-
Biochar is a highly porous carbon-rich substance, created through pyrolysis of various feedstocks at different temperatures[1,2]. Animal or plant waste is the primary source of biochar, which undergoes pyrolysis in an oxygen-deprived environment[3]. Compared to unheated biomass, biochar has a higher carbon content, lower nutritional value, a large surface area, and high cation exchange capacity[4,5]. The use of biochar can greatly enhance crop productivity and soil quality by improving soil water retention, nutrient uptake, pH, and cation exchange capacity, promoting microbial diversity, and reducing greenhouse gas emissions[6−9]. Over the past decade, biochar has gained significant attention as a cost-effective soil amendment for the management and restoration of infertile soil[10]. The International Biochar Initiative estimates that by 2050, up to 80% of crop and forestry residues could be utilized as raw materials for biochar production and energy generation[7,11].
Properties of biochar are influenced by the components of the feedstock and the pyrolysis temperature[12]. The impact of biochar is influenced by factors such as pyrolysis temperature, raw material qualities, soil pH, irrigation practices, fertilization methods, and the surface area of biochar. Among these factors, pyrolysis temperature plays a critical role in determining the quality of biochar compared to others[13]. Higher pyrolysis temperatures generally yield biochar with increased carbon content, decreased volatile matter, enhanced stability, and improved resistance to decomposition[14,15]. However, excessively high temperatures can result in the loss of beneficial characteristics and the formation of undesired compounds. Therefore, it is essential to identify optimal pyrolysis conditions for each feedstock to achieve the desired characteristics of biochar. Various feedstocks, including organic manure, wheat straw, plant wood, sewage sludge, paddy husk, weeds, and peanut shells, can be used to produce biochar[16].
The fast-growing legume tree Gliricidia sepium was employed in this study as the feedstock for making biochar. Native to Central and South America as well as Mexico, this species was introduced to Africa and Asia and has now become naturalized in Sri Lanka[17]. This semi-deciduous arboreal tree grows between 3 to 15 m in height with a trunk diameter of up to 30 cm[18]. Due to its numerous beneficial properties, it is widely cultivated in tropical and subtropical agroforestry systems, serving as living fences and shade trees[19]. To study the physical and chemical properties of biochar and its impact on climate change and agriculture, several methods and technologies such as scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX), Raman spectroscopy, and (X-ray diffraction) XRD have been employed[7,20]. SEM-EDX is an effective technique for studying the structural characteristics and quantifying the elemental composition of biochar at different pyrolysis temperatures, providing insights into changing trends in biochar structures[13,21,22]. The objectives of this study were to evaluate the nutritional and structural properties of Gliricidia sepium biochar at different pyrolysis temperatures using SEM – EDX technique.
-
Biochar was produced from well-matured 20-year-old Gliricidia sepium trees, which were cultivated at the Rathmallagara research station of the Coconut Research Institute in Sri Lanka. The process involved cutting the wood into small pieces, crushing them, and subjecting them to oven-drying at a temperature of 60 °C for 72 h. Subsequently, the dried wood was pyrolyzed using a Muffle Furnace (Model P330, Nabertherm, Germany) at six different temperatures: 325, 350, 400, 500, 600, and 700 °C. The pyrolysis process was carried out in a controlled environment with a limited oxygen supply, maintaining a heating rate of 7 °C per min for a duration of 3 h. The resulting biochar, labeled according to its carbonaceous composition, was then stored under room temperature conditions until further investigations were conducted.
Characterization of biochar properties
-
The nutritional and structural properties of the produced biochar were evaluated using the SEM-EDX spectroscopy technique (Model: Zeiss EVO LS15). SEM-EDX spectroscopy techniques are widely used for analyzing the nutritional and structural properties of various materials[23]. SEM-EDX analysis is relatively fast compared to other elemental digestion methods, making it a time-efficient option for elemental analysis[24]. This technique allows for non-destructive analysis, preserving the integrity of the samples. They provide high-resolution imaging capabilities, enabling detailed visualization of the sample's surface structure[13,24].
The principle of SEM-EDX techniques involves the operation of an SEM in conjunction with EDX. As shown in Fig. 1 the SEM microscope functions by generating a beam of electrons emitted from an emitter-cathode within an electron gun[25,26]. This beam is then accelerated and focused by an anode and a series of electromagnetic lenses. Once the electron beam strikes the sample, electrons, secondary electrons, and X-rays are ejected from the sample. The EDX detector gathers these emitted backscattered electrons, secondary electrons, and X-rays, converting them into a signal transmitted to a display akin to a television screen[26].
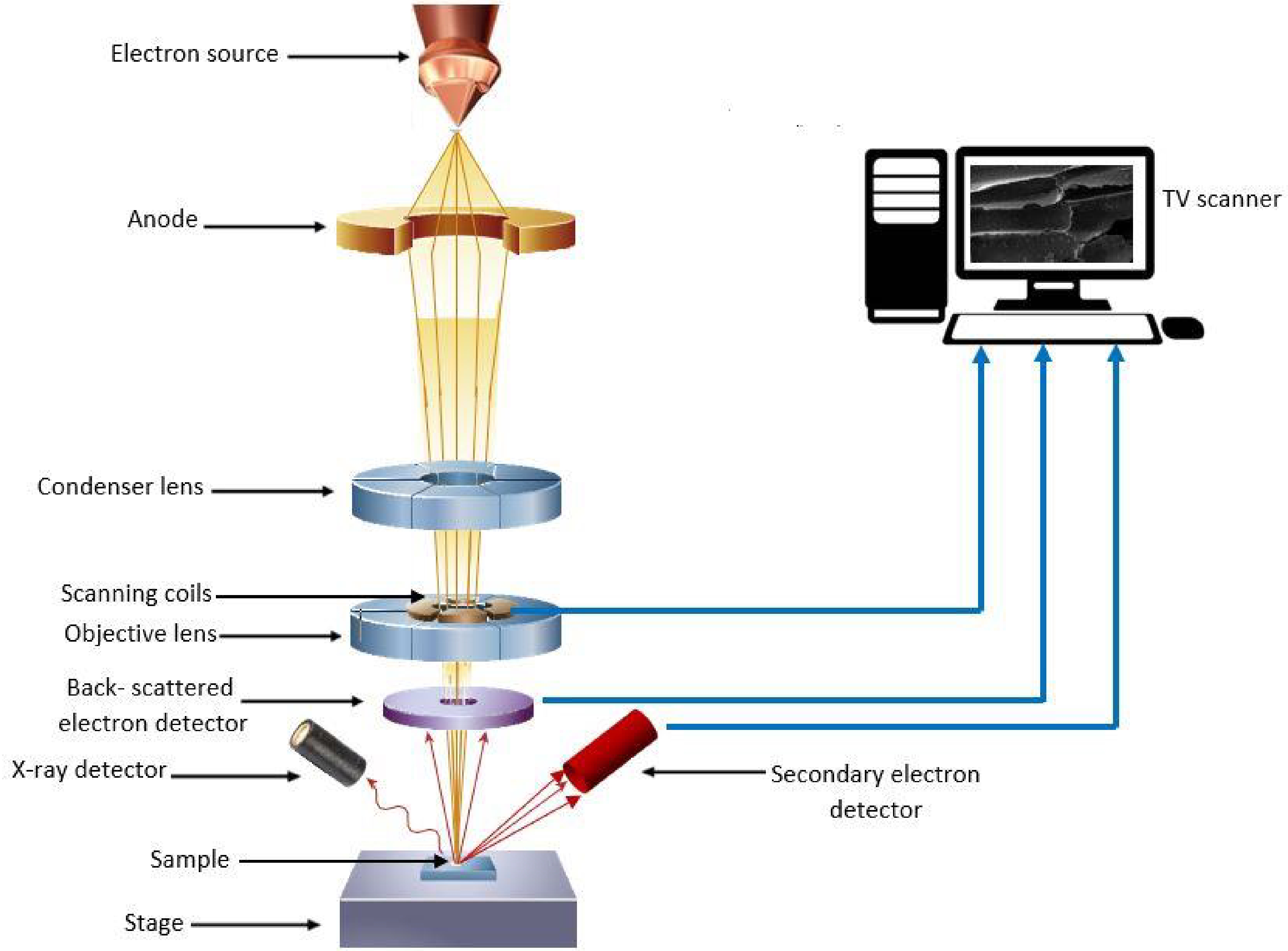
Figure 1.
Working principle of scanning electron microscope with energy dispersive X-ray spectroscopy analysis. (Adopted from Inkson[26]).
By examining the energy spectrum, the EDX can determine what elements are present in the sample and estimate the proportions of each element[27]. Together, the SEM provides high-resolution imaging of the sample's surface, while the EDX provides information about the elemental makeup of the sample. This analysis was conducted at the laboratory situated in the Faculty of Science, University of Peradeniya, Sri Lanka. The samples were examined using the SEM-EDX, employing the same magnification level as the initial step and the measurements were recorded as weight percentages with three replicates.
Statistical analysis
-
R program (version 4.1.3) was used to perform all statistical analysis. The mean values of the data were subjected to the one-way analysis of variance (ANOVA) at a significance level of 5%. It was used to determine whether there are any statistically significant differences between the means of biochar yield / nutrient content with six temperature levels. This was followed by Tukey's honestly significant difference (HSD) comparison test to perform statistical comparisons. Principal component analysis (PCA) was done to identify the principal components that explain the majority of the variance in the dataset.
-
The data indicated that the nutritional properties of Gliricidia sepium biochar are greatly influenced by the pyrolysis temperature. Figure 2a illustrates the changes in biochar yield at different pyrolysis temperatures. The highest biochar yield of 38.17% was observed at a temperature of 325 °C, while the lowest yield of 27.43% was recorded at 700 °C. These results indicate a statically significant (p < 0.05) decrease in biochar yield as the pyrolysis temperature increases. Figure 2b illustrates that the carbon (C) content in the biochar experienced a significant (p < 0.05) increase from 55.86% to 76.12% as the pyrolysis temperature rose from 325 to 600 °C. The results indicate a substantial augmentation in the C content of the biochar with escalating pyrolysis temperatures. Figure 2c demonstrates a decreasing trend in the oxygen (O) content of the biochar as the production temperature increases up to 500 °C. The highest O yield of 27.88% was obtained at 325 °C and the lowest yield of 12.08% was recorded at 500 °C, with a significant (p < 0.05) difference observed among treatments.
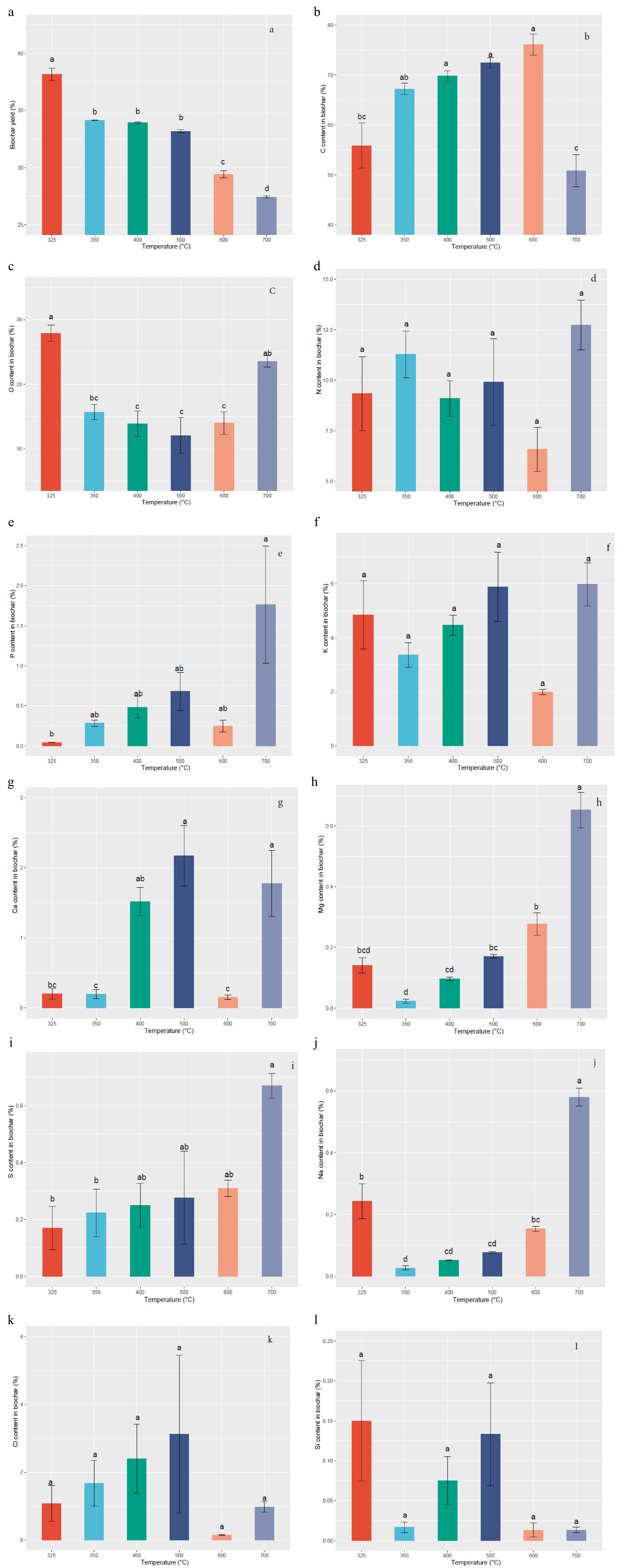
Figure 2.
Variation of Gliricidia sepium biochar nutrient content with different pyrolysis temperatures. (a) Biochar yield, (b) C content, (c) O content, (d) N content, (e) P content, (f) K content, (g) Ca content, (h) Mg content, (i) S content, (j) Na content, (k) Cl content, (l) Si content. Means with different letters represent significant differences at p < 0.05 level.
Although the increase in pyrolysis temperature did not exhibit a significant trend of increasing nitrogen (N) yield, an unexpectedly high N content of 12.72% was observed at 700 °C (Fig. 2d). As depicted in Fig. 2e, increasing the pyrolysis temperature from 325 to 700 °C resulted in an augmentation of phosphorus (P) content from 0.04% to 1.76%, respectively, with a significant (p < 0.05) difference observed among treatments. The potassium (K) content in the biochar exhibited a significant (p < 0.05) decreasing trend with increasing pyrolysis temperature, as shown in Fig. 2f. The highest K yield of 5.98% was recorded at 700 °C. As the pyrolysis temperature increased from 325 to 500 °C, the calcium (Ca) content exhibited a range from 0.20% to 2.17% (Fig. 2g), with significant (p < 0.05) differences observed among the six different pyrolysis temperatures. According to Fig. 2h, the highest magnesium (Mg) content of 0.65% was achieved at a production temperature of 700 °C, while the lowest Mg content of 0.02% was recorded at 350 °C, with significant (p < 0.05) differences observed among the six different pyrolysis temperatures. The sulfur (S) element exhibited a gradual increasing trend with the increment in production temperatures, varying from 0.17% to 0.67% (Fig. 2i) in response to temperature changes from 325 to 700 °C, with significant (p < 0.05) differences observed among treatments.
As depicted in Fig. 2j, the biochar derived from Gliricidia sepium exhibited a significant difference (p < 0.05) in increased sodium (Na) content with rising pyrolysis temperature, primarily attributed to minimal volatilization losses. The highest Na yield of 0.58% was observed at 700 °C, while the lowest Na yield of 0.02 % was recorded at 350 °C. From Fig. 2k, it is revealed that the highest chlorine (Cl) content of 3.12% was obtained at 500 °C, while the lowest Cl content of 0.15% was recorded at 600 °C. However, no significant (p < 0.05) difference was observed among treatments. In Fig. 2l, the treatments did not exhibit significant variations (p < 0.05) in silicon (Si) content with changes in production temperature, indicating 0.15% as the highest Si content at 325 °C, while the lowest Si content of 0.01% occurred at 700 °C.
Based on nutritional analysis results, the optimal number of principal components to retain in PCA were determined by employing a scree plot to identify the variance they encompassed, as illustrated in Supplemental Fig. S1. Based on the scree plot result, two major principal components that explained that 66.4% of the total variability was identified. PCA was then run, and based on its results, these key components were delineated as follows: the foremost principal component, contributing 42.9% to the total variance was associated with carbon content, while the second principal component, responsible for 23.5% of the variance, was related to biochar yield (Supplemental Fig. S2). The angle between two vectors indicated the correlation between their respective variabilities. A smaller angle suggested a positive correlation, while a larger angle suggested a negative correlation. A 90° angle indicated no correlation between the two characteristics.
Structural properties of Gliricidia sepium biochar
-
Biochar demonstrates significant variations in terms of chemical composition, structural characteristics, and morphology, which are influenced by both the temperature and the type of feedstock used. When considering morphological characteristics, using the SEM technique allows for a thorough investigation of surface morphology and structural changes in biochar. Figure 3 illustrates SEM images of biochar produced at 400 °C, captured at different magnifications: (a) ×250, (b) ×500, (c) ×1,000, and (d) ×3,000. Notable changes were observed on the surface of Gliricidia sepium biochar produced at 400 °C temperature due to the formation of voids. Under a magnification of ×3,000, the observed pores range from 1 to 3 µm in length at lower pyrolysis temperatures of 400 °C.
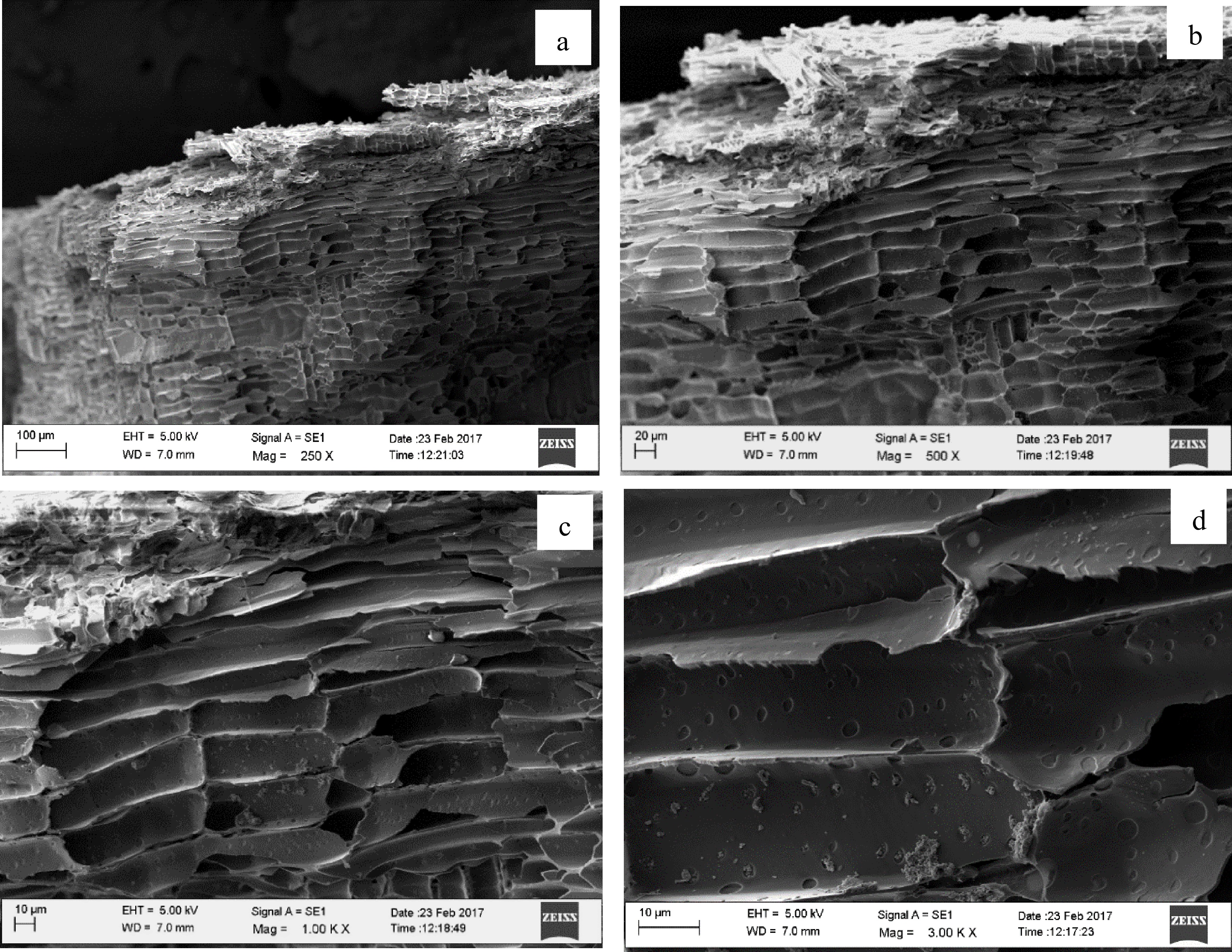
Figure 3.
Scanning electron microscopy (SEM) micrographs of Gliricidia sepium biochar produced at 400 °C. (a) Under ×250 magnification, (b) Under ×500 magnification, (c) Under ×1,000 magnification, (d) Under ×3,000 magnification.
Correlation analysis among measured nutritional properties of Gliricidia sepium biochar
-
The correlation analysis results for the measured characteristics of Gliricidia sepium biochar are presented in Fig. 4. The graphical representation reveals the presence of both positive and negative correlations among variables, which are characterized by varying absolute values of correlation coefficients. Pyrolysis temperature exhibits the strongest positive correlation with Mg. Additionally, it shows fewer positive correlations in the following nutrients. Na, S, P, Ca, K, and N. The biochar yield shows the most pronounced negative correlation with pyrolysis temperature, while this negative correlation weakens when considering Si, Cl, C, and O. When the pyrolysis temperature increased the biochar yield decreased. That is mainly due to the increased release of volatile components with increasing temperatures. The biochar yield of Gliricidia sepium biochar exhibits negative correlations with Mg, S, Na, P, Ca, and N. Conversely, silicon Si, O, Cl, and K show positive correlations with the biochar yield. However, C does not show any relationship with the biochar yield.
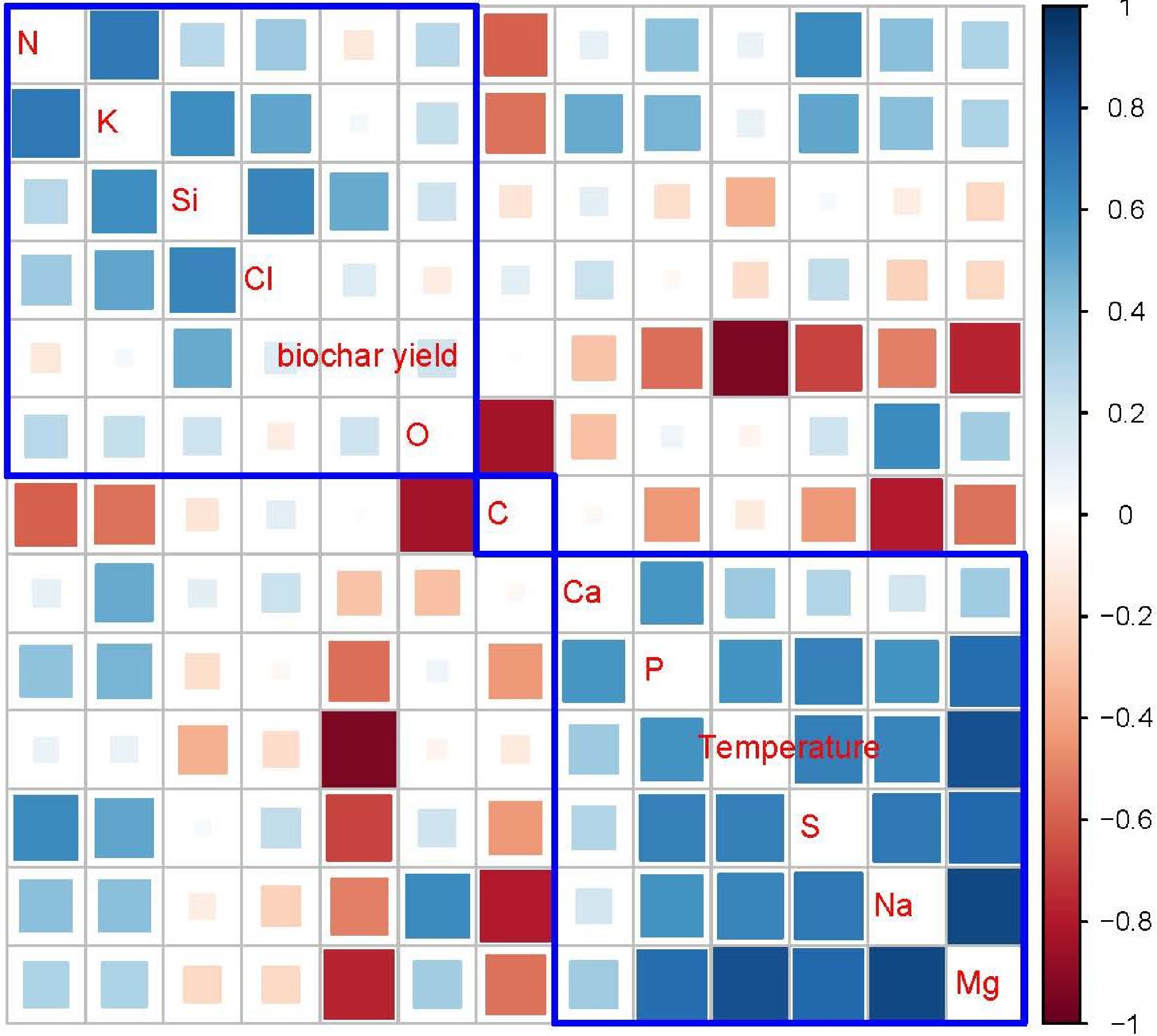
Figure 4.
Graphical display of the correlation matrix, using the 'corrplot' package of R-software. Red color indicates the negative correlations between shown variables; blue color indicates the positive correlations; white color indicates no correlation. The areas of the squares show the absolute value of corresponding correlation coefficients. Parameters covered by blue lines show similar correlation variation patterns.
-
The results from this study revealed that the pyrolysis temperature has a significant influence on the yield and nutritional properties of biochar derived from Gliricidia sepium. The highest biochar yield was observed at 325 °C, which aligns with the findings of Cantrell et al.[28] that lower pyrolysis temperatures tend to maximize biochar yield due to reduced thermal decomposition of the biomass. However, the results of this study indicate a decrease in biochar yield as the pyrolysis temperature increases, due to the increased decomposition of wood components like lignocelluloses and the greater release of volatile substances at higher temperatures[29]. As the pyrolysis temperature increased from 325 to 600 °C, a notable increase in C content was identified in this study. Several factors can be attributed to this result, including the polymerization of organic compounds, which could also contribute to the enhanced C yield observed at higher temperatures[30].
The O content exhibited a decreasing trend as the production temperature increased up to 500 °C, with the highest O yield recorded at 325 °C and the lowest yield recorded at 500 °C. The reason for this result is that as the pyrolysis temperature increases, it promotes the volatilization process, resulting in a lower O yield in the final product[13,31]. Additionally, when the pyrolysis temperature is increased, it affects dehydration, aromatization, decarboxylation, condensation, and polymerization in woody biomass, leading to changes in the affinity levels of chemical bonds between C and O elements[32]. This could cause a decrease in the final product's O content. When studying the final N content in Gliricidia sepium biochar, the increase in pyrolysis temperature did not show a significant trend of increasing N content. However, high N content was observed at 700 °C. This is identified as the main reason for these results, due to the processes of dehydration and decarbonization occurring during high temperatures, leading to an increase in N content in biochar[33]. Results showed that increasing the pyrolysis temperature from 325 to 700 °C led to a rise in the P content in biochar. This can be attributed to the reduction in volatilization of phosphorus-containing compounds, such as phosphine (PH3) and phosphorus oxides (P2O5), at higher temperatures. Therefore, increasing the pyrolysis temperature results in an increase in P content in biochar[34]. The K content in the produced biochar exhibited an increasing trend with increasing pyrolysis temperature, with the highest K yield recorded at 700 °C. As the pyrolysis temperature increases, the decomposition of complex-K and graphite layer-K occurs, and KCl is volatilized, resulting in a high K concentration towards the end product[35].
As the pyrolysis temperature increased from 325 to 500 °C, the Ca content in the produced biochar exhibited a significantly increasing trend. This increase in Ca levels in woody biochar at higher pyrolysis temperatures is attributed to the breakdown of Ca-containing organic compounds present in the biomass[36]. Consequently, Gliricidia sepium biochar demonstrates a high Ca content at elevated temperatures. According to the results, the highest Mg content was achieved at a production temperature of 700 °C, while the lowest Mg content was recorded at 350 °C. As the temperature rises during the pyrolysis process, chemical reactions occur, resulting in the breakdown of organic compounds containing Mg and the liberation of high concentrations of Mg ions within the biochar[37]. Therefore, increasing the pyrolysis temperature leads to an increase in Mg content in the biochar. The S content in biochar exhibited a gradual increasing trend with the increment in production temperatures.
The highest S content was recorded at 700 °C, and the lowest S yield was recorded at 325 °C in the produced biochar. When pyrolysis temperature increases, the organic compounds present in the biomass undergo thermal decomposition, causing the release of volatile S compounds and leading to a higher concentration of S in the biochar at high temperatures[38]. When examining the Na content in produced biochar, the highest Na yield was observed at 700 °C. As the pyrolysis temperature rises Na ions are released, which then become incorporated into the biochar structure, thereby increasing the concentration of Na[39]. Consequently, the Na content in the final product is higher due to minimal volatilization losses. The highest chlorine (Cl) content was obtained at 500 °C in Gliricidia sepium biochar. Raising the pyrolysis temperature can reduce the volatilization of Cl, leading to a higher Cl content in the final biochar product[40]. This was the reason for the high Cl yield in the end product. The highest Si content was observed in the biochar produced at 325 °C, while the lowest Si content occurred in the biochar produced at 700 °C. According to the study by Bolan et al.[41], lower temperatures (around 325 °C) are ideal for obtaining a high Si content in woody biochar. Therefore, the highest Si content was obtained in the Gliricidia sepium biochar when it is produced at lower temperatures.
Immature cuttings and leaves of the plant Gliricidia sepium are easily used as green manure or fodder sources in agriculture. However, the mature stems of this plant cannot be directly used for agricultural purposes on farmland. Based on the present findings, it can be stated that the Gliricidia sepium plant has a high potential for producing biochar from its mature stems due to its high yield. In addition to biochar's role as a soil amendment, it can also provide significant nutritional value for agricultural use. Biochar can significantly improve soil nutrient levels. When farmers use biochar to enhance soil characteristics and increase nutrient availability, it should be produced at temperatures that provide maximum biochar yield and optimal nutrient availability in proportion to the amount of nutrients required by the soil.
Although the Gliricidia sepium plant can be utilized to produce biochar for agricultural applications, we believe that extensive further research is necessary in this area. Additional research is needed to further expand our understanding, particularly to determine the optimal pyrolysis duration for biochar production, to investigate the nutrient transfer capacity of biochar when applied to soil, and to explore the effects of these biochar applications for reducing heavy metal concentrations in polluted soils.
-
This study aimed to evaluate the nutritional and structural properties of Gliricidia sepium biochar at different pyrolysis temperatures using the SEM – EDX technique. The results demonstrated that the pyrolysis temperature significantly impacted the nutritional value of the biochar and its potential applications in agriculture. According to the experiment findings, when the pyrolysis temperature is increased, the biochar yield decreases. If the objective is to maximize the production of biochar through pyrolysis, a preference would be given to a process with low temperature and a slow heating rate. Furthermore, the composition of the biochar, including nutrients such as C, O, P, Ca, Mg, and Na varied significantly depending on the pyrolysis temperature. SEM-EDX techniques offer a powerful means to investigate the structural and nutritional composition of biochar, thereby aiding in its identification and characterization. The production of biochar from Gliricidia sepium, resulted in a nutrient-rich source that can be effectively used as a soil amendment.
-
The authors confirm contribution to the paper as follows: study conception and design: Nuwarapaksha TD, Atapattu AJ; data collection: Udumann SS, Dissanayaka DMNS; analysis and interpretation of results: Nuwarapaksha TD, Dissanayaka DMNS; draft manuscript preparation: Nuwarapaksha TD, Udumann SS, Shanmugalingam Vinujan, Atapattu AJ. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
We would like to express our appreciation to the technical staff of the Agronomy Division of the Coconut Research Institute of Sri Lanka, Lunuwila, 61150, Sri Lanka.
-
The authors declare that they have no conflict of interest.
- Supplemental Fig. S1 Scree plot analysis showing percentage of explained variations in response to number of principal components for Gliricidia sepium biochar production.
- Supplemental Fig. S2 PCA for the parameters influencing Gliricidia sepium biochar production; relationship between principal component I and II.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Nuwarapaksha TD, Dissanayaka DMNS, Udumann SS, Vinujan S, Atapattu AJ. 2024. Exploring the impact of pyrolysis temperature on nutrient composition of Gliricidia sepium biochar: a comprehensive study. Technology in Agronomy 4: e016 doi: 10.48130/tia-0024-0014
Exploring the impact of pyrolysis temperature on nutrient composition of Gliricidia sepium biochar: a comprehensive study
- Received: 05 November 2023
- Revised: 03 May 2024
- Accepted: 09 May 2024
- Published online: 04 July 2024
Abstract: Biochar is a carbon-rich, highly porous substance produced through pyrolysis at different temperatures using various feedstocks. The performance of biochar can vary based on the properties of the raw materials and the production process. The objective of this study was to evaluate the nutritional and structural properties of Gliricidia sepium biochar at different pyrolysis temperatures. Gliricidia sepium biochar was generated at temperatures ranging from 325 to 700 °C under a limited oxygen supply, with a heating rate set at 7 °C per minute, and a pyrolysis duration of 3 h. Nutritional and structural properties of the produced biochar were examined using a scanning electron microscope (SEM) combined with energy dispersive X-ray spectroscopy (EDX) technique. Results demonstrated a significant influence of pyrolysis temperature, particularly at lower heating rates, on the nutritional properties and structure of Gliricidia sepium biochar. A maximum biochar yield of 38.17% was achieved at 325 °C, underscoring the preference for lower pyrolysis temperatures in biochar production, while the minimum biochar yield of 27.43% was observed at 700 °C. Different biochar treatments exhibited significant differences (p < 0.05) in the yields of Carbon (C), Oxygen (O), Phosphorus (P), Calcium (Ca), Magnesium (Mg), Sulphur (S), and Sodium (Na). Conclusively, the production of biochar from Gliricidia sepium yielded a nutrient-rich resource that holds great potential as a soil amendment and future studies should need to explore the effects of pyrolysis duration, nutrient transfer capacity, and heavy metal composition of Gliricidia sepium biochar to further expand our understanding of its characteristics and applications.













