-
Population growth and urbanization have resulted in increasing water demand and consequently accelerating fresh water scarcity[1]. On average, 320 gallons of water were used per day per household in the USA[2]. Reusing residential graywater could substantially reduce or eliminate the use of fresh water for landscape irrigation. Reclaimed wastewater has been used to irrigate turfgrass and proven to be effective in situations where transportation of the water is reasonable and soils are capable[3,4]. A large portion of residential graywater can be reclaimed for direct use at the local level; however, concerns over its reliability with regard to chemical composition have prevented the implementation of effluent water use on a national or global scale[5,6].
The major concern regarding recycled graywater (effluent water) use is due to its high salt content[5] which can cause salinity stress and ion stress or toxicity. It is well documented that saline soil conditions have detrimental effects on turfgrasses and other crops[4,7]. Turfgrass breeders have been working for decades to identify and breed salt-tolerant turfgrass species and cultivars to combat the issue of elevated salinity from effluent and gray water sources[8−11]. In addition to salinity, the boron (B), sodium (Na), and chloride (Cl) can build up in the soil and plant tissue which are potentially toxic to turfgrasses, therefore, their levels need to be monitored when using recycled or graywater irrigation[12−15]. As more and more areas of the world are experiencing issues related to water scarcity, identifying turfgrass species and cultivars that perform well under effluent water are critically important for improving the sustainability of turfgrass[10]. Perennial ryegrass (Lolium perenne L.) is a commonly used cool-season turfgrass in lawns, athletic fields, and golf courses, and it is also widely used for overseeding in warm-season grass during the winter season[16]. This study was designed to subject 11 lawn-height perennial ryegrass cultivars to summer effluent water irrigation in the Willamette Valley of Oregon (USA) where summer precipitation is scarce. The goals were to evaluate turfgrass performance and determine soil chemical properties and plant tissue B, Na, and Cl under effluent water irrigation compared to freshwater irrigation.
-
A field study was conducted at Lewis-Brown Horticulture Farm in Corvallis, OR, USA on a Chehalis silty clay loam soil. Corvallis is located in the Willamette Valley, OR, USA and has a warm-summer Mediterranean climate (Csb) according to the Köppen-Geiger climate classification with wet winters and warm, dry summers. The weather data during the study period is provided in Table 1. The average annual precipitation in Corvallis is 108.5 cm, which occurs almost exclusively in a nine-month period from autumn to spring[17], therefore, irrigation with harvested rainwater[18] and residential effluent water are viable alternatives to using the limited potable water resources for urban lawns in Corvallis or regions with a similar climate. Perennial ryegrass cultivars were sown at the rate of 54 g·m−2 to ensure uniform establishment on research plots, and a 25-3-10 (N-P-K) fertilizer (Wil-Gro 5 Iron, Wilbur-Ellis Company, Aurora, CO, USA) was applied at a rate of 100 kg·N·ha−1 at seeding on 12 October 2015.
Table 1. Corvallis, OR, USA weather data obtained from Bureau of Reclamation Hydromet/AgriMet System.
Month-year Evapotranspiration
(mm)Precipitation
(mm)Mean temperature
(°C)Max temperature
(°C)Min temperature
(°C)Mar-16 56 188 9.1 12.7 5.3 Apr-16 104 76 12.3 19.4 8.1 May-16 150 20 14.7 19.6 11.6 Jun-16 203 13 17.2 26.3 11.4 Jul-16 226 10 18.9 24.3 15.4 Aug-16 244 3 20.6 29.4 15.1 Sep-16 135 15 16.1 21.3 10.9 Mar-17 46 180 8.6 12.8 2.7 Apr-17 79 89 9.6 12.8 6.8 May-17 145 41 14.3 23.2 8.7 Jun-17 183 38 17.2 28.1 11.1 Jul-17 254 0 19.9 24.7 17.3 Aug-17 224 5 21.3 29.6 17.2 Sep-17 130 51 17.9 24.2 11.1 Experimental design was an 11 by 2 strip-plot organized as a randomized complete block design with three replications conducted over two years. Factors included 11 perennial ryegrass cultivars and fresh (control) versus effluent water summer irrigation. The 11 perennial ryegrass cultivars were 'Premium', 'Pillar', 'Pepper', 'Brightstar SLT', 'Estelle', 'Gray Fox', 'Allstar 3', 'Mighty', 'SR4660ST', 'Zoom', and 'Manhattan 6'. These cultivars were commercially produced by local companies in the Willamette Valley at the time the experiment was initiated. The sub-plot size was 2.3 m2. Effluent water treatment was applied twice weekly in June, July, August, and September in 2016 and 2017. Synthetic effluent water was manufactured for this experiment using a mass of constituents of Na, Cl, and B concentrations found in effluent-quality wastewater used for irrigation of the Heritage Golf Course, Westminster, CO, USA[3] and Whispering Palms turfgrass study, Davis, CA, USA[19]. To achieve these values of B, Na, and Cl for this experiment, water softener coarse salt (NaCl) (Compass Minerals International, Inc., Overland Park, KS, USA) at 6.03 × 104 mg·L−1, 20 Mule Team Borax Natural Laundry Booster (Na2B4O7·10H2O) (Henkel AG & Company, KGaA, Düsseldorf, Germany) at 4.15 × 103 mg·L−1, Arm & Hammer Super Soda Booster (Na2CO3) (Church & Dwight Co., Inc., Ewing, NJ, USA) at 4.31 × 103 mg·L−1, and trace amounts of ethylenediaminetetraacetic acid (EDTA; C10H16N2O8) (Fisher Scientific, Pittsburgh, PA, USA) at 35.2 mg·L−1 were utilized. The simulated concentrations of B, Na, Cl, and EDTA used for this study were 2.1 mg·B·L−1, 111 mg·Na·L−1, 168 mg·Cl·L−1, and 0.33 mg·EDTA·L−1. Assuming 38 mm irrigation per week, mass loadings over the four-month period were calculated and distributed as twice-weekly sprays at a high concentration to avoid any significant differences in irrigation rates. Concentrated spray applications were watered-in with uniform 2.5 mm overhead irrigation to prevent evaporative loss and any potential acute salinity damage. Both treatments received overhead irrigation at the same rate and frequency. Irrigation was applied at 4.7 mm daily, and 2.5 mm twice weekly following effluent water applications, for a total of 38 mm per week.
Turfgrass maintenance
-
The turfgrass was mowed as needed at a mowing height of 5 cm and clippings were removed to help prevent annual bluegrass (Poa annua L.) infestation. Annual nitrogen rate for the two trial years was 244 kg·N·ha−1 applied via a 25-3-10 fertilizer (Wil-Gro 5 Iron, Wilbur-Ellis Company). Selective herbicides were used to maintain plots as predominantly perennial ryegrass. Prograss SC (42% ethofumesate) was applied at 4.6 kg·ha−1 (1.9 kg·a.i.·ha−1) on December 1 of 2015, January 6, February 2, September 27, November 4, and December 6 of 2016, and January 23 of 2017. TZone SE (7.72% triclopyr BEE, butoxyethyl ester, 0.66% sulfentrazone, 29.32% 2,4-D, 2-ethylhexyl ester, and 2.22% dicamba acid) was applied at 4.6 kg·ha−1 on 19 September 2016. Barricade 65WG (65% prodiamine) was applied at 0.56 kg·ha−1 (0.36 kg·a.i.·ha−1) on 24 July 2017.
Response variables
-
Response variables included visual turf color and density, along with soil and tissue elemental analyses. Data were collected on a monthly basis with the exception of soil and tissue samplings which took place at the conclusion of the study in September of 2017.
Turf color and density were visually assessed using a 1–9 scale with 6 being the minimum acceptable level. In turf color, a 1 rating was given to straw-brown turf, and 9 was given to dark green turf. In turf density, a 1 rating equals the lowest density (open canopy), and 9 equals maximum density. Turf color and density were evaluated two to three days after an effluent water application.
Soil cores (12 per plot) were collected using a 19-mm-diameter probe to a 15-cm depth with the top 2.5 cm of root and thatch material removed. Aggregate soil samples were analyzed by Oregon State University Soil Health Laboratory (Corvallis, OR, USA) for pH, electrical conductivity (EC), Na, Cl, and B. Tissue samples were collected using a self-propelled push lawn mower (Honda, Minato, Tokyo, Japan), dried, and sent to the same laboratory for analyzing Na, Cl, and B concentrations.
Statistical analyses
-
Data were subjected to analysis of variance using SAS 9.4 Proc Mixed (SAS Institute Inc., Cary, NC, USA). Due to the significant year effect and interactions between year and some of the remaining factors, data were analyzed separately for each year. Factors in the final analyses included rating date, replication, irrigation water, and cultivar for field measurements, and replication, irrigation water, and cultivar for soil and tissue analyses. Fisher's Protected Least Significant Difference (LSD) at the 0.05 probability level was used to determine treatment difference.
-
Cultivar consistently had significant effects on turf color and density (Table 2). 'Premium' perennial ryegrass received the highest turf color rating, whereas 'Pepper' had the lowest color rating in both years. 'Pepper' had lower than acceptable color in 2017, but was not statistically different than 'Manhattan 6' (Table 3). 'Pillar' had a lower color rating than 'Premium', 'Allstar 3', 'SR4660ST', and 'Zoom' in 2016 but had the highest color rating that was not significantly different from 'Premium', 'Estelle', 'Allstar 3', 'SR4660ST', and 'Zoom' in 2017 (Table 3). All perennial ryegrass cultivars had acceptable turf density ranged from 7.3 to 7.7 in 2016 and 6.1 to 6.8 in 2017 when averaged over each summer and between two irrigation water treatments (Table 3). 'Premium' produced the highest density in both years (Table 3). 'Allstar 3' also had high turf density ratings that were statistically similar to those of 'Premium' in both years (Table 3).
Table 2. Analysis of variance and means table for visual turf color and density ratings affected by irrigation water, cultivar, and date in Corvallis, OR, USA in 2016 and 2017.
Source of variation df Turf color
(1‒9)aTurf density
(1‒9)b2016 2017 2016 2017 Pr > F Replication 2 NS * NS *** Irrigation water 1 NS *** NS *** Fresh 7.3 6.5 7.5 6.6 Effluent 7.4 6.1 7.5 6.3 Cultivar 10 *** *** * * Date 2 ** *** NS *** Irrigation water × cultivar 10 NS NS NS NS Irrigation water × date 2 NS *** NS *** Cultivar × date 20 * NS NS NS Irrigation water × cultivar × date 20 NS NS NS NS a Turf color ratings were visually assessed on a 1‒9 scale with 1 being straw-brown turf, 6 being the minimum acceptable color, and 9 being dark green turf. b Turf density ratings were visually assessed on a 1‒9 scale with 1 being the lowest density (open canopy), 6 being the minimum acceptable density, and 9 being the highest density. NS Not significant at the 0.05 probability level. * Significant at the 0.05 probability level. ** Significant at the 0.01 probability level. *** Significant at the 0.001 probability level. Table 3. Visual turf color and density for 11 perennial ryegrass cultivars evaluated in Corvallis, OR, USA in 2016 and 2017. Mean values represent data points averaged across replication, date, and irrigation water.
Cultivar Turf color (1−9)ab Turf density (1−9)ac 2016 2017 2016 2017 Premium 7.63A 6.67A 7.69A 6.83A Pillar 7.31CD 6.67A 7.56ABC 6.58AB Pepper 6.93E 5.81D 7.56ABC 6.31BCD Brightstar SLT 7.19D 6.22BC 7.33D 6.33BCD Estelle 7.39BC 6.42AB 7.50ABC 6.36BCD Gray Fox 7.26CD 6.25BC 7.56ABC 6.53ABC Allstar 3 7.46B 6.42AB 7.61AB 6.72AB Mighty 7.38BC 6.28BC 7.47BC 6.11CD SR4660ST 7.47B 6.50AB 7.57ABC 6.72AB Zoom 7.44B 6.47AB 7.39CD 6.44ABCD Manhattan 6 7.36BC 6.08CD 7.43BC 6.08D a Means followed by the same uppercase letter were not significantly different at the 0.05 probability level. b Turf color ratings were visually assessed on a 1‒9 scale with 1 being straw-brown turf, 6 being the minimum acceptable color, and 9 being dark green turf. c Turf density ratings were visually assessed on a 1‒9 scale with 1 being the lowest density (open canopy), 6 being the minimum acceptable density, and 9 being the highest density. Summer effluent water irrigation
-
Effluent water irrigation applied in the summer did not have significant effects on turf color or density compared to freshwater irrigation in the first year of the study. In the second year, irrigation water and its interaction with date were significant (Table 2). Effluent water irrigation had similar effects on turf color and density in 2017 (Fig. 1). Reductions in turf color (Fig. 1a) and density (Fig. 1b) with effluent water irrigation were observed in June and July 2017. In August of 2017, effluent water irrigation produced turf color and density comparable to freshwater irrigation (Fig. 1).
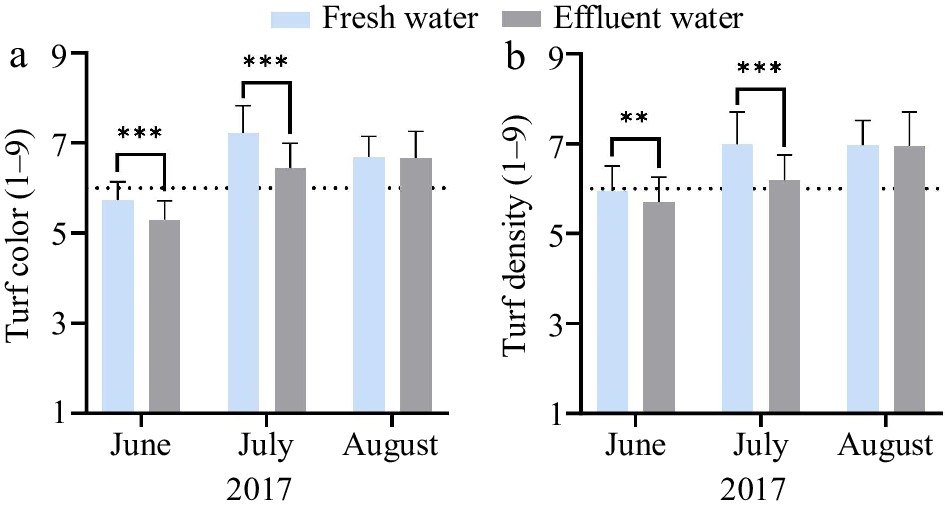
Figure 1.
The effects of summer irrigation with fresh versus effluent water on (a) turf color and (b) turf density varied by rating dates in 2017. Turf color ratings were visually assessed on a 1‒9 scale with 1 being straw-brown turf, 6 being the minimum acceptable color (indicated by the dotted line), and 9 being dark green turf. Turf density ratings were visually assessed on a 1‒9 scale with 1 being the lowest density (open canopy), 6 being the minimum acceptable density (indicated by the dotted line), and 9 being the highest density. Error bars indicate standard deviations. ** Significant at the 0.01 probability level. *** Significant at the 0.001 probability level.
Soil analysis
-
While effluent water irrigation did not affect soil pH, it was found to have significant effects on EC as well as soil B, Na, and Cl contents measured at the conclusion of the study (Table 4). Effluent water irrigation in the summer resulted in an EC of 0.24 dS·m‒1 which was statistically higher than the freshwater control of 0.15 dS·m‒1 (Table 4). The concentrations of B, Na, and Cl were found to be significantly higher in the soil of effluent water irrigation treatment compared to the freshwater irrigation treatment, all of which were 4 to 5 times higher than the freshwater control (Table 4). The main effect of perennial ryegrass cultivar and its interaction with irrigation water were not significant in any of the soil chemical properties tested in this study (Table 4).
Table 4. Analysis of variance and means table for soil pH, electrical conductivity (EC), soil boron (B), sodium (Na), and chloride (Cl) concentrations on 11 perennial ryegrass cultivars under fresh versus effluent water summer irrigation at the conclusion of a two-year study in Corvallis, OR, USA.
Source of variation df pH EC
(dS·m−1)B
(ppm)Na
(ppm)Cl
(ppm)Pr > F Replication 2 NS NS NS NS NS Irrigation water 1 NS * * * * Fresh 6.3 0.15 0.9 74 6 Effluent 6.2 0.24 4.3 326 30 Cultivar 10 NS NS NS NS NS Irrigation water × cultivar 10 NS NS NS NS NS NS Not significant at the 0.05 probability level. * Significant at the 0.05 probability level. Plant tissue analysis
-
Irrigation water had significant effects on Na and Cl ion concentrations in the leaf tissues (Table 5). Significantly higher concentrations of Na and Cl ions, and marginally higher (probability of 0.057) B ions were detected in the tissue samples from effluent water irrigation plots compared to freshwater irrigation plots (Table 5). Perennial ryegrass cultivars had different levels of tissue B regardless of irrigation water source, ranging from 8 ppm from 'Estelle' to 29 ppm from 'Allstar 3' (Table 6).
Table 5. Analysis of variance and means table for leaf tissue boron (B), sodium (Na), and chloride (Cl) concentrations on 11 perennial ryegrass cultivars under fresh versus effluent water summer irrigation at the conclusion of a two-year study in Corvallis, OR, USA.
Source of variation df B (ppm) Na (ppm) Cl (ppm) Pr > F Replication 2 NS NS NS Irrigation water 1 0.0568a ** * Fresh 15 874 5782 Effluent 21 7592 9506 Cultivar 10 * NS NS Irrigation water × cultivar 10 NS NS NS a Significant at the 0.1 probability level with a probability of 0.0568. NS Not significant at the 0.05 probability level. * Significant at the 0.05 probability level. Table 6. Leaf tissue boron (B) concentrations for 11 perennial ryegrass cultivars at the conclusion of a two-year study in Corvallis, OR, USA. Mean values represent data points averaged across replication and irrigation water.
Cultivar B (ppm)a Premium 22ABC Pillar 15BCD Pepper 18BCD Brightstar SLT 18BCD Estelle 8D Gray Fox 21ABC Allstar 3 29A Mighty 14BCD SR4660ST 18BCD Zoom 13CD Manhattan 6 23AB a Means followed by the same uppercase letter were not significantly different at the 0.05 probability level. -
Increasing concerns about fresh water scarcity and conservation are limiting the use of turfgrass in urban landscapes, especially in the western and southern USA. In these arid and warm regions, water conservation agencies have implemented incentive programs to remove turfgrass lawns, including Southern California, Southern Nevada, and Florida[20−23]. Effluent water irrigation provides an ideal solution for the amenity use of turfgrass without the concerns of food safety when effluent water irrigation is used for growing food crops.
Perennial ryegrass is generally considered not drought tolerant[24], therefore, an alternative source to freshwater irrigation is critically important. The intent of this field study was to compare effluent versus fresh water irrigation on 11 different lawn-height perennial ryegrass cultivars during the summer drought in the Willamette Valley of Oregon. Our results suggested that effluent water has the potential for irrigating perennial ryegrass during the summer drought periods. There was no significant interaction between the irrigation water and perennial ryegrass cultivar, indicating that cultivars performed well with freshwater irrigation also performed well with effluent water irrigation. Only small reductions in turf color and density were observed with effluent water irrigation in the second year of the study. The low soil EC in this study suggested that the two years of effluent water irrigation did not contribute to soil salinity or salinity stress to the plants. However, it is possible that the reductions in turf color and density could be attributed to ion toxicity.
Effluent water irrigation had no effect on turf quality (color and density) in the first year, but resulted in statistically lower turf quality compared to freshwater irrigation in June and July, but not August of the second year. The average ratings among the 11 cultivars were slightly under the acceptable level regardless of irrigation water type only in June of 2017 (Fig. 1). Irrigation was not applied in May, but evapotranspiration exceeded precipitation in May (Table 1) causing the lower turf color and density observed in June. Nevertheless, the majority of the perennial ryegrass cultivars used in this study provided acceptable turf quality when averaged over each summer (Table 3). Additionally, the differences in the turf color and density were expected to be associated with genetic traits, considering all the cultivars were subjected to the same level of fertility and irrigation rate. 'Premium', 'Allstar 3', and 'SR4660ST' consistently performed well in both years regardless of irrigation water source and were among the highest ranked cultivars in turf color and density (Table 3). 'Pepper' had low turf color among other cultivars but had relatively high turf density (Table 3).
Soil B, Na, and Cl concentrations of the effluent water treated plots were more than four times higher than the freshwater control, which likely resulted in higher EC compared to the control (Table 4). Isweiri et al. observed that long-term effluent water irrigation on fairways with perennial ryegrass and Kentucky bluegrass (P. pratensis L.) mixtures also resulted in higher EC values compared to fairways irrigated with fresh water, but remained well below the critical threshold level of 4 dS·m–1 for perennial ryegrass[25]. In our study, the EC value for the effluent water treatment was 0.24 dS·m–1 compared to 0.15 dS·m–1 for the freshwater treatment (Table 4), which is well below 4 dS·m–1. The buildup of salts to potentially toxic levels depends on concentration in irrigation water, amount of water applied, annual precipitation, and soil characteristics[26]. Annual rainfalls occurring between autumn and the following spring could result in lowering or slowing down the increase of EC and concentrations of salts for the effluent water irrigation treatment. This region of the Pacific Northwest is characterized as a cool-humid climate with an average annual precipitation of 108.5 cm, which occurs almost exclusively between autumn and the next spring[17,27], suggesting potential for leaching ions that would otherwise accumulate in the soil from effluent water applications. Our speculation was supported by a greenhouse study that also raised the concerns about the high salts from detergents and personal care products in the graywater can accumulate in soil and leach through soil to reach groundwater[12].
Higher levels of Na and Cl were also observed in the leaf tissues in response to effluent water irrigation (Table 5). The average tissue Na level of 874 ppm in the grass clippings of 11 perennial ryegrass cultivars irrigated with fresh water in our study is consistent with the Na concentration reported in the literature for perennial ryegrass[28]. In contrast, our results also indicated that the tissue Na level was nearly nine times higher in the effluent water treatment compared to the freshwater control. In Kentucky bluegrass, a study sampling golf courses under long-term recycled water irrigation has shown that increasing Na concentration up to 4,500 ppm in the shoots produced acceptable turf quality but was linearly correlated with decreasing turf quality[13]. Similarly, we observed a high level of tissue Na at 7,592 ppm in perennial ryegrass with effluent water irrigation, which could help explain the reduced turf color and density. Krishnan and Brown reported in a greenhouse study that salt tolerant perennial ryegrasses, including PST-2MNG (experimental code for 'Gray Fox'), accumulated about 40% less Na ion than the nearly 20,000 ppm with 'Linn' when subjected to salt stress and demonstrated that Na exclusion in the leaf tissue is one of the major salt tolerance mechanisms[14]. In the current study, we evaluated 11 newer perennial ryegrass cultivars than 'Linn' and did not observe differences in tissue Na concentrations among them, suggesting that these cultivars exhibited comparable salt tolerance.
The high concentration of Na and Cl accumulated in the shoot of cool-season turfgrass is a common issue with recycled wastewater[13,15]. Even though the reduction in turf quality was small in scale (Table 2 & Fig. 1), our tissue test results suggested that effluent water could have negative effects on turfgrass growth. Long-term field trials need to be conducted to verify whether the Na, Cl, and B will buildup in the plant tissue as effluent water irrigation continues to be applied. However, the toxicity levels of Na and Cl have not been described in perennial ryegrass. Research has shown that B was accumulated in the grass leaf tips, and that routine mowing could remove B and reduce injury[29]. When turfgrass is continuously growing, mowing is expected to remove grass clippings containing high levels of Na, Cl, and B, whether their concentrations will reach equilibrium and are not detrimental to perennial ryegrass remains unknown, and future research is warranted.
While many trees, shrubs, and groundcovers are sensitive to Cl, the toxic level and injuries of Cl in turfgrass have not been observed or reported in the literature, suggesting higher tolerance and great potential to utilize effluent water irrigation in turfgrass. On the other hand, the accumulation of Na in soil and leaf tissue could be problematic. Studies have shown Na to be directly toxic to plants; however, its most frequent negative effect is on soil structure[30,31]. Sodium causes breakdown of clay particles, thus decreasing soil aeration and infiltration[26]. Irrigation sources with lower sodium absorption ratio (SAR), the relative proportion of sodium to calcium plus magnesium ions in the water, should be preferred for turf and other landscape applications, particularly on clay soils[26]. Even though effluent water irrigation for two summers in our area, which has low baseline soil EC, did not lead to soil salinity or any significant change in soil pH, long-term studies are needed to continue monitoring EC levels for the safe use of effluent water. Furthermore, Negahban-Azar et al. suggested that graywater should be used as needed based on the evapotranspiration rate and not over applied as a disposal method[12].
-
The results of this study demonstrated the potential of perennial ryegrass cultivars with high turf quality for use as lawns under effluent water irrigation. Although, small reductions in turf density and color were observed in June and July of 2017, the majority of the perennial ryegrass cultivars had acceptable turf color and density ratings when averaged over each summer. Soil analyses showed the buildup of B, Na, Cl, and elevated EC with effluent water applications. The accumulation of Na and Cl was also observed in the plant leaf tissue with effluent water applications. Effluent water irrigation is a viable option for growing perennial ryegrass in the dry summer of Willamette Valley. There is still a need for well-documented research on the long-term effects of effluent water irrigation on turfgrass species and cultivars, particularly in areas that are relatively new to water scarcity. Future research is warranted to determine whether turfgrass provides sufficient filtrating of wastewater for safe groundwater and compare turfgrasses to bare soil and other landscape and groundcover plants.
-
The authors confirm contribution to the paper as follows: study conception and design: Olsen CJ, Kowalewski AR; data collection: Olsen CJ, Gould MA; analysis and interpretation of results: Wang R, Kowalewski AR; draft manuscript preparation: Wang R, Olsen CJ. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
This research was funded by Western Canada Turfgrass Association (No. 3118).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang R, Olsen CJ, Gould MA, Kowalewski AR. 2023. Field evaluation of perennial ryegrass cultivars for use with effluent water irrigation. Grass Research 3:23 doi: 10.48130/GR-2023-0023
Field evaluation of perennial ryegrass cultivars for use with effluent water irrigation
- Received: 27 August 2023
- Accepted: 02 November 2023
- Published online: 28 November 2023
Abstract: Fresh water is a scarce resource that needs to be conserved. Landscape irrigation, a large portion of the outdoor water use, can be accomplished with water of less-than-potable quality. The use of effluent water generated from residential graywater in landscapes would go a long way toward conserving potable water for other essential uses. The objectives of this study were to evaluate the effect of effluent versus fresh water irrigation on the performance of 11 lawn-height perennial ryegrass (Lolium perenne L.) cultivars in the Willamette Valley of Oregon, USA, and determine the effects of effluent water irrigation on soil and tissue analyses. A two-year field trial was established in October 2015 on native soil, and the experimental design was an 11 by 2 strip-plot design with three replications. Synthetic effluent water (water-softening salt, two laundry detergents, and a chelating agent) was applied twice-weekly over perennial ryegrass plots in the summers of 2016 and 2017 and compared to a freshwater control. Small reductions in turf color and density were observed with effluent water irrigation only in June and July of 2017. Our results suggest that effluent water is a viable alternative to freshwater irrigation in the Willamette Valley, where there is little to no precipitation during summer. However, the accumulation of Na, Cl, and B in the soil and plant tissue indicates that future research is warranted to determine any long-term effects from effluent water irrigation on turfgrass and soil health.
-
Key words:
- Sustainability /
- Wastewater /
- Soil












