-
Vitellaria paradoxa (shea tree) is an economically and ecologically important tree capable of adapting to oligotrophic shallow soils and seasonally dry savanna environments in the Sudano-Sahelian region[1−4]. In particular, the seeds of V. paradoxa are used to produce 'shea butter' that is used locally as an edible oil and in traditional medicine as well as being commercially exploited for export, thereby earning the name 'the emerald of vegetable oil'[5]. The economic importance of V. paradoxa has prompted researchers to investigate its introduction, silviculture, agroforestry ecosystem, ecology, productivity and exploitation[6−9].
Introduction and plantation history of V. paradoxa in China: From December 1963 to February 1964, Premier Zhou En-lai and delegation were shown the fruits and products of V. paradoxa In Ghana, Yi Chen, the Chinese Foreign Minister at the time, brought seeds of V. paradoxa to the botanist Cai Xitao. Based on his field experience in Yunnan, Xi-tao Cai recognized the potential for developing V. paradoxa cultivation trial at Yuanjiang Research Station, which features savanna-like vegetation with regularly burnt grassland and dry thickets. In 1982, the research station closed, and approximately 12 hectares of successfully planted shea trees were handed over to the Yuanjiang County Forestry Bureau[10]. These trees have been growing now for over 54 years.
Internationally, shea trees is best known for its commercial value estimated at multi millions of dollars across cosmetic, pharmaceutical and chocolate industries[11]. Pouliot & Elias[7] estimated that shea trees can potentially funnel about USD150 million to the local economy and contribute up to 12% of total household income in Burkina Faso. Moreover, shea trees are also an important tree species in the agroforestry system in Africa, which integrates ecological and economic benefits[9,12]. To date, most research has focused on its chemical components, fats, proteins and amino acids[13−15]. Studies on V. paradoxa ecology and fruit yield have been carried out in countries where this species naturally occurs[16,17]. Although the natural range of this monotypic species extends across over 20 African countries in a narrow belt of well-drained soils, ranging from the savannas of eastern Senegal to the high plateau of east Africa and into south-eastern Uganda[13,18], V. paradoxa has not yet been reported as successfully established outside of Africa, ultimately limiting its output and development. Therefore, a better understanding of factors influencing the productivity of V. paradoxa planted outside Africa is necessary for cultivation and utilization.
In order to improve the yield and further accelerate the development of V. paradoxa outside Africa, understanding the relations between fruit yield and geographical condition are important and of significant interest to the scientific community. We aim to develop a model to understand and predict fruit yield in response to the dendrometric and geographical characteristics at the plantation site, and explore how shea butter trees grown outside Africa have adjusted to the introduced area and how selected variables affect fruit yield.
-
The research was carried out at the Yuanjian dry-hot valley, located in Yuanjiang County, Yuxi City, Yunnan Province, China (N: 23.601496, E: 101.970145) (Fig. 1). The altitude is about 450 m and annual sunshine duration is 2,239.5 h. The mean annual precipitation is 780−850 mm, most of it (about 80%−85%) falling in the rainy season (May to October)[19]. The vegetation is similar to 'Savanna vegetation', presenting an expansive grassland with scattered trees and shrubs[20]. Moreover, other cultivated species are mainly Mangifera indica, Borassus flabellifer, Litchi chinensis and Delonix regia.
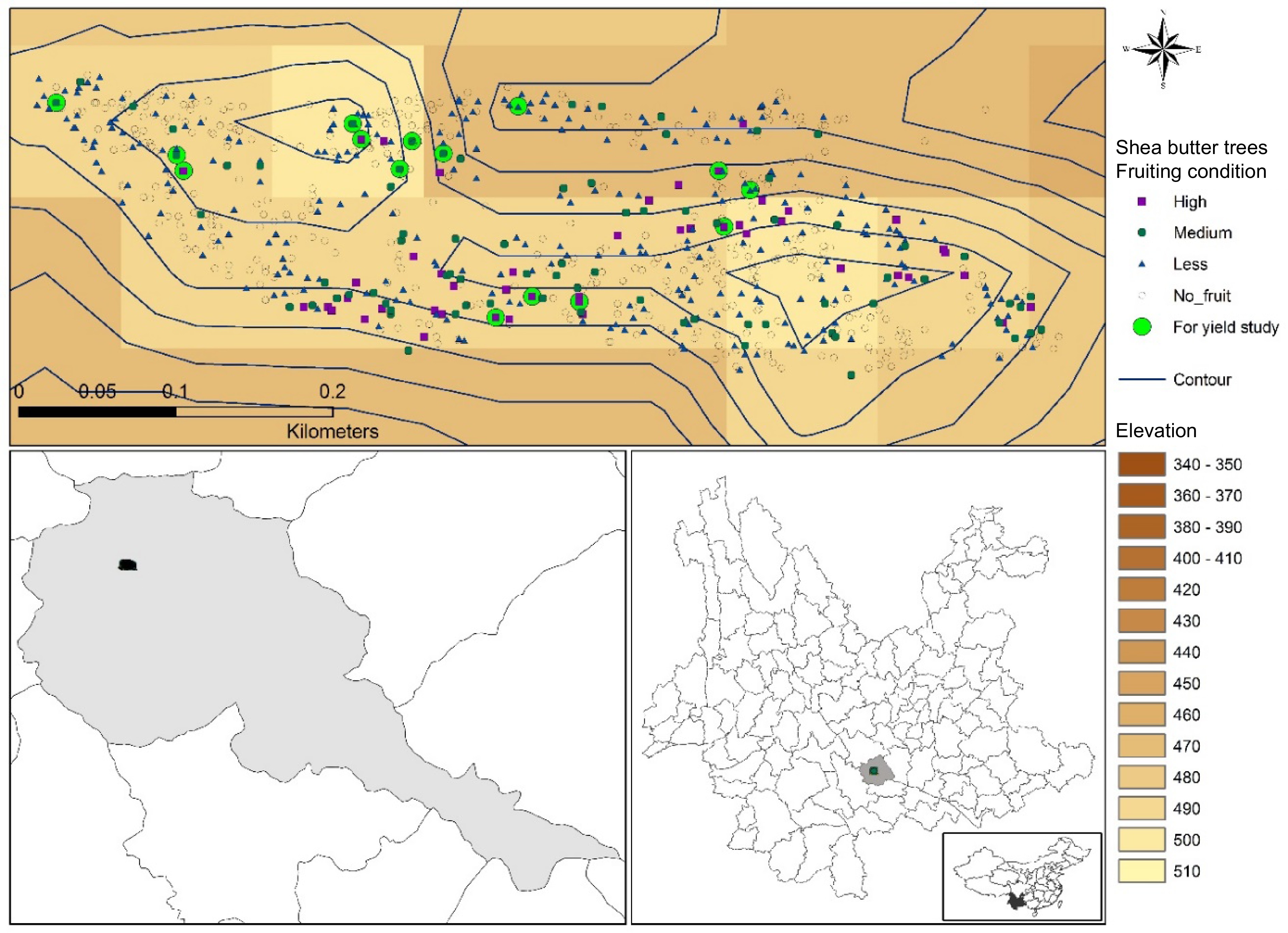
Figure 1.
Plantation area of shea butter in Yunnan, China. Shea butter distribution in the plantation site were mapped along with fruiting trees and fruiting intensity.
Temperature records obtained from a meteorological station about a kilometer from the shea butter plantation site show warming trends (particularly after 2000) (Fig. 2a). Average temperature change per decade was +0.19 °C from 1973 to 2018, while after 2000 change per decade was +0.58 °C. The mean temperature at the area was 24.36 ± 0.52 °C from 1973 to 2018, while the minimum temperature was 19.09 ± 0.41 °C and the maximum temperature 30.71 ± 0.7 °C. Average annual precipitation was 851.07 ± 192.99 mm, with precipitation levels peaking from 1991 to 1993 (Fig. 2b). After the year 2000, annual precipitation variation was about 200 mm, with no discernible pattern of increase or decrease.

Figure 2.
(a) Temperature and (b) precipitation trend from weather data between 1973 to 2018 recorded at the Yuanjiang, China meteorological station.
Experimental design and field data collection
-
Shea butter trees were planted across approximately 8.86 hectare of land. We were informed that originally the trees were spaced 6 m apart when Chinese scientists first introduced the shea trees to Yuanjiang, China. We collected data of all trees on the site and the geo-location of each tree was positioned and mapped precisely (Fig. 1). We measured the DBH, basal diameter, height and crown diameter of the trees. Among all measured trees, some showed different growth characteristics, such as multiple trunks. Such trees referred to as outliers in the Grubbs test (p < 0.01) were removed. Each tree was carefully observed for fruiting condition and recorded. We assigned 'high' for a tree with a higher amount of fruit, 'less' for fewer fruit, and 'medium' for in-between, with 'no fruit' used for the trees that did not fruit in the survey year. Because alternating between high and low fruit yields (or no fruit) in consecutive years is common in large fruiting perennial trees, we accordingly supplemented our observations on fruiting by surveying fruiting conditions of 2018 and 2019 and conducting informal interviews with farmers. Interviewed farmers perform agricultural labor at the Yuanjiang Station year-round. They conduct annual weeding and possess a systematic understanding of the growth habits of shea tree; some of them provided key information regarding the recent growth conditions of the trees and assisted us in fruit yield surveys. We calculated Growing Degree Days (GDDs) using temperature datasets from the same time period. Our best threshold range was between 10 and 25 °C, where for computing the GDDs, we used 18 °C as the optimal base temperature. For each year of observation, the total amount of heat accumulation after bud formation till fruit maturity was estimated. Heat accumulation and precipitation data were plotted against fruit yield data to determine the heat accumulation and precipitation required for fruit growth in the plantation site shea tree[20]. The Kruskal-Wallis test was conducted to check if differences between dendrometirc characteristics of recorded trees yielded a different amount of fruit.
We selected five sites within the plantation area across east-, north-, west- and south-facing slopes as well as the hilltop. Three fruit-containing shea trees at each of the selected sites were randomly marked for recording. Next, three trees were randomly selected from each sample site to collect all the fruit on each tree and measure fruit yield per tree. Then, the fruits of different trees were weighed on scales separately. We selected five different sample sites to evenly distribute study points facing all ordinal directions with the consideration that the different sunlight times could affect fruit yield.
Statistical analyses
-
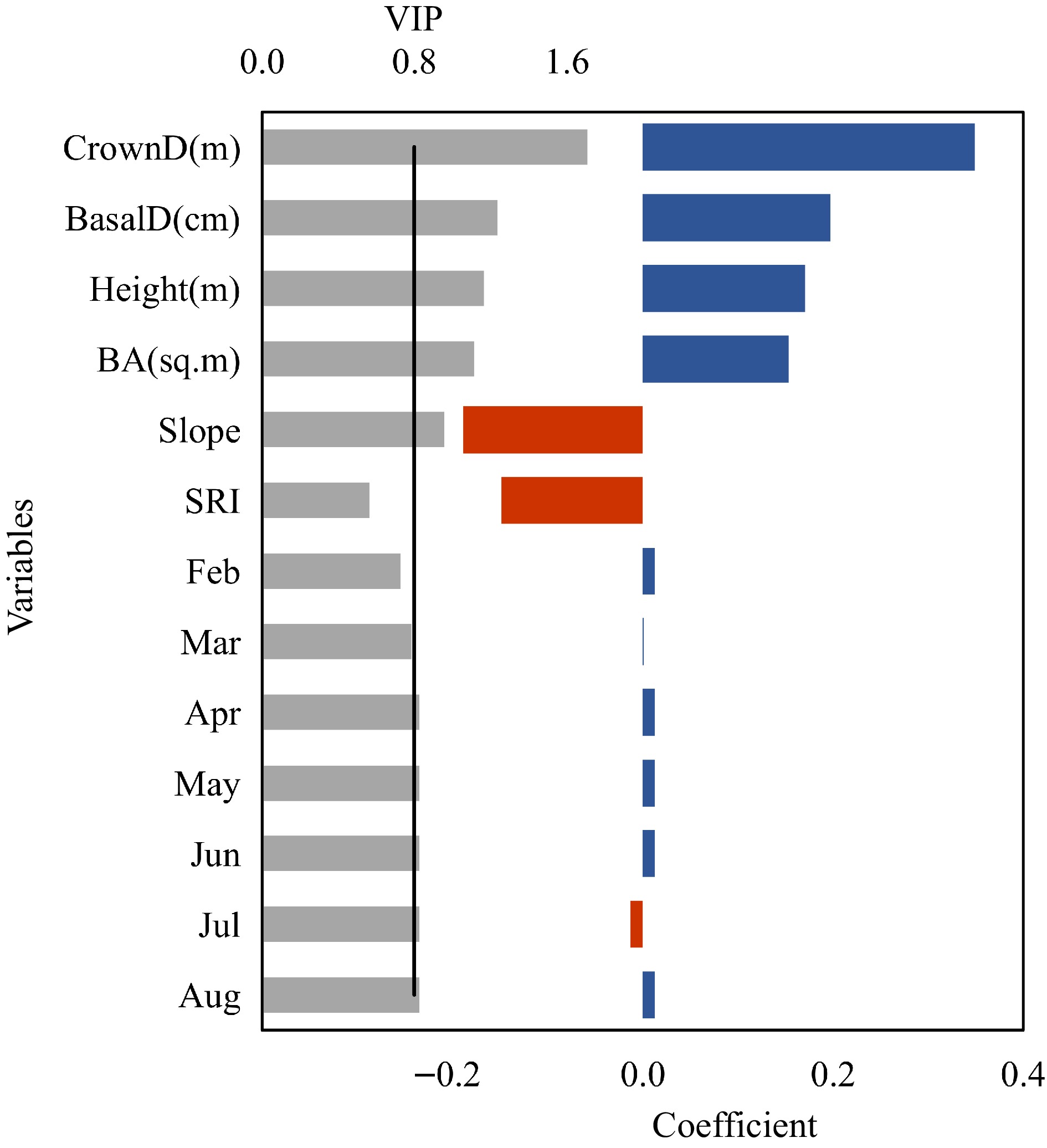
The relationship between fruit yield to dendrometric and geographical factors was evaluated using partial least square (PLS)[21,22]. Fruit yield from 15 trees were used as response variables (Y), while tree DBH, basal diameter, height, crown diameter, solar radiation index (SRI), slope, and monthly temperature during active growth period of shea tree during observation years were used as predictors (X). Monthly temperature for each site was calculated using lapse rate method. The temperature lapse in the studied mountain is approximately −0.6 °C for each 100 m elevation gain. The SRI was calculated as absolute radiation index (ARI) is related to the latitude of the surface and thus appropriate to compare the solar radiation received by areas located at sites of the same latitude. In this paper, fruit yield parameters refer to the fresh weight of fruit per tree weighed in the field. The number of significant PLS factors to build the model was determined by a cross-validation method in which 25% of randomly selected data were used to validate the calibration model used by 75% of the data set. The Q2 cumulated index measures the global goodness of fit and predictive quality of the models. Ideally, the cumulated index should be close to 1, while a lower value suggests that the yield varies significantly depending on the selected predictor variables. Calculated R2Y and R2X correspond to correlations between the explanatory (X) and dependent (Y) variables with the components generated by PLS regression. A parameter called Variable Importance in the Projection (VIP) was assessed to determine the importance of different variables in the model both with respect to dependent and other independent variables[23]. VIP scores are based on a weighted sum of squares of the PLS loading and calculated for each variable taking into account the amount of explained Y variance. The major advantage of this is that there will be only one VIP-vector, summarizing all factors and Y-variables, thereby enabling us to identify predictor variables influencing the prediction models. Independent variables with VIP ≥ 0.8 have been suggested to discriminate between relevant and irrelevant predictors for explaining fruit yield[24−26]. Standardized model coefficients were used to interpret the results of the regression[26].
PLS regression first determines latent factors, a variant of principal components, to reduce the dimensionality of the independent (and dependent) variables[24]. Partial least square regression is thus beneficial for highly auto-correlated variables, such as closely related data collected from the field. It then uses these factors as independent factors in a linear model to explain variation in the dependent variables[27]. Additionally, PLS models confer several advantages, such as their capability to handle collinearity, detect outliers, analyze multiple responses and easily interpret visual data[28,29]. As some predictor variables in our study are derived from primary measurements, it is legitimate to expect collinearity problems in the data, which leads to poor prediction performance.
-
We found that the mean annual accumulated temperature during 1973 to 2018 was 8,853.1 ± 201.1 °C when daily mean temperatures above or equal to 10 °C were combined. Heat accumulation increased in recent years compared to earlier decades, indicating very likely changes in the phenology. Similarly, number of rainy days recorded ranged from 81 to 170 d for the same period. Number of days receiving rainfall was found to be increasing in recent years compared to earlier decades. During field work, we observed well established shea trees at the Yuanjiang Research Station and collected dendrometric characteristics of shea trees and are presented in Table 1 (n = 794).
Table 1. Tree and site characteristics in the study site.
Statistic Height (m) DBH (cm) Basal
diameter (cm)Crown
diameter (m)Altitude (m) Minimum 5.2 17.2 17.5 2.4 451 Maximum 14.5 40.3 52.5 10.6 509 Mean 9.3 28.2 32.4 6.1 487 Standard deviation (n-1) 1.7 4.9 5.9 1.1 10.1 Number of trees (n) = 794. Of the 794 trees measured in 2018, 395 trees were not bearing fruit. The remaining 399 trees bore fruit, and we categorized these trees based on the amount of the fruit produced. In the 'less' category, we recorded 273 trees that had few fruits. In the 'medium' and 'high' categories, we recorded 79 trees that bore a significant amount of fruit and 47 trees that bore a higher number of fruits, respectively. In 2019, 12 trees were felled and out of the remaining 782 trees, 365 were not bearing fruit. One hundred and seventy three trees bore 'less' fruit, while in 'medium' and 'high' category 142 and 102 trees were recorded (Table 2). We recorded phenological events in the shea tree, which showed that flower bud appeared during early April, fruit appeared during late May and matured by September (Fig. 3). Leaf phenology began in February and the whole of this period was referred to as the active growth period of shea tree in Yuanjiang Research Station. Fruit transverse diameter measured 29.3 ± 0.9 mm, while longitudinal diameter was 33.8 ± 1.06 mm (n = 20). We selected 15 trees randomly to study fruit yield, and the fruit yield ranged from 6.0 to 76.8 kg per tree (average 33.95 ± 21.31 kg) in 2018 and from 16.25 to 86.0 kg per tree (average 42.3 ± 22.61 kg) in 2019.
Table 2. The number of fruiting trees at different levels in 2018 and 2019.
Year 'no fruit' 'less' 'medium' 'high' Total 2018 395 273 79 47 794 2019 365 173 142 102 782 In 2019, 12 trees were felled. 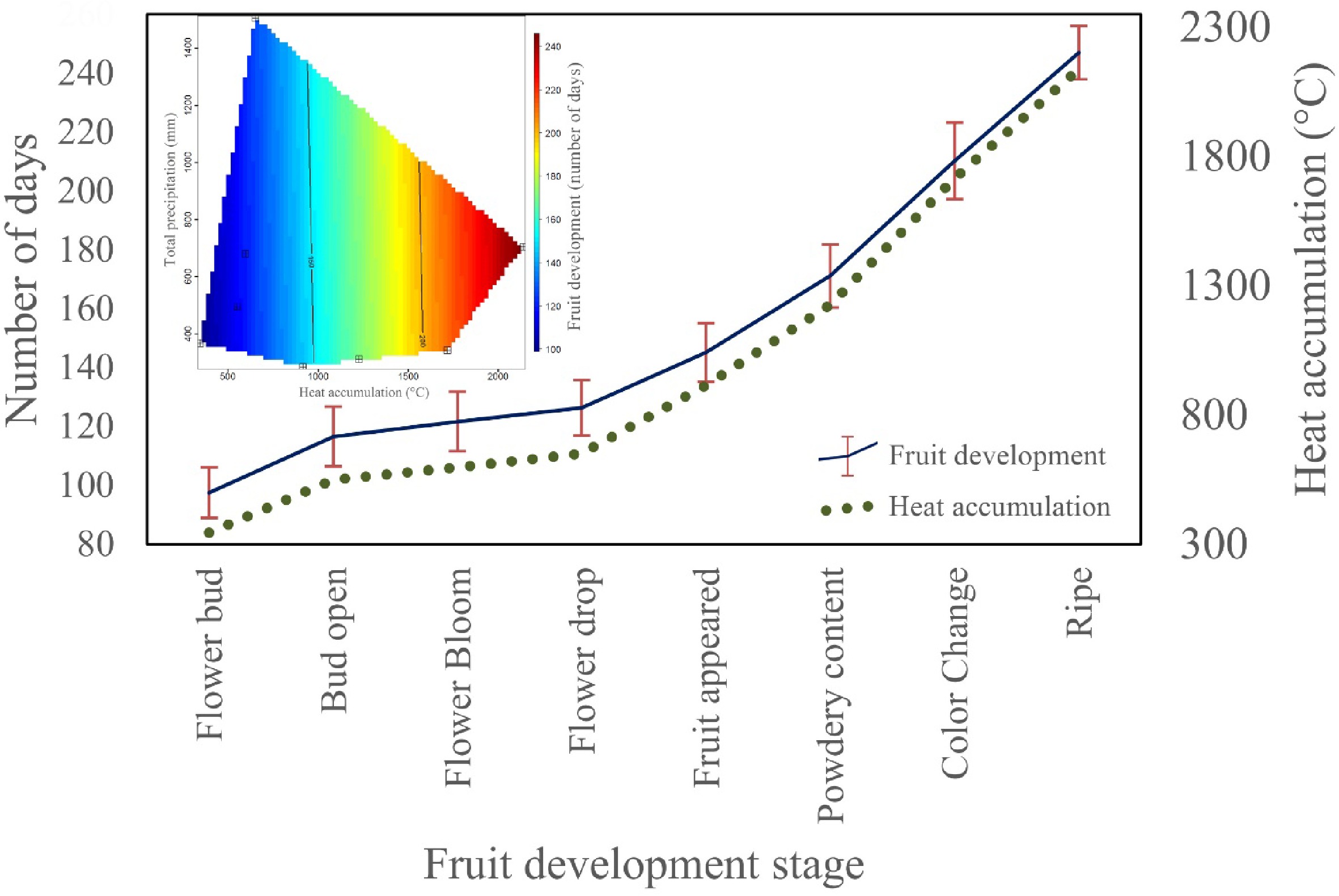
Figure 3.
The response of phenological stage to heat accumulation and precipitation. Variation in color reflects change in the phenological stages from flower to fruit.
Fruit phenology of shea tree at Yuanjiang Research Station
-
We recorded the phenology stage of shea trees, sampled from the whole plantation area. Each phenology stage was plotted against the median value of number of days required to reached that particular stage (Fig. 3). Standard deviation for each stage was calculated and plotted. Heat accumulation and total precipitation during different phenological stages was plotted to understand their relationship. Phenology development stages closely follow heat accumulation, indicating about 350 °C was necessary for bud formation, about 900 °C for fruit formation, about 1,700 °C for color change in the fruit (beginning of biological maturation of the fruit) and above 2,000 °C for fruit maturation (Fig. 3). Similarly, total accumulated precipitation of about 600 mm was good for fruit maturation when heat accumulation reached about 2,000 °C.
Fruiting in relation to dendometric character
-
The Kruskal-Wallis test revealed that apart from crown diameter, there was no significant difference in dendrometric characteristics between different categories of fruiting conditions as well as trees with 'no-fruit' (Table 3). Results indicated that differences in the fruiting condition can be attributed to crown diameter, while remaining tree measurements were not significantly related to the fruiting condition.
Table 3. Summary of Kruskal-Wallis test showing dendrometric characters for various fruiting conditions.
Variable p-value (2018) p-value (2019) BA (m2) 0.38 0.09 Basal diameter (cm) 0.11 0.12 Crown diameter (m) 0.04* 0.02* DBH (cm) 0.38 0.09 Height (m) 0.23 0.49 * Significant at p < 0.05. A correlation analysis between all the dendrometirc variables revealed that tree height was correlated with DBH, basal area and basal diameter. Similarly, DBH was highly correlated with basal diameter and basal area. All correlations were significant at p < 0.05. Remaining variables were not correlated with each other (Table 4). A multicollinearity test among the explanatory variables indicated that DBH had highest VIF value (Table 4) and could affect the results of the regression. Although PLS regression deals with collinearity issues, we removed DBH from the PLS regression. However, monthly temperatures were retained in the PLS analysis although high correlation among monthly temperatures were well established.
Table 4. Correlation matrix of dendrometric and geographical variables (explanatory and response) used in PLS regression and results of multi-collinearity test among explanatory variables.
Variables BA Basal D Crown D DBH Height Slope SRI Yield BA 0.85 0.49 0.93 0.77 −0.44 0.18 0.46 Basal D 0.26 0.92 0.84 −0.17 0.21 0.55 Crown D 0.31 0.23 −0.43 −0.42 0.50 DBH 0.76 −0.35 0.29 0.50 Height −0.26 0.24 0.42 Slope −0.18 −0.14 SRI −0.27 VIF 12.77 11.64 2.80 17.57 4.05 1.91 1.85 VIF 6.51 6.33 2.59 3.60 1.78 1.82 Variables in bold represent the response variable while all others were explanatory variables. Variable highly correlated (> 0.65) are italicized. BA, basal area; D, diameter; DBH, Diameter at breast height; SRI, solar radiation index. VIF stands for variance inflation factor that quantifies the severity of multicollinearity. Fruit yield prediction models
-
We generated PLS models using 14 dendrometric, geographical and fruit yield variables. Out of 30 replicated models, we selected nine models based on the regression coefficient (R2) value (Fig. 4). However, we selected best model that showed least RMSE and higher R2. The model described 74% of the variation in the predictor block (R2X) that in turn explained 67% of the variations in fruit yield parameters (R2Y). The predictive quality of the models according to cross-validation was substantially high as indicated by the Q2 value (52%). The median value of Q2 suggested that the fruit yield varied, depending on several of the selected predictor variables. The goodness of model fit statistics show R2 = 0.67 and RMSE = 11.58.
PLS analysis revealed that most of the variables used in the model were important variables contributing to the model and explaining fruit yield. Among these important variables, crown diameter was the most influential variable in explaining fruit yield. The standard coefficient of the model revealed that the larger the crown diameter, the higher the fruit yield per tree (Fig. 4). Similarly, basal diameter, basal area and tree height also positively affected the fruit yield.
Regarding geographic variables, slope was a major influential factor in the model, though it showed a strong negative influence. The model revealed that the monthly average temperature during the active growth period of shea tree was important for fruit yield. February and March temperatures were not important as revealed by a VIP value less than 0.8, while average temperatures of other months were important. All temperature variables except that of July positively affect the fruit yield (Fig. 4). However, the magnitude of the contribution of temperature variable were not high compared to dendrometirc variables.
When considering all nine selected models, six models revealed crown diameter as the most influential variable (Fig. 4). However, basal diameter was important in all nine models. However, basal diameter influence the models least compared to crown diameter. Across all the model dendrometirc variables shows consistency in the influence direction (positive or negative), while other variables show difference across these models. Hence, dendrometric variables were more reliable.
-
To our knowledge, we presented a healthy growth and well established small population of shea trees outside of Africa. It is not clear whether the individuals introduced to Yuanjiang originated from a single mother tree or from a heterogeneous population in Ghana. However, they have grown well, and now 54 years later, the plantation produces a significant amount of fruit. We documented 844 and 832 strong and healthy shea trees in 2018 and 2019 respectively. According to a respondent at the Yuanjiang Research Station, the total number of shea trees planted in 1964 exceeded 1,800. Tree counts in 1981 and 2010 documented 1,702 and 1,074 trees, respectively[10], and by 2019, only 832 trees remain, with their total area reduced from 12 ha to 8 ha. Though trees have grown, produced viable seeds that drop and regenerate in the area, it is not allowed to expand[30]. Caretakers and farmers tell us that disease and wind damage are responsible for the death of some trees.
In addition to their fruit production, these V. paradoxa trees have important historical and research values. Most of the shea trees planted in 1964 reached fruiting stage after 8 years, and the fresh weight of fruits per tree is comparable to V. paradoxa growing in Burkina Faso, West Africa[16]. Fruit yields at the Yuanjiang Research Station are comparable to those of African countries; however, fruit size , is relatively large compared to those reported from Ghana[31,32]. Although the current plantation site is comparatively smaller, dendrometric characteristics varied across different elevations, aspects and slopes; such variation is typical in hilly terrain and attributable to micro-climatic variations and soil conditions[16,27,33]. Buba et al.[34] reported such variation in dentrometric characteristics from different parts of Africa, including within the same as well as different populations and in different land uses. Several studies mentioned the relationships between dendrometric characteristics and fruit production in shea tree as well as other trees[16,35,36]. Studies have shown that tree diameter at breast height, tree height, basal diameter, basal area and crown diameter are the most commonly used measurements[33,37]. However, in our study, no differences were detected for all dendrometric variables except for crown diameter in fruiting condition. These tree characteristics explain the growth and phenology of V. paradoxa at the Yuanjiang Research Station.
Several studies in Africa focus on the fruit yield of shea butter trees while examining tree growth characteristics. These studies have shown either weaker relationships between dendrometric characteristics and fruit yield, or stronger relationships, particularly with tree height, DBH and crown area/diameter[16,35,38]. Our findings were consistent with African research regarding the influence of dendrometric characteristics, such as crown diameter, basal diameter and basal area, on fruit yield[16,17,35,39]. In addition, we analyzed geographical factors, as Yunnan Province is a mountainous region, and factors such as slope and temperature difference with elevation might play important roles in tree growth and fruiting[40,41]. These geographical factors contributed to the model; however, strong correlation could not be found. Temperature across the active growing period shows a weak influence in the model. Fruiting from only two seasons and temperature estimation that is used in the model might not fully explain the influence of temperature on the fruit yield. Our model indicates that the higher the temperature during the early growth period, fruit yield would be higher and high temperature during fruit development and maturation might cause less fruit yield. However, in our model influence of temperature is not very strong, therefore, further detailed study on phenology of shea tree and climatic influence is required to develop climate based fruit models. In our model, slope is influential, this might be attributed to moisture and wind distribution because soil water mostly drains downward in slopes that could minimize water availability affecting fruit yield[31,42]. Wind intensity is comparatively stronger at higher elevations potentially causing flower and fruit drop before harvest, and pre-harvest winds are reported to reduce the fruit yield of individual V. paradoxa trees by half or even more in Africa[38,43]. Finally, other unknown factors may exist that influence shea fruit yield beyond ones considered in this study[43].
As we only had access to two-years worth of data, more longer-term data would be useful to quantify the inter-annual variability in V. paradoxa fruit yields. Variations in fruit yield have been noted by the caretaker of the plantation at the Yuanjiang Research Station. Inter-annual variability has been commonly reported for V. paradoxa and may be attributable to weather variability in recent years[1,38,44]. We also noticed approximately 50% of the trees in the plantation not bearing fruit in the survey years. The caretaker also mentioned that some trees bear fruit in alternating years, which could be due to different ecological gradients than in the natural range of shea tree. It is thus critical to understand ecological, physiological or other factors that may be responsible for bearing fruit in alternating years[43]. Hence, research regarding detailed phenology of shea trees in Yuanjiang Research Station will provide a theoretical basis and scientific reference for shea management and possibly pave the way for their introduction and planting as a species useful in retarding desertification in tropical dry valley areas. Understanding these dynamics will be key for effective management and developing commercial-level extraction of the product from shea trees in China.
In Yuanjiang, shea trees are planted at only one site, precluding the opportunity to compare growth characteristics and fruit yields across different climatic conditions. Our data could not support or explore the causes of such phenomena, as the climatic conditions and elevation of the plantation area is within the comfort zone of shea trees. Studies carried out in the natural range of shea trees have reported that temperature has less influence compared to the pattern of rainfall in the fruiting of this tree, and results from a past study showed that shea productivity in Cote d'Ivoire increased with mean monthly rainfall[43,45,46]; however, heavy rainfall cause flowers to drop, decreasing total fruit yield[47]. Allal et al.[48] reported that shea tree distribution is likely to expand during the warming period in Africa, which is linked to effects related to current climate change. Even the small naturalized population in Yunnan could expand its niche in the context of climate change. Yuanjiang in its adjoining areas in Honghe Prefecture will experience a novel climate by 2050[49], potentially seeing increased dry and warm conditions. Shea trees could be a valuable economic tree in such a harsh climate and it would be an important tree for the management of dry-lands. Moreover, recent literature recommends selecting species based on economic and ecological value to enhance restoration efforts[50,51]. Shea trees could thus fill an important gap in land management planning. Promoting alien tree species for restoration might be debatable, but researchers such as Davis et al.[51] argued considering economic and ecological value rather than the origin of the selected tree for land management. Finally, no harm has been noted yet with regards to the shea tree plantation at the Yuanjiang Research Station, and its economic value is self-evident.
Fostering a small population of shea trees, classifying it as a dry-land management species, and exploiting its economic potential are all appropriate goals, particularly on the north and northeast slopes of low altitudes in dry-hot valleys. The research results from this study can be used to produce management guidelines for shea butter plantations. Including those enabling shea trees to have dendrometric traits (such as crown diameter) that are positively connected with high fruit yields. Although the identity of the mother trees or tree populations at Yuanjiang is unknown, high-yielding healthy trees can be selected for the collection of seed materials for further plantation and management. Furthermore, future research can continue based on this theoretical foundation and scientific reference for the introduction, planting, management of germplasm resources and construction of agroforestry ecosystems in shea trees to bolster ecological and economic benefits in dry tropical areas.
-
The authors confirm contribution to the paper as follows: study conception and design: Zhao G , Wu L , and Ranjitkar S; data collection: Li T, Wang X, Qin H, Ayemele AG, and Han X; data analysis: Ranjitkar S, Cunningham AB, and Zhang S; draft manuscript preparation: Zhao G, and Ranjitkar S. All authors reviewed the results and approved the final version of the manuscript.
This research is supported by the Yunnan Provincial Science and Technology Department (202003AD150004), The Yunnan Provincial Key Laboratory Project (E03L081261), Postdoctoral Research Program in Yunnan Province and the CGIAR research program on climate change (FTA-FP5). We would like to acknowledge support from the farmers at Yuanjiang Research Station for their participation and cooperation in the study. We thank Prof. Sheng-ji Pei for providing planting history information and initial research advice. Dr. Peter Lovett is also thanked for his advice.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhao G, Ranjitkar S, Ayemele AG, Li T, Wang X, et al. 2023. From Ghana to the dry-hot valleys of China: assessing factors influencing fruit yield in agroforestry species Vitellaria paradoxa after 54 years of cultivation outside Africa. Circular Agricultural Systems 3:7 doi: 10.48130/CAS-2023-0007
From Ghana to the dry-hot valleys of China: assessing factors influencing fruit yield in agroforestry species Vitellaria paradoxa after 54 years of cultivation outside Africa
- Received: 05 January 2023
- Accepted: 12 May 2023
- Published online: 28 November 2023
Abstract: Although distributed across the Sudano-Sahelian region as an agroforestry system tree species, Vitellaria paradoxa has yet to be reported as successfully established outside of Africa, significantly limiting its yield and further exploitation. In this paper, in order to assess a well-established population of V. paradoxa in the Yuanjiang dry-hot valley of China and examine the relationships between morphological-geological factors and fruit yield, we monitored dendrometric traits and fruiting across 844 shea trees located on different aspects, and applied partial least square regression to build a yield model based on dendrometric and geographical variables. Results revealed climatic resemblance of the introduction site in Yuanjiang to the natural habitat in Ghana, and the growth performance and fruit yield were also comparable, but accumulated precipitation of about 600 mm was better for fruit yield when heat accumulation reached about 2,000 °C. Apart from crown diameter (p < 0.05), dendrometric parameters (basal diameter, basal area and tree height) had positively weak relationships with fruit yield. On the contrary, aside from north and northeast aspect, other aspects showed a strong negative influence. The findings presented that growth and productivity of V. paradoxa increased with dendrometric parameters and monthly average temperature on shady and semi-shady slope, providing a theoretical basis for the development of shea tree and construction of agroforestry system in dry tropical areas outside Africa.