-
Rice (Oryza sativa L.) is a popular staple food worldwide, especially in east and southeast Asia, due to its palatability and availability[1]. There are significant differences in nutritional value among different cultivars of rice[2]. Compared with polished rice, brown rice contains abundant proteins, lipids, fiber, trace elements, and bioactive components, which are mainly present in the germ and bran layers[3]. However, the adhesion of bran layers can result in poor texture, unpleasant bran odor, low digestibility and long cooking time, thereby reducing consumer satisfaction[4]. Germination is a traditional and widely accepted method to improve the palatability and nutritional quality of brown rice.
During germination, beneficial bioactive compounds, including polyphenols, flavonoids, vitamins, and non-protein amino acids such as γ-aminobutyric acid (GABA), are synthesized and accumulated due to the activation of endogenous enzymes. GABA, a four-carbon non-protein amino acid widely present in organisms, serves as a major inhibitory neurotransmitter in the central nervous system of mammals[5]. In many countries, it is permitted as a food additive due to its safety, stability and physiological functions of alleviating anxiety, enhancing memory, regulating sleep, and activating immune cells[6]. For plants, external stresses such as drought, salinity, abnormal temperatures, and pathogen infection can promote its synthesis[7].
Germinated grains are widely recognized as a safe and sustainable source of GABA. In recent years, there has been growing interest in utilizing physical fields, such as low temperature plasma, high pressure treatment, pulsed electric field, microwave, radio frequency, and ultrasonic wave, to enhance nutrient enrichment during seeds germination and sprouts growth[8]. Magnetic field (MF) treatment, in particular, has emerged as a promising green and efficient method for improving germination rates and promoting sprout growth[9]. For instance, pretreating brown rice with a 10 mT static magnetic field (SMF) for 60 min was able to enhance the germination index, including the germination rate (158.8%), bud length (87.3%), and fresh weight (16.5%), as well as facilitate the hydrolysis of starch into reducing sugar (8.2%)[10]. Moreover, this SMF pretreatment resulted in a significant increase in GABA content from 16.43 to 25.64 mg/100 g, thereby improving its nutritional quality[11]. Chemical treatment has been extensively utilized in research on seed germination due to its stability and efficiency[12]. Among these treatments, salt stress is a commonly employed technique for enriching GABA. In germinated black rice, 50−200 mmol/L NaCl treatment has been found to increase the levels of phytochemicals, particularly GABA and anthocyanin[13]. However, it is important to note that salt stress can have negative effects on germination rate, leading to ionic toxicity and oxidative damage caused by the excessive accumulation of reactive oxygen species (ROS)[14]. Conversely, in germinated brown rice, treatment with 0−200 mmol/L CaCl2 could enhance the content of GABA, total phenolics, and other bioactive substances, as well as increase the antioxidant activity of the rice kernels[15]. Furthermore, CaCl2 treatment was able to mitigate the detrimental effects of NaCl stress on the membrane structure of soybeans and promote their growth. The combined usage of CaCl2 and NaCl was found to improve the activity of GABA synthetase in soybeans and increase the GABA content[16]. Additionally, KCl, an important plant growth regulator, was able to alleviate the inhibitory effects of NaCl on growth and significantly increase the germination rate in Lycium barbarum L.[17]. In conclusion, NaCl treatment can inhibit the growth of plants, while CaCl2 and NaCl treatments may promote the growth. All of them have the potential to enhance GABA content in germinated brown rice. Moreover, studies have demonstrated that SMF treatment could alleviate the adverse effects of NaCl treatment on the germination and growth processes of chickpeas and tomatoes, leading to improved seed germination rate, seedling growth speed, and fruit yield[18,19]. Therefore, SMF pretreatment and its combination with chloride salt treatment may offer a more effective means of accumulating GABA and other nutrients, while also preventing damage caused by salt stress on rice and maintaining its vitality.
The present study aimed to choose one rice cultivar with higher GABA contents both before and after germination from nine common rice cultivars. Additionally, the study investigated the impacts of a 10 mT SMF treatment with varying durations and different concentrations of NaCl, CaCl2, and KCl culture solutions on the physiological metabolism and GABA content of germinated brown rice. The GABA content served as the ultimate indicator for optimizing the germination conditions. And the changes in physiological metabolism, key nutrients, and enzyme activities related to GABA synthesis during the 0−48 h germination process were studied. The objective of this study was to establish a theoretical foundation for improving the yield and GABA content of germinated brown rice by optimizing the germination conditions.
-
There were nine cultivars of brown rice for testing, harvested in 2021 and stored at −20 °C. Cultivars of Zhennuo29 (I), SimiaoZayou (II), and Huarun2 (III) were provided by Anhui Muma Lake Agricultural Group Co., Ltd. Nanjing3908 (IV), Nanjing9108 (V), Nanjing46 (VI), NanjingJinggu (VII), Huaidao5 (VIII), and Ningxiangjing9 (IX) were provided by Jiangsu Academy of Agricultural Sciences. Suitable brown rice cultivars are selected based on germination and GABA content. The morphology of brown rice before and after germination is shown in Supplemental Fig. S1. GABA reagents were purchased from Yuan Ye Biotechnology Co., Ltd. (Shanghai, China). The reagents for sodium hypochlorite, calcium chloride, sodium chloride, potassium chloride, phenol, and sodium tetraborate decahydrate were all of analytical grade.
SMF pretreatment and chloride salt treatment during germination of brown rice
-
Intact brown rice seeds with germ were chosen and washed with distilled water to remove impurities, before being soaked in a 1% NaClO solution for 15 min to sterilize. Subsequently, brown rice seeds were thoroughly rinsed with distilled water until pH was neutral and soaked in deionized water at 30 °C for 6 h to absorb moisture. They were taken out and exposed to a 10 mT SMF for varying durations (0, 20, 40, and 60 min) before being laid flat on a sprouting tray.
Seeds were germinated in a dark environment controlled at 25 °C and sprayed with 10 mL of differing concentrations of NaCl (0, 20, 40, 60, and 80 mmol/L), CaCl2 (0, 2, 4, 6, and 8 mmol/L), and KCl (0, 2, 4, 6, and 8 mmol/L) solutions every 8 h for 36 h. After germination, some seeds were preserved at −80 °C, while others were freeze-dried (LGJ-12A; Jipu Electronics Technology Co., LTD., Shanghai, China) and ground into powder for further analysis. The study also used response surface methodology (RSM) with a Box-Behnken design to assess the impacts of NaCl concentration (A), CaCl2 concentration (B), and KCl concentration (C) on the germination process (Supplemental Table S1).
Determination methods of indexes
Determination of germination rate, sprout length, fresh weight and dry weight
Germination rate (%)
-
One hundred brown rice seeds were randomly selected. Samples with bud length over 0.2 mm were recorded as germinated. Germination rate (%) = Number of germinated brown rice /100 × 100%.
Fresh weight
-
Fresh weight was measured by a precision electronic balance and 100 grains were taken as a parallel group.
Dry weight
-
Dry weight was measured by drying seeds at 105 °C until constant weight and 100 grains were taken as a parallel group.
Determination of GABA content
-
The colorimetric method was employed for the determination of GABA content[20]. Initially, 1.0 mg/mL GABA solution was prepared and diluted to concentrations of 0.01, 0.02, 0.05, 0.10, 0.15, and 0.2 mg/mL to serve as the standard solutions for measurement. Then, 1.00 g of germinated brown rice flour was precisely weighed and placed into a 10 mL centrifuge tube, to which 5 mL of distilled water was added. The mixture was then shaken for 4 h at 60 °C and 150 r/min followed by centrifugation at 4 °C for 15 min at 8,000 r/min. 0.5 mL of the supernatant was taken, 0.2 mL of 0.2 mol/L borate buffer (pH = 9.0) was added, followed by 1.0 mL of 6% phenol, and 0.4 mL of NaClO with an effective chlorine content of 5.5%. After shaking well, the solution was heated in a 60 °C water bath for 5 min, followed by a 10-min ice bath. Finally, 2.0 mL of 60% ethanol was added to the mixture, and the sample analyzed at a wavelength of 640 nm using a UV-visible spectrophotometer (UV-2802, Unicore Shanghai Instruments Co., Ltd., Shanghai, China).
Determination of total starch and amylose content
Total starch content
-
Using kit, obtained from Shanghai Macklin Biochemical Co., Ltd (Shanghai, China). The soluble sugar and starch in the sample can be separated by 80% ethanol, and the starch can be decomposed into glucose by acid hydrolysis. The glucose content was determined by anthrone colorimetric method.
Amylose content
-
Fifty mg of the germinated brown rice power was weighed and 0.5 mL of 95% ethanol solution was added, followed by the addition of 4.5 mL of 1 mol/L NaOH solution. The mixture was then thoroughly shaken, heated in 100 °C for 10 min and 45 mL water was added. This resulting solution was referred to as the test solution. 0.5 mL test solution was taken and 9 mL water, 0.1 mL acetic acid and 0.2 mL reagent (0.2 g of I2 and 2 g of KI and making up to 100 mL by water). The mixture was shaken and placed for 10 min before the absorbancy at 620 nm was measured[21].
Determination of water soluble protein and sugar content
-
One gram fresh germinated brown rice flour was taken 10 ml water added and ground until homogenized. The mixture was then shaken for 2 h at 30 °C and 150 r/min, followed by centrifugation at 4 °C for 15 min at 8,000 r/min. After extration, water soluble protein content was determined using the Coomassie brilliant blue method[22]. Water soluble sugar content was determined by phenol-sulfuric acid method[23].
Determination of GAD activity
-
One g germinated brown rice was taken and 5.0 mL 0.07 mol/L potassium phosphate buffer with pH 5.8 added, containing 2.0 mol/L β-mercaptoethanol, 2.0 mmol/L EDTA, 0.2 mmol/L PLP, then ground into homogenate in an ice bath, and centrifuged at 4 °C, 10,000 r/min for 20 min. The supernatant was the crude enzyme extract. Two hundred μL crude enzyme solution was added to 100 μL substrate solution (l% Glu, pH 5.8). After a 2 h reaction at 40 °C, the enzyme activity was halted by placing it in a 90 °C water bath for 5 min. The sample was then centrifuged and the supernatant taken to measure the GABA content after passing through a 0.45 µm filter (water phase). The 1.0 mg GABA generated every 1 h was determined as an enzyme activity unit[24].
Determination of relative gene expression
-
The rice samples were pre-cooled in liquid nitrogen, ground to a fine powder in liquid nitrogen, and immediately transferred to enzyme-free tubes. RNA extraction was performed using the TAKARA Plant RNA Extraction Kit (Catalog No. 9769). cDNA synthesis was carried out using the TAKARA RT-PCR Mater Mix Kit (Catalog No. RR036A). The samples were analyzed using the SYBR Premix Ex TaqTM kit (TAKARA Catalog No. RR420A). The primers used in this study are listed in Supplemental Table S2. Following the method described by Ma et al.[25], the samples were subjected to 40 cycles of denaturation at 95 °C for 30 s, followed by annealing and extension at 95 °C for 3 s and 60 °C for 30 s, respectively.
Statistical analysis
-
Results were presented as the means ± standard deviations (SD) with at least three replicates. Data was analyzed with SPSS Statistics 25 software at a 0.05 level of significance. Graphs were drawn with Origin 2021 software.
-
Table 1 presents the changes in physiological indexes before and after germination of brown rice, including germination rate, sprout length, fresh weight, and dry weight. After 36 h of germination, the sprout length of brown rice ranged from 1.27−2.25 mm, with all cultivars, except for cultivar I (glutinous rice) having a sprout length of over 1.5 mm and a germination rate of over 98%. The sprout length of the indica rice cultivars (II and III) measured at 2.25 and 2.18 mm, respectively, exhibiting a statistically significant increase compared with the tested japonica rice cultivars (IV−IX), which ranged from approximately 1.52 to 1.89 mm. Additionally, the sprout length of the indica rice cultivars surpassed that of the glutinous rice, which measured approximately 1.27 mm. Furthermore, the fresh weight and dry weight per 100 grains of the indica rice cultivars were significantly lower when compared to the other rice cultivars. The germination rate, sprout length, fresh weight, and dry weight of variations IV−VII and IX exhibited similar values, however cultivar VIII had larger grains (Supplemental Fig. S1) and significantly higher fresh weight and dry weight compared to other japonica rice cultivars.
Table 1. Germination rate, sprout length, fresh weight and dry weight of different cultivars.
Cultivar Germination rate (%) Sprout length (mm) Fresh weight (g/100 grain) Dry weight (g/100 grain) I Zhennuo29 87.33 ± 2.51b 1.27 ± 0.07d 3.26 ± 0.07b 1.95 ± 0.01bc II Simiaozayou 98.33 ± 1.53a 2.25 ± 0.24a 2.48 ± 0.05f 1.78 ± 0.02d III Huarun2 98.67 ± 1.15a 2.18 ± 0.19a 2.47 ± 0.04f 1.79 ± 0.02d IV Nanjing3908 98.00 ± 1.00a 1.66 ± 0.24bc 3.15 ± 0.04cd 1.92 ± 0.02c V Nanjing9108 98.33 ± 1.53a 1.61 ± 0.11c 3.06 ± 0.04e 1.92 ± 0.01c VI Nanjing46 98.67 ± 0.58a 1.68 ± 0.13bc 3.21 ± 0.03bc 1.98 ± 0.02b VII Nanjingjinggu 98.33 ± 1.53a 1.52 ± 0.10c 3.12 ± 0.03de 1.93 ± 0.02c VIII Huaidao5 98.00 ± 1.00a 1.89 ± 0.14b 3.51 ± 0.04a 2.29 ± 0.02a IX Ningxiangjing9 99.00 ± 1.00a 1.53 ± 0.10c 3.10 ± 0.04de 1.94 ± 0.02c Different lowercase letters in the same column represent significant difference between cultivars (p < 0.05). The GABA content of nine different types of brown rice (before germination) was illustrated (Fig. 1), ranging from 11.54 to 21.66 mg/100 g . The GABA content of japonica rice (average 17.91 mg/100 g) was found to be higher compared to glutinous rice (11.54 mg/100 g) and indica rice (average 14.58 mg/100 g). After germination, the GABA content increased significantly, ranging from 23.64 to 49.20 mg/100 g. Specifically, cultivar V exhibited notably higher GABA content both before and after germination, with values of 21.66 mg/100 g and 49.20 mg/100 g, respectively. Moreover, the increase in GABA content for cultivar V was substantially greater than that observed in other cultivars, with an increase of 27.53 mg/100 g, representing a 127.10% increase. Consequently, Nanjing9108 (V) was selected as the subsequent experimental cultivar.
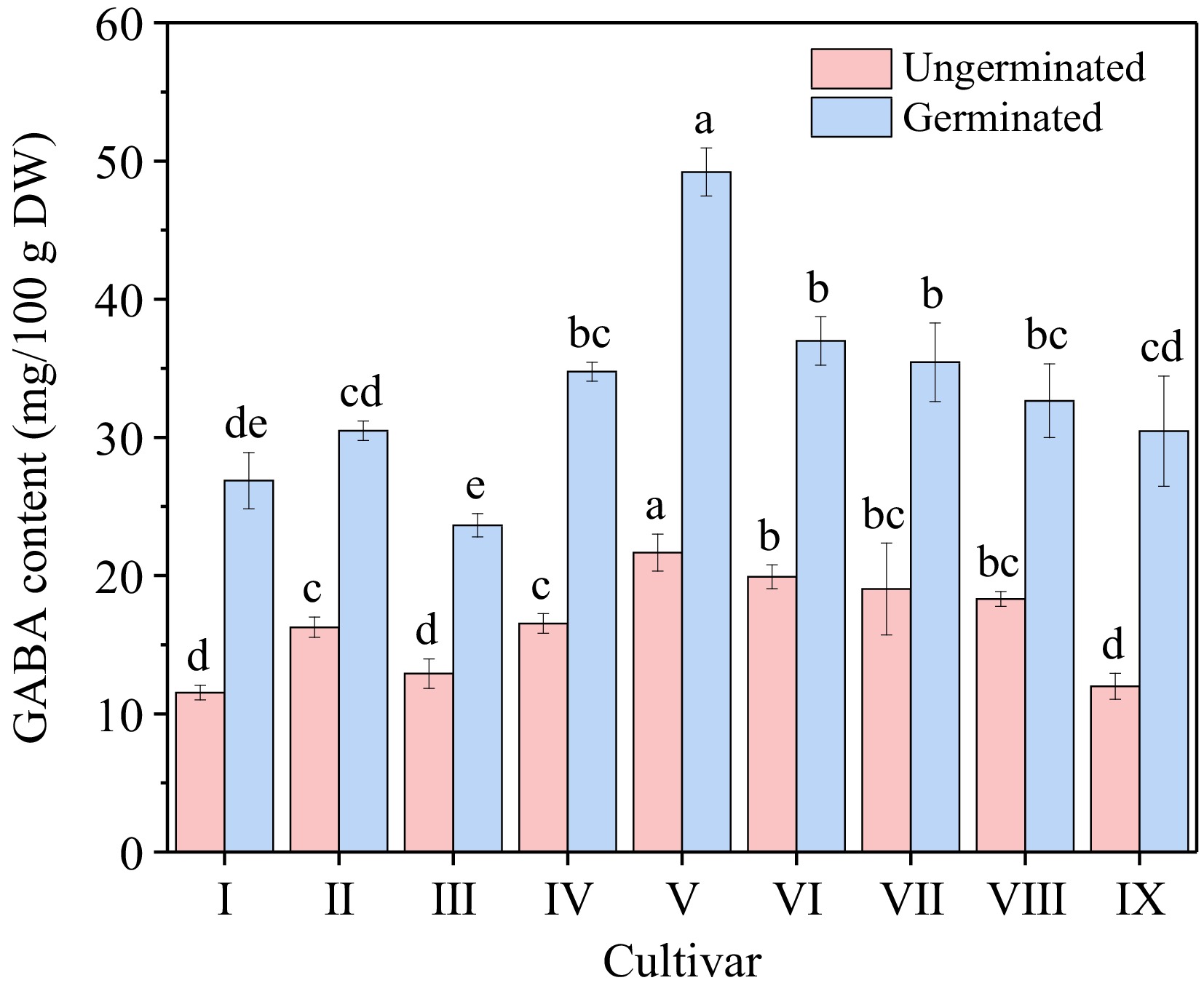
Figure 1.
GABA content of different cultivars before and after germination. Cultivar I to IX refers to Zhennuo29, Simiaozayou, Huarun2, Nanjing3908, Nanjing9108, Nanjing46, Nanjingjinggu, Huaidao5 and Ningxiangjing9, respectively. Different lowercase letters in the columns with same color represent significant difference between cultivars (p < 0.05).
Effects of SMF on germination indexes and GABA content of germinated brown rice
-
The germination rate of brown rice exhibited a substantial rise with the extension of SMF treatment duration, as seen in Fig. 2b. Additionally, the sprout length (Fig. 2c) and the fresh weight (Fig. 2d) were observed to be positively correlated with the prolonged treatment time. Conversely, a decrease in dry weight was observed (Fig. 2e). The early growth of brown rice was accelerated, the consumption of nutrients was raised, and water absorption was improved. GABA content in germinated brown rice, as depicted in Fig. 2f, showed an initial increase followed by a subsequent decrease with the prolongation of SMF pretreatment duration. Following treatment durations of 20, 40, and 60 min, there was an increase in GABA content of 9.43%, 13.88%, and 4.63%, respectively, in comparison with the control group (Fig. 2f). Hence, by considering GABA content as the primary criterion, a 40 min duration of SMF pretreatment was chosen. Under these conditions, the germination rate, sprout length, and fresh weight of brown rice exhibited respective increments of 2.67%, 15.87%, and 7.69% compared with the control group, while the dry weight decreased by 4.35%.
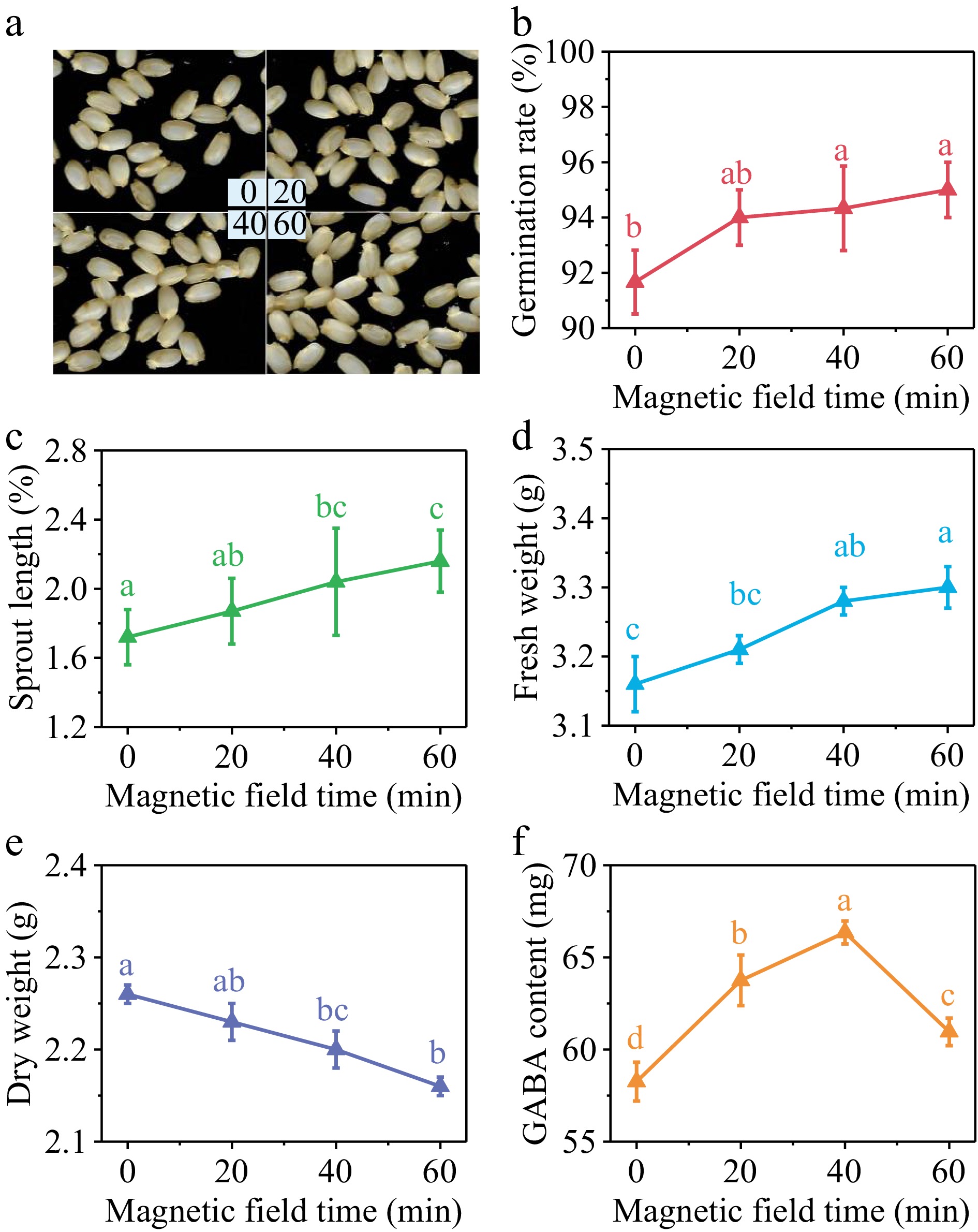
Figure 2.
(a) Photos, (b) germination rate, (c) sprout length, (d) fresh weight, (e) dry weight and (f) GABA content in germinated brown rice treated by 10 mT SMF for 0, 20, 40, and 60 min. Different lowercase letters represent significant difference between treatments (p < 0.05).
Effects of chloride salts under SMF pretreatment on germination indexes and GABA content of germinated brown rice
Effects of NaCl concentration on germinated indexes and GABA content of germinated brown rice
-
With the increase of NaCl concentration, germination rate (Fig. 3b) and sprout length (Fig. 3c) showed a decreasing trend. Both germination rate and sprout length reached the lowest value when NaCl concentration was 80 mmol/L, decreasing by 4.3% and 19.8% compared with the control group. Specifically, the fresh weight reached its highest value at the NaCl concentration of 80 mmol/L, representing a 2.5% increase compared to the control group. the dry weight reached its peak at the NaCl concentration of 40 mmol/L, measuring 2.17 g/100 grains, which corresponds to a 2.2% increase compared with the control group. Exposure to NaCl stress had a modest inhibitory effect on the germination and initial growth of brown rice.
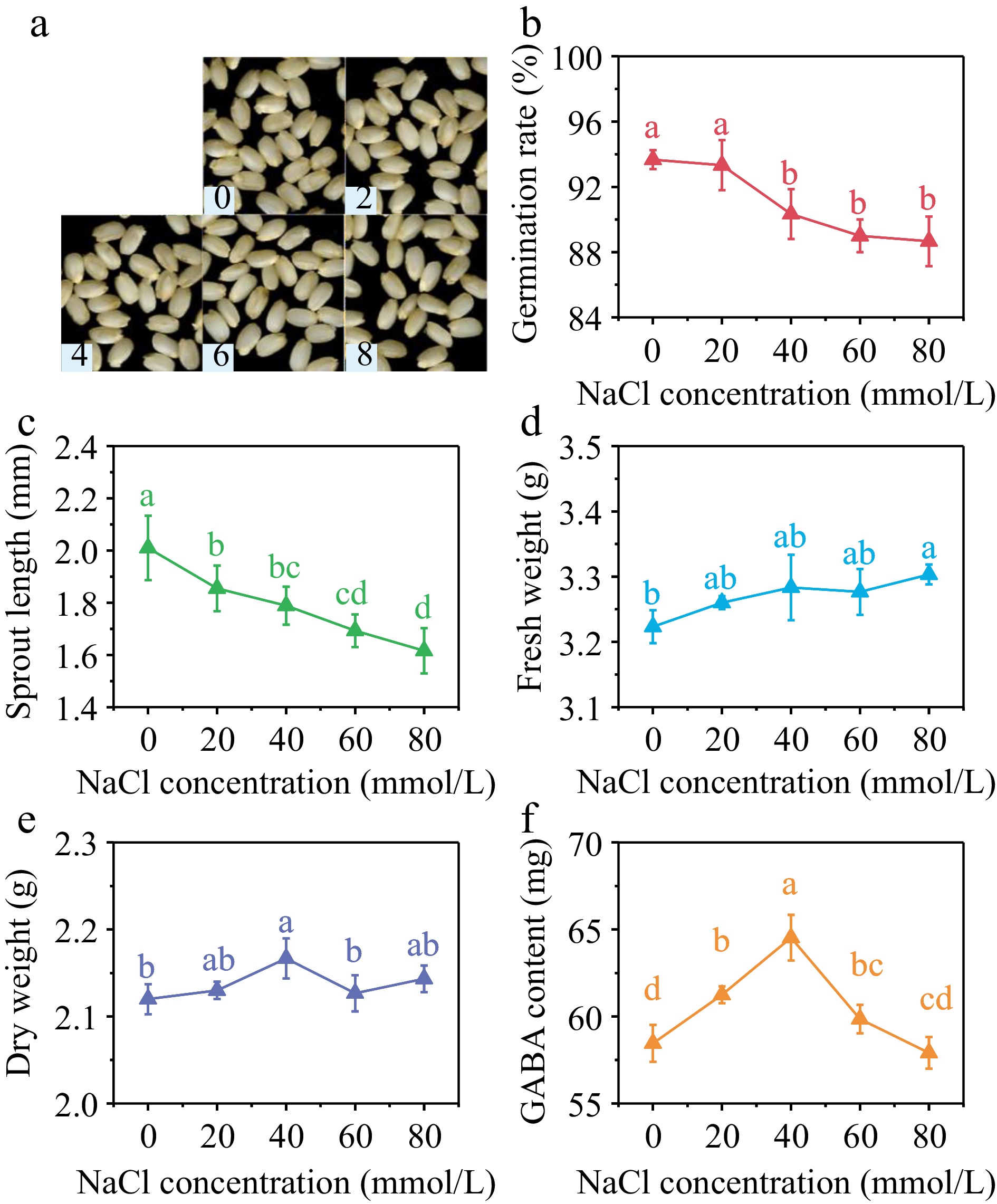
Figure 3.
(a) Photos, (b) germination rate, (c) sprout length, (d) fresh weight, (e) dry weight and (f) GABA content in germinated brown rice cultivated by 0, 20, 40, 60, and 80 mmol/L NaCl under 40 min, 10 mT SMF pretreatment for 40 min. Different lowercase letters represent significant difference between treatments (p < 0.05).
As can be seen from Fig. 3f, GABA content in germinated brown rice showed a trend of first increasing and then decreasing with the increase of NaCl concentration. When NaCl concentration was 20, 40, and 60 mmol/L, GABA content in brown rice after 36 h of germination reached 61.25, 64.53, and 59.8 mg/100 g, respectively. Compared with the control group, the increase was 4.78%, 10.39%, and 2.39%, respectively.
Effects of CaCl2 concentration on germinated indexes and GABA content in germinated brown rice
-
The addition of CaCl2 at various concentrations did not result in significant impact (p > 0.05) on the germination rate (Fig. 4b), sprout length (Fig. 4c), and fresh weight (Fig. 4d) of brown rice after 36 h germination. The dry weight reached its lowest level at the CaCl2 concentration of 6 mmol/L, which was 2.16 g per 100 grains (Fig. 4e), representing a 2.70% decrease compared to the control group. This indicates that under this treatment, brown rice had a higher water absorption rate, faster growth, and greater consumption of dry matter such as starch. However, overall, the low concentration of CaCl2 had a smaller impact on the germination and early growth of brown rice under SMF pretreatment.
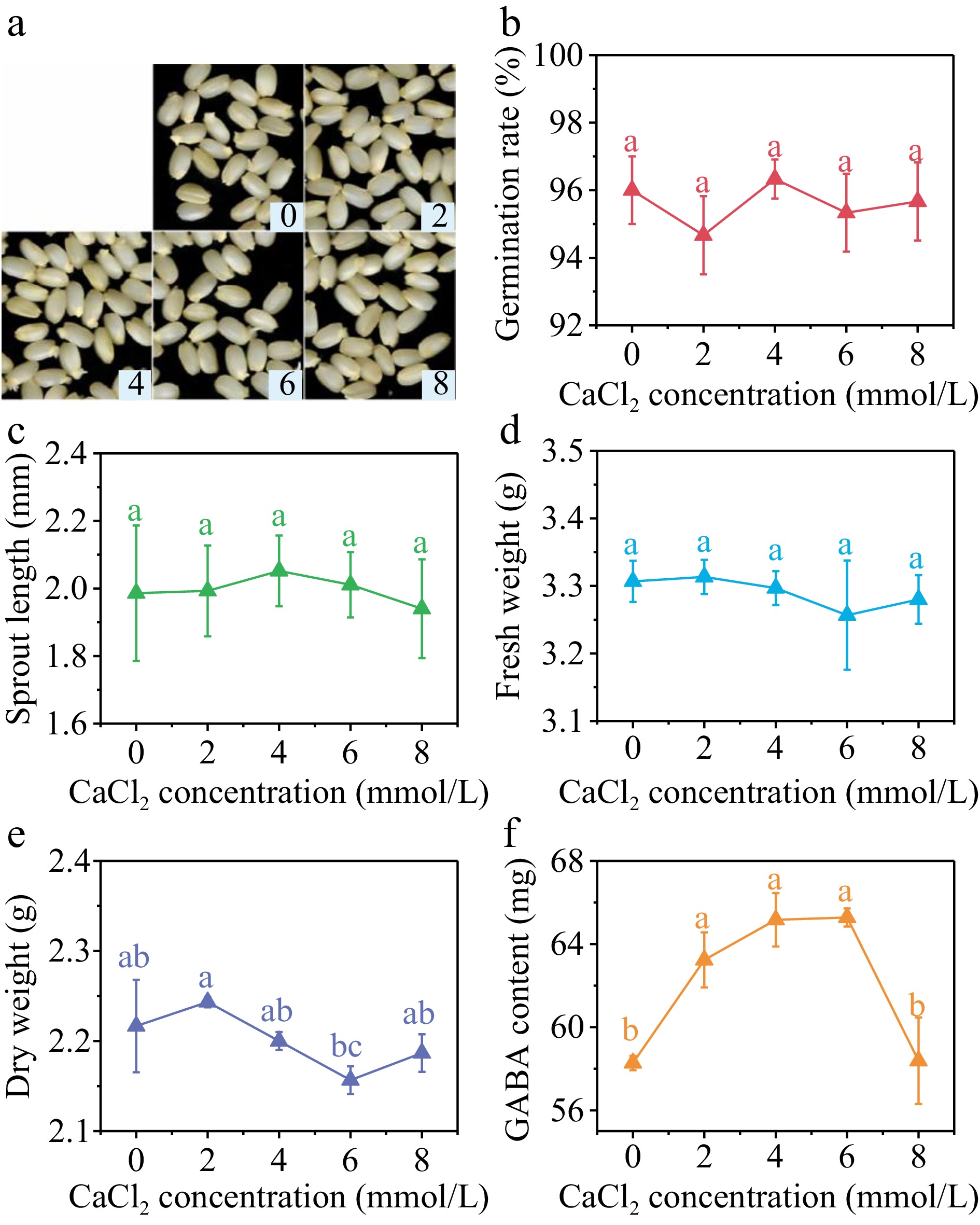
Figure 4.
(a) Photos, (b) germination rate, (c) sprout length, (d) fresh weight, (e) dry weight and (f) GABA content in germinated brown rice cultivated by 0, 2, 4, 6, and 8 mmol/L CaCl2 under 40 min, 10 mT SMF pretreatment for 40 min. Different lowercase letters represent significant difference between treatments (p < 0.05).
The GABA content in germinated brown rice exhibited a pattern of initial increase followed by subsequent decrease with the rise in CaCl2 concentration. The GABA content of brown rice was found to be significantly higher than that of the control group at CaCl2 concentrations of 2, 4, and 6 mmol/L. Specifically, the GABA content rose by 8.52%, 11.83%, and 12.02% at these respective concentrations. Nevertheless, no statistically significant disparity in GABA levels was seen when comparing the control group with the CaCl2 concentration of 8 mmol/L.
Effects of KCl concentration on germinated indexes and GABA content in germinated brown rice
-
Different concentrations of KCl did not have a significant impact on the germination rate of brown rice (Fig. 5b). However, the sprout length (Fig. 5c) and fresh weight (Fig. 5d), as well as the GABA content (Fig. 5f), showed a trend of initial increase followed by decrease. The sprout length reached its maximum value when the KCl concentration was 4 mmol/L, increasing 11.83% compared with the control group. In contrast, the fresh weight reached its peak at the KCl concentration of 2 mmol/L, showing a 3.83% increase compared with the control group. Additionally, the dry weight reached its minimum value at the KCl concentration of 2 mmol/L. Suitable concentration of KCl can enhance the absorption of water and facilitate the initial development of sprouts. Nevertheless, an excessive concentration could potentially result in ion toxicity within seeds, thereby impeding their growth.
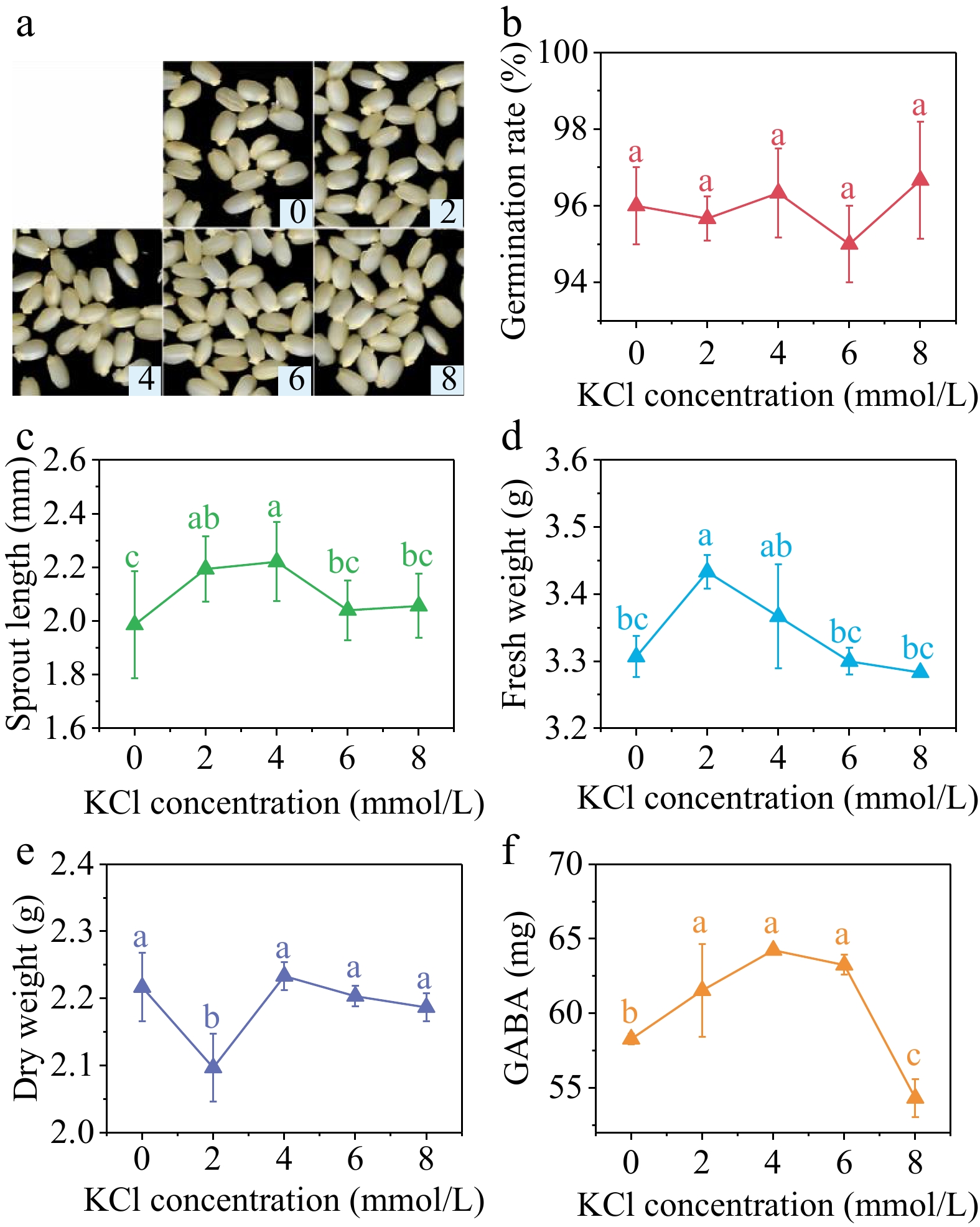
Figure 5.
(a) Photos, (b) germination rate, (c) sprout length, (d) fresh weight, (e) dry weight and (f) GABA content in germinated brown rice cultivated by 0, 2, 4, 6, and 8 mmol/L KCl under 40 min, 10 mT SMF pretreatment for 40 min. Different lowercase letters represent significant difference between treatments (p < 0.05).
The GABA content of germinated brown rice was significantly higher than the control group at KCl concentrations of 2, 4, and 6 mmol/L, with increases of 5.58%, 10.22%, and 8.52% respectively. However, at a KCl concentration of 8 mmol/L, the GABA content was significantly lower than the control group.
Optimization of culture medium composition
-
The Box-Behnken design and results are shown in Supplemental Table S1. The codings of different levels (−1, 0, 1) are shown in Supplemental Table S1. A second-order polynomial regression model was used to fit the data in Supplemental Table S3, and the obtained second-order polynomial regression equation is Y = 68.69 − 0.8037A + 0.7150B + 1.08C − 1.17AB + 0.4850AC + 0.5175BC − 2.98A2 − 0.6325B2 + 0.0000C2.
To test the validity of the model, variance analysis of the response surface regression model for the data in Supplemental Table S3 was conducted, and the results are shown in Supplemental Table S4. By analyzing the multivariate quadratic regression model obtained from the Box-Behnken experiment, response surface and contour maps for different factors were obtained (Supplemental Fig. S2). Using Design-Expert 8.0 software, the optimal combination of process parameters was determined, and the predicted optimal culture medium concentrations for GABA enrichment were found to be NaCl 37.23 mmol/L, CaCl2 4.71 mmol/L, and KCl 5.75 mmol/L, with a GABA content of 69.783 mg/100 g. To validate reliability of this model, verification experiments were conducted, and the GABA content in germinated brown rice was found to be 69.42 mg/100 g, with an error of 0.52%, which is consistent with the predicted value, indicating a high level of model reliability.
Germination kinetics during 0-48 h germination
-
According to Table 2, it can be seen that during the germination process, SMF treatment (treatment 1) increased the germination rate and fresh weight of germinated brown rice compared to the control group. The group treated with SMF and combined salt medium (treatment 2) had a growth trend similar to the control group.
Table 2. Changes in growth index, starch, protein and sugar during germination.
Treatment Germination
time (h)Germination
rate (%)Fresh weight
(g/100 grains)Dry weight
(g/100 grains)Starch
(g/100 g)Amylose
(g/100 g)Soluble protein
(g/100 g)Soluble sugar
(g/100 g)Control 0 0.00 ± 0.00aD 3.17 ± 0.02bC 2.13 ± 0.03aA 68.04 ± 2.67aA 11.65 ± 0.21aC 3.41 ± 0.50bCD 6.53 ± 0.65aA 12 12.33 ± 1.53aC 3.23 ± 0.02aB 2.07 ± 0.01aB 61.58 ± 0.60aB 13.23 ± 0.98aA 3.90 ± 0.26bBC 5.64 ± 0.13aB 24 54.67 ± 3.06aB 3.22 ± 0.02bBC 2.03 ± 0.03aBC 57.49 ± 1.37abC 13.04 ± 0.13aAB 3.08 ± 0.22cD 4.54 ± 0.17aC 36 86.33 ± 0.58bA 3.32 ± 0.02aA 2.03 ± 0.01aBC 54.38 ± 2.03aC 12.75 ± 0.26bABC 4.02 ± 0.30bB 3.66 ± 0.24aD 48 89.67 ± 2.08bA 3.29 ± 0.05aA 2.01 ± 0.02aC 53.99 ± 1.66aC 11.76 ± 1.05aBC 5.36 ± 0.05aA 3.85 ± 0.62aCD Treatment 1 0 0.00 ± 0.00aD 3.21 ± 0.02aB 2.12 ± 0.02aA 68.82 ± 9.09aA 7.50 ± 0.88bA 5.05 ± 0.40aA 7.01 ± 0.96aA 12 16.67 ± 2.52aC 3.23 ± 0.01aAB 2.05 ± 0.01bB 61.08 ± 2.35aAB 9.88 ± 1.11bB 5.03 ± 0.43aA 5.70 ± 0.59aAB 24 61.00 ± 3.61aB 3.29 ± 0.05aAB 2.03 ± 0.01aBC 60.17 ± 2.63aAB 11.77 ± 0.72aA 4.14 ± 0.10bC 5.24 ± 0.93aB 36 92.67 ± 1.15aA 3.34 ± 0.03aA 2.04 ± 0.01aBC 56.68 ± 1.93aB 11.60 ± 0.89bB 4.29 ± 0.17abBC 3.57 ± 0.31aC 48 96.33 ± 0.58aA 3.34 ± 0.12aA 2.01 ± 0.02aC 53.51 ± 2.38abB 12.72 ± 0.85aA 4.74 ± 0.16bAB 3.34 ± 0.16aC Treatment 2 0 0.00 ± 0.00aD 3.21 ± 0.02aC 2.12 ± 0.02aA 68.82 ± 9.09aA 7.50 ± 0.88bC 5.05 ± 0.40aA 7.01 ± 0.96aA 12 12.00 ± 3.00aC 3.26 ± 0.02aB 2.09 ± 0.02aAB 57.22 ± 0.41bB 11.96 ± 0.97abB 5.42 ± 0.04aA 5.53 ± 0.24aB 24 56.33 ± 3.21aB 3.32 ± 0.02aA 2.05 ± 0.03aBC 55.83 ± 0.8bB 12.58 ± 1.19aAB 5.23 ± 0.37aA 4.92 ± 0.29aBC 36 86.67 ± 2.52bA 3.34 ± 0.03aA 2.03 ± 0.02aC 53.94 ± 1.12aB 13.93 ± 0.31aA 4.50 ± 0.17aB 3.97 ± 0.24aC 48 92.33 ± 1.15bA 3.27 ± 0.02aB 2.01 ± 0.01aC 49.77 ± 1.78bB 12.65 ± 0.91aAB 4.11 ± 0.09cB 4.05 ± 0.27aC Control: brown rice seeds were soaked for 6 h at 30 °C, not exposed to SMF and culture medium was DI water. Treatment 1: brown rice seeds were exposed to 10 mT SMF for 40 min and culture medium was DI water. Treatment 2: brown rice seeds were exposed to 10 mT SMF for 40 min and culture medium was combined salt solution. Different lowercase letters in the same column represent significant difference between treatments (p < 0.05). Different uppercase letters in the same column among the same treatment represent significant difference between different germination time (p < 0.05). The total amount of starch exhibited a consistent decline throughout the process of germination. During the 0−36 h germination stage, the starch content in the control group decreased from 68.04 to 54.38 g/100 g, with a faster rate of decrease compared with the 36−48 h stage (54.38 to 53.99 g/100 g). In treatment 1 group, there was no significant change (p > 0.05) in the total starch content when compared with the control group at 48 h. However, in the treatment 2 group it reduced by 7.83%. After SMF treatment, the content of amylose in brown rice decreased significantly by 55.33% immediately. Throughout the 0−36 h germination period, there was a noticeable upward trend in the ratio of amylose to dry weight. In the control group, the content of water-soluble protein generally increased during the 0−48 h germination period. In treatment 1 and treatment 2 groups, the water-soluble protein content was 48.09% higher after SMF treatment but decreased by 11.57% and 23.32% compared with the control group after 48 h of germination. The content of water-soluble sugars gradually decreased by 41.04%, 52.35%, and 42.23% after 48 h germination in contol, treatment 1 and treatment 2 group, respectively.
The GABA content of three different treatment groups showed a rapid enrichment during 0−36 h of germination, with an increase of 33.45%, 34.70%, and 51.27% at 36 h compared with 0 h, respectively. However, there was no significant difference (p > 0.05) in GABA content between 36 and 48 h of germination in the same group. At 12 h of germination, the GABA content in the salt treatment group was lower than that in SMF treatment group, while at 36 h, the GABA content in treatment 2 group was significantly higher than that in treatment 1 group.
The glutamic acid decarboxylase (GAD) activity of the salt-treated group reached its peak at 36 h and then declined. Meanwhile, the SMF treatment up-regulated the gene expression of GAD and GABA transaminase (GABA-T), with the gene expression of GABA-T reaching the highest level at 48 h of germination. The addition of salt treatment down-regulated the gene expression of GABA-T in the late stage of germination, effectively reducing the decomposition of GABA.
-
Germination is an environmentally friendly way to enrich GABA in brown rice, and it can also improve the edible quality[26]. The GABA content in different varieties of brown rice varies, with generally lower content in indica and glutinous rice, and higher content in japonica rice. The GABA content significantly increases after 36 h of germination, with japonica rice showing a higher increase compared to indica and glutinous rice (Fig. 1). The accumulation of GABA in brown rice, as well as the change of activity and gene expression of GAD, primarily occurs in the embryo of the grain, and rice with larger embryos typically have higher GABA content[27]. Akita et al.[28] studied 158 rice varieties, with embryo weights ranging from 0.28 to 0.76 mg for indica rice and 0.41 to 0.81 mg for japonica rice. The overall higher embryo weight in japonica rice may be the reason for its higher GABA content. However, Yao[29] studied 181 rice varieties and found no significant difference in GABA content between japonica and indica rice. However, it was observed that the GABA content in early-maturing rice was significantly greater than that in both medium-maturing and late-maturing rice. These results suggest that the duration and conditions of growth play a crucial role in influencing the GABA content in rice. Furthermore, it is noteworthy that this experiment revealed a notable disparity in GABA levels among cultivars within the same series (IV and V), potentially attributable to the distinct growth conditions specific to each variety.
This study found that the GABA content in germinated brown rice can be further increased under the treatment of SMF and combined salt culture medium. In rice and other grain plants, the main metabolic pathway of GABA is known as the GABA shunt: Glutamic acid (GA) is conversed to GABA through the catalytic action of glutamic acid decarboxylase (GAD), which is then catalyzed by GABA-T to produce succinic acid[30]. The enrichment of GABA in brown rice by SMF may be mainly due to its ability to promote seed growth and regulate the activity of related synthetic enzymes in the early stages of germination and growth (Fig. 6). Studies suggest that SMF can activate the antioxidant defense system of plants and enhance early seed vitality by altering plant metabolism[31]. This study found that SMF treatment effectively promotes germination and early growth of brown rice, increasing germination rate, shoot length, and fresh weight, while reducing dry matter (Fig. 2). It has also been reported that SMF has no significant effect on the antioxidant enzyme activity of brown rice[11]. A study conducted by Jin et al.[32] demonstrates that SMF modulates the growth of Arabidopsis roots through its involvement in the auxin signaling system. At the same time, SMF can enhance GAD activity and inhibit GABA-T gene expression to promote GABA enrichment, which has also been verified in germinated brown rice[33] and maize[34]. In addition, the magnetic field can affect the synthesis and cell proliferation of DNA and RNA, activating the cellular stress response as a protective mechanism that induces gene expression in the stress response[35].
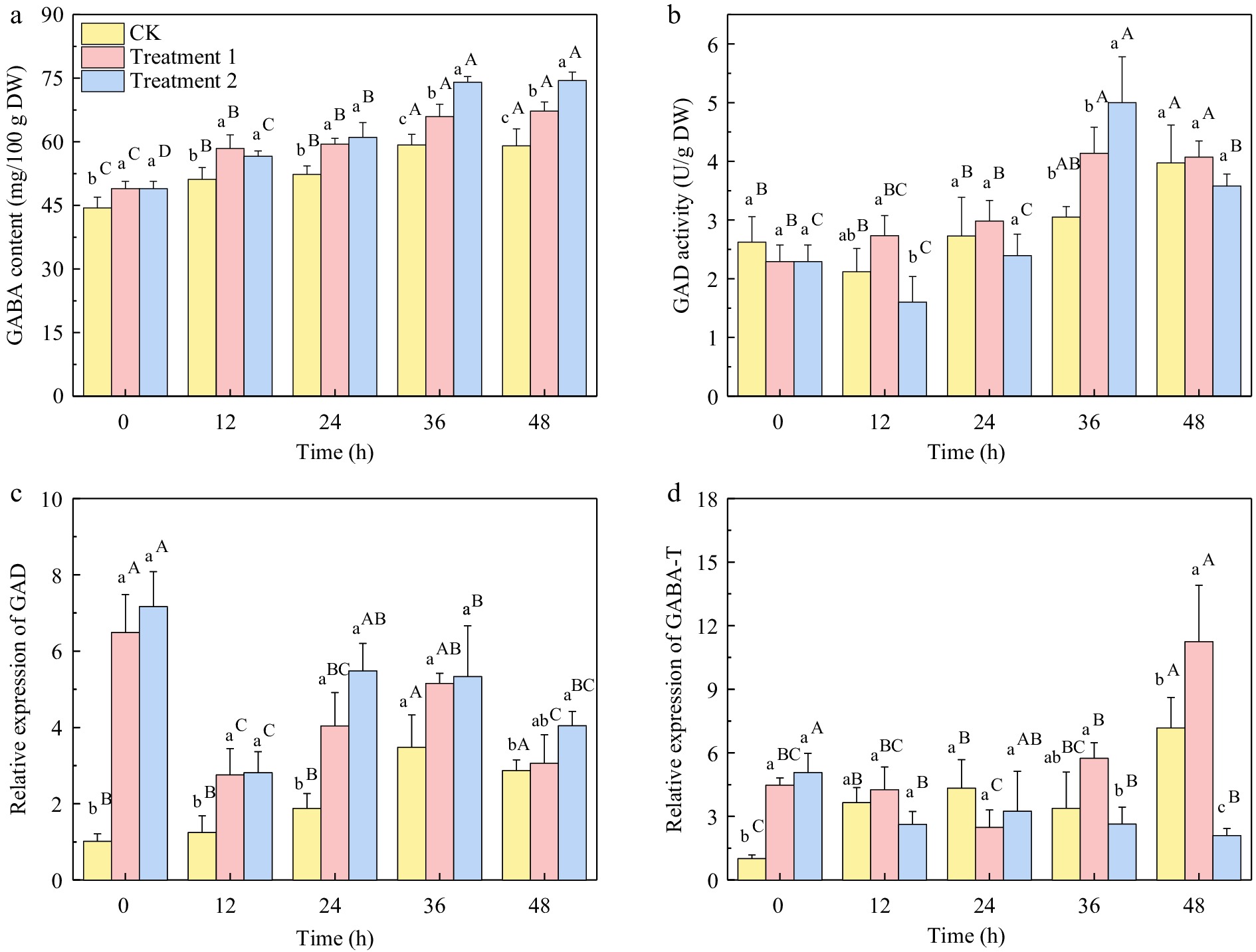
Figure 6.
(a) GABA content, (b) GAD activity, (c) relative gene expression of GAD, and (d) GABA-T under different treatments of brown rice germinated for 0−48 h. CK: brown rice seeds were soaked for 6 h at 30 °C, not exposed to SMF and culture medium was DI water. Treatment 1: brown rice seeds were exposed to 10 mT SMF for 40 min and culture medium was DI water. Treatment 2: brown rice seeds were exposed to 10 mT SMF for 40 min and culture medium was combined salt solution. Different lowercase letters represent significant difference between treatments (p < 0.05). Different upper letters represent significant difference between different germination time among the same treatment (p < 0.05).
The treatment of salt can further enrich GABA on the basis of SMF. NaCl is a widely distributed salt in nature and is also used as a seasoning in daily life. The appropriate salt concentration helps to enrich nutrients in the process of plant growth[36]. In soybeans, NaCl treatment can increase the content of GABA and phenolic substances[37]. NaCl at a concentration of 60 mM can cause accumulation of ROS and reduction of antioxidant enzyme activities in Cicer arietinum[38]. NaCl at concentrations more than 100 mM can decrease germination index, speed of germination and dry weight of rice[39]. A concentration between 20−60 mM was chosen in this resarch. In this study, SMF pretreatment promoted growth metabolism, while NaCl inhibited growth. The combined use of NaCl and SMF treatment resulted in a stress state and activated the GABA synthesis pathway. Thus, the GABA content was increased and no significant adverse effects on germination rate and appearance of brown rice were caused (Fig. 3). Ca2+ is an important second messenger that can be combined with calcium-modulated protein (CaM) to change the composition and activity of CaM, activate proteinases in plants, and regulate related physiological activity. GAD, which regulates GABA synthesis in the GABA shunt, is a CaM. The presence of Cl− in soil is extensive, thereby many researches put efforts to investigate the impact of CaCl2 on plant growth. Some studies focused on higher concentrations (> 100 mmol/L) of CaCl2, and found that it slightly improved the germination and growth of brown rice[40]. Germinated brown rice is a kind of food product. In order to reduce the presence of additives, treatments with a lower concentration of CaCl2 was chosen. Potassium, an element with high reactivity, is abundantly found in nature in the form of various salts. The K+ has the ability to initiate the activation of enzymes that are essential for many plant growth processes. Additionally, K+ plays a crucial role in safeguarding seeds by regulating the antioxidant defense system[41]. This study employed a diluted solution of potassium chloride (KCl) to facilitate the growth of the subject and enhance the process of synthesizing metabolic compounds. Low concentration KCl was used in this study, which can promote growth and anabolism of brown rice.
Researchers also discovered that the addition of Ca2+[16] or K+[42] can impede the excessive buildup of reactive oxygen species (ROS) by activating protective mechanisms like the AsA-GSH pathway and the acetaldehyde enzyme system. As a result, this effectively alleviates the harm caused by salt stress and restores the adverse effects of NaCl on plant growth and yield attributes. Furthermore, previous studies have demonstrated that the application of SMF enhances the transmembrane flux of Ca2+ and enhances the permeability of cellular membranes[43]. Hence, the use of SMF combined salt treatment has the potential to reduce the use of salt in production and save resources. This study innovatively combined three chloride salt culture medium and introduced SMF pretreatment. It was found that GABA content was significantly increased and GABA-T gene expression was significantly down-regulated under the optimized condition , indicating that the combined culture medium of different chloride salts would not lead to excessive ion concentration and inhibit GABA synthesis.
-
In brief, among nine rice cultivars, cultivar V exhibits the highest GABA content both before and after germination. This particular characteristic renders variety V a potential candidate for the purpose of GABA enrichment. The application of SMF treatment has been found to facilitate the early germination of brown rice, enhance the activity of GAD and promote the enrichment of GABA. The addition of combined salt solution can further increase the GABA content of brown rice treated with SMF by salt stress, and can down-regulate the activity of GABA-T in the late germination stage, inhibiting GABA degradation. Furthermore, the combined use of these two treatments did not impede the synthesis of GABA or the growth of germinated brown rice related to the presence of elevated ion concentrations. Magnetic field treatment could also enhance the carbon framework structure of starch and reduce the content of amylose, but during the germination process, the content of amylose gradually returns to normal, indicating that the change in starch structure caused by the magnetic field is reversible.
-
The authors confirm contribution to the paper as follows: study conception and design: Zhu Y ,Yang R; data collection: Xie C, Wang P, Li D; analysis and interpretation of results: Zhu Y, Tan S; draft manuscript preparation: Zhu Y, Yang R. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This work was supported by Anhui Provincial Key Research and Development Plan (2023n06020001) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
-
The authors declare that they have no conflict of interest.
- Supplemental Table S1 Coded values of parameters for Box–Behnken design.
- Supplemental Table S2 The primers of GABA-T, GAD and actin.
- Supplemental Table S3 Response surface design and experimental results.
- Supplemental Table S4 Analysis of variance (ANOVA) for the response surface regression model to GABA content.
- Supplemental Fig. S1 Morphology changes of different cultivars of brown rice before and after germination.
- Supplemental Fig. S2 Effects of interaction between NaCl and KCl (A), CaCl2 and KCl (B) and NaCl and CaCl2 (C) on GABA content.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhu Y, Tan S, Xie C, Li D, Wang P, et al. 2024. Effects of magnetic field pretreatment and chloride salt stress on physio-biochemical changes and γ-aminobutyric acid accumulation in germinated brown rice. Food Materials Research 4: e015 doi: 10.48130/fmr-0024-0006
Effects of magnetic field pretreatment and chloride salt stress on physio-biochemical changes and γ-aminobutyric acid accumulation in germinated brown rice
- Received: 09 December 2023
- Revised: 21 February 2024
- Accepted: 15 March 2024
- Published online: 07 May 2024
Abstract: Germinated brown rice is a staple food with high nutritional value and market prospects. Gamma-Aminobutyric Acid (GABA), abundantly present in germinated brown rice, has attracted significant attention due to its multiple active functions on the human body. This study aimed to enrich GABA in germinated brown rice by using static magnetic field pretreatment and NaCl, CaCl2 and KCl stress. After selecting Nanjing9108, which had the highest GABA content among the nine cultivars, a single-factor experiment was conducted and optimized the pretreatment condition as 10 mT static magnetic field for 40 min. Under this condition, the GABA content in brown rice germinated for 36 h was 66.35 mg/100 g, which was 13.88% higher than the control group. Simultaneously, the germination rate and early growth of germinated brown rice were also promoted. The optimal combination of culture medium for GABA enrichment obtained by response surface experimental design was NaCl 37.23 mmol/L, CaCl2 4.71 mmol/L, and KCl 5.75 mmol/L, with a GABA content of 69.783 mg/100 g. Under this condition, the changes in nutrients and the expression of glutamic acid decarboxylase (GAD) and GABA transaminase (GABA-T) related genes during the 0-48 h germination process of brown rice were studied. The relative expression of GAD was promoted and the relative expression of GABA-T was inhibited, resulting in the accumulation of GABA. This indicates that the combination of static magnetic field and salt treatment is an effective method to increase the GABA content in germinated brown rice.
-
Key words:
- Germination /
- Brown rice /
- γ-Aminobutyric acid /
- Chloride salt /
- Static magnetic field













