-
Sunflower seed is an important economic oil seed worldwide after soybean, palm and rapeseed[1]. Global sunflower seed production was estimated to be over 50 million tons in 2022/2023[2]. It is primarily consumed as vegetable oil and meal, either alone or in the blends of different nuts and other components[3]. Sunflower seeds not only have potential as a protein source but also have high nutritional value in the human diet due to their excellent nutritional quality and relatively low content of anti-nutrient factors, which provide a large number of antioxidants, minerals, and unsaturated fatty acids.
Roasting is a great way to prepare the seeds for snacking. During the roasting process, not only is the typical nutty aroma formed, but with the high-temperature heating, nutrients change. Sunflower seeds are susceptible to oxidation during roasting and storage[4]. The rate of oxidation and rancidity of fat are highly dependent on the roasting time and temperature. Thus optimum roasting temperature and time are necessary for addition to desirable flavor and nutritional quality. To improve the nutritional quality of sunflower seeds, seed germination technology has been extensively used as the nutritional components of entire seeds are increased during germination, such as free amino acids, dietary fiber, minerals, phenolic compounds and antioxidant capacity[5,6]. Therefore, the germinated and ungerminated sunflower seeds were used as materials for investigating the effects of roasting conditions on metabolite and flavoromic profiles of sunflower seeds.
Metabolomics and Flavouromics are novel omics studies used for considering overall targeted and non-targeted compounds in food[7]. Metabonomics has been used in many research fields, including medicine[8,9], microbiology[10], horticulture[11] and nutrition[12], to understand metabolic reactions and to identify metabolites/biomarkers associated with given conditions or treatments. In the last few years, flavor profiling has been used in many fields. One of the most promising applications in profiling of volatile organic compounds is the HS-SPME-GC-MS. This is a useful technique that allows the collection of sensory analysis to detect the overall aromatic spectrum of samples. It is a new technology that enables the acquisition of the sensory analysis for the detection of the overall aromatic profile of samples. PCA and AHC which give an overview of useful data to detect outliers and evaluate the relationships between samples and variables and between variables themselves.
As far as we know, it is the first application of metabolite and flavor profiling being applied for metabolite and flavor analysis of sunflower seed roasted at different temperatures and times. Detailed untargeted metabolite and flavor of sunflower seeds can provide the opportunity to better understand the relevant roasting process. The aim is to find the effects of roasting conditions on the metabolite and flavor profiles of sunflower seeds.
-
Sunflower seeds were bought in a local Thai market. The seeds were placed in aluminum foil bags and then stored in the refrigerator at 4 °C. The seeds were then germinated for further analysis.
Reference standards and reagents
-
Internal standard for semi-quantitative of fraction I was tetracosane, 5α-cholestan-3ß-ol was used as an internal standard for fraction II, phenyl-β-D-glucopyranoside was for fraction III, and p-chloro-L-phenylalanine was for fraction IV. Retention time standards were undecane, hexadecane, tetracosane, triacontane, octatriacontane. The reagents were bought from Sigma (Sigma-Aldrich, St. Louis, MO, USA).
Ethyl decanoate was used as an internal standard for volatile compounds. The reference standards used for volatile compounds identification experiments were prepared with 0.5% (weight/volume) concentration of acetone. N-alkanes (C6−C26) were used as the criterion for the calculation of the retention index. All the reference standards for volatiles identification were purchased from Sigma-Aldrich, USA.
Preparation of RGSF and RUSF
-
Soak 400 g seeds in 2 l of distilled water at room temperature for 8 h. The soaked sunflower seeds were placed in an incubator with humidity type KBF 240 (Binder, Tuttlingen, Germany) in darkness at 25 °C for 24 h. Germinated and ungerminated seeds were baked in an oven (Model UF55, Memmert, Thailand) at 125, 135, and 145 °C for 30−60 min. The seeds were cooled to room temperature, husked and ground by the grinder (Panasonic, Japan), and packaged in the aluminum bag. Raw sunflower seed was used as a control sample.
Metabolite profiling
-
The procedures for extraction and fractionation of sunflower flour followed a previous method with some modifications[13]. Fraction I contained fatty acid methyl esters (FAMEs) and hydrocarbons, fraction II contained free fatty acids (FFAs) and sterols, fraction III contained silylated sugars and sugar alcohols, and fraction IV contained organic acids and amino acids. GC-FID analysis was run by an Agilent Technologies HP 6890+ equipped with FID (320). Obtain and integrate GC-FID data using HP-ChemStation A.06.03 (Hewlett Packard, Polo Alto, CA, USA). The capillary column was DB-1 (60 m × 0.32 mm, 0.25 μm) (J&W Scientific, Agilent, USA). The flow rate of helium was 1.8 ml/min. The splitless injection was performed at 280 °C. The initial column temperature was set at 100 °C, then it rose to 320 °C at 4 °C/min (maintained for 15 min). The components of sunflower seed were identified by comparison of retention time between the analyte chromatographic peak and the reference standard chromatographic peak.
Flavor profiling
-
One mg/ml ethyl decanoate was dissolved in 10% methanol. Ethyl decanoate was added to sunflower seed powder in the proportion of 100 μg/2 g. The sample was incubated for 20 min at 60 °C for the equilibration of the volatiles. A 50/30 μm DVB/CAR/PDMS SPME fiber (57348-U, Supelco) was inserted into the headspace bottle, and the headspace extraction was carried out at 60 °C for 30 min.
The volatile components were analyzed by an Agilent 7890A gas chromatograph equipped with a 5975C mass spectrometer. A DB-1 (60 m × 0.25 mm, 0.25 μm) MS column was used for GC-MS. The volatiles were thermally desorbed for 20 min at 250 °C in a splitless mode. Helium entered the column at a constant flow rate of 1.5 ml/min. The initial oven temperature was 50 °C for 1 min. Next, the temperature rose to 100 °C at 5 °C/min (for 5 min), then to 140 °C at 4 °C/min (for 5 min), then to 180 °C at 5 °C/min (for 2 min), and finally to 250 °C at 10 °C/min (for 7 min). The source temperature of mass spectrometer was 230 °C, the transmission line temperature was 225 °C, and the quadrupole temperature was 150 °C. The ionization voltage was 70 eV and the scanning range was m/z 50-550.
Pure component mass spectra were automatically extracted from highly complex GC-MS data files using AMDIS (Automated Mass Spectral Deconvolution and Identification System, version 2.66, USA). These purified spectra were used for a search in a mass spectral library. The volatile compounds were identified according to the NIST library (NIST 11, Version 2.0, Gaithersburg, USA) and comparison mass spectra with reference standards. Based on the internal standard area, the relative concentration of each compound was calculated.
Statistical analysis
-
PCA and AHC analysis were used to analyze the correlation between and within groups. The data were subjected to one-way analysis of variance (ANOVA) by XLSTAT version 2016.7 (Addinsoft, NY, USA). Differences between means were assessed with Tukey's range test at a 95% significance level (p < 0.05).
-
This study investigated the metabolic and flavor profiles of 19 samples (including 18 roasting treatment samples and the raw sample) obtained from RUSF and RGSF at 125, 135, and 145 °C for 30, 45, and 60 min. A total of 169 metabolites and flavor compounds identified by GC-FID and HS-SPME-GC-MS were analyzed using PCA (Spearman correlation) to determine the main sources of variation in the dataset (Fig. 1). PC1 and PC2 explained 44.29% of the data variability of 169 compounds. The clustering of RUSF grouped to the right was separated from the clustering of RGSF grouped to the left. The aldehydes (2-methylbutanal, 3-methybutanal, isobutanal, 2-phenyl-2-butenal), pyrazines (2,3-dimethylpyrazine, 3-ethyl-2,5-dimethylpyrazine, 2-methylpyrazine) and 2-heptanone were grouped to the top side on PC2 (positively associated with RGSF at the higher temperature). The amino acids and reducing sugars were grouped to the left side (negatively associated with RGSF).
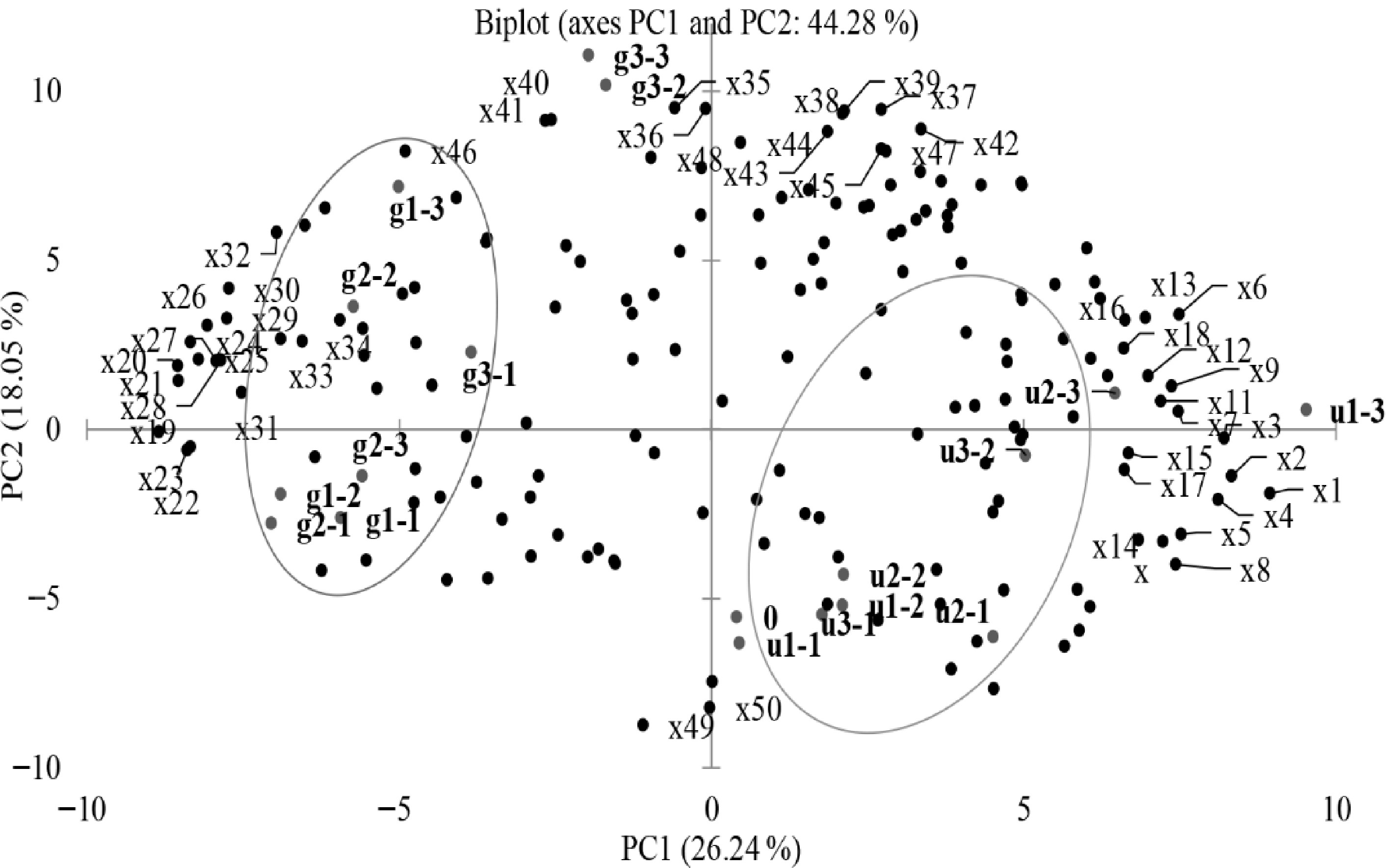
Figure 1.
Biplot of metabolic and flavor profiles of RUSF and RGSF. Compound codes are explained in Table 1 (loading score higher than or equal to 0.7). The sample names represent RUSF described as: u1-1 = 125 °C 30 min, u1-2 = 125 °C 45 min, u1-3 = 125 °C 60 min, u2-1 = 135 °C 30 min, u2-2 = 135 °C 45 min, u2-3 = 135 °C 60 min, u3-1 = 145 °C 30 min, u3-2 = 145 °C 45 min, u3-3 = 145 °C 60 min. The sample names represent RGSF described as: g1-1 = 125 °C 30 min, g1-2 = 125 °C 45 min, g1-3 = 125 °C 60 min, g2-1 = 135 °C 30 min, g2-2 = 135 °C 45 min, g2-3 = 135 °C 60 min, g3-1 = 145 °C 30 min, g3-2 = 145 °C 45 min, g3-3 = 145 °C 60 min
To examine the distribution of the individual metabolite and flavor compound among different roasting conditions, a heatmap diagram combined with AHC was applied for the selected 50 compounds which had a loading value higher than 0.7 (Fig. 2). The 50 compounds are shown in Table 1. Amino acids and reducing sugars were higher in RGSF compared to RUSF because of the increase of these metabolites during germination. Meanwhile, RGSF appears to have higher levels of pyrazines (2-methylpyrazine 2-ethyl-3,5-dimethyl-pyrazine, and 3-ethyl-2,5-dimethylpyrazine). The grouping result of AHC followed the PCA which grouped the samples into two groups. The most volatile compounds were clustered to the left. The majority of metabolites, Maillard reaction substitutes (amino acids and reducing sugars), products (pyrazines), and Strecker degradation products (3-methylbutanal, 2-methylbutanal, and isobutanal) clustered to RGSF group. The observed differentiation between RUSF and RGSF reflected the volatile compounds were formed more in RUSF compared to RGSF. The groups of shorter roasting times were separated from those of longer roasting times both in RUSF and RGSF. The results indicated that the metabolic and flavor profiles were affected by temperature and time significantly.
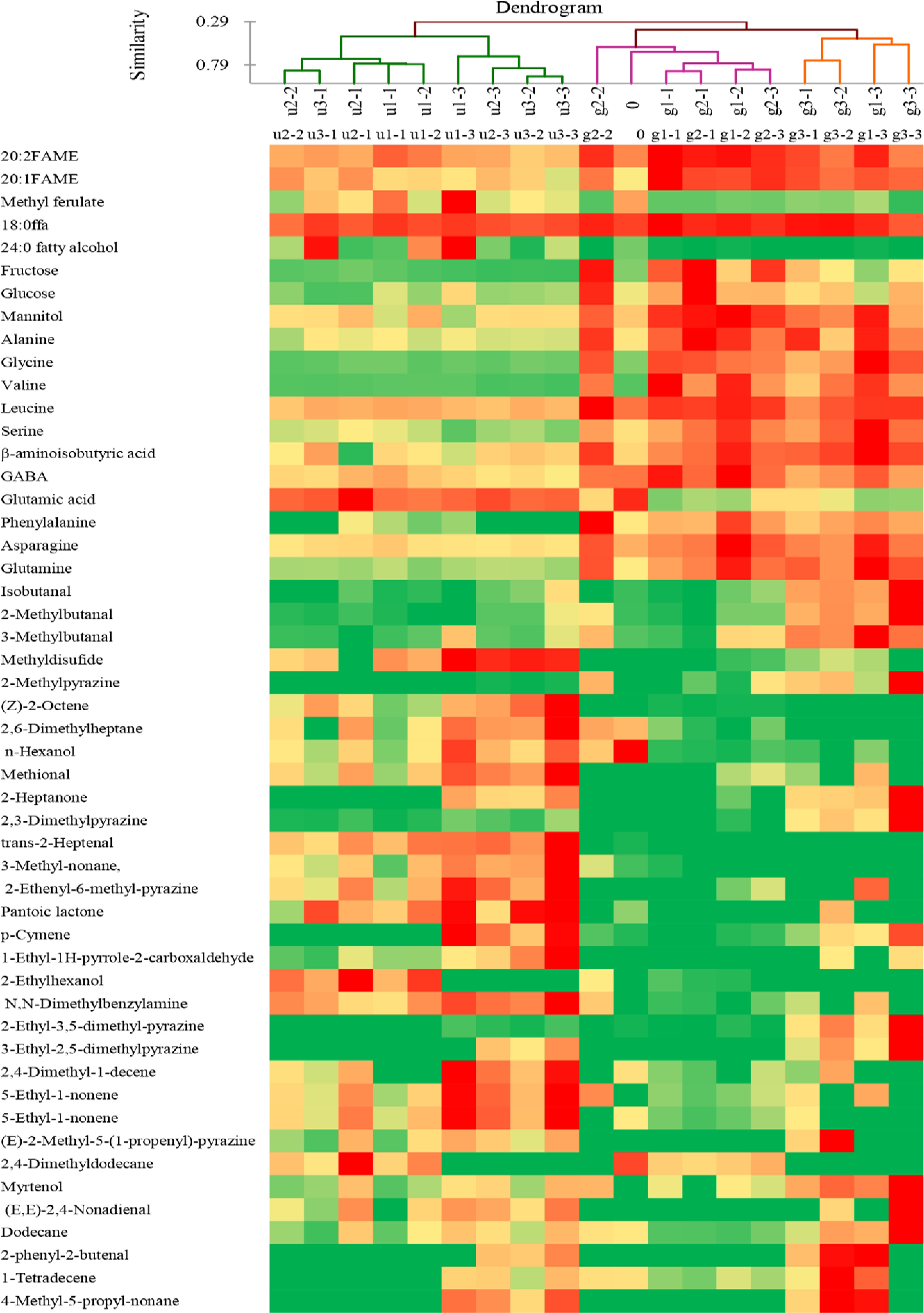
Figure 2.
Heatmap and AHC dendrogram of metabolic and flavor profiles of RUSF and RGSF. The compounds with loading scores higher than 0.7 were presented in the heatmap. Metabolite levels correspond to the color temperature. The higher the temperature (red), the higher the content of the corresponding compound.
Table 1. Varimax rotated factor loadings of the significant principal components of the metabolic and flavor profiles of RUSF and RGSF.
PCA code Compounds PC1 PCA code Compounds PC1 x1 trans-2-Heptenal 0.960 x27 Valine −0.850 x2 3-Methyl-nonane 0.894 x28 Mannitol −0.842 x3 Pantoic lactone 0.881 x29 Glycine −0.832 x4 5-Ethyl-1-nonene 0.871 x30 Glutamine −0.828 x5 (Z)-2-Octene 0.807 x31 20:1FAME −0.806 x6 1-Ethyl-1H-pyrrole-2-carboxaldehyde 0.804 x32 β-Aminoisobutyric acid −0.745 x7 N,N-Dimethylbenzylamine 0.802 x33 Glucose −0.739 x8 24:0 fatty alcohol 0.798 x34 18:0ffa −0.701 x9 Methional 0.791 x10 Glutamic acid 0.776 PC2 x11 2-Ethenyl-6-methyl-pyrazine 0.772 x35 Myrtenol 0.846 x12 Methyldisufide 0.751 x36 2-Ethyl-3,5-dimethyl-pyrazine 0.844 x13 (E)-2-Methyl-5-(1-propenyl)-Pyrazine 0.746 x37 p-Cymene 0.840 x14 Methyl ferulate 0.735 x38 2-Heptanone 0.837 x15 2,6-Dimethylheptane 0.717 x39 3-Ethyl-2,5-dimethylpyrazine 0.831 x16 2,4-Nonadienal,(E,E)- 0.711 x40 3-Methylbutanal 0.813 x17 n-Hexanol 0.710 x41 2-Methylbutanal 0.813 x18 2,4-Dimethyl-1-decene 0.709 x42 4-Methyl-5-propyl-nonane 0.789 x19 Fructose −0.948 x43 2-Phenyl-2-butenal 0.782 x20 Asparagine −0.916 x44 1-Tetradecene 0.754 x21 Leucine −0.914 x45 Dodecane 0.738 x22 20:2FAME −0.900 x46 2-Methylpyrazine 0.732 x23 GABA −0.894 x47 2,3-Dimethylpyrazine 0.731 x24 Serine −0.893 x48 Isobutanal 0.714 x25 Alanine −0.879 x49 2,4-Dimethyldodecane −0.778 x26 Phenylalanine −0.865 x50 2-Ethylhexanol −0.732 Changes of representative metabolites in fraction I-IV from RUSF and RGSF
-
Changes in the representation of different metabolites observed during the roasting process are shown in Figs 3−6. FAMEs detected in fraction I were produced by transesterification of lipid extract. In our previous study, FAMEs decreased significantly (p < 0.05) after germination. Therefore, the contents of FAMEs were lower in RGSF compared to those in RUSF (Fig. 3). In RUSF, roasting resulted in increases in C16:0, C18:0, and C18:1, and a decrease in C18:3. Roasting temperature and time influenced the composition of C18:2 to a small extent in RGSF, but the decrease of C18:2 occurred in RUSF. The results indicated that roasting resulted in a loss of the content of unsaturated fats. Previous studies also showed that higher baking temperatures of soybeans and hazelnuts resulted in higher relative percentages of saturated fat acids, while lower relative percentages of polyunsaturated fatty acids[14]. C16:0 increased at 125 °C, an even more increase was observed at 135 and 145 °C in RUSF and RGSF. C18:0 showed the same behavior. The results indicated that roasting resulted in a loss of the content of unsaturated fats (C18:2 and C18:3) and an increase of C18:1. This difference might be attributed to hydrolytic and oxidative degradation of the lipid fraction during roasting. An increase of C18:1 has been also reported for 15 min microwave roasting of sunflower seeds[15]. However, microwave heating for 9 min did not remarkably affect the fatty acid composition of sunflower seeds[16].
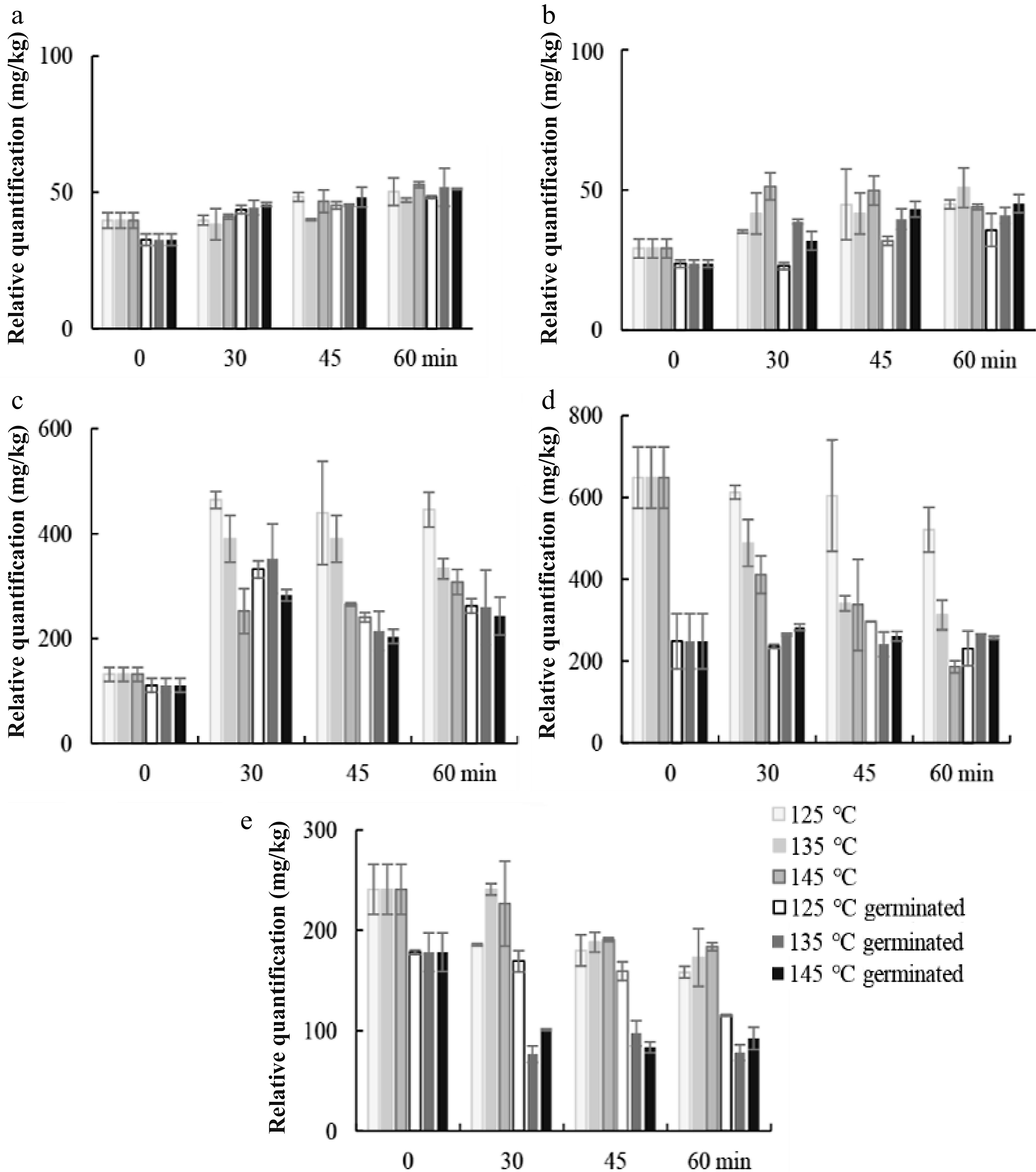
Figure 3.
Changes in relative quantification of representative compounds in fraction I. (a) C16:0 FAME, (b) C18:0 FAME, (c) C18:1 FAME, (d) C18:2 FAME, and (e) C18:3 FAME during roasting of ungerminated and germinated sunflower seeds at 125, 135, and 145 °C.
Peroxide value (PV) and FFA are evidence of autoxidation and hydrolytic rancidity, respectively[17]. The content of FFAs of sunflower seeds submitted to the different roasting conditions are presented in Fig. 4. The predominant FFAs in sunflower seed were C16:0, C18:0, C18:1, C18:2, and C18:3. C18:1, C18:2, and C18:3 was more affected by roasting than C16:0 and C18:0. Roasting at 145 °C for 60 min caused the reduction of FFAs especially for unsaturated FFAs. The longer the roasting time, the less the content of C18:1, C18:2, and C18:3. The higher the temperature, the more the reduction of C18:1, C18:2, and C18:3. Sesame seeds roasted at high temperature long term increases more lipid oxidation products[18]. As FFAs increased at the first 24 h of germination in our previous study, FFAs content in germinated sunflower was higher compared to that in RUSF during roasting. The lower decrease of C18:3 in RGSF compared to that in RUSF may be due to the higher content of α-tocopherol present in germinated seeds, slow down the lipid oxidation rate. However, the higher decrease of C18:1 was observed in RGSF. The results were similar to a previous report[15].
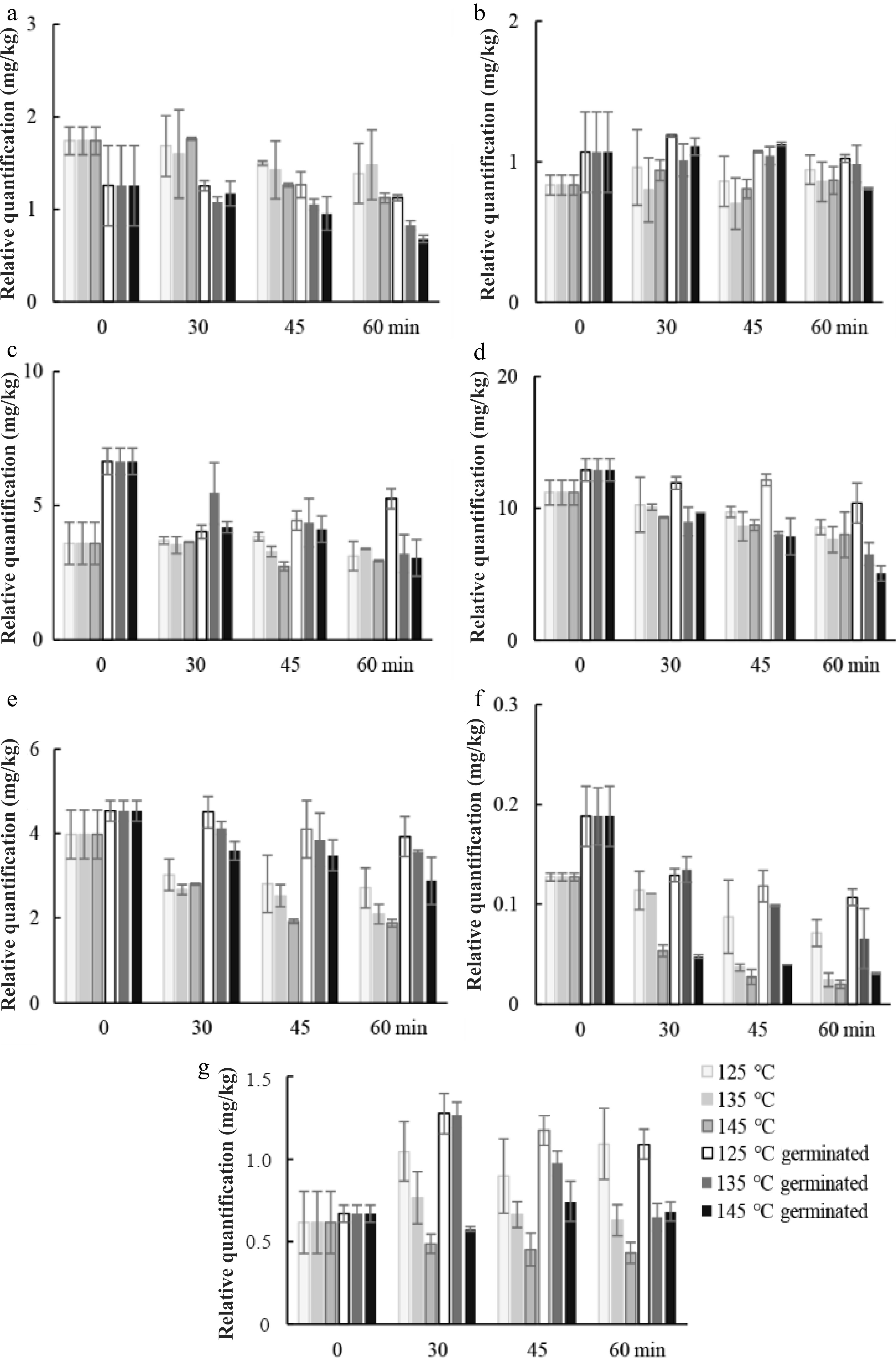
Figure 4.
Changes in relative quantification of representative compounds in fraction II. (a) C16:0 ffa, (b) C18:0 ffa, (c) C18:1 ffa, (d) C18:2 ffa, (e) C18:3 ffa, (f) α-tocopherol, and (g) β-sitosterol during roasting of germinated and ungerminated sunflower seeds at 125, 135, and 145 °C.
The effect of roasting on α-tocopherol content of the sunflower seeds is shown in Fig. 4. α-Tocopherol was the predominant tocopherol in the sunflower seeds. Germination increased α-tocopherol. As the results revealed, α-Tocopherols in ungerminated and germinated sunflower seeds exposed to roasting significantly (p < 0.05) decreased with temperature and time. The result was in agreement with those of Vaidya & Eun[19]. As a-tocopherol is not stable, higher temperatures and long-time heating resulted in a large loss of α-tocopherol. The highest content of α-tocopherol was observed at 125 °C for 30 min in RGSF. The value of α-tocopherol in different oils reported in the previous study was comparable to this study. Alpha-Tocopherol reduced around 20% after 15 min by microwave heating[15]. The highest rate of loss of α-tocopherol during heating was reported in the sunflower seeds[4].
Sterols, mainly found in the cell membranes are steroid alcohols, they are hydrophobic, hence, typically associated with the lipid fraction in plants. The sterols found in sunflower oils include β-sitosterol, stigmasterol, and campesterol. The change observed for β-sitosterol is shown in Fig. 4. An increase of β-sitosterol was observed in RUSF and RGSF at 125 and 135 °C less than 45 min, and increased more at 125 °C in RGSF compared to that in RUSF. While β-Sitosterol significantly decreased (p < 0.05) at 145 °C in RGSF and RUSF.
Fructose and glucose concentration was significantly higher (p < 0.05) in RGSF as a result of germination (Fig. 5). Roasting at 145 °C for a longer duration (45 and 60 min) might cause a higher reduction of fructose and glucose as the result of Maillard reaction. Sucrose decreased in RUSF and RGSF except for 125 °C of roasting, while mannitol increased during roasting.
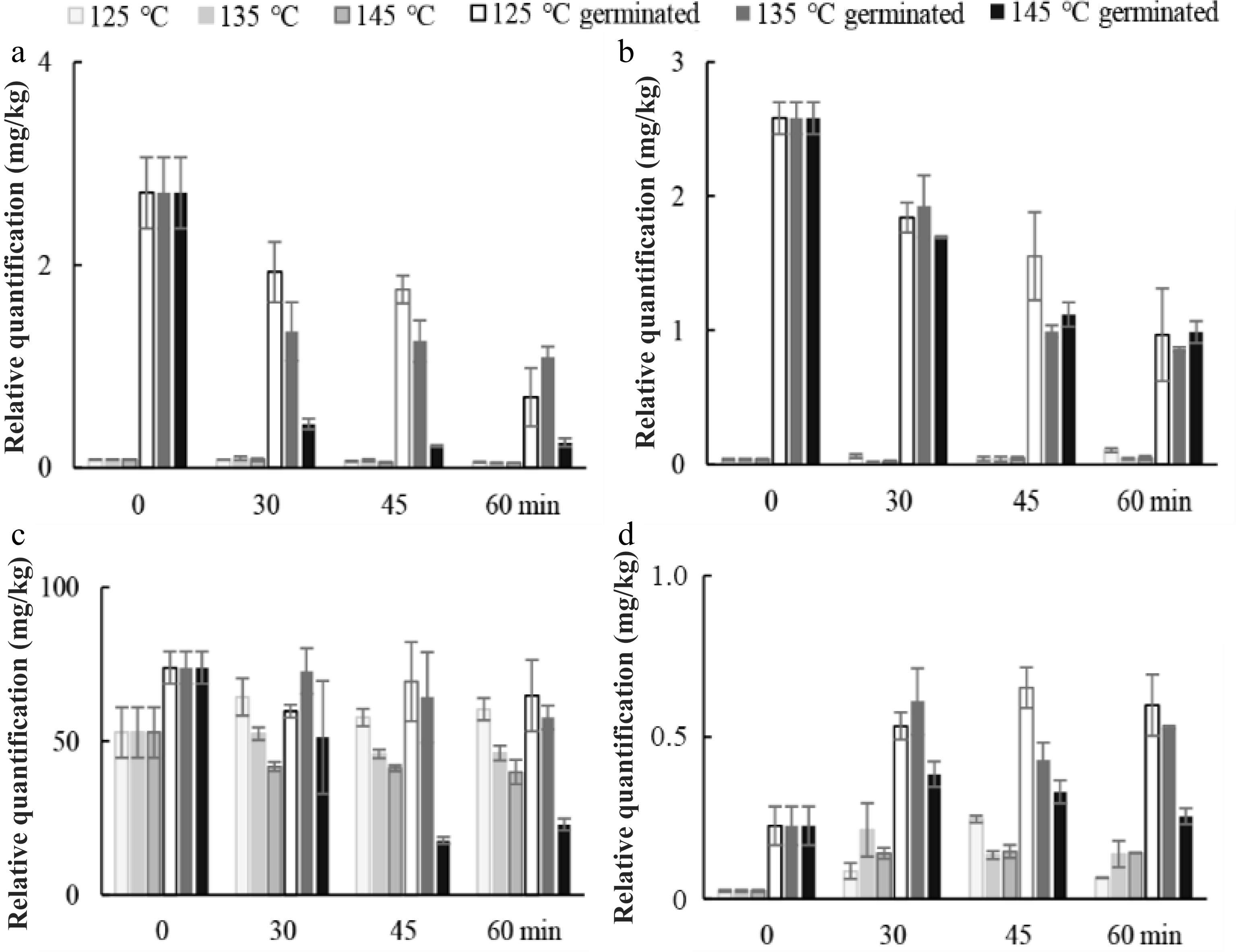
Figure 5.
Changes in relative quantification of representative compounds in fraction III. (a) fructose, (b) glucose, (c) sucrose, (d) mannitol during roasting of ungerminated and germinated sunflower seeds at 125, 135, and 145 °C.
The effects of roasting conditions on amino acid profiles and GABA of sunflower seeds are shown in Fig. 6. Free amino acids (alanine, glycine, serine, and phenylalanine) initially at low levels in RUSF, were highly decreased during roasting, especially at 145 °C. Roasting resulted in the reduction of alanine, glycine, threonine, phenylalanine, serine, asparagine, and GABA content in both RUSF and RGSF at three temperatures. This fact might be related to their inherent thermal stabilities. The decrease in amino acids may be due to their heat destruction[20]. The statement also agree with those reported by Damame et al.[21]. However, the increase of leucine and threonine content was observed during roasting. Therefore, possibly protease enzymatic activity could be taking place during this step, despite this not having been described previously. Lower temperature resulted in a lower loss of amino acid. The processes of boiling and roasting generally have very high total amino acid content compared with the fresh safflower seeds[22]. The reason for the variation in results among studies is not known. The amino acid content of RGSF was significantly (p < 0.05) higher than those of RUSF because of the increase of amino acids during germination. The content of alanine, glycine, phenylalanine, serine, asparagine, and GABA was higher at 125 °C compared to the other two temperatures. A 56%−72% decrease in GABA content was observed for RUSF. The overall changes of GABA at lower temperatures were much less than those observed at the higher temperature. GABA was largely involved in Maillard reaction during baking, resulting in GABA trace levels in wheat bread samples[23]. Whereas, the drying temperature did not significantly affect the GABA content of cooked germinated brown rice, except at the temperature of 130 °C for hot air drying[24]. Germination markedly improved GABA content in the RGSF. The content of GABA was greater higher at 125 °C than that at 135 and 145 °C. Roasting for 45 min at 125 °C resulted in the lowest reduction in GABA content.
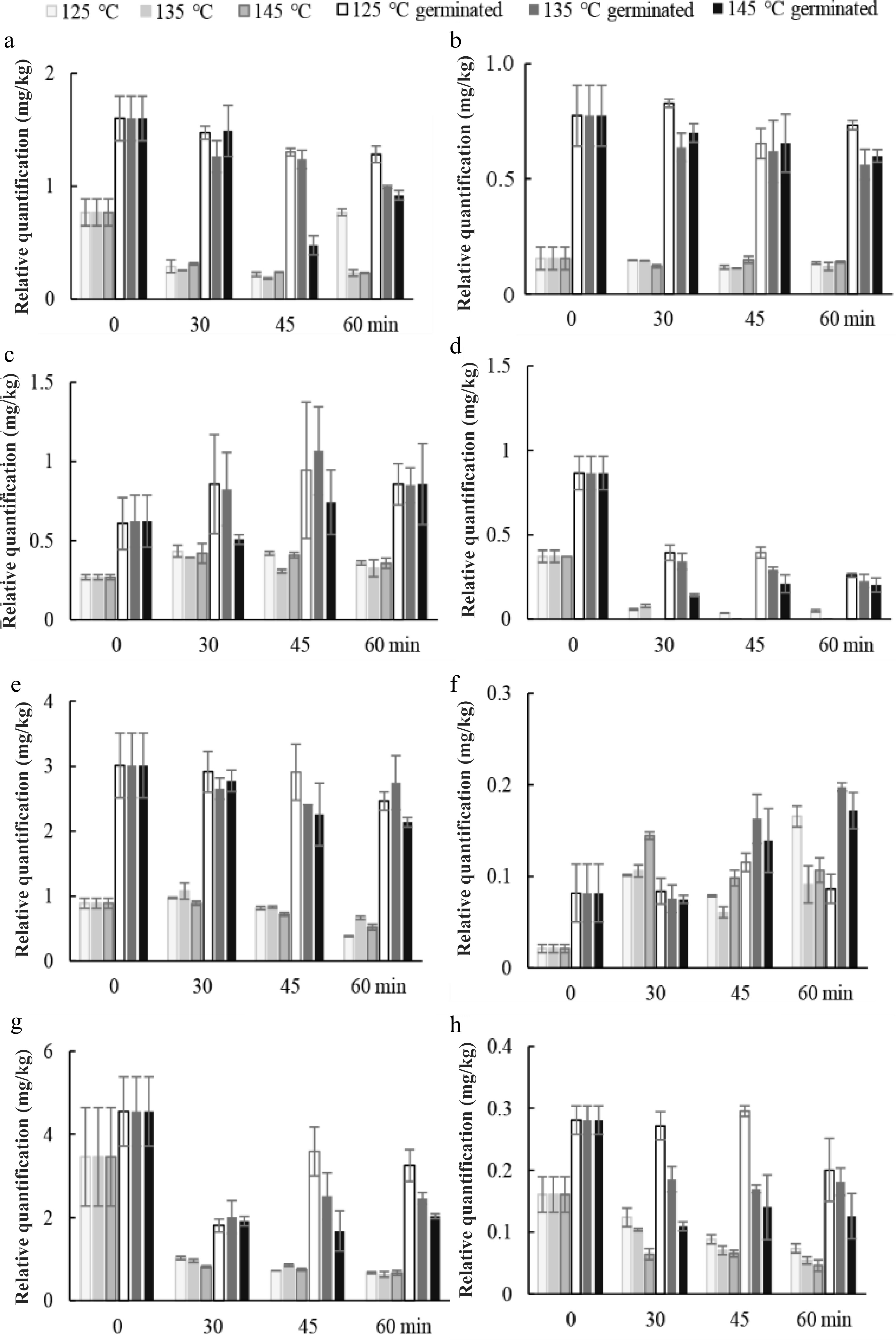
Figure 6.
Changes in relative quantification of representative compounds in fraction IV. (a) alanine, (b) glycine, (c) leucine, (d) phenylalanine, (e) serine, (f) threonine, (g) asparagine, and (h) GABA during roasting of germinated and ungerminated sunflower seed at 125, 135, and 145 °C.
Changes in flavor profiles of RUSF and RGSF
-
Flavoromics investigates sample constituents considered collectively and opens new perspectives for correlating the particular sensory attributes of food with its chemical composition. Tables 2 & 3 show concentrations (μg/g) of volatile components in RUSF and RGSF at 125, 135 and 145 °C for 30, 45, and 60 min. The main volatile components in RUSF and RGSF were 2-methylbutanal, pyridine, α-pinene, β-pinene, furfural, hexanal, pyrazines. The main volatile compounds in raw sunflower seeds were α-pinene, hexanal, furfural, octane, γ-butyrolactone. Table 4 shows the key odorants content obtained from RUSF and RGSF. 2,5-Dimeththylpyrazine, 2,3-dimethylpyrazine, 3-ethyl-2,5-dimethylpyrazine, 2-ethyl-3-methylpyrazine, and 2-ethyl-3,5-dimethylpyrazine, found in the RUSF and RGSF were to be considered representative of the active compound, suggesting that nutty, roast characteristics might contribute greatly to the aroma of roasted sunflower seeds.
Table 2. Concentration (μg/g) of volatile components in RUSF roasted from 125, 135, and 145 °C for 30, 45, and 60 min.
Components RI Relative concentration (μg/g) 0 125 °C 135 °C 145 °C 30 min 45 min 60 min 30 min 45 min 60 min 30 min 45 min 60 min Isobutanal 629 0.21 ± 0.06 0.10 ± 0.03 0.17 ± 0.04 − 0.35 ± 0.09 − 0.36 ± 0.05 − 0.48 ± 0.06 1.22 ± 0.16 3-Methylbutanal 646 0.28 ± 0.07 0.21 ± 0.06 0.31 ± 0.08 1.60 ± 0.43 − 0.19 ± 0.03 0.31 ± 0.04 0.18 ± 0.02 0.26 ± 0.04 0.74 ± 0.10 2-Methylbutanal 662 0.54 ± 0.14 0.27 ± 0.07 − − 0.55 ± 0.15 0.37 ± 0.05 0.77 ± 0.10 0.25 ± 0.03 0.77 ± 0.10 2.11 ± 0.28 3-Penten-2-one 711 0.45 ± 0.12 0.04 ± 0.01 0.32 ± 0.09 0.19 ± 0.05 0.59 ± 0.16 0.25 ± 0.03 0.42 ± 0.06 0.23 ± 0.03 0.23 ± 0.03 0.67 ± 0.09 Pyridine 717 0.11 ± 0.03 1.51 ± 0.41 1.19 ± 0.32 3.13 ± 0.84 − 0.74 ± 0.10 2.66 ± 0.36 0.92 ± 0.12 2.81 ± 0.38 2.7 ± 0.36 Toluene 751 0.24 ± 0.07 0.15 ± 0.04 0.29 ± 0.08 0.27 ± 0.07 0.19 ± 0.06 0.36 ± 0.05 0.40 ± 0.05 0.17 ± 0.02 0.18 ± 0.03 0.63 ± 0.08 Hexanal 757 1.35 ± 0.36 0.68 ± 0.18 1.35 ± 0.36 3.35 ± 0.09 1.74 ± 0.47 1.00 ± 0.13 1.96 ± 0.26 0.81 ± 0.11 2.50 ± 0.34 8.49 ± 1.14 Octane 796 2.67 ± 0.72 0.13 ± 0.03 0.54 ± 0.15 5.50 ± 1.48 1.77 ± 0.47 0.60 ± 0.08 1.15 ± 0.16 1.09 ± 0.15 − − Furfural 800 0.95 ± 0.25 0.57 ± 0.15 0.87 ± 0.23 2.98 ± 0.80 1.92 ± 0.52 1.21 ± 0.16 3.46 ± 0.47 3.63 ± 0.49 5.09 ± 0.68 8.18 ± 1.10 2,3,3-Trimethyl-1,4-pentadiene 805 − 0.12 ± 0.03 0.34 ± 0.09 1.21 ± 0.32 0.87 ± 0.23 0.41 ± 0.06 0.89 ± 0.12 − 0.85 ± 0.11 1.97 ± 0.26 Z-2-Octene 810 − 0.04 ± 0.01 0.26 ± 0.07 0.59 ± 0.16 0.61 ± 0.16 0.35 ± 0.05 0.67 ± 0.09 − 0.75 ± 0.10 1.07 ± 0.14 2,6-Dimethylheptane 828 0.67 ± 0.18 − − 1.34 ± 0.36 − 0.25 ± 0.03 0.86 ± 0.12 0.12 ± 0.02 0.40 ± 0.05 1.02 ± 0.14 Ethylcyclohexane 830 0.54 ± 0.13 0.22 ± 0.06 0.41 ± 0.11 1.72 ± 0.46 0.68 ± 0.18 0.40 ± 0.05 0.85 ± 0.11 0.25 ± 0.03 0.56 ± 0.07 1.63 ± 0.22 n-Hexanol 849 1.94 ± 0.52 1.23 ± 0.33 0.31 ± 0.08 0.20 ± 0.05 0.63 ± 0.17 0.35 ± 0.05 0.56 ± 0.07 0.16 ± 0.02 0.29 ± 0.04 0.04 ± 0.01 γ-Butyrolactone 850 1.15 ± 0.31 0.30 ± 0.08 0.90 ± 0.24 2.25 ± 0.60 1.36 ± 0.37 0.78 ± 0.10 1.72 ± 0.23 0.37 ± 0.05 1.43 ± 0.19 3.19 ± 0.43 Methional 854 − 0.13 ± 0.04 0.16 ± 0.04 0.59 ± 0.16 0.42 ± 0.11 0.54 ± 0.07 0.64 ± 0.08 0.39 ± 0.05 0.51 ± 0.07 0.64 ± 0.09 2-Methyloctane 863 0.47 ± 0.13 − 0.36 ± 0.10 1.26 ± 0.34 − 0.33 ± 0.04 0.73 ± 0.10 0.29 ± 0.04 0.36 ± 0.05 0.97 ± 0.15 2-Heptanone 866 − − − 0.42 ± 0.11 − − 0.24 ± 0.03 − 0.23 ± 0.03 0.54 ± 0.07 3-Methyl-octane 870 0.26 ± 0.07 0.05 ± 0.01 0.24 ± 0.06 0.81 ± 0.22 0.34 ± 0.09 0.20 ± 0.03 0.49 ± 0.07 0.14 ± 0.02 0.29 ± 0.04 0.96 ± 0.13 2,5-Dimethylpyrazine 885 0.20 ± 0.05 0.99 ± 0.27 0.98 ± 0.28 1.71 ± 0.46 2.47 ± 0.66 0.81 ± 0.11 2.56 ± 0.34 0.54 ± 0.07 5.17 ± 0.69 10.19 ± 1.37 2,3-Dimethylpyrazine 891 − − − − − − − − 0.55 ± 0.07 1.30 ± 0.17 n-Nonane 899 0.28 ± 0.07 0.06 ± 0.02 0.22 ± 0.06 0.70 ± 0.19 0.37 ± 0.10 0.16 ± 0.02 0.51 ± 0.07 0.14 ± 0.02 0.33 ± 0.04 0.96 ± 0.13 Benzaldehyde 926 0.35 ± 0.09 0.11 ± 0.03 0.16 ± 0.04 0.60 ± 0.16 0.63 ± 0.17 0.36 ± 0.05 0.40 ± 0.05 0.08 ± 0.01 0.87 ± 0.12 0.75 ± 0.10 α-Pinene 930 7.57 ± 2.03 7.05 ± 1.89 12.50 ± 3.4 12.70 ± 3.4 10.79 ± 2.9 6.56 ± 0.88 13.71 ± 1.8 4.72 ± 0.63 10.20 ± 1.4 21.85 ± 2.9 Camphene 943 0.34 ± 0.09 0.06 ± 0.02 0.48 ± 0.13 0.20 ± 0.05 0.41 ± 0.11 0.28 ± 0.04 0.53 ± 0.07 0.17 ± 0.02 0.36 ± 0.05 0. 96 ± 0.13 Thujane-2,4(10)-diene 946 − 0.04 ± 0.01 − 0.61 ± 0.16 − − − 0.04 ± 0.00 0.14 ± 0.02 0.35 ± 0.05 Phenol 951 0.27 ± 0.07 0.08 ± 0.02 0.25 ± 0.07 0.55 ± 0.15 0.24 ± 0.06 0.11 ± 0.01 0.29 ± 0.04 0.06 ± 0.01 − − 1-Octen-3-ol 959 0.27 ± 0.06 0.06 ± 0.02 − − 0.27 ± 0.06 0.17 ± 0.02 − 0.38 ± 0.05 0.06 ± 0.01 − β-Phellandrene 965 0.24 ± 0.07 0.15 ± 0.04 0.39 ± 0.10 1.83 ± 0.49 0.48 ± 0.13 0.20 ± 0.03 0.52 ± 0.07 0.13 ± 0.02 0.37 ± 0.05 0.80 ± 0.11 3-Methylnonane 968 0.13 ± 0.03 0.04 ± 0.01 0.14 ± 0.04 0.50 ± 0.13 0.19 ± 0.05 0.10 ± 0.01 0.26 ± 0.04 0.05 ± 0.01 − − β-Pinene 970 0.76 ± 0.20 0.16 ± 0.04 0.98 ± 0.26 1.66 ± 0.45 0.85 ± 0.23 0.53 ± 0.07 1.15 ± 0.15 0.37 ± 0.05 1.25 ± 0.17 3.00 ± 0.40 2-Ethyl-3-methylpyrazine 974 0.14 ± 0.04 0.36 ± 0.10 0.54 ± 0.15 0.88 ± 0.24 1.63 ± 0.44 2.27 ± 0.31 2.86 ± 0.38 0.18 ± 0.02 1.96 ± 0.26 3.89 ± 0.52 2-Pentylfuran 976 0.33 ± 0.09 0.21 ± 0.06 0.14 ± 0.04 1.53 ± 0.41 0.54 ± 0.14 0.13 ± 0.02 0.30 ± 0.04 0.07 ± 0.01 0.53 ± 0.07 1.17 ± 0.16 3(E)-3-methyl-3-nonene 981 0.18 ± 0.05 0.07 ± 0.02 0.16 ± 0.04 0.58 ± 0.15 0.29 ± 0.08 0.13 ± 0.02 0.31 ± 0.04 0.07 ± 0.01 0.16 ± 0.02 0.74 ± 0.10 Pantoic lactone 987 0.12 ± 0.03 0.05 ± 0.01 0.06 ± 0.02 − 0.14 ± 0.04 0.07 ± 0.01 0.19 ± 0.03 − 0.32 ± 0.04 0.49 ± 0.07 4-Decene 989 0.32 ± 0.09 0.18 ± 0.05 0.34 ± 0.09 − 0.51 ± 0.14 0.26 ± 0.04 1.03 ± 0.14 0.22 ± 0.03 0.56 ± 0.08 1.84 ± 0.25 Phenylacetaldehyde 1007 0.27 ± 0.07 0.34 ± 0.09 0.77 ± 0.21 1.26 ± 0.22 0.47 ± 0.13 0.13 ± 0.02 0.27 ± 0.04 0.95 ± 0.13 1.25 ± 0.17 1.29 ± 0.17 p-Cymene 1011 0.11 ± 0.03 0.05 ± 0.01 − 0.37 ± 0.10 0.23 ± 0.06 0.07 ± 0.01 0.16 ± 0.02 − 0.33 ± 0.04 0.70 ± 0.09 D-Limonene 1020 0.27 ± 0.07 0.16 ± 0.04 0.28 ± 0.08 1.15 ± 0.31 0.44 ± 0.12 0.20 ± 0.03 0.47 ± 0.06 0.10 ± 0.01 0.17 ± 0.02 0.39 ± 0.05 1,4-Butanediol diacetate 1041 0.92 ± 0.25 0.15 ± 0.04 0.89 ± 0.24 0.71 ± 0.18 1.26 ± 0.30 0.49 ± 0.07 1.50 ± 0.20 0.22 ± 0.03 0.69 ± 0.09 1.68 ± 0.23 2-Ethyl-3,5-dimethylpyrazine 1053 − 0.19 ± 0.05 0.19 ± 0.04 0.36 ± 0.10 0.30 ± 0.08 0.12 ± 0.02 0.53 ± 0.07 0.07 ± 0.01 1.11 ± 0.15 2.09 ± 0.28 N-allyl- cyclopentanecarboxamide 1056 1.52 ± 0.41 0.58 ± 0.16 1.37 ± 0.37 − 1.71 ± 0.46 1.02 ± 0.14 − 0.67 ± 0.09 − − 2,4-Dimethyl-1-decene 1065 0.25 ± 0.07 0.12 ± 0.03 0.23 ± 0.06 0.89 ± 0.24 0.27 ± 0.07 0.17 ± 0.02 0.57 ± 0.08 0.11 ± 0.02 0.38 ± 0.05 1.01 ± 0.14 Nonanal 1081 0.32 ± 0.09 0.21 ± 0.06 0.51 ± 0.14 0.79 ± 0.21 0.24 ± 0.06 0.54 ± 0.07 0.63 ± 0.08 0.44 ± 0.06 0.56 ± 0.07 1.06 ± 0.14 6-Ethyl-3-octanone 1083 0.38 ± 0.10 − − 1.25 ± 0.34 0.48 ± 0.13 0.26 ± 0.03 0.73 ± 0.10 0.16 ± 0.03 0.39 ± 0.05 1.19 ± 0.16 2,6-Dimethylundecane 1120 − − − 1.64 ± 0.45 − − 1.09 ± 0.15 − 0.54 ± 0.07 3.97 ± 0.53 6,6-Dimethylundecane 1140 0.42 ± 0.11 0.22 ± 0.06 0.44 ± 0.12 1.99 ± 0.53 0.84 ± 0.22 0.35 ± 0.05 1.26 ± 0.17 0.19 ± 0.03 0.65 ± 0.09 1.94 ± 0.26 5-Methylundecane 1157 0.28 ± 0.08 0.10 ± 0.02 0.39 ± 0.10 0.74 ± 0.20 0.66 ± 0.18 0.19 ± 0.02 0.51 ± 0.07 0.09 ± 0.01 0.26 ± 0.02 0.74 ± 0.10 3-Methylundecane 1171 − − − 0.68 ± 0.18 − − 0.48 ± 0.06 − 0.26 ± 0.03 0.70 ± 0.09 1-Dodecene 1189 0.27 ± 0.07 0.13 ± 0.03 0.17 ± 0.05 0.45 ± 0.12 0.58 ± 0.16 0.19 ± 0.03 0.49 ± 0.07 0.010 ± 0.01 0.26 ± 0.03 0.67 ± 0.09 Dodecane 1201 0.35 ± 0.10 0.13 ± 0.04 0.34 ± 0.09 0.73 ± 0.19 0.65 ± 0.19 0.20 ± 0.03 0.55 ± 0.07 0.13 ± 0.02 0.31 ± 0.04 0.80 ± 0.11 3-Methyltridecane 1368 − − − − 0.09 ± 0.02 0.10 ± 0.01 − − − − 1-Tetradecene 1388 0.14 ± 0.04 0.07 ± 0.02 0.14 ± 0.04 0.25 ± 0.07 0.40 ± 0.11 0.10 ± 0.01 0.31 ± 0.04 0.12 ± 0.02 0.13 ± 0.01 0.44 ± 0.06 Tetradecane 1399 0.13 ± 0.03 0.06 ± 0.02 0.02 ± 0.00 − 0.19 ± 0.05 0.09 ± 0.01 0.03 ± 0.00 0.01 ± 0.00 0.03 ± 0.01 0.31 ± 0.04 Octyl methacrylate 1424 0.18 ± 0.03 − 0.17 ± 0.05 0.33 ± 0.09 0.35 ± 0.09 0.11 ± 0.01 0.29 ± 0.04 0.06 ± 0.01 0.14 ± 0.02 0.40 ± 0.04 7-Methyltridecane 1429 0.20 ± 0.05 0.08 ± 0.02 0.16 ± 0.04 0.38 ± 0.10 0.40 ± 0.11 0.12 ± 0.02 0.33 ± 0.04 0.06 ± 0.02 0.116 ± 0.02 0.41 ± 0.05 Calarene 1433 0.31 ± 0.08 0.12 ± 0.03 0.33 ± 0.09 0.62 ± 0.17 0.57 ± 0.15 0.20 ± 0.03 0.42 ± 0.06 0.08 ± 0.01 0.24 ± 0.02 0.70 ± 0.09 Chamigrene 1456 0.12 ± 0.03 − 0.12 ± 0.03 − 0.20 ± 0.05 0.07 ± 0.01 − − − − 5,6-Dipropyldecane 1465 0.46 ± 0.12 0.18 ± 0.05 0.39 ± 0.10 − 0.86 ± 0.23 0.27 ± 0.04 − 0.14 ± 0.02 − − β-Bisabolene 1499 0.27 ± 0.07 0.03 ± 0.01 0.33 ± 0.09 − 0.42 ± 0.11 0.20 ± 0.03 0.27 ± 0.04 0.07 ± 0.01 0.21 ± 0.03 0.48 ± 0.06 Table 3. Concentrations (μg/g) of volatile components in RGSF roasted from 125, 135, and 145 °C for 30, 45, and 60 min.
Components RI Relative concentration (μg/g) 0 125 °C 135 °C 145 °C 30 min 45 min 60 min 30 min 45 min 60 min 30 min 45 min 60 min Isobutanal 629 0.21 ± 0.06 0.06 ± 0.02 0.40 ± 0.11 2.39 ± 0.60 − − 0.55 ± 0.15 2.41 ± 0.65 2.78 ± 0.70 5.77 ± 1.32 3-Methylbutanal 646 0.28 ± 0.04 0.22 ± 0.03 1.22 ± 0.17 5.28 ± 0.75 0.04 ± 0.01 1.80 ± 0.25 1.17 ± 0.17 2.85 ± 0.40 2.58 ± 0.37 3.12 ± 0.44 2-Methylbutanal 662 0.54 ± 0.08 0.29 ± 0.04 1.07 ± 0.15 5.47 ± 0.77 − 2.54 ± 0.36 1.06 ± 0.15 5.11 ± 0.72 6.81 ± 0.96 14.02 ± 1.98 3-Penten-2-one 711 0.45 ± 0.06 0.09 ± 0.01 0.11 ± 0.02 0.40 ± 0.06 − − 0.46 ± 0.06 0.25 ± 0.03 0.22 ± 0.03 − Pyridine 717 0.11 ± 0.02 0.03 ± 0.00 − − − − − 1.06 ± 0.15 0.35 ± 0.05 1.41 ± 0.20 1-Pentanol 744 0.19 ± 0.03 0.03 ± 0.01 0.03 ± 0.01 0.09 ± 0.02 − 0.22 ± 0.04 0.05 ± 0.01 − 0.13 ± 0.02 0.93 ± 0.16 Toluene 751 0.24 ± 0.04 0.11 ± 0.02 0.06 ± 0.01 0.12 ± 0.02 0.09 ± 0.02 0.60 ± 0.10 0.16 ± 0.03 0.09 ± 0.02 0.12 ± 0.02 0.84 ± 0.14 Hexanal 757 1.35 ± 0.23 0.82 ± 0.14 − 1.19 ± 0.20 0.54 ± 0.09 1.33 ± 0.23 1.48 ± 0.25 0.79 ± 0.13 1.29 ± 0.22 3.75 ± 0.64 2-Methylpyrazine 795 − − 0.40 ± 0.07 2.80 ± 0.48 1.50 ± 0.25 8.58 ± 1.46 4.52 ± 0.77 6.63 ± 1.12 7.82 ± 1.33 24.15 ± 4.10 Octane 796 2.67 ± 0.45 0.24 ± 0.04 0.18 ± 0.03 − 0.28 ± 0.05 0.91 ± 0.15 0.32 ± 0.05 − − − Furfural 800 0.79 ± 0.10 0.37 ± 0.05 0.36 ± 0.05 3.69 ± 0.47 − − 0.53 ± 0.07 1.33 ± 0.17 21.17 ± 2.70 95.00 ± 12.09 Z-2-Octene 810 − 0.12 ± 0.02 0.10 ± 0.01 − 0.04 ± 0.01 − 0.10 ± 0.01 − − − Furfuryl alcohol 825 − 1.41 ± 0.18 0.24 ± 0.03 − 3.64 ± 0.46 0.13 ± 0.02 − 1.56 ± 0.20 1.27 ± 0.16 − 2,6-Dimethylheptane 828 0.67 ± 0.08 0.17 ± 0.02 − − 0.05 ± 0.01 0.80 ± 0.10 0.14 ± 0.02 − − − Ethylcyclohexane 830 0.54 ± 0.07 0.22 ± 0.03 0.14 ± 0.02 0.76 ± 0.10 0.12 ± 0.01 0.60 ± 0.08 0.25 ± 0.03 − 0.85 ± 0.11 − n-Hexanol 849 1.94 ± 0.49 0.06 ± 0.01 0.03 ± 0.01 0.16 ± 0.04 0.04 ± 0.01 0.54 ± 0.14 0.10 ± 0.02 0.08 ± 0.02 − − γ-Butyrolactone 850 1.15 ± 0.29 − − 0.53 ± 0.13 − − − 0.36 ± 0.09 0.70 ± 0.18 2.24 ± 0.57 Methional 854 − − 0.35 ± 0.09 1.09 ± 0.28 − − 0.45 ± 0.11 0.31 ± 0.08 − − 2-Methyloctane 863 0.47 ± 0.12 0.20 ± 0.05 − − 0.09 ± 0.02 1.70 ± 0.43 − − − − 2-Heptanone 866 − − 0.07 ± 0.02 0.30 ± 0.04 − − − 0.24 ± 0.06 0.26 ± 0.07 1.03 ± 0.20 3-Methyloctane 870 0.26 ± 0.07 0.01 ± 0.03 0.08 ± 0.02 0.32 ± 0.08 0.06 ± 0.02 1.06 ± 0.27 0.15 ± 0.04 − 0.32 ± 0.08 − Heptanal 875 0.13 ± 0.03 0.08 ± 0.02 − 0.01 ± 0.01 0.03 ± 0.01 0.04 ± 0.00 0.02 ± 0.00 − − 0.02 ± 0.00 2-Acetylfuran 878 − − − 0.36 ± 0.04 − − − 0.33 ± 0.05 1.07 ± 0.12 − 2,5-Dimethylpyrazine 883 0.20 ± 0.03 0.06 ± 0.01 2.13 ± 0.30 13.15 ± 1.86 − − 0.44 ± 0.06 13.1 ± 1.85 25.71 ± 3.64 72.55 ± 10.28 Ethylpyrazine 885 − − 0.49 ± 0.07 2.84 ± 0.40 − − − 2.37 ± 0.34 3.99 ± 0.56 − 2,3-Dimethylpyrazine 891 − − 0.27 ± 0.04 1.82 ± 0.26 − − − 1.63 ± 0.23 2.82 ± 0.40 9.58 ± 1.26 n-Nonane 899 0.28 ± 0.04 0.10 ± 0.01 0.11 ± 0.02 0.48 ± 0.07 0.06 ± 0.01 0.76 ± 0.11 0.14 ± 0.02 0.22 ± 0.01 0.33 ± 0.05 − Benzaldehyde 926 0.35 ± 0.05 0.14 ± 0.03 0.20 ± 0.03 1.34 ± 0.19 0.07 ± 0.01 0.58 ± 0.08 0.21 ± 0.12 0.86 ± 0.15 1.62 ± 0.23 − α-Pinene 930 7.57 ± 1.07 3.76 ± 0.53 3.58 ± 0.60 11.23 ± 1.59 4.65 ± 0.66 38.85 ± 5.49 10.93 ± 1.55 7.37 ± 1.04 10.93 ± 1.55 33.03 ± 4.67 Camphene 943 0.34 ± 0.05 0.18 ± 0.03 0.17 ± 0.02 0.66 ± 0.09 0.15 ± 0.02 1.65 ± 0.23 0.42 ± 0.06 0.42 ± 0.05 0.33 ± 0.06 3.24 ± 0.46 Thujane-2,4(10)-diene 946 − 0.06 ± 0.01 0.11 ± 0.02 0.45 ± 0.06 0.03 ± 0.00 0.48 ± 0.07 0.17 ± 0.02 0.54 ± 0.08 0.87 ± 0.12 − Phenol 951 0.27 ± 0.04 − − 0.18 ± 0.03 − − − 0.11 ± 0.02 0.28 ± 0.04 − 1-Octen-3-ol 959 0.27 ± 0.05 − − 0.20 ± 0.04 − − − 6.18 ± 1.14 13.38 ± 2.01 − β-Phellandrene 965 0.24 ± 0.05 0.10 ± 0.01 0.08 ± 0.02 0.32 ± 0.06 0.10 ± 0.02 1.21 ± 0.22 0.30 ± 0.06 0.16 ± 0.03 − − 3-Methylnonane 968 0.13 ± 0.02 0.07 ± 0.01 − − − 0.40 ± 0.07 − − − − β-Pinene 970 0.76 ± 0.14 0.37 ± 0.07 0.62 ± 0.11 − 0.40 ± 0.07 4.15 ± 0.76 1.02 ± 0.19 0.26 ± 0.05 − − 2-Ethyl-3-methylpyrazine 974 0.74 ± 0.11 0.06 ± 0.01 0.99 ± 0.18 6.84 ± 1.26 − − 0.21 ± 0.04 3.70 ± 0.68 4.11 ± 0.72 − 2-Pentylfuran 976 0.33 ± 0.06 0.16 ± 0.02 0.36 ± 0.07 − 0.05 ± 0.01 0.44 ± 0.08 0.27 ± 0.05 1.70 ± 0.31 − − 3(E)-3-methyl-3-nonene 981 0.18 ± 0.02 0.08 ± 0.01 0.14 ± 0.03 0.47 ± 0.09 0.04 ± 0.01 0.48 ± 0.08 0.14 ± 0.03 0.27 ± 0.05 0.68 ± 0.13 − Pantoic lactone 987 0.12 ± 0.02 − − − − − − − 0.44 ± 0.05 4-Decene 989 0.32 ± 0.06 0.26 ± 0.05 − − 0.04 ± 0.01 1.50 ± 0.28 0.24 ± 0.05 − − − Phenylacetaldehyde 1007 0.27 ± 0.04 0.14 ± 0.02 0.78 ± 0.11 5.15 ± 0.69 0.04 ± 0.01 0.61 ± 0.09 0.41 ± 0.06 1.11 ± 0.16 1.13 ± 0.11 0.77 ± 0.11 p-Cymene 1011 0.11 ± 0.02 − 0.12 ± 0.02 0.88 ± 0.12 − 0.27 ± 0.04 0.18 ± 0.03 0.49 ± 0.07 1.15 ± 0.16 3.73 ± 0.50 D-Limonene 1020 0.27 ± 0.04 0.10 ± 0.01 0.13 ± 0.02 0.26 ± 0.03 0.05 ± 0.01 0.77 ± 0.11 0.26 ± 0.04 0.83 ± 0.12 − 0.69 ± 0.10 2-Ethyl-3,5-dimethylpyrazine 1053 0.36 ± 0.05 0.02 ± 0.00 0.73 ± 0.10 6.59 ± 0.93 − − 0.10 ± 0.01 5.84 ± 0.83 16.60 ± 2.35 30.74 ± 4.85 N-allyl- cyclopentanecarboxamide 1056 1.52 ± 0.22 0.65 ± 0.09 0.63 ± 0.09 − 0.38 ± 0.05 − 0.93 ± 0.13 1.31 ± 0.19 2.82 ± 0.40 − 3-Ethyl-2,5-dimethylpyrazine 1059 − − − 0.66 ± 0.09 − − − 0.53 ± 0.08 1.78 ± 0.25 3.83 ± 0.54 2,5-Diethylpyrazine 1063 − − − 0.27 ± 0.04 − − − 0.07 ± 0.01 0.49 ± 0.07 − 2,4-Dimethyl-1-decene 1065 0.25 ± 0.04 0.10 ± 0.01 − − 0.07 ± 0.01 − 0.16 ± 0.02 0.11 ± 0.02 0.51 ± 0.07 − 1-Methyl-4-isopropenylbenzene 1072 − − − − − − − 0.55 ± 0.08 3.04 ± 0.43 6.89 ± 0.81 Nonanal 1081 0.32 ± 0.07 0.15 ± 0.03 0.15 ± 0.02 0.92 ± 0.19 0.10 ± 0.02 0.76 ± 0.16 0.24 ± 0.04 0.35 ± 0.07 1.96 ± 0.42 − 2-Acetyl-3-methylpyrazine 1085 − − − − − − − − 1.85 ± 0.39 3.08 ± 0.65 5-Ethyl-1-nonene 1086 − 0.18 ± 0.04 0.16 ± 0.03 0.79 ± 0.17 0.11 ± 0.02 1.00 ± 0.19 0.25 ± 0.05 0.40 ± 0.09 − − 2,6-Dimethylundecane 1120 − − − 1.20 ± 0.20 − 1.20 ± 0.25 − − − − Verbenol 1128 − 0.38 ± 0.10 0.47 ± 0.12 2.62 ± 0.42 0.23 ± 0.06 1.92 ± 0.49 0.64 ± 0.16 1.11 ± 0.28 2.87 ± 0.65 4.07 ± 1.04 2,3-Diethyl-5-methylpyrazine 1133 − − − 0.49 ± 0.13 − − − 0.31 ± 0.08 1.33 ± 0.34 1.79 ± 0.46 2,6-Diethyl-3-methylpyrazine 1135 0.39 ± 0.10 0.15 ± 0.04 − − 0.11 ± 0.04 − 0.23 ± 0.01 0.85 ± 0.22 2.69 ± 0.57 6,6-Dimethylundecane 1140 0.42 ± 0.11 0.22 ± 0.05 0.22 ± 0.06 1.24 ± 0.32 0.10 ± 0.02 1.56 ± 0.09 0.33 ± 0.08 0.46 ± 0.12 1.26 ± 0.15 − 5-Methylundecane 1157 0.28 ± 0.03 0.10 ± 0.02 0.10 ± 0.02 0.30 ± 0.08 0.09 ± 0.02 0.68 ± 0.15 0.14 ± 0.04 0.28 ± 0.07 0.83 ± 0.21 − 2-Methyl-5-[(1E)-1-propenyl]pyrazine 1160 − − − − − − − 0.45 ± 0.12 1.85 ± 0.47 − Myrtenal 1170 − − − − − − − 0.18 ± 0.05 0.73 ± 0.10 − 3-Methylundecane 1171 − − − 0.64 ± 0.12 − 0.48 ± 0.16 − 0.20 ± 0.05 0.79 ± 0.15 1.93 ± 0.49 1,4-Butanediol diacetate 1177 0.92 ± 0.23 0.03 ± 0.01 0.04 ± 0.01 0.75 ± 0.19 0.04 ± 0.01 0.12 ± 0.03 0.05 ± 0.01 0.25 ± 0.06 1.31 ± 0.33 − Myrtenol 1178 − 0.19 ± 0.05 0.21 ± 0.02 0.67 ± 0.17 − 0.44 ± 0.11 0.17 ± 0.02 0.50 ± 0.01 0.80 ± 0.02 1.26 ± 0.32 2,5-Dimethyl-3-(2-methylpropyl)-pyrazine 1181 − − − 0.39 ± 0.02 − 0.43 ± 0.11 − − 0.37 ± 0.09 − 1-Dodecene 1189 0.27 ± 0.07 0.06 ± 0.02 0.11 ± 0.03 0.79 ± 0.15 0.09 ± 0.01 0.41 ± 0.10 0.13 ± 0.03 0.24 ± 0.04 1.04 ± 0.26 − Dodecane 1201 0.35 ± 0.09 0.10 ± 0.01 0.09 ± 0.02 0.75 ± 0.19 0.10 ± 0.03 0.40 ± 0.10 0.15 ± 0.02 0.27 ± 0.07 0.88 ± 0.19 2.03 ± 0.36 3-Methyl-2(E)-undecene 1203 0.12 ± 0.02 − − − 0.04 ± 0.01 − 0.03 ± 0.00 − 0.34 ± 0.05 − 3-Methyltridecane 1368 − − − − − − − 0.07 ± 0.01 0.32 ± 0.04 − 1-Tetradecene 1388 0.14 ± 0.02 0.07 ± 0.01 0.10 ± 0.01 0.48 ± 0.07 0.05 ± 0.01 0.15 ± 0.02 0.07 ± 0.01 0.11 ± 0.02 0.73 ± 0.10 − Tetradecane 1399 0.13 ± 0.02 − − 0.20 ± 0.03 0.01 ± 0.00 − − 0.02 ± 0.00 0.21 ± 0.03 − Octyl methacrylate 1424 0.18 ± 0.03 0.07 ± 0.01 0.07 ± 0.01 0.47 ± 0.05 0.05 ± 0.01 0.20 ± 0.03 0.08 ± 0.01 0.14 ± 0.02 0.56 ± 0.08 − 7-Methyltridecane 1429 0.20 ± 0.03 0.07 ± 0.01 0.08 ± 0.01 0.56 ± 0.08 0.06 ± 0.01 0.24 ± 0.02 0.08 ± 0.01 0.16 ± 0.03 0.65 ± 0.12 Calarene 1433 0.31 ± 0.04 0.12 ± 0.02 0.09 ± 0.01 0.74 ± 0.10 0.12 ± 0.02 0.56 ± 0.15 0.19 ± 0.02 0.23 ± 0.03 0.78 ± 0.11 − Chamigrene 1456 0.12 ± 0.02 0.04 ± 0.01 − − − − − − 0.23 ± 0.03 − 5,6-Dipropyldecane 1465 0.46 ± 0.05 0.16 ± 0.02 0.17 ± 0.03 0.37 ± 0.05 0.14 ± 0.02 − 0.18 ± 0.03 − 0.40 ± 0.06 − β-Bisabolene 1499 0.27 ± 0.04 0.10 ± 0.01 0.09 ± 0.02 0.83 ± 0.12 0.15 ± 0.02 0.64 ± 0.09 0.20 ± 0.03 0.19 ± 0.04 0.77 ± 0.11 0.26 ± 0.04 Table 4. Key odorants content obtained from RUSF and RGSF which were roasted at 125, 135, and 145 °C for 30, 45, and 60 min.
Compounds Odor quality RUSF seeds RGSF seeds Pyrazines 2,5-Dimethylpyrazine Roasty, flowery, cocoa 5,7,9,10 3,4,8.9,10 2,3-Dimethylpyrazine Nut, peanut, cocoa, meat 9,10 3,4,8,9,10 3-Ethyl-2,5-dimethylpyrazine Potato, roast N 4,8,9,10 2-Ethyl-3-methylpyrazine Nutty, cereal like 1,2, 3,4,5,6,7,8,9 1,3,4,7,8,9 2-Ethyl-3,5-dimethylpyrazine Nutty 2,3,4,5,6,7,8,9,10 1,2,3,4,7,8,9,10 Aldehydes 2-Methylbutanal Cocoa, almond 1,2,5,6,7,8,9,10 1,2,3,4,6,7,8,9,10 3-Methylbutanal Malt 1,2,4,6.7.8,9,10 1,2,3,4,5,6,7,8,9,10 Hexanal Green, fatty 1,2,3,4,5,6,7,8,9,10 1,2,4,5,6,7,8,9,10 Furfural Bread, almond, sweet 7,8,9,10 4,9,10 Heptanal Fat, citrus, rancid N N Nonanal Fatty, green 1,3,4,6,7,8,9,10 1,4,6.8.9 Benzaldehyde Almond, sugar N N Phenylacetaldehyde Flowery, honey like 1,2,3,4,5,6,7,8,9,10 1,2,3,4,5,6,7,8,9,10 Ketones 2-Heptanone Soap N N ɣ-Butyrolactone Creamy 1,4,5,7,9,10 10 Alcohols 1-Pentanol Balsamic 5 10 1-Hexanol Resin, flower, green 1,2,5,7 1,6 Furfuryl alcohol Sweet, caramellic N 5 Pyridine Burnt, smoky 4,7,9,10 N Sulfur compounds Dimethyl disulfide Onion, cabbage, putrid N 9,10 Methional Cooked, potato 2,3,4,7,8,9,11,12,13 3,4,7,8 Others α-Pinene Pine, turpentine 1,2,3,5 ,6,9,11,12,13 1,2,3,4,5,6,7,8,9,10 2-Acetylfuran Peanut, sweet N N Number representation: 1 (raw seed), 2 (125 °C 30 min), 3 (125 °C 45 min), 4 (125 °C 60 min), 5 (135 °C 30 min), 6 (135 °C 45 min), 7 (135 °C 60 min), 8 (145 °C 30 min), 9 (145 °C 45 min), 10 (145 °C 60 min). The dominant pyrazines presented in RUSF were 2-ethyl-3,5-dimethylpyrazine, 2,6-diethyl-3-methylpyrazine, 2,3-dimethylpyrazine, 2-ethyl-3-methylpyrazine, 2,5-dimethylpyrazine. While the major pyrazines in the RGSF samples were 2-methylpyrazine, 2,3-dimethylpyrazine, 2,5-dimethylpyrazine, 2-ethyl-6methylpyrazine, 2-methyl-5-ethylpyrazine and 2-ethyl-3,5-dimethylpyrazine. These compounds formed during Maillard reaction, under conditions similar to those used in this study has been reported in a previous study[25]. The reducing sugars and amino acids increased during germination that leads to a significant increase of pyrazines in RGSF compared to RUSF. Heating L-threonine could form 2,5-dimethylpyrazine. Dehydration of heating L-serine following decarbonylation could form products or intermediates methylpyrazine and ethylpyrazine[26]. The content of 2-methylpyrazine, 2,5-dimethylpyrazine, 2,3-dimethylpyrazine, 2-ethyl-3,5-dimethylpyrazine at 60 min were 24.15, 72.53, 9.58, and 30.74 μg/g in RGSF, respectively. During roasting ungerminated sunflower seeds, 2,3-dimethylpyrazine required a minimum reaction time of 45 min, corresponding to a temperature of at least 145 °C. When heated at 145 °C for 45 and 60 min, the concentrations of 2,3-dimethylpyrazine were 0.55 and 1.30 μg/g, respectively. 2,5-Dimethylpyrazine, 2-ethyl-3-methylpyrazine, 2-ethyl-3,5-dimethylpyrazine increased as time and temperature increase. The concentration range of 2,5-dimethylpyrazine was 0.20 to 10.19 μg/g, the concentration in the raw material was the lowest, and the highest concentration was found after roasting at 145 °C for 60 min. The concentration of 2-ethyl-3-methylpyrazine ranged from 0.14 (raw material) to 3.89 μg/g (145 °C for 60 min). 2-ethyl-3,5-dimethylpyrazine was 0.19 μg/g of seed roasting at 125 °C for 30 min, reached to the highest concentration at 145 °C for 60 min (2.09 μg/g). The formation of the 2,6-diethyl-3-methylpyrazine requires a minimum reaction time of 45min at the corresponding temperature of 125 and 135 °C, a minimum reaction time of 30 min at 145 °C. It indicated that the heat treatment of seeds was necessary for the formation of these compounds. These pyrazines had also been reported in previous studies on the typical flavor of other roasting seed, such as pumpkin seed[25], perilla seeds[27]. The results of previous studies indicated that the minimum roasting temperature required for the formation of these compounds in pumpkin seeds was 100 °C, and the temperature required for the formation of pyrazine was higher than 150 °C.
Aldehydes mainly contribute to the overall flavor of roasted oilseed. They mainly came from the lipid oxidation and degradation or Strecker reaction[28]. Fourteen aldehydes were identified in the RUSF which varied with different roasting conditions. The concentrations of isobutanal, 2-methylbutanal, 3-methylbutanal, and phenylacetaldehyde increased significantly during the roasting process, especially at 60 min. 2-Methylbutanal and 3-methylbutanal was responsible for a pleasing odor in many roasted foods[29]. The concentration of these compounds were significantly higher (p < 0.05) in RGSF compared to those in RUSF. Benzaldehyde contributed to bitter aroma[30], showed higher content in sunflower seeds at the 145 °C for 60 min. Hexanal detected in the RGSF (0.55−3.75 μg/g) was lower than that in the RUSF (0.48−8.49 μg/g). Higher temperature and long-time heating led to the large accumulation of undesirable flavor notes, such as hexanal, nonanal, and pyridine, especially in RUSF. Meanwhile, reduction of unsaturated fats (C18:2 and C18:3) were observed in RUSF and RGSF. The short chain branched aldehydes might be attributed to hydrolytic and thermal oxidative decomposition of unsaturated fatty acids[31].
Aliphatic alcohols, mostly formed by the decomposition of hydroperoxides of fatty acids or the reduction of aldehydes[32,33]. The major alcohols in RGSF included furfuryl alcohol, hexanol, 1-octen-3-ol, verbenol, myrtenol. 1-Pentanol, hexanol, 1-octen-3-ol, and 3-methyl-2-propyl-1-pentanol were identified in RUSF. In RGSF, furfuryl alcohol began to appear and reached max at 30 min at all temperatures, and decreased with the increasing time. Hexanol decrease with prolonged time may be due to the formation of hexanal. Hexanol showed the same behavior in RUSF, the highest concentration was observed at 125 °C. 1-Octen-3-ol which contributes to an herbaceous aroma is generated from thermal decomposition of methyl linoleate hydroperoxide. It began to appear at 125 and 135 °C for 60 min and 145 °C for 45 min and reached maximum at 145 °C for 60 min (13.38 μg/g), but the content in RUSF decreased significantly with roasting temperature and time.
Besides, terpenes including α-pinene and β-pinene were also important constituents of aroma. α-Pinene was the most abundant volatile component in RGSF. The concentration of α-pinene ranged from 3.58 to 38.85 μg/g in RGSF, 7.05 to 21.85 μg/g in RUSF and 7.57 μg/g in raw seed. β-Pinene has a woody-green pine-like smell. It reached max at 135 °C for 60 min with a concentration of 4.15 μg/g. In RUSF, β-pinene concentration increased from 0.76 to 3.00 μg/g with increasing temperature and time.
-
The germination process leads to structural modification and synthesis of new compounds. The content of fructose, glucose, amino acids, and GABA increased during germination. In addition, aroma precursors, such as reducing sugars and free amino acids are formed during germination. Therefore, germinated sunflower seeds are used in the roasting process to improve the nutritional quality of sunflower seed. To obtain the high nutritional quality of roasted sunflower seeds, the roasting temperature and time should not exceed 135 °C and 45 min. The best roasting temperature was suggested to roast at 125 °C. Metabolomics and flavormics technologies were able to differentiate the roasted sunflower seeds based on the roasting temperature and time. The roasting temperature and time had a significant effect on the metabolic and flavor profiles of RUSF and RGSF. Differences in these flavors between RUSF and RGSF may have been driven by differences in concentrations of precursor substances which increased due to the germination process. Pyrazines have a positive correlation with amino acids and reducing sugars. The amino acids, reducing sugars, pyrazines, and aldehydes could be identified as the biomarker to predict the flavor in the roasting process.
-
The authors confirm contribution to the paper as follows: study conception and design: Ge Y, Guo S, Lan W, Chen X; material preparation, data collection and analysis: Guo S, Ge Y; draft manuscript preparation: Guo S; manuscript revision: Ge Y, Guo S, Lan W, Chen X. All authors read and approved the final manuscript.
-
The data that support the findings of this study are available from the corresponding author upon reasonable request.
-
Dr. Weijie Lan and Dr. Xiao Chen's valuable comments and suggestions are acknowledged.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Guo S, Lan W, Chen X, Ge Y. 2024. Exploring the metabolic and flavoromic variations of germinated sunflower seed during roasting conditions. Food Materials Research 4: e012 doi: 10.48130/fmr-0024-0004
Exploring the metabolic and flavoromic variations of germinated sunflower seed during roasting conditions
- Received: 03 September 2023
- Revised: 25 December 2023
- Accepted: 16 January 2024
- Published online: 07 April 2024
Abstract: The metabolite and flavor characteristics of roasted germinated sunflower seeds (RGSF) were evaluated and compared with those of roasted ungerminated sunflower seeds (RUSF) by gas chromatograph-flame ionization detector (GC-FID) and headspace solid phase microextraction combined with gas chromatography-mass spectrometry (HS-SPME-GC-MS). During roasting, α-tocopherol, β-sitosterol, fructose, and glucose content were higher at 125 °C compared to those at 135 and 145 °C in RGSF, and lower reductions of alanine, glycine, phenylalanine, serine, asparagine, and γ-aminobutyric acid (GABA) content at 125 °C in RGSF. Considering their nutritional value, it is suggested that sunflower seeds are roasted at 125 °C. The dominant volatile compounds in RGSF were α-pinene, furfural, pyrazines, 1-octen-3-ol, and 2-methylbutanal. High-temperature heating for long term led to a large accumulation of unpleasant odors like pyridine, hexanal and nonanal, especially in RUSF. To examine the distribution of the individual metabolites and flavor compounds among different roasting conditions. A heatmap diagram combined with agglomerative hierarchical clustering (AHC) analysis and principal component analysis (PCA) showed that most Maillard reaction substitutes (amino acids and reducing sugars), products (2-methylpyrazine 2-ethyl-3,5-dimethyl-pyrazine, and 3-ethyl-2,5-dimethylpyrazine), and Strecker degradation products (3-methylbutanal, 2-methylbutanal, and isobutanal) contributed to separate RGSF from RUSF.
-
Key words:
- Sunflower seed /
- Metabolic profiles /
- Flavoromic profiles /
- Roasting processing












