al. 2023. Methyl jasmonate enhances salt stress tolerance associated with antioxidant and cytokinin alteration in perennial ryegrass. Grass Research 3:6 doi: 10.48130/GR-2023-0006
Methyl jasmonate enhances salt stress tolerance associated with antioxidant and cytokinin alteration in perennial ryegrass
- Received: 09 March 2023
- Accepted: 11 April 2023
- Published online: 28 April 2023
Abstract: Jamonic acid (JA) and its derivatives such as methyl jasmonate (MJ) regulate stress tolerance but of the mechanism of MJ’s impact on turfgrass salt stress tolerance are not fully understood. The objectives of this experiment was to investigate the responses of antioxidant and hormone metabolism to exogenous MJ in perennial ryegrass (Lolium perenne L.) subjected to salt stress. The MJ at 0, 25, 50, and 100 µM were applied to the ryegrass seedlings, and then the treated grass was exposed to salt stress at 250 mM NaCl. The grass plants serving as control were irrigated with regular water without MJ. Salt stress induced an increase in leaf electrolyte leakage (EL), and malondialdehyde (MDA) and reduction in turf visual quality rating and chlorophyll content. Exogenous MJ treatments suppressed EL and MDA, promoted chlorophyll content. The MJ treatments also improved superoxide dismutase (SOD) and catalase (CAT) and ascorbate peroxidase (APX) activity relative to salt treatment alone. Salt stress enhanced leaf gibberellin A4 (GA4) content but decreased indole-3-acetic acid (IAA), zeatin riboside (ZR), and isopentenyl adenosine (iPA). Exogenous MJ at 50 and 100 uM increased IAA, ZR, and iPA content under salt stress. Our data suggest that MJ treatment may enhance salt stress tolerance of perennial ryegrass by promoting the selected hormone level and antioxidant enzyme activity, and protecting cell membrane and photosynthetic function in perennial ryegrass.
-
Key words:
- Abiotic stress /
- Antioxidant /
- Hormone /
- Turfgrass.