-
Prior to the invention of refrigeration, meat curing was an ancient method of preserving foodstuffs that is still widely used today, effectively preventing the deterioration of meat after slaughter and prolonging the shelf life of food for a short period of time[1]. Nitrates or nitrites, sugar, ascorbic acid and other substances are usually added to cured meat products in addition to salt to enhance the color of cured meat. Nitrites, which give cured meat products a bright red color and prevent the production of botulinum toxin, have not yet emerged as a complete substitute for nitrites, despite their proven carcinogenicity[2]. The pigments in cured meats are dominated by the bright red nitrosomyoglobin produced by the reaction of nitric oxide from the decomposition of nitrates or nitrites with Mb[3]. Due to the carcinogenicity of nitrites, antioxidants such as ascorbic acid are added to cured meats to enhance nitrite coloration and inhibit excessive oxidation of Mb in order to increase the amount of nitrosoMb in the meat while limiting nitrite content[4].
The color of cured meat products is closely related to the quality of the raw meat. Animals with high Mb content, such as beef and lamb, are bright red, while animals with low Mb content, such as chicken and freshwater fish, are lighter in color. The content and activity of pigment-related enzymes such as high iron Mb reductase in meat products also affects the quality of meat. In recent years, there have been more studies on the effect of meat aging on meat color, and elevated temperatures during meat preparation soften the meat, and protein hydrolysis of must myogenic fibers and cytoskeletal proteins may affect the permeability of the muscle body to Mb as time increases[5]. In addition, during the curing and maturation of meat, the Melad non-enzymatic browning reaction produces brown polymers that affect the color of cured meat products, Starowicz & Zielinski showed that an increase in acrylamide concentration was associated with a darker color of the product[6]. The Maillard reaction also affects the production of a brown substance from fat and the formation of an eventual yellow fat due to factors such as lipid oxidation. Due to the huge market demand for cured meat products, packaging and storage of cured meat has become extremely important. This paper mainly reviews the above related issues affecting the color and luster of cured meat products to provide more theoretical basis for the color and luster changes and regulation in the processing and marketing of cured meat products.
Myoglobin
-
Oxidation of Mb is a major internal factor affecting the color of cured meat[7]. As a result of the bloodletting of livestock animals after the completion of all slaughtering operations, this leads to a significant loss of hemoglobin, the higher the percentage content of Mb in the muscle tissue of animals that have been fully bled, the greater the redness value[8].
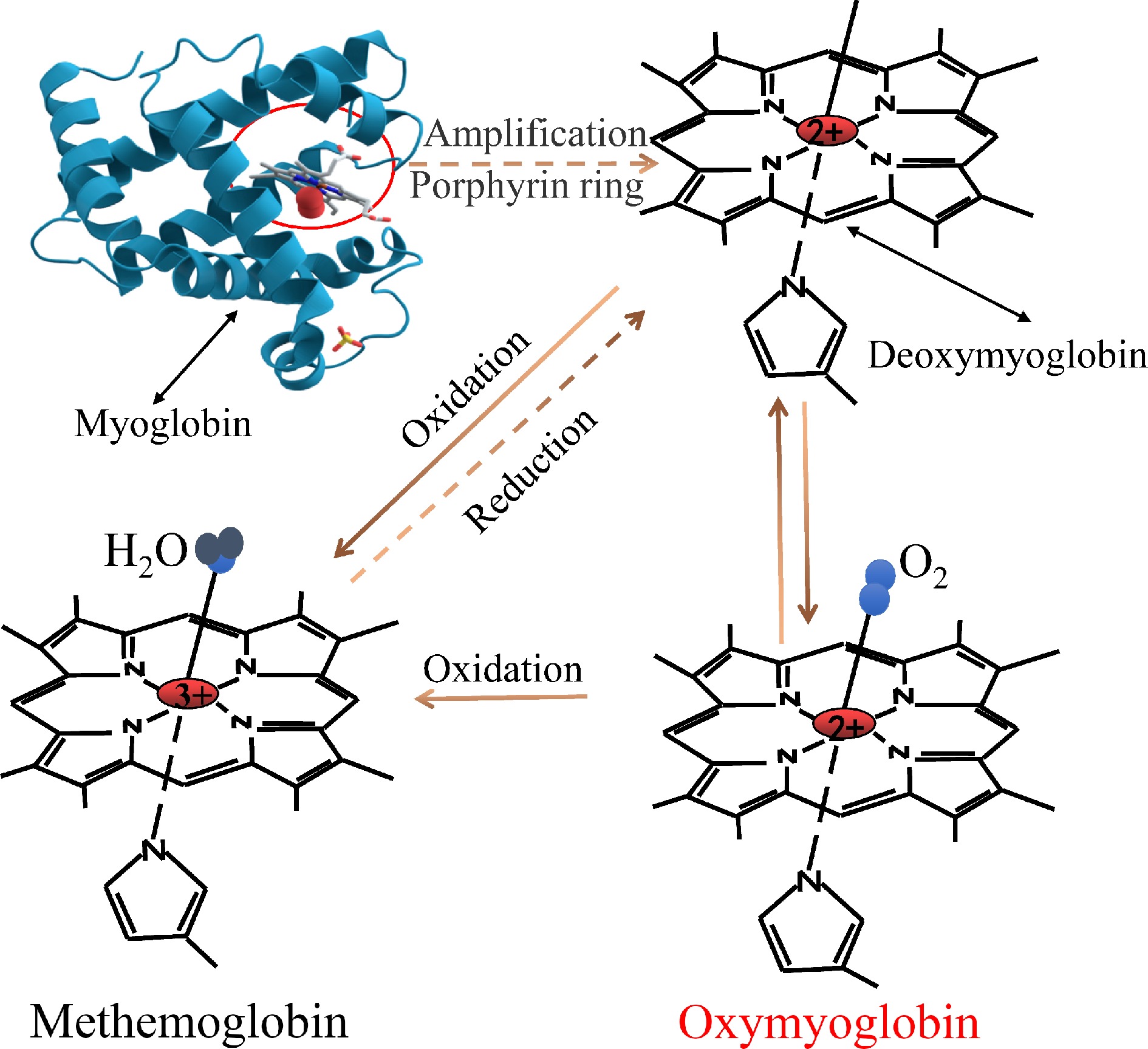
Mb is found primarily in the myoplasm and is responsible for the storage and distribution of oxygen to myocytes. The three-dimensional structure of Mb as seen microscopically is a single globular protein in the form of a flattened pike, consisting of a heme cofactor and bead protein. The amino acids of the hydrophilic group side chains of Mb are distributed almost on the outer surface, and the hydrophobic groups wrapped around the interior protect heme from oxidation by the external environment, allowing the central heme to bind oxygen, and the outer bead protein chains confer water solubility to the heme group, enabling the protein to maintain its function[9]. Heme consists of four pyrrole substituents and an iron atom in the center, and the resonance properties of the conjugated double bonds in the group determine the ability of Mb to absorb visible light, thus fulfilling its role as a pigment (Fig. 1)[7].
Mb itself is purple in color, and upon contact with O2 in the air it can quickly covalently combine with it to form bright red oxygenated Mb, which has a fresh bright red appearance. As the oxidation time continues to increase, Fe2+ in the heme cofactor is oxidized to Fe3+, and Mb or MbO2 becomes Metmyoglobin (Met-Mb). Mancini & Hunt[10] used MetMb as an indicator of the deterioration of fresh meat, if the content of MetMb is less than 20%, the meat color is bright red, up to 30% the meat color is dark, up to 50% the meat is reddish-brown, and up to 70% the meat turns completely brown. The key to maintaining bright red meat color is to prevent or reduce the production of MetMb substances, while the formation of high iron Mb in meat is also affected by a variety of factors in meat processing, and the reduction pathway and mechanism of high iron Mb are still the focus of the current meat color research work. During the storage of meat, the three forms of Mb are constantly transforming into each other, their relative content determines the color of the meat[11].
When curing meat, as the temperature increases, the color of the meat changes from dark red to pinkish gray and finally light brown. These color changes are related to Mb, and the most significant change in the surface of the meat occurs in the brightness, which is especially prominent between 42 and 56 °C[12]. Meat brightness also decreased with temperature, and the darker meat color also indicated thermal denaturation of heme proteins, so the brightness observed at lower temperatures may be related to the precipitation of other structural proteins that produce modifications within the muscle thereby affecting the meat color[12].
Raw meat
-
The quality of meat is closely related to the functional properties of meat in cured meat processing (Table 1). Meat with a bright red uniform color and less drip loss is more likely to be preferred by consumers. There are three main factors that affect the color of raw meat (1) pH; (2) pigment content in the meat; (3) aging of the meat[13]. Numerous studies have shown a negative correlation between brightness (L*) values and pH. Meat with high pH has weaker light scattering, higher transmittance of muscle fibers, longer light paths through the tissues, and increased selective absorption of light by Mb and its derivatives, so the meat will look darker; while meat with a low pH has a high reflectance and light scattering, reduced water-holding capacity and tenderness, increased steaming and dripping losses, and a pale meat color[14]. L* closely related to water content and mobility of water molecules hemoglobin, and cytochrome c, which together influence the color of raw meat. The effect of pH on the brightness of the meat after maturation and curing depends on the initial color of the raw meat, and aging of the meat can also result in the loss of heme pigments leading to lightening of the meat color. For light-colored meat, pH is the main determinant of brightness; however, for dark-colored meat, pH changes may not be the only cause of the increase in L* values during ageing[15].
Table 1. Effect of raw meat quality on myoglobin.
Factor Influence Refs Species Mountain animals and marine mammals have high myoglobin content [16] Age With the increase of age, the content of myoglobin increased and the meat color became darker [17] Motion Highly athletic areas with high oxygen consumption, high myoglobin content and dark meat color [18,19] Feeding method Antioxidants such as polyphenols are added to the feed to help increase the redness value [20] Pre-slaughter stress Redness (a*) values were lower and yellowness (b*) values were elevated in pork with pre-slaughter stress than in the control group [21] Sexuality The pH value of female lambs at 4 h after death was significantly lower than that of male lambs, with higher tenderness and more stable color [21] At present, the direction of research on Mb before and after slaughter of livestock at home and abroad is mainly based on the feeding method, slaughter age, selection site, sex and breed. The final pH of meat and the rate of pH decline are influenced by ante- and post-mortem biochemical events that act on the structural components of muscle cells and their associated connective tissues.
Related enzymes and proteins in raw meat
-
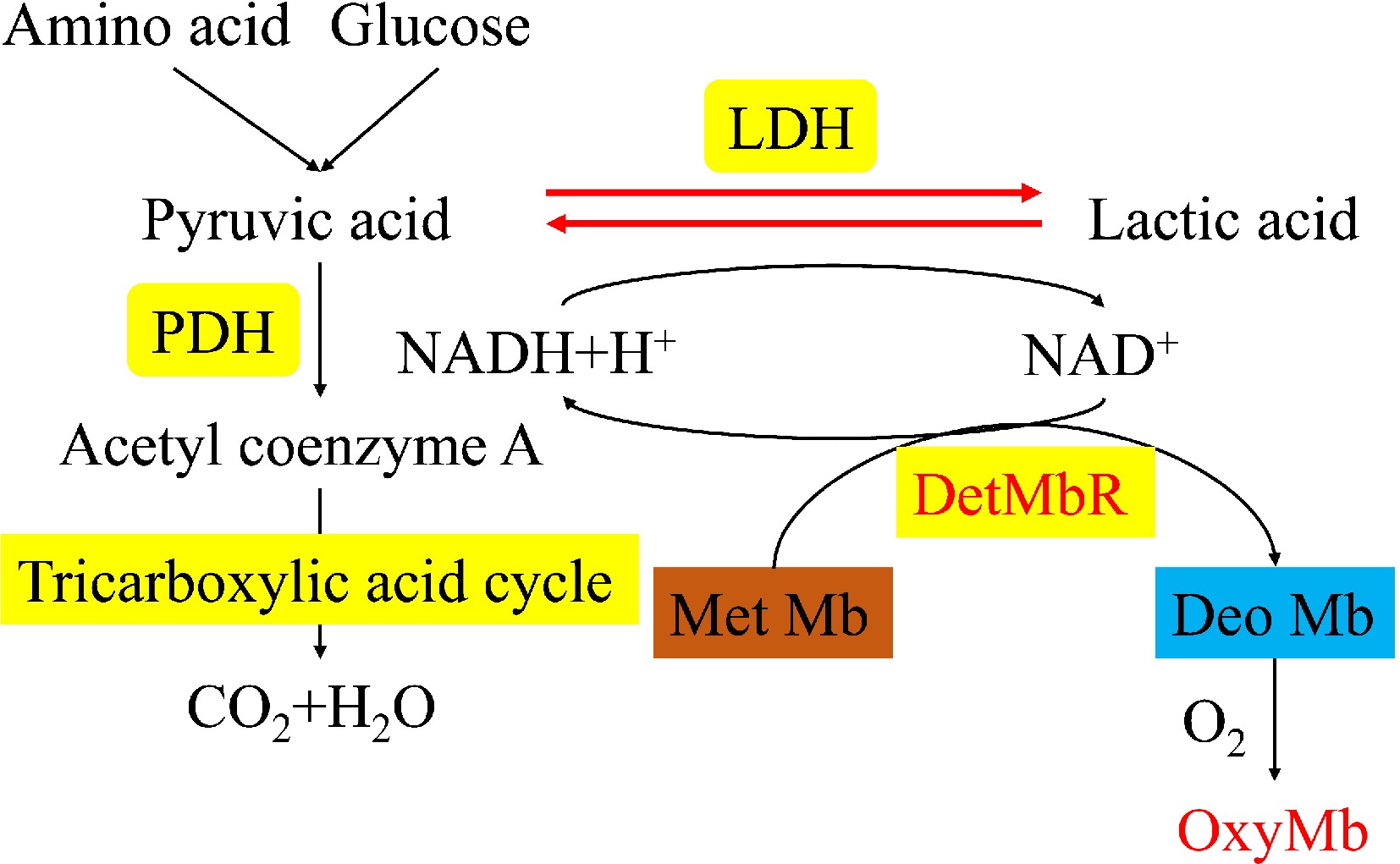
Met-Mb reductase is an enzyme that regulates protein oxidation in the muscle of living animals, and its activity gradually decreases after slaughter. A large number of studies have shown that the activity of Met-Mb reductase helps raw meat to maintain a bright color during curing and storage. Nicotinamide adenine dinucleotide (NADH) can provide electrons for Met-Mb reduction through enzymatic, non-enzymatic and mitochondrial-mediated pathways to form deoxy / oxidized Mb. In postmortem muscles, NADH-dependent Met-Mb reductase is still active. Processes affecting high iron myoglobinase activity or NADH regeneration may affect color stability[12]. Lactate dehydrogenase (LDH) and pyruvic acid dehydrogenase (PDH) belong to the class of enzymes related to mitochondrial function, and both are involved in the tricarboxylic acid cycle, which is a key enzyme in anaerobic glycolysis in living organisms (Fig. 2)[8]. Lactate dehydrogenase reacts more slowly in post-mortem anaerobic state in intramuscular tissues[22].
-
Color-production tends to be closely related to the pigment Mb, while brightness is related to the structural properties of the muscle, and together these depend on physical achromatic aberrations. Krzywicki pointed out that the attenuation of the reflection of light falling on the surface of meat is the sum of two properties: (1) Absorption by pigments; (2) achromatic refraction and reflection caused by the structural properties of the muscle[23]. The three main mechanisms leading to changes in light scattering from meat are: (1) Changes in the spacing of the myofilament lattice within myofibrils, which also leads to changes in myofilament diameter and changes in the diameter of the entire myofibril; (2) changes in the length of myonodes in relation to the diameter of myofibrils; (3) changes in the composition and distribution of the proteins in the sarcoplasm, where sarcoplasmic proteins are bound to myofilaments or are free-floating. When light is incident on the surface of a non-transparent material containing pigments, some may be reflected back due to the smoothness or roughness of the surface. Surface moisture may reflect or scatter incident light, and surface roughness characteristics may cause specular reflections. The amount of moisture on the surface of meat may be related to the pH and water-holding capacity of the meat, but surface liquids are also affected by the temperature and humidity of the ambient atmosphere, over-packaging, package contents, and time since cutting[24]. Jacques considered the membranous structures of the cell (myosin and cell membranes associated with transverse tubules, sarcoplasmic reticulum, mitochondria, and lysosomes) to be the primary source of light scattering[25]. White adipose tissue and tissues with high collagen content (e.g., epidermis) also had higher light scattering coefficients, but the difference in achromatic aberration observed in Pale Soft Exudative Meat (PSE) and Dry, Firm and Dark (DFD) meat was not significant, and Jeacocke's study suggested that the part responsible for light scattering was the lipid component of the structure and that the effect of light scattering was only 10%[26].
When the pH of cured meat is high, the meat has a higher water-holding capacity, the muscle fibers have a larger diameter, and the distance between the bright bands of the muscle fibers is longer, allowing more light to be transmitted to its interior, resulting in a darker color of the meat[27]. Low pH causes lateral contraction of myofibrils and myofibrillar lattices, leading to lower water holding capacity and more incoherent scattering. The extent to which sarcoplasmic and extracellular spaces undergo fluid migration and increased or decreased protein concentration, thereby altering the refractive, reflective, light-scattering, and achromatic color properties of muscle (Fig. 3)[28]. When cured meat products are frozen and processed due to oxidation induced a high degree of water loss, resulting in a significant increase in the interstitial space between fiber bundles and individual muscle fibers, and when the pH decreases it shrinks the diameter of the muscle fibers, the extracellular space increases, which promotes light scattering, an increase in brightness, and a lighter meat color. The process is semi-reversible, and muscle fibers are exposed to extensive pH cycling and undergo swelling and scattering. In final low pH (pHu) muscle, Mb head denaturation and sarcoplasmic protein denaturation may outweigh the effects of high pHu muscle. If the muscle has a small drip loss, there are no significant gaps between the muscle fibers, there is little change in lattice spacing when the muscle is maintained at physiological pH, the muscle fibers are swollen, and there is less scattering of light, the muscle exhibits dark muscle color. Hughes et al. suggested that the integrity of the z-lines may be related to color differences, as lighter, lower pH muscles have more intact z-lines, higher pH muscles have more myocardin and related proteins, and darker meat has shorter muscle segments than lighter meat, which may be the result of degradation of myocardin 'springs'[29].
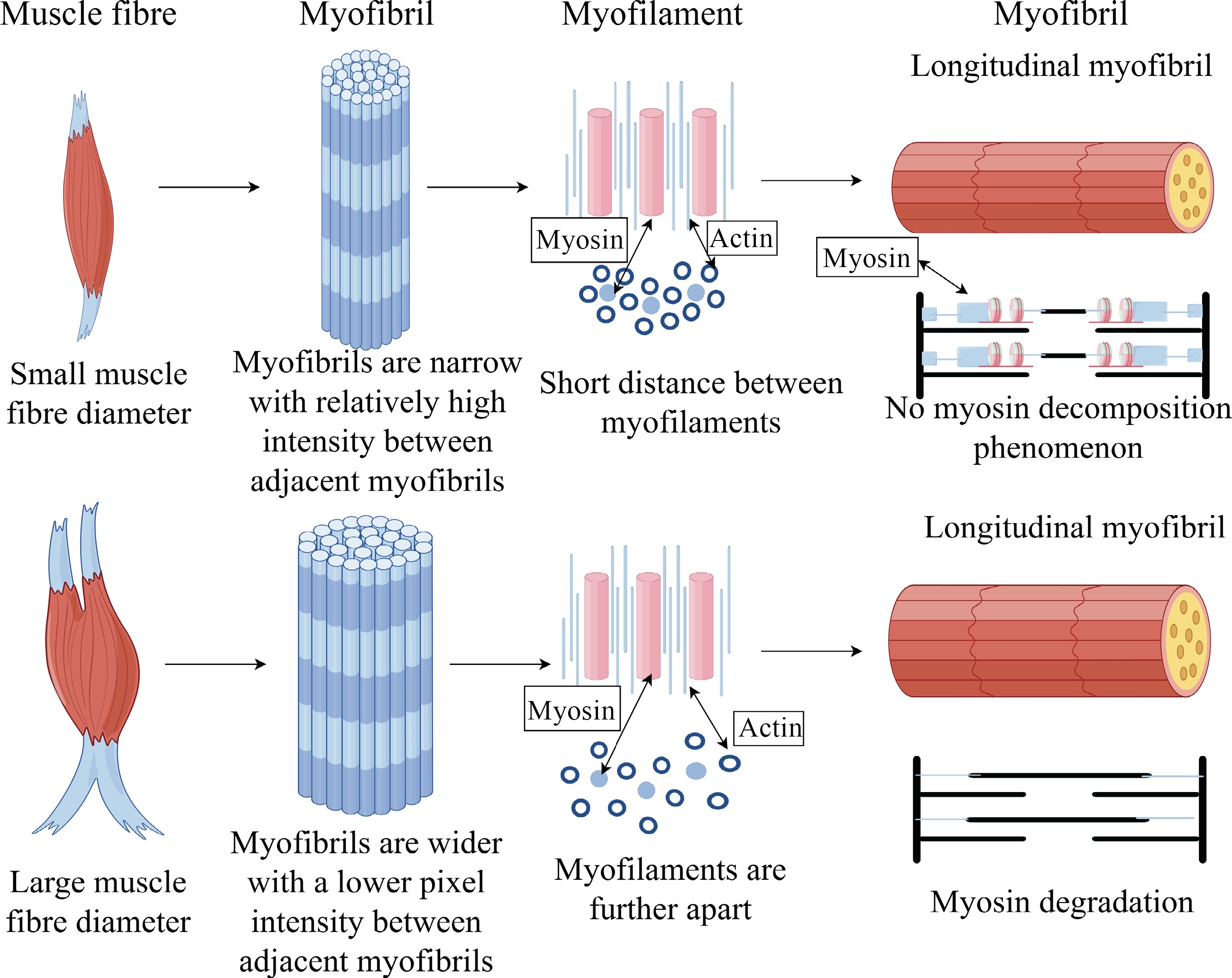
Figure 3.
Effect of transverse and longitudinal microstructure difference of muscle fibers on achromaticity.
Temperature also affects the physical structure of the muscle fibers and changes the color of the meat. When the temperature is too high, the meat loses a lot of water in the form of gravy, the rigidity of the myofibrillar structure increases due to the denaturation of the proteins, the structure of the meat is gradually compressed, and the contraction of the muscle fibers creates large gaps between the fibers, which can increase the light scattering[30]. At the muscle fiber level, cross sections can show a granular appearance, where contraction of myofibrils and reduced fiber diameter can allow for more light scattering, resulting in a lighter meat coloration[31]. The contraction of myogenic fibers may be due to protein denaturation, disruption of water-binding sites on proteins, which increases hydrophobicity and disruption of the myofilm to form a denser protein structure, and speculation that the cause of achromatic aberrations may be due to: (1) increased hydrolysis of proteins at higher temperatures, and (2) formation of aggregated sarcoplasmic proteins.
-
Lipids provide essential fatty acids and fat-soluble vitamins in addition to energy for the body. Lipids are easily oxidized and degraded, and moderate lipid oxidation produces a large amount of flavor during the maturation stage of meat products, transforming the white fat into an attractive pale yellow color. However, excessive oxidation produces peroxidation products such as aldehydes and oxysterols, which are toxic to cells[32].
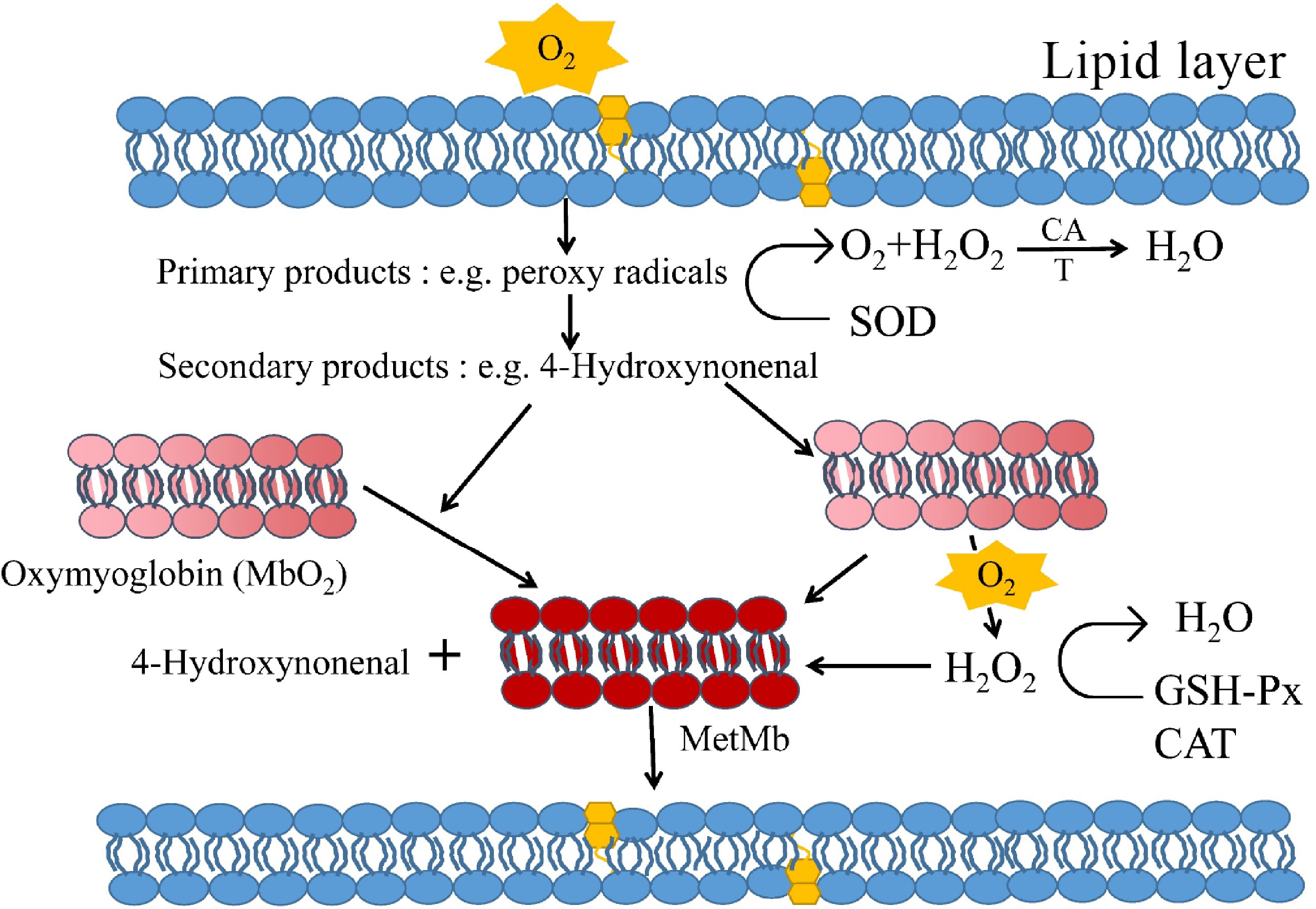
Lipids oxidized (important fatty acids such as: Arachidonic acid, linoleic acid, and α-linolenic acid etc.) in three main ways: (1) autoxidation; (2) enzyme-catalyzed oxidation; and (3) photo-oxidation. Autoxidation is the most important continuous free radical chain reaction for lipid oxidation, and enzymatic and photo-oxidative mechanisms differ from autoxidation only in the initiation of hydroperoxide formation[33]. Autoxidation is the main process of interaction between unsaturated fatty acids and oxygen, and unsaturated fatty acids first react with molecular oxygen to produce hydroperoxides through a free radical mechanism (Fig. 4)[34,35]. Hydroperoxides are extremely unstable and they decompose rapidly to form a large number of secondary compounds such as hydrocarbons, aldehydes, ketones, alcohols, esters and acids, of which aldehydes are the most important decomposition products[36]. The main aldehydes produced by lipid oxidation include n-alkanes, trans-2 olefins, 4-hydroxytrans-2 olefins, and malondialdehyde, which can oxidize Mb to react to produce high iron Mb and accelerate the oxidation of Mb, which causes a gradual change in the hue of meat color to brown[37]. Numerous studies have shown that Mb correlates with lipid oxidation[38], and high iron Mb has been shown to be pro-oxidant, with the catalytic ferrous and ferric ions in hemoglobin transferring an electron to the peroxide in the presence of peroxides, leading to further fragmentation of the peroxide to trigger lipid oxidation[39]. In summary, the causes of lipid oxidation in cured meat are: (1) fatty acids, which are the main substrates for the development of lipid oxidation; (2) prooxidants such as heme proteins, metals, prooxidant enzymes, or antioxidants such as vitamins, antioxidant enzymes, and polypeptides, which are determinants of oxidation; and (3) factors such as the type of muscle, the species or breed, the feeding system, the anatomical location, or the diet that the animal receives. Therefore, strategies to inhibit lipid oxidation not only minimize rancidity but also improve Mb stability[40]. Xia et al. showed that the yellowness of fat particles in meat products is also closely related to lipid oxidation[41]. The effect of lipid oxidation on the formation of yellow pigment in meat is related to the non-enzymatic browning reaction of lipid oxidation products with amines in proteins or phospholipids[42]. In recent years, many studies have focused on the replacement of animal fats with fats of plant origin rich in unsaturated fatty acids and have examined the stability of the products during storage, being used by the food industry as a strategy to better cover the dietary and health needs of modern consumers.
-
The Maillard reaction was discovered by Louis C. Maillard in 1912, when he observed the formation of browning in heated solutions containing sugars and amino acids. The Maillard reaction begins with the reaction between the carbon groups of various sugars and the amino groups of amino acids, followed by the formation of intermediary compounds or carbonyl compounds, which then reacts with the amino acids through a variety of ways, condensation, polymerization, degradation, cyclization etc., the color and aroma are formed[43]. The formation of the brown product of the Maillard reaction is mainly attributed to melanin, however, the chemical structure of melanin is not known because melanin is a complex heterogeneous polymer. Hayase et al. proposed two hypotheses about the chemical structure of melanin: (1) that the melanin backbone is formed from sugar degradation products that branch with amino compounds; and (2) that melanin is a polymer of low-molecular-weight pigments formed from sugars and amino acids, and that the blue pigment, which is formed from xylose and glycine, is transformed into a brown color by polymerization[44]. Since a variety of low molecular weight pigments can be produced during the process of the Maillard reaction, and the color recognition is cumulative. Therefore, it is important to study the changes in low molecular weight pigments to understand the browning or pigmentation produced by the Maillard reaction in meat products. Murata showed that the color contribution of furpipate in the Maillard browning reaction was estimated to be 25%, that of decarboxylated furpipate was 3%, and that 5-hydroxymethylfurfuryl ester and its decarboxylated derivatives contributed to the total pigmentation at 43% and 18%, respectively, and that the hue became brighter when the reaction conditions were controlled to be suitable for the formation of low molecular weight pigments with a specific maximum specific absorption[45].
The browning or hyperpigmentation of the Maillard reaction is mainly attributed to melanin, but its chemical structure has not been clarified. With advances in instrumental analysis, a variety of low molecular weight pigments have recently been identified, and a variety of pigments can cumulatively impart color even when the concentration of individual pigments is low. Since the reaction conditions can affect the formation of pigments, if the conditions of the reacting substances are changed so that some of them that are polymerized to form melanin become cross-linkers between proteins, it means that the hue of the reacting solution or meat product can be changed. Geng et al. investigated the effects of pH and free amino acids on meladic browning in the drying of Japanese squid. The results showed that the browning of dried squid was significantly inhibited at a pH of 5.5 and that the inhibition of arginine production was more effective than the attenuation of meladic reactivity between ribose and arginine, which mitigated meladic browning of dried squid at acidic pH[46].
-
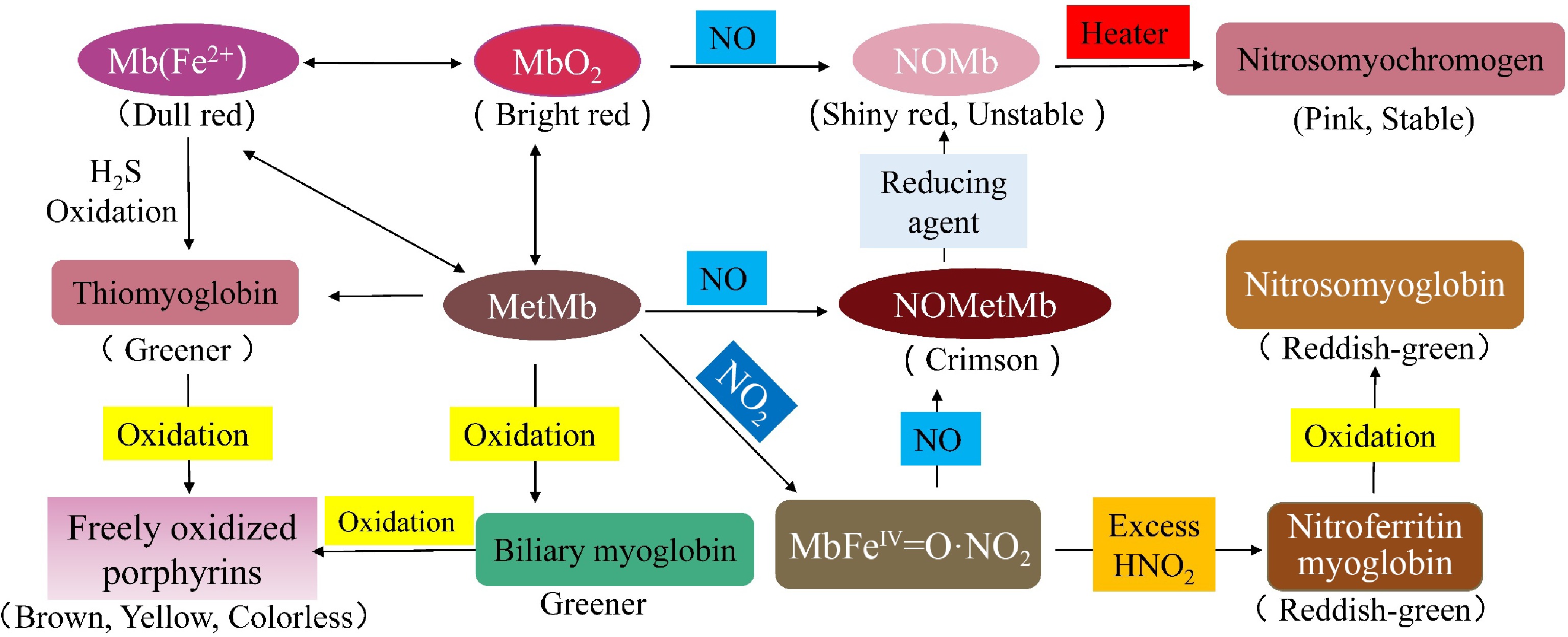
Nitrite can be further reduced to ammonium by nitrite reductase to prevent nitrite poisoning, and Nicotinamide adenine dinucleotide (NADH) is reoxidized to NAD+. NADH reduces nitrite to NO and typical bright pink nitroso Mb (MbFe (II) NO) (Fig. 5)[47]. During oxidation of nitrosomyoglobin, brown sub-myoglobin (MbFe(III)) and nitrate are produced, which can be used for further reactions. Nitrosomyoglobin is unstable and releases sulfhydryl groups when heated to produce stable bright red nitrosomyochromogen. During processing and curing, peroxynitrite can react with high iron Mb to form a protein cage product intermediate, MbFeIV = O·NO2, which is further broken down to nitro-high iron Mb, which is partially reduced to greenish-red nitro-myoglobin by the action of reducing agents such as ascorbic acid[47]. In addition, in the process of curing meat, due to contamination, microbial decomposition of products such as hydrogen peroxide or hydrogen sulfide will be generated with Mb to produce bilirubin or sulfur Mb, which affects the color of meat products[48].
Nitrite, as a coloring agent for meat products, can effectively inhibit the growth of certain spoilage bacteria and pathogenic bacteria, excessive nitrites entering the bloodstream can deprive tissues of oxygen, with a short incubation period, and in severe cases causes death from respiratory failure. The strong carcinogenicity of traditional meat coloring agents has made the search for new, safe meat coloring agents with reduced toxicity to humans a focus of attention[49].
Antioxidants
-
The red color of meat products is an important quality attribute, and the intensity of the red color is not only related to the concentration of hemoglobin, but also affected by the process and storage conditions, among which lipid oxidation is the most detrimental in negatively affecting the color of meat products. The addition of antioxidants to cured meat has two main effects: (1) it effectively mitigates the browning that occurs on the surface of meat products due to lipid oxidation and inhibits the transformation of oxygenated Mb to high iron Mb; and (2) excess antioxidants can react with sodium nitrite to produce N2 or N2O, which reduces the reaction of NO with Mb, and diminishes the coloring effect of nitrite[50]. Natural antioxidants are phenolic compound-containing substances extracted from plants, animal tissues, spices, and botanicals. These antioxidants have shown good performance in inhibiting lipid oxidation, but lack sufficient resources in the natural environment, making large-scale production impossible. Natural antioxidants such as raw synthols, tea polyphenols, and ascorbic acid are the most widely used in meat products and their safety is more acceptable to consumers. Rohlik et al[51] found that the novel natural antioxidant lycopene molecule is relatively stable and its slightly acidic nature inhibits the growth of microorganisms, which effectively reduces lipid oxidation and stabilizes the red color of meat products during storage.
NaCl
-
Sodium chloride is an essential multifunctional ingredient in meat products, which is added to improve sensory characteristics, microbial safety and technical functions, such as water retention capacity[52]. Binding of chloride ions to myofilm proteins (actin, myocardin) increases the negative net charge, resulting in electrostatic repulsion leading to swelling of the myofilm, and an increase in the spacing of the myofibrils leading to lateral swelling of the myofibrils, while sodium chloride increases the solubility of myocardin, leading to structural changes in myofibrils such as extraction of the a-band and z-line and disruption of the m-line structure. This leads to changes in the refractive index within muscle fibers and sarcoplasm[53]. Offer & Trinick showed that selective binding of chloride ions to muscle fibers increased electrostatic repulsion and eliminated lateral structural constraints[54]. Interestingly, the addition of salt also has an effect on the iridescence of meat; salt-induced photonic structure changes the color of the muscle by altering the lattice distance or refractive index, and cooked cured products (e.g., pastrami, corned beef) and raw cured meats (e.g., Black Forest ham) are more likely to be iridescent and interfere with the color in a more vivid way than fresh meat[55].
Higher concentrations of salt solution will accelerate the oxidation of Mb and promote the formation of high iron Mb, turning the cured meat grey-red or grey-brown, but high osmolality will also lead to a reduction in the moisture content of the cured meat, which will darken the color of the meat, and lower moisture content and water activity can inhibit the growth of microorganisms in the product, which will in turn affect the flavor and result in a poorer texture. Liu et al. found that short-term NaCl treatment reversibly altered the structure of Mb, whereas long-term high NaCl treatment induced irreversible denaturation of Mb and relatively low bioavailability of Mb in vivo, and saline treatment altered the structure of heme and the hydrophobic lumen of Mb, leading to reduced Mb digestibility[56]. Gouvêa et al. found that cured meat sun-dried with reduced moisture compared to fresh meat, cured meat presented higher values of the color indices L*, a*, and c*, lower values of the index color b*, and presented higher scores for sensory characteristics[57]. A study by Soladoye et al. also found that NaCl may be involved in the oxidation cycle of Fe from Fe2+ to Fe3+ and consequently promotes the involvement of transition metal elements in a chemical reaction similar to the Fenton reaction, leading to the production of more ROS radicals[58].
-
Cured meat has a certain shelf life, and the oxidation of lipids and proteins and the growth and reproduction of microorganisms will affect the color and luster of meat products in the market circulation due to the influence of factors such as air, light and processing temperature[59].
Cured meat products are usually sold in deboned or sliced packages; however, cured products require the complete removal of oxygen from the interior of the package, as gas in the presence of light facilitates photo-oxidation of the product, which accelerates the discoloration of the meat product. The most commonly used preservation packaging methods are vacuum packaging and modified atmosphere packaging. The gases in gas-conditioned packaging are usually carbon dioxide (which has antimicrobial activity) and nitrogen (filler gas), which provides a good package appearance. Vacuum packaging has the advantage of high barrier will delay the deterioration of cured meat and color deterioration, is the most commonly used packaging method. In order to avoid exposing meat products to sunlight, products usually use transparent films with light-blocking properties, and common methods include printing, coloring, coating with polyvinylidene chloride, gold, and adding aluminum foil layers. Antimicrobial and smart packaging has become a new food-safe packaging technology, edible films and coatings also have good mechanical properties, gas and moisture barriers by foaming, impregnating, spraying, casting, painting, individually wrapping, or rolling have a positive effect on the color of cured meat products[60].
Other packaging methods used in the market today to protect the color of cured meats include air-conditioned packaging, smoking, and active packaging. Gas-conditioned packaging is commonly available in high oxygen-conditioned packaging and CO gas-conditioned packaging. High-oxygen environment can promote the formation of bright red oxygenated Mb on the surface of cured meat products, but with the increase of storage time, excessive oxidation will lead to browning and loss of color in the late storage period. Active packaging technology adds antioxidants, antimicrobial agents, and other active agents in packaging materials that can effectively inhibit the corruption and discoloration of meat products, which has become a hot spot of research. Yang et al. utilized grape seed extract in hyperoxic packaging and significantly inhibited lipid and Mb oxidation of raw meat patties and reduced premature browning of late meat patties[61].
-
In conclusion, the formation of color of cured meat products is a complex physicochemical process, which is not only related to the pigment content of raw meat and enzymes, but also related to the structure of muscle fibers and achromatic aberration. Sodium chloride, nitrites, and antioxidants added to cured meats alter color and participate in lipid oxidation and the Meladic reaction in meat products. In addition, the way in which the cured meat products are packaged during the distribution process can also affect the color of the cured meat products. Therefore, the color of cured meat products can be improved by regulating the above factors to make it more acceptable to consumers. The color of cured meat products is also related to the quality of meat, pH, water retention and other microstructures, which is an important indicator of the freshness of cured meat products. At present, most of the domestic and foreign studies on meat color of cured meat are not comprehensive and in-depth, and there is no unified standard for judging meat color of cured meat products, and the traditional methods such as sensory evaluation are subjective, and the instruments used to measure the meat color of cured meat are single and the detection methods are relatively outdated. Therefore, the study of color of cured meat products and the development of detection methods remain a hot trend in meat color research in the future.
-
The authors confirm contribution to the paper as follows: study conception and design: Han J, Wang Y, Tian J; Manuscript revision: Han J, Wang Y; data curation and analysis: Hao S; supervision, project administration and funding acquisition: Tian J, Zhang K, Jin Ye. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The research was financially supported by the funds: Science Foundation of China (Grant Nos 31960514 and 32160589); Science and Technology Projects of Inner Mongolia Autonomous Region (Grant Nos 2019GG239 and 2022YFDZ0020); Inner Mongolia Natural Science Foundation Project, (Grant No. 2021MS03090); Central government guides local science and technology development fund projects (Grant No. 2022ZY0133); Key Project of Bayannur National Agricultural High Tech Industry Demonstration Zone for the 'Science and Technology Revitalization of Mongolia' Action (Grant No. NMKJXM202205); Inner Mongolia Agricultural University High Level Talent Research Launch Project (Grant No. NDYB2019-37).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Han J, Wang Y, Wang Y, Hao S, Zhang K, et al. 2024. Effect of changes in the structure of myoglobin on the color of meat products. Food Materials Research 4: e011 doi: 10.48130/fmr-0024-0003
Effect of changes in the structure of myoglobin on the color of meat products
- Received: 10 November 2023
- Revised: 03 January 2024
- Accepted: 23 January 2024
- Published online: 03 April 2024
Abstract: Color is an important factor in determining a consumer's desire to buy and an important indicator of meat quality. Processing and storage processes affect the color of meat products. Therefore, research on how to improve the color of meat products can not only improve the quality of meat products but also enhance a consumer's desire to buy. Nitrosomyoglobin is known to be the main substance that exerts color in meat products. Meanwhile, meat products undergo a series of chemical and physical changes during the curing process that also affect the color of cured meat products. This paper reviews the six main factors currently affecting the color of cured meat products: (1) Quality of raw meat and the content of myoglobin; (2) physical structure of the muscle and achromatic aberration; (3) lipid oxidation; (4) Maillard reaction; (5) additives; and (6) packaging methods. In addition, the article also explores the relationship between pH, temperature, water retention and color in cured meat products, in order to provide more ideas for the study of color in cured meat products.
-
Key words:
- Cured meat /
- Color /
- Myoglobin /
- Nitrite /
- Light scattering