-
Coconut (Cocos Nucifera L.) is one of the most symbolic species in tropical regions, holding significant economic and cultural importance[1]. Due to its versatile applications, it is often referred to as the 'tree of life'. Undoubtedly, one of the key factors that make coconuts so alluring is coconut water[2]. The coconut tree is a symbol representing life and fertility, as it thrives in harsh conditions and continues to yield[3]. As one of the most vital products of the coconut tree, coconut water is regarded as the source of life and a sacred gift. Coconut water, derived from the liquid endosperm of the coconut fruit, it emerges when the fruit is approximately five months old, and it reaches its peak for consumption at around 18 months[4]. At this stage, the coconut water volume is at its maximum, and its sweetness is notably elevated. As the coconut fruit matures, the sensory characteristics of the coconut water undergo changes, its volume reduces gradually, and transforms into solid endosperm (coconut meat)[5]. During coconut germination, the array of nutrients present in coconut water and meat provides the required energy, nutrition, and moisture for sprouting coconuts, while also maintaining a stable internal environment to ensure favorable conditions for coconut survival[6]. When the fruit reaches full maturity at around 12 months, the coconut water acquires a mild carbonated beverage-like flavor[7]. Coconut water also serves as a rich source of antioxidants, electrolytes, and nutrients, comprising a variety of vital nutritional elements, such as vitamins, minerals (potassium, phosphorus, magnesium, calcium), amino acids, glucose, fructose, among others[8]. Consequently, coconut water not only fulfills the roles of hydration and quenching thirst but also contributes to the maintenance of electrolyte balance, reduction of high blood pressure and cardiovascular diseases, defense against free radical damage, and acceleration of food digestion[9]. Current reviews on coconut water have predominantly focused on its applications as a beverage and food additive. Coconut water is indispensable in these areas but its application in other fields is often neglected, as coconut water also finds extensive applications in biotechnology and the health industry. In this review, our main focus has been on the applications of coconut water in the medical and biotechnology fields. This review can provide a comprehensive overview of the existing research, including the potential applications of coconut water in biotechnology and healthcare. This review paper will also highlight gaps in existing research where further investigation is needed. This consolidated information can serve as a valuable resource for coconut industry stakeholders.
-
As a significant beverage and cultural symbol in the tropical world, coconut water holds a great importance in many countries. Coconut water provides refreshment and nutrition to humans in a pure, additive-free manner. It earned global popularity and is known as a natural health drink[10]. Its lower calorie and sugar content have gradually positioned it as an alternative to sugary beverages and other traditional drinks[11]. Coconut water contains various electrolytes such as potassium, sodium, calcium, and magnesium, which assist the body in reestablishing hydration and electrolyte balance[12]. Therefore, whether facing scorching heat or intense workouts, coconut water's rapid electrolyte and hydration replenishment combats dehydration and fatigue[4].
The importance of coconut water extends beyond its function as a beverage. Coconut water finds its role as a food addictive in culinary endeavors, cocktails, and desserts[13]. Its fresh taste and subtle sweetness enhance the flavors of food and beverages. People utilize coconut water to craft several types of juices, alcoholic concoctions, pastries, imparting a distinctive coconut essence[14], with the Hainanese 'Qing Bu Liang' standing out as an exceptional example[15].
Furthermore, coconut water bears significant cultural symbolism. In certain regions' traditional cultures, coconut water frequently assumes a prominent role in celebrations, rituals, blessings, and festive occasions[16]. Whether in tropical regions or elsewhere, coconut water has become an integral part of people's pursuit of a healthy lifestyle. With the increasing emphasis on health and natural foods, coconut water continues to attract more attention and appreciation. As coconut water gains popularity in the global market, various processing facilities have employed multiple methods to extract and package coconut water, allowing for long-distance transportation to satisfy people's cravings worldwide[17].
-
Coconut water is comprised of sugars, minerals, vitamins, amino acids, enzymes, volatile aromatic compounds, and various biochemical compounds. The makeup of fresh coconut water is influenced by several factors such as the cultivation region, affecting soil, environmental conditions, and fertilizer usage, as well as the coconut variety, cultivar, and the harvesting stage's maturity[18]. Various analytical techniques have been employed to study the components of coconut water for different purposes, such as examining quality changes during diverse sterilization processes. These techniques include nuclear magnetic resonance (NMR), gas chromatography (GC), and High-Performance Liquid Chromatography (HPLC) coupled with mass spectrometry (MS)[19]. The biochemical structure of coconut water is affected by the stage of maturity and hence the variety[20]. Necessary contents in coconut water which are beneficial for human beings are listed in Table 1.
Table 1. Nutritional components in coconut water.
Nutrient Benefits Reference Electrolytes (potassium, sodium, magnesium, and calcium) Help regulate fluid balance and muscle function [21] Vitamins (C, B1, B2, B3, B5, B6, B9, and B12) Essential for overall health and well-being [22] Minerals (iron, manganese, copper, and zinc) Play important roles in metabolism, immunity, and other bodily functions [10] Amino acids Building blocks of proteins [7] Enzymes Help with digestion and other metabolic processes [23] Antioxidants Protect cells from damage [4] Tender Coconut Water (TCW) is called the 'fluid of life'. It is a highly nutritious beverage derived from palm trees, serving as a natural isotonic drink with similar osmotic pressures closely resembling our body's blood plasma[4]. The rich array of macro and micro nutrients in tender coconut water has the potential to reduce lipid levels and provide protective benefits to both the heart and liver[24]. It has been used worldwide to treat oral rehydration, childhood illnesses, intestinal flu, and cholera[8]. Coconut has been used in traditional Indian medicine for thousands of years to treat a variety of ailments, such as gonorrhea, bronchitis, fever and gingivitis[25]. Ayurvedic medicine (antient India) describes coconut as medicine that increases semen, promotes digestion, and clears the urinary path[26]. In Sri Lanka, where coconut is a staple food, there are many references to its medicinal uses[27]. Coconut water is a better choice than plain water or fruit drinks at relieving symptoms of diseases that cause dehydration[10]. It has a great impact to the health of human race and animals; the beginning of these research was started in 1942. This early research indicated that the biological value of coconut water was determined by proteins, amino acids. Also, the medical application of coconut water, for example, the feasibility of parenteral injection of coconut water and the applications in pediatrics (feeding infants)[20]. Coconut water has been widely used over the past few decades to treat infectious diseases, dysentery, influenza, and other diseases that cause dehydration[10], as listed in Table 2. Most of the experiments were conducted using rats but those positive results clearly indicated the future perspective. Recently, in-vivo and in-vitro research showed that coconut water has the potential to lower blood sugar levels, similar to standard oral diabetes medications. Coconut water could have a significant impact on the treatment of diabetes[28]. Nursing mothers who regularly drink coconut water can help baby's uptake calcium. Coconut water also contains vitamin-C, vitamins-B and B9[10]. The amount of vitamin-C in coconut water decreases as the coconut matures. Vitamin-B9 is important for preventing anemia in pregnancy and mitochondrial toxicity caused by methanol metabolites[29]. Furthermore, coconut water has antioxidant properties, and a mixture of coconut water and mauby bark syrup from Trinidad and Tobago has been shown to reduce hypertension in humans[30]. Coconut water was used as the sperm extender of equine sperm[31] and rabbit sperm[32], which ensures a high pregnancy percentage, and large, healthy litters. Coconut antimicrobial peptide-1, abbreviated as CnAMP1, is classified as an antimicrobial peptide, which is naturally present in green coconut[24]. Antimicrobial peptides are compact proteins that constitute a component of the innate immune system found in various organisms, including plants. Their primary function involves protecting the organism against microbial infections[33]. Interestingly, scientists from Indonesia found that isolated probiotics from coconut water, which could potentially contribute to the recovery of Covid-19 patients. This study suggests that coconut water may have a role in assisting Covid patients[34].
Table 2. Clinical applications of coconut water.
Objects Induction method/dose Result Reference Male Sprague-Dawley rats Mature coconut water; 1,000 mg/kg 53.14% reduction of blood glucose level [35] Male albino rats (Sprague-Dawley) Tender coconut water (TCW),
4 mL/100 g/daySignificantly reduced the oxidative stress induced by isoproterenol and exerted significant antithrombotic effects. [36] Male Wistar rats TCW from West African Tall coconut,
20 ml/kg body weightCoconut water reduced the toxicity of chloramphenicol by increasing enzyme level [37] Carbon tetrachloride (CCl4)-intoxicated female rats TCW, 2−6 mL/kg TCW significantly lowered the generation of free radical. [38] Male Wistar rats Coconut endocarp (CNE), 3.33 mg CNE graetly reduced the systolic blood pressure in rats (from 185 to 145 mmHg). [39] White Wistar male rats TCW, 8 mL/200 gr BW rats/day TCW can elevate hematocrit, hemoglobin and erythrocyte levels in rats. [40] Adult male Sprague-Dawley rats Coconut water, Intraperitoneal
injection of (60 mg/kg).Coconut water can be protective against diabetic retinopathy by decreasing oxidative stress and anti-inflammatory activities in the retina. [41] Mouse breast cancer cell line (4T1) Coconut water and vinegars,
2.00 mL/kgCoconut water vinegar slower the spread of breast cancer by killing cancer cells, and boosting the immune system. [42] Male Wistar rats Coconut water, Normal rat diet +
0.75% EG + 10% Coconut waterCoconut water prevented kidney stones by stopping crystals from forming and reducing urine crystals. It also protected the kidney damage and prevented oxidative stress caused by free radicals. [43] Healthy physically active male Coconut water enriched with sodium (SCW), 3,000 ml/trial SCW did not cause nausea and stomach upset. Coconut is a great alternative to commercial sports drinks for rehydrating. [21] COVID-19 patient Lactic acid bacteria from coconut water Extracted probiotics from coconut water, which could potentially contribute to the recovery of Covid-19 patients. [34] -
Coconut water is a liquid endosperm that provides suitable nutrients and bio-stimulants for the formation, development, and later germination of coconut zygotic embryo. Due to the natural ability to regulate the growth of plant tissue, this special form of endosperm has high potential for the application in plant tissue culture as an additional supplement to promote morphogenesis and rapid proliferation of plant cells and tissues as Fig. 1 indicated.
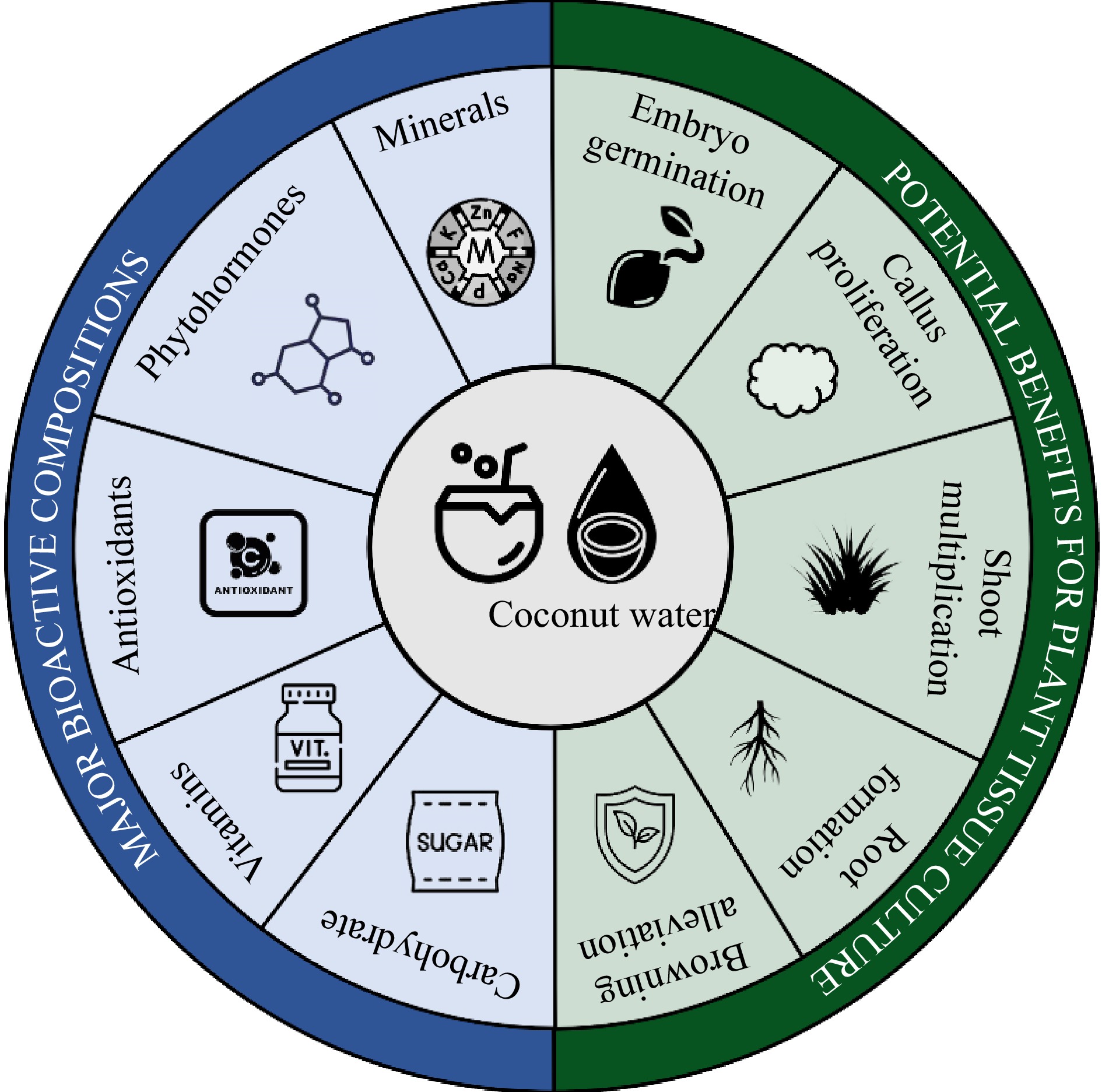
Figure 1.
Major bioactive compositions in coconut water and their potential benefits for plant tissue culture.
The application of coconut water in tissue cultures was firstly introduced by Van Overbeek et al., stating that this special additive was critical for the development of immature zygotic embryos of Daruta stramonium[44]. Since then, increasing numbers of studies have been established, adapting the supplementation of coconut liquid endosperm in plant tissue and cell culture[10,45,46]. As a stand-alone culture medium, coconut water can be used as the primary culture medium, providing a suitable environment for the growth of plant tissues. Coconut water's composition is highly varied depending on the coconut's maturity and origin, it may need to be appropriately diluted or supplemented with other components to achieve ideal results. Coconut water can be added as a supplement to other basal media like MS medium[47] or B5 medium[48]. By adding coconut water to the basal medium, researchers can enhance the growth-promoting effects and improve the regeneration capacity of certain plant tissues.
According to multiple studies to date, the stimulating activities of coconut water are mainly derived from the notable contents of natural phytohormones, mainly auxin and cytokinin[49]. Coconut's liquid endosperm has been found to contain indole-3-acetic acid (IAA), an endogenous auxin involved in a variety of plant growth and development processes[22]. IAA is biosynthesized in the meristematic regions located at the shoot apex and subsequently transported to the root tip in order to promote cell division and elongation. IAA can also drastically affect plant orientation by promoting cell division to one side of the plant in response to sunlight and gravity. In plant tissue culture, IAA and its analogues could also be applied as a stimulus for cell dedifferentiation and reprogramming, leading to the formation of embryogenic callus and later somatic embryos. The presence of this type of hormone in coconut water has led to some research into the potential use of coconut water as a stimulant for callogenesis, somatic embryogenesis, plantlet elongation and rooting. Specifically, a study by Sarathchandra et al.[50] has found that green coconut water alone was capable to induce callus formation from nodal segments of Camellia sinensis, however, the efficiency was significantly lower compared to other treatments with exogenous auxin. Another attempt on Musa paradisiaca revealed that coconut water was highly sufficient in promoting elongation and rooting of shoot tip-derived plantlets with similar rooting efficiency as 1.5 mg·L−1 α-naphthaleneacetic acid (NAA)[51]. Similar results were observed on Capsicum annuum, coconut water alone or in combination with silver nitrate exhibited significantly higher efficiency to obtained rooted plantlets compared to indole-3-acetic acid (IBA)[52]. The addition of coconut water at 10%–20% (v/v) was also found to promote callogenesis and somatic embryogenesis when combining with different exogenous auxins in various plant species, including Spicata oleracea[53], Lycopersicon esculentum[54], Phoenix dactylifera[55], Cocos nucifera[56], and Triticum aestivum[57]. As a result, the auxinic activity of coconut water has been proven to be highly beneficial in combination or replacement for exogenous auxin during micropropagation and tissue culture regeneration.
Apart from auxin, a diverse composition of different cytokinin compounds such as N6-isopentenyladenine (2-iP), kinetin, zeatin, and their derivatives, has also been identified in coconut water[58]. These naturally occurring hormones, in combination with auxin, create a hormonal balance within plant cells and tissues, which are critical to various aspects of plant development, such as cell division and differentiation, gene activation, tissue organization, embryogenesis, seed germination, and shoot branching. With these specialized activities, coconut water is highly suggested for in-vitro plant cultivation, especially during shoot regeneration and multiplication. In fact, this additive has been noted to be crucial for indirect shoot regeneration in Ariocarpus kotschoubeyanus even in very small amounts (0.05%–0.15%) with 10–14 shoots per explant compared to no shoot formation in the control treatment[59]. In another study, Prando et al. found that coconut water could improve both shoot multiplication and elongation in Corylus avellana[60]. Sembiring et al. also reported an interesting observation that coconut water stimulated not only shoot multiplication and elongation but also the formation of potato tubers in in-vitro conditions[61]. Strawberry micropropagation was also subjected using coconut water with and observed 4-fold increase in shoot multiplication rate compared to the control treatment[62]. In addition to the studies mentioned above, there have been a number of other studies that have shown the benefits of coconut water in tissue culture of various plant species, such as Actinidia deliciosa[63], Cyamopsis tetragonolobust[64], Musa acuminata[65], Ananas comosus[66], Dianthuus caryophyllus[67], and Olea europaea[68]. However, application of coconut water does not always positively influence the development of plant tissue in in-vitro conditions. A study on Elaeis guineensis showed that multiple shoot abnormalities had been recorded from zygotic embryo in culture medium containing 15% coconut water[69], whereas this additive was found to inhibit germination of zygotic embryo Xiem Green Dwarf coconut at any concentration[70]. Thus, it is required to have specific investigation to determine the influence of coconut water in tissue culture for each plant species.
Other than phytohormones, coconut water is also a great source of nutrients and bioactive compounds[10,22]. Ionic minerals, such as potassium (K+), chloride (Cl−), calcium (Ca2+), and magnesium (Mg2+) are commonly found in coconut water[8]. These ionic minerals are the macronutrients which are highly essential for plant cell metabolism. In addition, supplying this highly nutritious additive to culture media could also provide high amounts of carbohydrates, acting as an alternative source of energy and carbon for in-vitro plants to live and continue developing. In fact, a study has shown that the application of 20% coconut water was able to completely replace sucrose in shoot culture of Ficus carica with no significant change in propagation efficiency[71]. Furthermore, this unique type of endosperm could also be utilized to reduce explant browning and promote further morphogenesis during plant tissue culture due to its rich contents of antioxidants and vitamins. This consideration was supported by Inpeuy et al., as coconut water significantly alleviated oxidative stress and reduce browning rate in Elaeis guineensis zygotic embryo explants[69].
It is essential to understand that the choice of culture medium, including the use of coconut water (Table 3), depends on the specific plant species, tissue type, and research objectives. Different plants have unique requirements for growth, and their optimal culture medium may vary. Additionally, the composition of coconut water is somehow considered as undefined since it can vary based on multiple factors such as coconut's maturity, variety, geographical location, as well as the methods of sterilization and storage[10,22] leading to different outcome in its application during plant tissue culture[55,72]. Thus, standardization and careful preparation of culture medium are essential to obtain the best outcomes for tissue culture in a particular plant species or tissue type with high consistency.
Table 3. Application of coconut water in tissue culture of different plant species.
Plant species Explant Callogenesis
and embryogenesisShoot regeneration
and multiplicationNotable effect of CW Reference Daruta stramonium Zygotic embryo _ Tukey's medium + 1% dextrose + 33.3% CW - Critical for the development and germination of zygotic embryo [44] Ipomea batatas Leaf MS + 0.5–2.0 mg·L−1 2,4-D + 6% sucrose
MS + 2.0 mg·L−1 2,4-D + 2.0 mg·L−1 kinetin + 20% CW + 6% sucroseHormone-free MS - Supported callus formation and multiplication [10,45,46] Agave cantala,
A. fourcroydes,
A. sisalanaStolon MS + 0.1 mg·L−1 2,4-D + 0.1 mg·L−1 BA + 10% CW + 2% sucrose MS + 0.075 mg·L−1 NAA + 0.1 mg·L−1 IBA + 0.5 mg·L−1 kinetin + 10% CW + 2% sucrose
Hormone-free MS- Supported callus growth and shoot regeneration [10,45,46] Camellia sinensis Nodal segment Hormone-free VW + 15% CW + 2% sucrose
MS + 1 mg·L−1 2,4-D + 1 mg·L−1 kinetin + 6% sucrose- Stimulated callogenesis in hormone-free medium [10,45,46] Musa paradisiaca Shoot tip N/A MS + 0.5 mg·L−1 NAA + 5% CW - Showed positive effect on shoot multiplication in combination with NAA
- Sufficiently stimulate rooting with similar result compared to 1.5 mg·L−1 NAA
- Accelerated the emergence of the first root but reduced shoot length and number of roots at any concentrations higher than 5%[10,45,46] Capsicum annuum Seed N/A Hormone-free MS + 3% sucrose
MS + 9.0 μM TDZ + 5.77 μM GA3 + 14.7 μM PAA + 10% CW
MS + 0.45 μM TDZ + 5.77 μM GA3 + 14.7 μM PAA + 10% CW
MS + 9.8 μM IBA + 10% CW + 30 μM AgNO3- Enhanced shoot formation and elongation
- Exhibit more efficient rooting effect than IBA
- Showed synergistic effect with AgNO3 during rooting process[10,45,46] Spinacia oleracea Young leaf MS + 9.3 μM kinetin + 2.3 μM 2,4-D + 15% CW + 3% sucrose MS + 9.3 μM kinetin + 0.05 μM 2,4-D + 2.9 μM GA3 + 15% CW + 3% sucrose - Promoted callogenesis and shoot regeneration [10,45,46] Lycopersicon esculentum Hypocotyl, leaf disk MS + 5 mg·L−1 IAA + 1.5 mg·L−1 kinetin + 12% CW + 3% sucrose MS + 5 mg·L−1 IAA + 1.5 mgl kinetin + 12% CW + 3% sucrose
MS + 0.1 mg·L−1 IAA + 3% sucrose- Improved callogenesis and shoot regeneration
- Significantly enhanced plantlet survival rate during acclimatization[10,45,46] Phoenix dactylifera Shoot tip MS + 100 mg·L−1 2,4-D + 3 mg·L−1 2iP + 3% sucrose + 1.5 g·L−1 AC
MS + 10 mg·L−1 NAA + 30 mg·L−1 2iP + 10–15% CW + 1.5 g·L−1 ACHormone-free MS + 10%–15% CW
½ MS + 0.1 mg/l NAA- Both co-autoclaved and filtered CW improved callogenesis and somatic embryogenesis
- Co-autoclaved CW showed slightly higher improvement in callogenesis and somatic embryogenesis[10,45,46] Cocos nucifera Zygotic embryo Y3 + 700 μM 2,4-D + 15% CW + 5% sucrose + 0.25% AC Y3 + 300–350 μM 2,4-D + 15% CW + 5% sucrose + 0.25% AC - Enhanced callus induction and somatic embryogenesis [10,45,46] Triticum aestivum Seed MS + 3 mg·L−1 2,4-D + 20% CW + 30 g·L−1 sucrose MS + 1.5 mg·L−1 BA + 10% CW + 30 g·L−1 sucrose - Enhanced callus induction and shoot regeneration [10,45,46] Ariocarpus kotschoubeyanus Epicotyl segment MS + 2 mg·L−1 zeatin + 3% sucrose MS + 1% CW + 3% sucrose
MS + 3% sucrose + 1% PEG + 1% AC
½ MS + 1 mg·L−1 IAA + 3% sucrose + 1% PEG- Crucial for shoot regeneration from callus explant even at very small amount [10,45,46] Corylus avellana Nodal segment N/A 80% DKW + 2 mg·L−1 BA + 2% glucose + 0.5% AC
80% DKW + 2 mg·L−1 BA + 0.01 mg·L−1 IAA + 0.5 mg·L−1 GA3 + 20% CW + 3% glucose- Improved shoot proliferation and growth [10,45,46] Solanum tuberosum Nodal segment N/A MS + 22.5% CW + 30 g·L−1 sucrose - Improved in vitro shoot multiplication and tuber formation [10,45,46] Fragaria × ananassa Shoot tip N/A 1/3 MS + 0.5 mg·L−1 BA + 0.1 mg·L−1 IBA + 10% CW + 30 g·L−1 sucrose - Shoot multiplication was 4-fold higher than the control [10,45,46] Actinidia deliciosa Seed N/A Hormone-free MS
MS + 2 mg·L−1 BA + 20% CW
½ MS + 0.02 g·L−1 IBA- Stimulated the highest rate of shoot multiplication when combined with BA [10,45,46] Cyamopsis tetragonolobust Seed N/A Hormone-free MS
MS + 2 mg·L−1 2,4-D + 20% CW
½ MS + 0.02 mg·L−1 IBA- Enhanced shoot multiplication [10,45,46] Musa acuminata Shoot tip N/A MS + 5 mg·L−1 BA + 3% sucrose
½ MS + 1 mg·L−1 NAA + 10% CW + 1.5% sucrose- Improved shoot regeneration and growth [10,45,46] Ananas comosus In vitro bud N/A MS + 1 mg/l IAA + 4 mg·L−1 BA + 10% CW
MS + 1 mg·L−1 IAA + 20% CW- Efficiently stimulated bud proliferation at the concentration of 10% when combined with IAA and BA.
- Induced the greatest plantlet growth at the concentration of 20% when added to cytokinin-free medium[10,45,46] Dianthus caryophyllus Shoot tip, nodal segment N/A MS + 1 mg·L−1BA + 10% CW + 3% sucrose
½MS + 1 mg·L−1 NAA + 3% sucrose- Enhanced shoot proliferation rate and average length of shoots [10,45,46] Olea europaea Zygotic embryo N/A MS + 10% CW + 3% sucrose - Exhibited significant interactive effect between olive genotypes and CW concentration during shoot multiplication.
- Improved overall proliferation and growth of in vitro shoot at the concentration of 10%[10,45,46] Elaeis guineensis Zygotic embryo N/A Hormone-free MS
MS + 0.5 mg·L−1 BA + 0.5 mg·L−1 kinetin + 15% CW + 3% sucrose- Significantly reduced explant browning rate
- Stimulated shoot proliferation when combined with other hormones[10,45,46] Cocos nucifera Zygotic embryo N/A Y3 + 5 μM BA + 3% sucrose + 0.25% AC
Y3 + 5 μM BA + 10% CW + 2% sucrose + 0.25% AC- Reduced germination rate of zygotic embryo compared to control
- Promoted post-germination growth off seedlings[10,45,46] Ficus carica Shoot tip N/A MS + 1 mg·L−1 BA + 20% CW - Replaced sucrose as an alternative carbon and energy source [10,45,46] Solanum tuberosum Nodal segment N/A MS + 30% 6-month-old CW - Exhibited different effects among various maturities and concentration due to differences in hormone contents.
- Significantly improved in vitro shoot growth at any concentrations for 6-month-old CW.
- Only posed positive effect at the concentration of 30% for younger maturities of CW[10,45,46] MS: Murashige and Skoog basal medium[10,45,46]; Y3: Eeuwens basal medium[70]; DKW: Driver and Kuniyuki basal medium[8]; VW: Vacin and Went basal medium[71]; 2,4-D: 2,4-dichlorophenoxyacetic acid; NAA: α-naphthaleneacetic acid; IAA: indole-3-acetic acid; IBA: indole-3-butyric acid; BA: 6-benzylaminopurine; TDZ: Thidiazuron; GA3: gibberellic acid; PEG: polyethylene glycol; CW: coconut water; AC: activated charcoal. From the above text, we can easily reach the conclusion that coconut water was generally used as an addition in the plant tissue culture medium during the callus maturation and multiplication stage. Most researchers used 10% to 20% CW in the culture medium, which gives the greatest development to the secondary callus or somatic embryos. Very few researchers employed it in the initiation stage of callus since the low sugar content in CW.
Coconut water has been used as an addition in the biotechnologies but there are still limitations of concern in the future. Firstly, the nutritional content of coconut water can vary depending on the coconut variety, maturity level, and environmental conditions. This can lead to unpredictable results in tissue culture experiments. Secondly, we have very limited understanding of active components of CW during tissue culture processes. While researchers have identified some beneficial components in coconut water, the specific mechanisms behind its effects are not fully understood. This knowledge gap hinders optimization of culture media and targeted applications. As an addition in the culture medium, efficient sterilization methods are crucial but can affect its beneficial properties of CW. Furthermore, finding the optimal concentration of coconut water for each plant species and culture system is crucial. Excessive amounts can have inhibitory effects, highlighting the need for careful optimization. These questions must be answered if we would like to further discover the potential of CW in tissue culture.
-
Coconut water has diverse and promising applications in medical addictives and biotechnology. It is widely recognized as a refreshing beverage and a versatile culinary ingredient, as its potential goes far beyond the realm of food and beverage. Our exploration has revealed the significant role of this natural gift for medical and biotechnological industry. The medical applications of coconut water are multifaceted. Its natural composition, rich in electrolytes, vitamins, and minerals, makes it an excellent option for rehydration and electrolyte balance, particularly in situations of dehydration and physical stress. Its antioxidant properties offer a unique advantage in combating oxidative stress and free radicals. Regarding biotechnology, coconut water versatility becomes evident as it serves as a nutrient-rich medium for cell and tissue culture. Its unique features make it an ideal candidate for plant cell/tissue culture and genetic research. In summary, coconut water is not merely a tropical thirst quencher; it is a reservoir of potential in the realms of medical science and biotechnology. As researchers continue to uncover its applications and benefits, the horizon of possibilities expands. This review underscores the importance of recognizing and harnessing the untapped potential of this natural wonder, not only for the betterment of our well-being but also for advancing the frontiers of medicine and biotechnology.
-
The authors confirm contribution to the paper as follows: Study design and writing: Mu Z, Tran BH, Xu H; Figure and table modification: Mu Z, Tran BH, Xu H, Yang Z, Xia W, Qamar UZ, Wang X; Review and editing: Luo J, Xiao Y, Mu Z. All authors reviewed the results and approved the final version of the manuscript.
-
All data used in this review paper were derived from Hainan University institutional repository and National University of Ho Chi Minh City institutional repository. All data were freely available and accessible without restrictions. Sources included: Hainan University institutional repository, National University of Ho Chi Minh City institutional repository.
This research was sponsored by the Scientific and Technological Cooperation Projects of Hainan Province (Grant No. ZDYF2020215), Hainan Yazhou Bay Seed Lab. + (JBGS + B21HJ0903), China Postdoctoral Science Foundation (Grant No. 2023M740951), Project for Science and Technology Innovation in Sanya City (Grant No. 2022KJCX53), the Postdoctoral Research Funding Project of Hainan Province, PhD Scientific Research and Innovation Foundation of Sanya Yazhou Bay Science and Technology City(HSPHDSRF-2023-12-002)and '111'Project (No. D20024).
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Zhihua Mu, Binh-Minh Tran, Hang Xu
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Mu Z, Tran BM, Xu H, Yang Z, Qamar UZ, et al. 2024. Exploring the potential application of coconut water in healthcare and biotechnology: a review. Beverage Plant Research 4: e018 doi: 10.48130/bpr-0024-0009
Exploring the potential application of coconut water in healthcare and biotechnology: a review
- Received: 11 December 2023
- Revised: 08 January 2024
- Accepted: 26 January 2024
- Published online: 22 May 2024
Abstract: Coconut (Coconut Nucifera L.) is one of the most important palms worldwide. Coconut water, its liquid endosperm, is a nutrient-rich beverage with a wide range of potential applications as a refreshing beverage, medicine and additives in biotechnology. It is a sterile, isotonic solution containing a variety of sugars, amino acids, vitamins, minerals, and phytohormones which showed a range of biological activities, including antioxidant, antimicrobial, anti-inflammatory, and immunomodulatory effects. It also provided a wide range of secondary metabolites. Coconut water has been used successfully for the tissue culture of many plants, including field crops, ornamentals, and medicinal plants. In this review, we have discussed, the chemical composition and biological properties of coconut water, as well as its potential applications in biotechnology and medicine. It was found that coconut water has therapeutic or preventive effects against many diseases, or has applications in medicine. In addition, in biotechnology such as plant tissue culture, coconut water can be used as an additive to enhance the growth of callus tissue. This review will also highlight the challenges and opportunities for future research on coconut water.
-
Key words:
- Coconut water /
- Tissue culture /
- Biotechnology /
- Healthcare /
- Coconut













