-
Blue honeysuckle is referred to as 'the third generation of small berries' primarily found in the cold regions of northeast China, northern Russia, and central Europe[1]. Blue honeysuckle is known to contain numerous bioactive substances, including polyphenols. It has been extensively studied for its diverse functional benefits, including its antidiabetic, anti-inflammatory, viral safe, anti-atherosclerotic effects, anticancer, and antioxidant properties[2]. Since its certification as a new resource food resource by the European Food Safety Authority (EFSA) in 2018, the cultivation area and yield of blue honeysuckle have experienced steady annual increases[3]. With a high moisture content of 80% to 85% and a delicate pericarp lacking protective tissue, blue honeysuckle is highly susceptible to mechanical damage, dehydration, and softening. Consequently, due to this inherent vulnerability, the fruit is ultimately susceptible to decay and a loss of its edible value. More than 95% of the global production of blue honeysuckle is currently processed into frozen fruit. To promote the diversified development of the blue honeysuckle industry and increase the market circulation rate of fresh fruits, it is crucial to address the issue of the shelf life of blueberries. This will contribute to the overall growth and sustainability of the industry. Currently, the methods for extending the shelf-life of fruits primarily involve low-temperature preservation, controlled atmosphere preservation, chemical preservation, coating film preservation, and irradiation preservation. Research has shown that low temperatures can prolong the preservation period of blue honeysuckle from 7 d to up to 14 d[4]. The storage methods of modified atmosphere (MA) and controlled atmosphere (CA) have been proven to be effective in preserving bioactive components, improving antioxidant capacity, and extending the fruit’ shelf life[5]. However, its effect on the storage duration of blue honeysuckle has not been thoroughly investigated. Therefore, further research is significantly needed to identify suitable storage methods that can effectively extend the shelf life of fresh blue honeysuckle.
The technology of controlled atmosphere preservation plays a pivotal role in the modern food preservation field. It has significant implications for reducing food waste, improving food quality, and meeting consumer demands. Controlled atmosphere preservation is a chemical-free technique of preservation, mainly divided into MA and CA[6]. CA involves the regulation of oxygen levels, carbon dioxide levels, moisture content, and temperature in the surrounding environment during the storage of fruits[7]. Recent studies have demonstrated that different concentrations of O2 and CO2 can significantly impact the respiration rate, shelf life, nutrient content, and biological capacity of fruit[8]. Maintaining a high concentration of oxygen has a positive impact on postharvest decay and the quality of blueberry, strawberry, and Chinese bayberry, while an increase in oxygen concentration results in decreased severity of decay[9]. Cantín et al. showed that blueberries were susceptible to softening and unpleasant flavors at a 24% concentration of CO2[10]. Dziedzic et al. found that high concentrations of CO2 (20%) and low concentrations of O2 (5%) increased antioxidant capacity in blue honeysuckle during the 14-d storage[5]. However, there has been a lack of systematic studies on the impact of long-term storage of blue honeysuckle under various O2 and CO2 concentrations. It is necessary to analyze the quality changes of blue honeysuckle under different concentrations of O2 and CO2.
Therefore, the aims of this study were: (1) to investigate the shelf life of fresh blue honeysuckle fruit under CA conditions; (2) to evaluate the effect of CA on various postharvest quality parameters, including physical properties (epicuticular wax coverage, color, moisture content, weight loss, and firmness), chemical properties (total soluble solids, acidity, pH, ethylene, malondialdehyde, abscisic acid, and ascorbic acid), biological properties (respiration rate), total phenolic contents (phenolics, flavonoids, and anthocyanins), and antioxidant capacity (measured using DPPH, ABTS, and FRAP assays); and (3) to investigate the correlation between bioactive components and the antioxidant capacity of blue honeysuckle during 28 d of storage.
-
Standard substances include gallic acid, abscisic acid (ABA), iron(II) sulfate heptahydrate (FeSO4·7H2O), ascorbic acid (ASA), and 6-Hydroxy-2,5,7,8-tetramethylchloromethane-2-carboxylic acid (Trolox). HPLC-grade reagents include potassium dihydrogen phosphate, methanol, water, and acetonitrile. Analytical reagents include hydrochloric acid, glacial acetic acid, folin-Ciocalteau, 2,2-Diphenyl-1-picrylhydrazine (DPPH), sodium carbonate, sodium acetate. 2,2'-hydrazine bis (3-ethylbenzothiazoline-6-sulfonic acid) diamine salt (ABTS) potassium chloride, ferric chloride hexahydrate (FeCl3·6H2O), and 2,4,6-triazine-s-triazine (TPTZ).
The blue honeysuckle cultivar 'Lanjingling' was harvested when they had reached a fully ripe and blue-black color at commercial maturity from the Xiangyang base station (latitude 39.93° N, longitude 116.04° E) at Northeast Agricultural University in Harbin, China.
Precooling and CA treatment
-
To reduce respiration and field heat in the blue honeysuckle, a gradient air-cooling process at low temperatures was used. For the CA treatment, 15 kg of blue honeysuckle were stored in three gas-adjusted fresh-preserved storehouses for monitoring the concentration of CO2 and O2 (GQX-300, Gasin-DH Co., Ltd., China) with three different CA conditions: 20% O2, 20% CO2 (CA1), 5% O2, 20% CO2 (CA2), and 5% O2, 10% CO2 (CA3). Another 5 kg of blue honeysuckle was stored in ambient air conditions as a control. The storage conditions were maintained at a temperature of −1 °C and a relative humidity of 95%. This study collected samples on days 0, 3, 7, 14, 21, and 28 after treatment, with three replicates used for each condition.
Physical, chemical, and biological properties
Physical properties
-
Postharvest fruit can be evaluated through various physical, chemical, and biological properties. In this study, we measured the epicuticular wax coverage, color, moisture content, weight loss, and firmness as physical properties. The epicuticular wax coverage was assessed using the previously established method[11], which involved grading the fruit into five levels: 0 (lack of wax coverage), 1 (0 < wax coverage ≤ 1/3), 2 (1/3 < wax coverage ≤ 2/3), 3 (2/3 < wax coverage < 1), and 4 (wax coverage = 1).
The color, moisture content, weight loss, and firmness were determined at each storage interval. The measurement methods and calculations of these indicators are based on previous studies[11].
Chemical properties
-
The chemical properties were assessed by determining the total soluble solids (TSS), acidity, pH, ethylene (ETH), malondialdehyde (MDA), abscisic acid (ABA), and ascorbic acid (ASA). TSS was determined using a Brix-acidity meter (PAL-BX/ACID7, ATAGO, Japan), while acidity was also assessed with the same instrument. And pH was determined utilizing a pH meter (S400-K, Mettler Toledo, Switzerland). To determine the ETH content, the sample was mixed with a 0.1 M phosphate buffer solution (pH 7.4) and subsequently subjected to centrifugation at 3000 g for 20 min. The Plant ETH ELISA Kit was utilized to quantify the production of ETH, by the provided instructions.
The MDA determination was carried out utilizing a previously established method as delineated by Li et al.[12]. Briefly, the sample (30 μL) was combined with 450 μL of 10% trichloroacetic acid solution, followed by centrifugation. Then, the supernatant (100 μL) was combined with an equal volume of 0.6% sulfuric acid. The resultant mixture was subjected to absorbance measurements at 450, 532, and 600 nm utilizing a microplate reader (Epoch 2, Bio Tek, USA).
A high-performance liquid chromatography equipped with a photodiode array (HPLC-PDA, Agilent, 1200, USA) was utilized to determine the contents of ABA and ASA in the sample. The chromatography column is a reversed-phase C18 column (150 mm × 4.6 mm, 5 μm, Shiseido, Japan) and the injection volume for both ABA and ASA was 10 μL. For the analysis of ABA, isocratic elution was employed with eluent flow (methanol, 0.7% acetic acid, and acetonitrile with a ratio of 55:40:5) for 20 min at a rate of 0.7 mL·min−1, while maintaining the column temperature at 30 °C[13]. The analysis was carried out at a wavelength of 254 nm. The ASA was conducted utilizing a methodology pioneered by Klimczak & Gliszczyńska-Świgło[14]. Elution was performed with a linear gradient profile, utilizing 5 mmol·L−1 KH2PO4 (solvent A) and methanol (solvent B). The proportions of solvent B at different time points were as follows: (t [min], B%): (0, 5), (6, 22), (9, 5). Detection was carried out at a wavelength of 245 nm and a flow rate of 0.8 mL·min−1.
Biological property
-
The biological property of respiration rate was also evaluated. This measurement was conducted utilizing a fruit and vegetable respirometer (SYS-GH30A, SAIYASI, China).
Total content of phenolic, flavonoid and anthocyanin
-
The total phenolic content (TPC) was estimated using the Folin-Ciocalteu method, following the procedure as delineated by Singleton et al.[15]. In brief, a reaction mixture was prepared by combining 20 μL of the diluted sample, 90 μL of water, and 10 μL of the Folin-Ciocalteu reagent. Following a 5-min reaction in the absence of light at a temperature of 25 °C, an addition of 80 μL of a sodium carbonate solution (75 g·L−1) was made. The measurement of absorbance was conducted at a wavelength of 765 nm.
The total flavonoid content (TFC) was determined using a modified colorimetric method based on the procedure described by Chang et al.[16]. Briefly, 30 μL of the sample or catechin standard solution was introduced. Afterward, 180 μL of water and 10 μL of a 5% NaNO2 solution was added. The solution was allowed to react at 25 °C for 6 min. Subsequently, 20 μL of 10% AlCl3 was added, and the reaction continued for an additional 6 min. The reaction was subsequently terminated by the addition of 60 μL of 4% NaOH, following which the absorbance was assessed at a wavelength of 510 nm after 15 min.
To determine the total anthocyanin content (TAC), we employed the spectrophotometric pH differential method, as described by Zhang et al.[17]. Briefly, samples (20 μL) were diluted with 0.025 M KCl and 0.4 M CH3COONa solutions, which had been adjusted to pH levels of 1.0 and 4.5 using HCl and CH3COOH, respectively. The samples were measured for absorbance at wavelengths of 510 and 700 nm.
Antioxidant capacity
DPPH
-
The DPPH of each extract was assessed utilizing the methodology delineated by Brand-Williams et al.[18]. Specifically, the diluted sample (5 μL) was mixed with DPPH solution (195 μL). The reaction liquid was shaken and then placed in a dark incubator at 25 °C for 2 h. The measurement of absorbance was conducted at a wavelength of 515 nm. A series of Trolox equivalent (TE) solutions with concentrations of 100, 200, 500, 800, and 1,000 μmol·L−1 were utilized to construct a calibration curve.
ABTS
-
The ABTS was conducted using the same methodology as in the previous research[19]. The antioxidant capacity was determined by constructing concentration-response curves for ABTS•+ using Trolox equivalent solutions (50, 100, 200, 500, and 800 μmol·L−1). The solution of ABTS•+ (190 μL) was diluted with methanol until it achieved an absorbance of 0.7 ± 0.02 at 734 nm, following which it was combined with 10 μL of the sample.
FRAP
-
The FRAP was conducted following the method by Benzie et al.[20]. Briefly, the FRAP working solution consists of 10 mM TPTZ, 20 mM FeCl3·6H2O, and 300 mM acetate buffer in a ratio of 1:1:10, v/v/v, which was measured at 593 nm as a control. Next, 10 μL of the sample was combined with 30 μL of water and then measured at the same wavelength for 30 min.
Statistical analysis
-
The values presented in the tables were presented as the mean ± standard deviation. ANOVA was utilized to analyze the data for each parameter using IBM SPSS Statistics 26. Partial Least Squares Discriminant Analysis (PLS-DA) was achieved via MetaboAnalyst 6.0. The principal component analysis (PCA) and correlation analysis were performed in Origin 2021. The significance of the correlation analysis was determined at a level of p ≤ 0.05, and the findings are elegantly presented in a visually appealing correlation plot.
-
The removal of natural wax from fruit has been discovered to speed up water loss and decay, diminish sensory and nutritional attributes, and reduce the shelf-life of postharvest fruits[21]. Supplemental Table S1 and Fig. 1 show the epicuticular wax coverage of control and CA-treated blue honeysuckle. All treated samples exhibited a declining trend during the 28-d storage, with the day 28 value decreasing by 61.12%, 39.46%, 40.41%, and 28.14% compared to the initial values, respectively. Similarly, significant changes have been observed in the epidermal wax of blueberries during storage[21]. Furthermore, different treatments have been observed to produce changes in blue honeysuckle storage. The CA treatment was beneficial in delaying the reduction of fruit epicuticular wax. After 3 d of storage, the epicuticular wax coverage of CA2 and CA3 was found to be significantly higher compared to the control. This finding is consistent with observed results in pear, where compositions and wax contents in CA storage demonstrated a more favorable effect compared to cold storage of fragrant pear. These results indicate that CA storage is more suitable than cold storage for maintaining the quality of fruit[22].
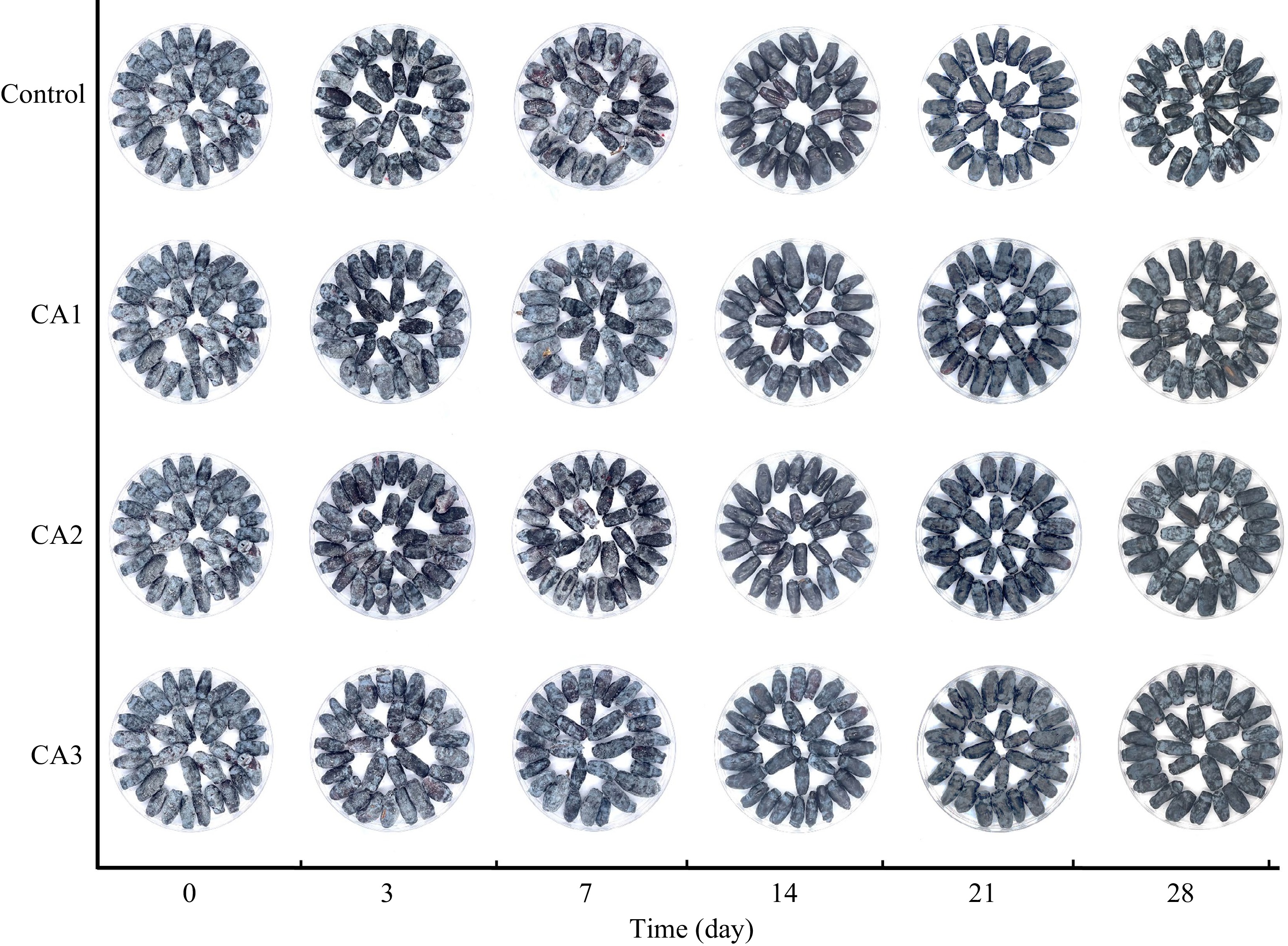
Figure 1.
Visual changes in fruit color and wax of blue honeysuckle in the control (air) and after CA treated during 28 d of storage at −1 °C. Control represents air. CA1 represent 20% O2 and 20% CO2, CA2 represent 5% O2 and 20% CO2, CA3 represent 5% O2 and 10% CO2.
The quality of fruit is closely related to its surface color, which directly affects consumers' purchase desire[23]. The control and CA groups showed an upward trend in ΔL values and a gradual decrease in Δa and Δb values during storage (Fig. 2a−c). The increase in ΔL values may be attributed to the presence of the natural wax layer in the fruit epidermis, which is consistent with findings reported for blueberries[21]. The blue honeysuckle fruit became greener and more yellowish during storage, as indicated by the slight decrease in Δa and Δb values. Moreover, as shown in Fig. 2d, the total color difference (ΔE) revealed that CA3 was more effective in protecting fruit color than other treatments, which was consistent with the epicuticular wax coverage index in this study. These findings suggest that postharvest CA treatment is beneficial in preserving the color of the fruit[24].

Figure 2.
Changes in (a) ΔL, (b) Δa, (c) Δb, and (d) ΔE values of blue honeysuckle in the control (air) and after CA treated during 28 d of storage at −1 °C. ΔL represents the difference in terms of lightness; Δa represents the red (+a) to green (−a); Δb represents the yellow (+b) to blue (−b); ΔE represents the total color difference. Control represents air. CA1 represent 20% O2 and 20% CO2, CA2 represent 5% O2 and 20% CO2, CA3 represent 5% O2 and 10% CO2. Each treatment was repeated in ten replicates and ten fruits of blue honeysuckle were used per replicate.
Moisture content, weight loss, and firmness are among the most important parameters evaluated when assessing the postharvest quality of small berries[5]. As depicted in Fig. 3a−b, the moisture content of blue honeysuckle declined during storage, with no significant difference observed between the control and CA-treated samples. The trend of weight loss was consistent with the moisture content, with blue honeysuckle showing less weight loss during 21 d of storage and a sharp loss in weight loss on the 28th d. Specifically, the weight loss values of CA1, CA2, and CA3 were 6.30%, 3.50%, and 10.83%, respectively, compared to 13.17% in the control group on the 28th d. Therefore, CA storage conditions may be useful in reducing the weight loss of blue honeysuckle during storage. These findings align with a prior study, which demonstrated that atmosphere packaging of fruit may reduce weight loss and extend shelf life[8]. Furthermore, the present study suggests that increasing CO2 and decreasing O2 levels are beneficial for reducing weight loss during CA storage, which aligns with a previous study on cranberries[25]. Regarding fruit firmness, as shown in Fig. 3c, the level increased for all samples, except for CA2, during the first 3 d of storage, but gradually declined in the CA2 sample thereafter. There are several reasons for the change in fruit firmness, including cell changes that lead to softening, as well as variations in external conditions such as environmental conditions[9,26]. Furthermore, the firmness of blue honeysuckle during CA storage was greater than that of the control. This phenomenon may be attributed to a decrease in the activity of enzymes responsible for breaking down the cell wall and causing softening, as well as an increase in lignification, which can result in fruit toughening. Additionally, a study conducted by Paniagua et al. showed that moisture loss is also a major factor contributing to firmness changes during postharvest storage of blueberries[27].
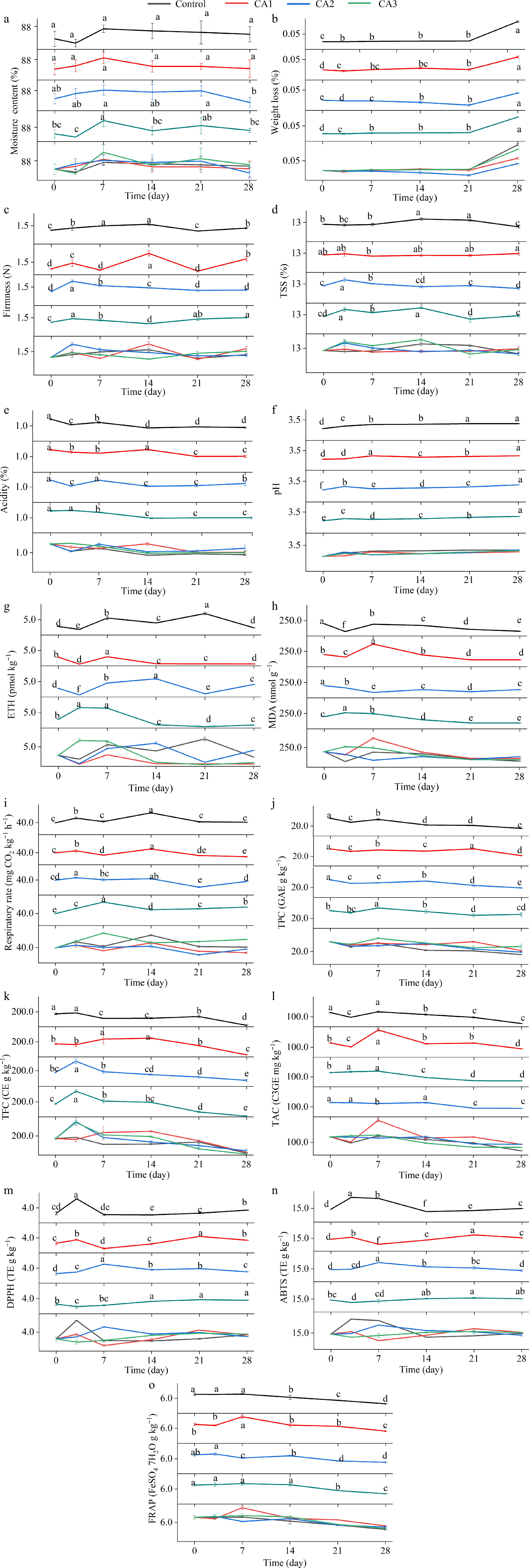
Figure 3.
Effect of CA treatment on (a) moisture content (%), (b) weight loss (%), (c) firmness (N), (d) TSS (%), (e) acidity (%), (f) pH, (g) ETH (pmol·kg−1), (h) MDA (nmol·g−1), (i) respiration rate (mg CO2 kg−1·h−1), (j) TPC (GAE g·kg−1), (k) TFC (CE mg·g−1), (l) TAC (C3GE mg·kg−1), (m) DPPH (TE g·kg−1), (n) ABTS (TE g·kg−1), and (o) FRAP (FeSO4·7H2O g·kg−1) of blue honeysuckle during 28 d of storage at −1 °C. The vertical bars represent the average with SD for three replicate samples (approximately 500 g fruit of blue honeysuckle were used per replicate sample), and different letters (a–o) above the line indicate significant differences between treatments using Duncan's multiple range test.
Chemical properties
-
Fleshy fruits are classified based on physiology into climacteric or non-climacteric types, while blue honeysuckle falls somewhere in between[28]. The quality of postharvest fruit can be measured by several critical indicators such as TSS, acidity, and pH[17]. Figure 3d shows that the TSS trend increased initially and then decreased during storage. Under CA condition, we observed an approximate 1.15-fold increase in TSS values from harvest day to after 3 d of storage. The acidity of blue honeysuckle decreased significantly during storage (Fig. 3e), but CA treatment effectively delayed the acidity decline rate. Our findings were in line with previous studies, which reported acidity levels ranging from 0.9% to 1.48%[29]. The pH values of blue honeysuckle ranged from 3.08 to 3.29 (Fig. 3f), which is consistent with the results reported by Gerbrandt et al.[29]. Furthermore, the current findings revealed a rise in pH values during storage.
ETH is a critical factor in determining fruit type, as it regulates fruit ripening and cell wall modification[28]. In Fig. 3g, we observe that blue honeysuckle produced very little ETH during storage and that the trends increased first and then declined, with a range of 0.42~7.01 pmol·kg−1. Furthermore, the low level of O2 was found to be effective in reducing ETH content compared to other treatments. The ETH content in both CA2 and CA3 decreased by 75.49% and 72.45%, respectively, compared to the control after 228 d of storage. Literature data suggest that blue honeysuckle produces very little ETH during storage in the air atmosphere[30], which was consistent with our results.
MDA is a well-known marker of senescence and membrane damage, resulting from the peroxidation of polyunsaturated fatty acids in cells[12]. In Fig. 3h, great changes in MDA content can be observed within 28 days of storage, with an initial increase followed by a decline. Under high oxygen conditions (CA1), the highest MDA value was observed, which was consistent with findings in peach[31]. Comparison between the control and CA-treated samples showed a sharper increase in MDA content in blue honeysuckle stored at CA1. These results suggest that elevated levels of oxygen result in an increased extent of membrane lipid peroxidation, leading to MDA accumulation, which can potentially damage membranes and cells[31].
ABA is considered to play a pivotal role in the ripening and aging of fruit., as it promotes the maturity of fruits and the softening of cell walls. The current study (Fig. 4a−c) shows that ABA content in blue honeysuckle during postharvest storage initially increases and then decreases, which is in line with previous research on mangoes[32]. Moreover, the loss of ABA content was delayed under CA storage conditions, especially under hypoxic conditions (CA3), which may be due to the reduced oxidative damage of fruit under hypoxia. Additionally, high levels of endogenous ABA were found to retard senescence in blueberries, and this may be a possible reason for the better fruit quality observed in blue honeysuckle under CA storage, as it may be related to endogenous ABA levels[33].
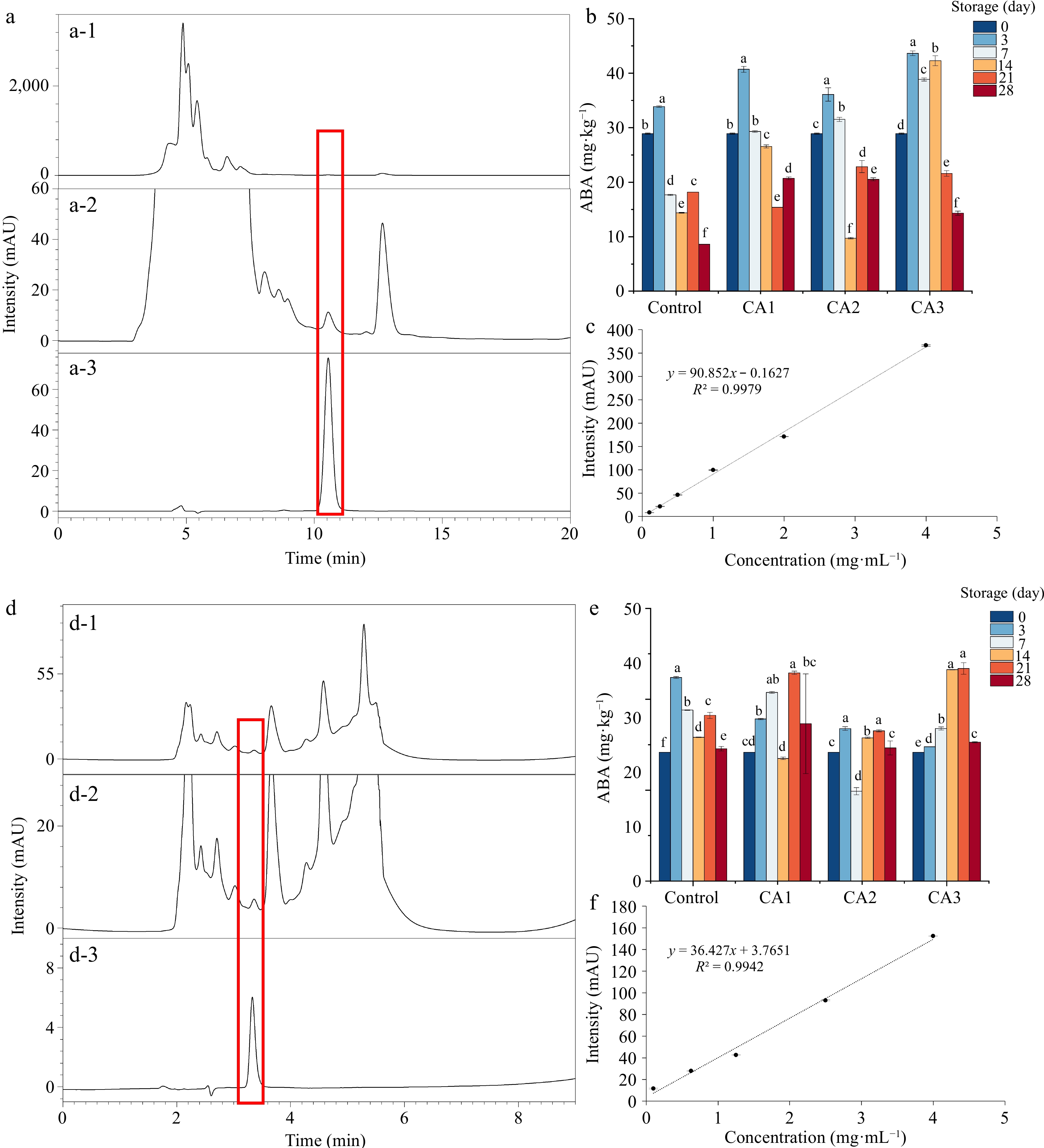
Figure 4.
Effect of CA treatment on ABA and ASA of blue honeysuckle during 28 d of storage at −1 °C. HPLC chromatograms of blue honeysuckle for (a-1) ABA and (d-1) ASA; enlarged views of HPLC chromatograms of blue honeysuckle for (a-2) ABA and (d-2) ASA; HPLC chromatograms of (a-3) ABA standard, (d-3) ASA standard; calibration curves for (c) ABA and (f) ASA; the content of (b) ABA in blue honeysuckle; and the content of (e) ASA in blue honeysuckle.
ASA is a vital vitamin present in fruits and vegetables that helps reduce damage caused by oxygen-free radicals[14]. However, ASA is susceptible to oxidation when combined with oxygen in the air, leading to a decline in fruit quality[34]. In blue honeysuckle, the ASA content initially increased, followed by a subsequent decrease over the storage period of 28 d (Fig. 4d−f). In the control, the decrease in ASA content started after 3 days of storage, whereas in other treatments, it started later. The study findings indicated that the CA treatment functioned as an air-protective layer, preventing ASA autoxidation. The highest ASA content was observed in CA1 and CA3, while the lowest amount was in control and CA2 on the 28th d after storage. The present findings are in line with those of Dziedzic et al.[5], who observed a decrease in ASA content in four blue honeysuckle cultivars stored in 20% CO2 and 5% O2 compared to the control.
Biological properties
-
Respiration stands as a pivotal metabolic process, serving as the primary source of energy for the intricate biochemical mechanisms within plants. In fresh fruit, respiration rate can be measured as the rate of CO2 production, which is an important indicator of postharvest shelf-life and metabolic activity of fruit[35,36]. As shown in Fig. 3i, the respiration rate increased gradually in the initial days of storage, reaching its peak before gradually decreasing. Specifically, the respiration rate of control and CA1 peaked after 14 days of storage. Meanwhile, CA2 reached its peak after 3 days (46.20 mg CO2 kg−1·h−1), and CA3 reached its peak after 7 days (78.01 mg CO2 kg−1·h−1). While higher O2 treatment reduces the time at which the peak respiration rate occurs, little information is available on the mechanism in other fruits. Additionally, the respiration rate of CA3 was found to be higher than that of the control, while CA1 and CA2 were lower than the control. This can be explained by the slowing down of respiration through the reduction of overall metabolic activity and oxidase activity (polyphenol, ASA, and glycolic acid oxidase) due to the decrease of available O2[35]. The results showed that increasing CO2 while maintaining a fixed O2 level reduced the respiration rate of fruits[8]. However, there is a lack of direct studies examining the impact of CO2 on respiration rate.
Polyphenolic contents
-
Phenolics, flavonoids, and anthocyanins are secondary metabolites known for their biological activities, which encompass antioxidant, antibacterial, and anti-inflammatory properties[1]. During the storage period in the control group, there was a slight decrease observed in the TPC (Fig. 3j). However, the TPC of CA-treated samples exhibited a significantly higher level compared to that of the control group. On day 28, the TPC in CA-treated fresh blue honeysuckle showed an increase of approximately 1.11−1.36 times compared to the control group. As illustrated in Fig. 3k, the TFC in the CA group showed a slight change before day 3 and decreased afterward, except for CA1. CA1 reached the maximum value of TFC on day 14 and then gradually decreased. Our study indicated that CA treatment effectively halted the decrease in TFC during the postharvest preservation of fresh blue honeysuckle, which aligns with a previous study conducted on wolfberry[37]. The TAC exhibited a consistent decrease across all groups over the 28-d storage period, as demonstrated in Fig. 3l. The trend of TAC content was unstable during the initial 7-d period of storage. After the conclusion of the experiment (28 d), the TAC in CA-treated blue honeysuckle was 1.81−2.55 times higher than that of the control. The present study suggested that the atmospheric condition was beneficial in protecting the anthocyanins in blue honeysuckle, especially under high CO2 conditions. This result was consistent with that found in strawberries[38].
Antioxidant capacity
-
Free radicals can cause damage to vital molecules, making their scavenging important for maintaining good health. DPPH and ABTS assays are commonly used for the rapid screening and evaluation of new antioxidant formulations[18,39]. As shown in Fig. 3m, the DPPH value increased sharply in the first 3 days and then stabilized until 28 d in the control and CA-treated groups, except for CA2, which showed a gradual increase and stabilization during 28 d of storage. There was a significant difference in the DPPH radical scavenging capacity between the control and CA-treated blue honeysuckle, with significantly higher values observed in the latter. The maximum value was reached on day 7, which was a 2.3-fold increase compared to the control. Our findings indicated that a high O2 atmosphere protected the DPPH radical scavenging ability of blue honeysuckle during long-term storage, which aligns with the conclusions of a prior study[5]. The ABTS value of all groups during storage showed an initial increase in the first 3 days and a gradual decrease until 28 d, except for CA3 (Fig. 3n). The ABTS•+ radical scavenging capacity of CA3-treated blue honeysuckle did not show any significant differences during storage, indicating that suitable gas conditions were conducive to protecting the ABTS•+ radical scavenging capacity[5].
The ferric ion-reducing antioxidant power (FRAP) is commonly utilized for assessing the antioxidant capacity of samples, based on the reduction of ferrous ions[20]. In postharvest blue honeysuckle fruit, the FRAP value decreased in the group treated with CA as compared to the control over the entire storage period (Fig. 3o). However, significantly higher FRAP values were observed in the CA-treated groups than in the control from day 14 to day 28. Specifically, the FRAP value in CA1 exhibited a 1.29-fold increase compared to the control group on day 28. These findings were consistent with a previous study that observed roughly 1.02 to 1.50 times higher FRAP values in different blue honeysuckle varieties after 14 d of CA storage[5].
In summary, the implementation of CA treatment resulted in a significantly improved antioxidant capacity. Furthermore, among the CA treatments, CA1 demonstrated the greatest effectiveness in preserving the fruit's antioxidant capacity.
Multivariate statistical analyses
-
In the present study, a comprehensive assessment of the effectiveness of CA treatments on blue honeysuckle using PLS-DA, PCA, and correlation analysis were conducted. As shown in Fig. 5a, the results of the VIP variable importance projection indicate that the VIP values of respiration rate, ethylene, TFC, color, pH, and acidity are greater than 1. This suggests that they can be considered the most discriminative indicators in the analysis. The model validation results suggest that the computational model demonstrates a high level of accuracy, with an accuracy rate of 1.2 (Fig. 5b). Furthermore, Fig. 5c illustrates that R2 is approximately 0.7 and Q2 exceeds 0.5, indicating the model possesses robust explanatory and predictive capabilities. The data obtained from the samples were summarized as PC1 (34.2%) and PC2 (13.1%), collectively explaining 47.3% of the total variability (Fig. 6a). To comprehensively understand the effect of CA on the apparent and internal quality of postharvest blue honeysuckle, correlations among various quality parameters, including wax coverage, color, moisture content, weight loss, firmness, TSS, acidity, pH, ETH, MDA, ABA, ASA, respiration rate, polyphenolic compounds, and antioxidant capacity were analyzed in control and CA-treated groups. This is the first study to conduct a correlation analysis in postharvest preservation of blue honeysuckle. The correlation heatmap (Fig. 6b), plotted with Pearson’s correlation coefficients (r), showed positive correlations between FRAP and TAC (r = 0.88), TPC (r = 0.76), TFC (r = 0.77), ABA (r = 0.60), MDA (r = 0.79), and wax coverage (r = 0.72). An inverse relationship was observed between pH and acidity (r = −0.73), ABA (r = −0.53), TPC (r = −0.73), FRAP (r = −0.52), and wax coverage (r = −0.78); weight loss and TAC (r = −0.55), TPC (r = −0.52), TFC (r = −0.60), FRAP (r = −0.63), and wax coverage (r = −0.63). In addition, a strong correlation was found between DPPH and ABTS (r = 0.73), as well as MDA (r = −0.79). However, correlations between firmness, color, and other indexes were not found in blue honeysuckle. Although Gonçalves et al. indicated a negative correlation between color and TAC in strawberries, the present results were not consistent with their findings, possibly due to species differences and pericarp waxiness[40].
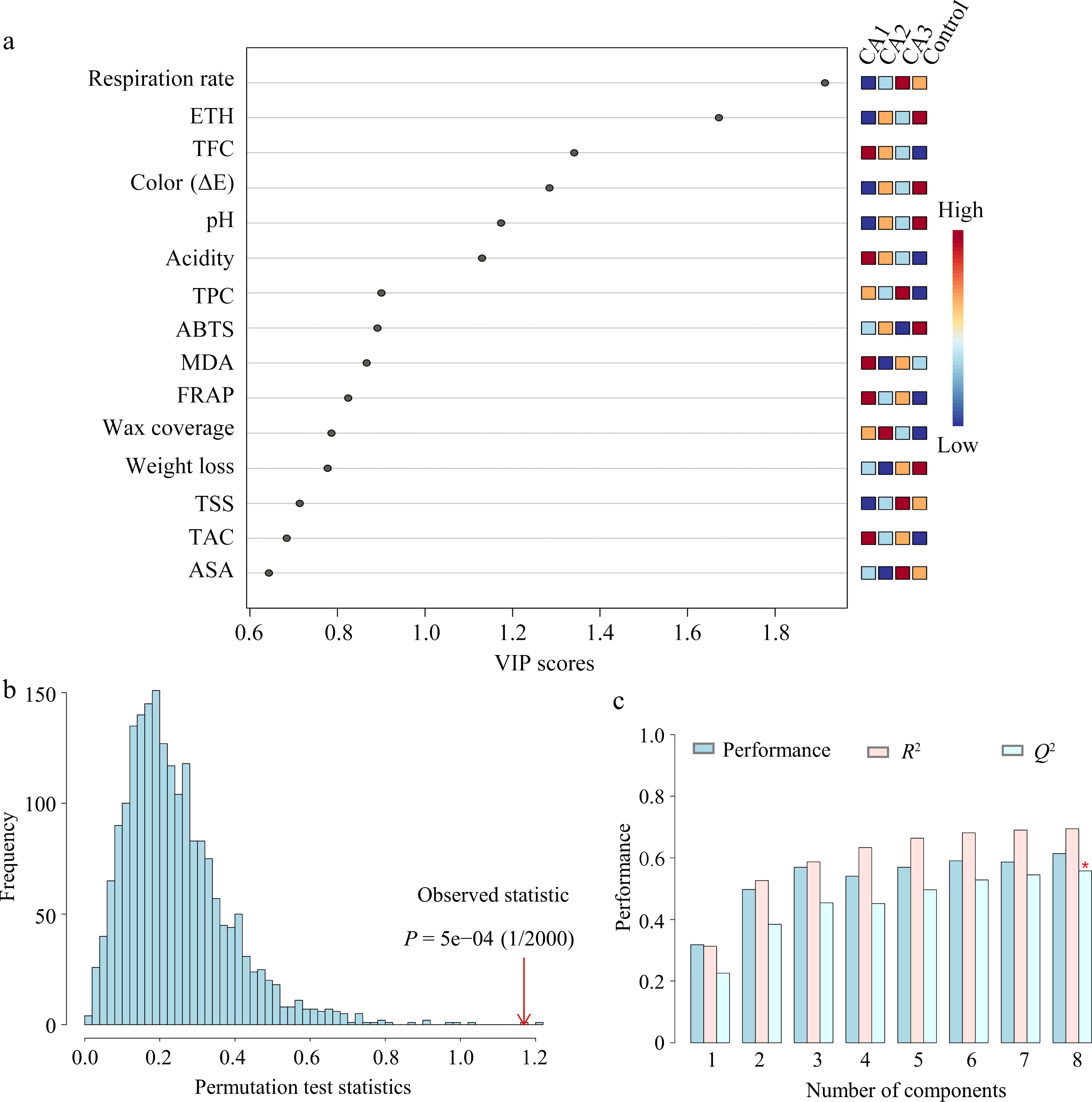
Figure 5.
Partial least squares discriminant analysis (PLS-DA) of the postharvest quality index of blue honeysuckle. Variable importance in (a) projection scores, (b) permutation test, and (c) model prediction evaluation chart.
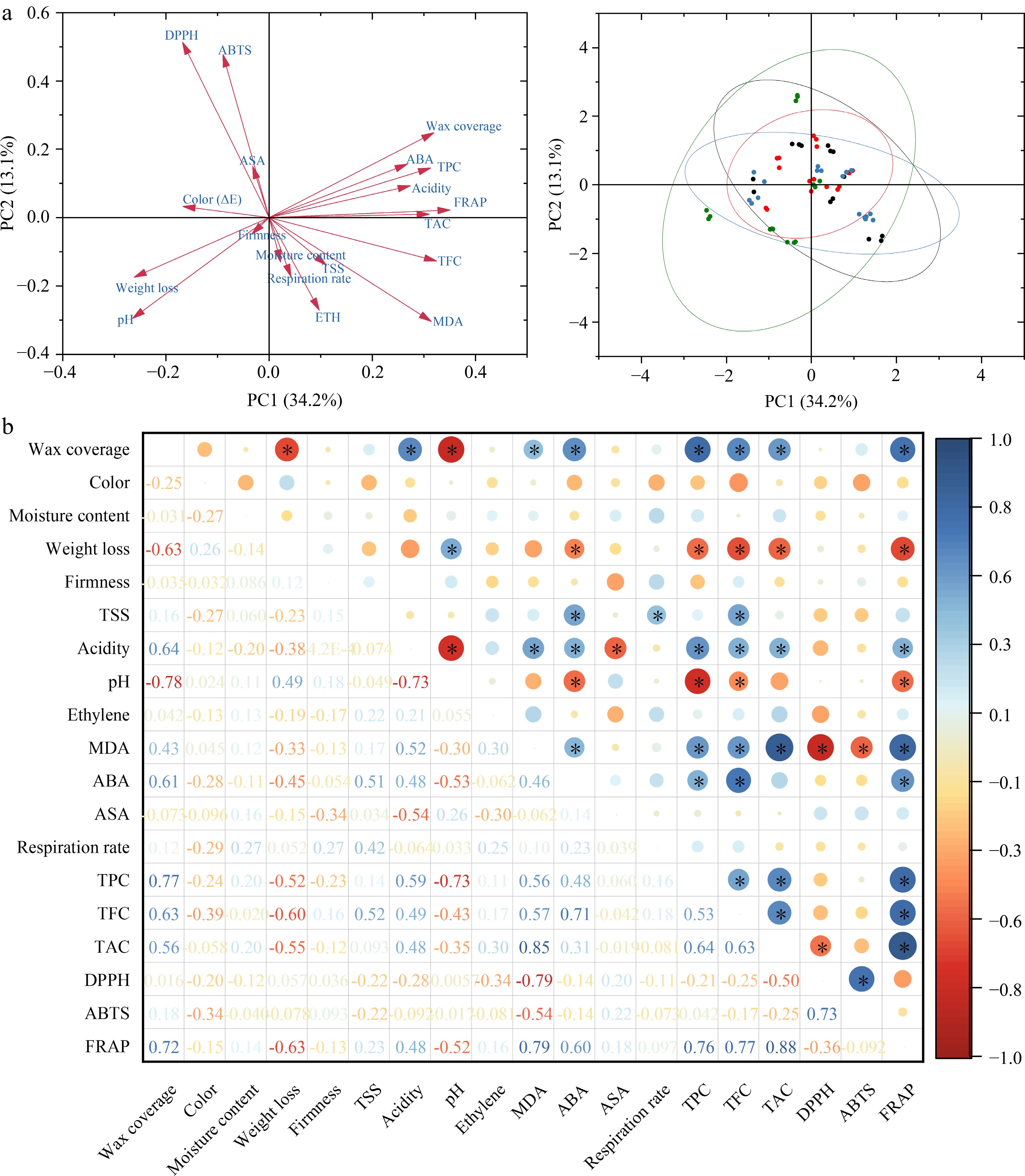
Figure 6.
(a) Principal component analysis and (b) correlation analysis between changes in wax coverage, color (ΔE), moisture content, weight loss, firmness, TSS, acidity, pH, ETH, MDA, ABA, ASA, respiration rate, TPC, TFC, TAC, DPPH, ABTS, and FRAP in fresh blue honeysuckle. (*) denote significant differences (p ≤ 0.05) between the control and CA-treated fresh blue honeysuckle fruit at each time point.
-
The present study demonstrates that controlled atmosphere treatment of blue honeysuckle effectively delayed the loss of surface wax and prevented the degradation of important bioactive components such as polyphenols, and ASA, thus maintaining the high antioxidant capacity of the fruit. Elevated concentration of CO2 will improve the quality of the postharvest blue honeysuckle. The treatment of 20% O2 and 20% CO2 resulted in reduced weight loss, ethanol content, and respiration rate while simultaneously increasing the epicuticular wax coverage index, firmness, TSS, ascorbic acid, polyphenols, and antioxidant capacity. Notably, this treatment led to an approximately 150% increase in total anthocyanin content compared to control conditions. Furthermore, the treatment of 5% O2 and 20% CO2 was observed to lead to a reduction in the antioxidant capacity of blue honeysuckle during its storage period, with DPPH, ABTS, and FRAP decreasing by 10.4%, 16.8%, and 6.7%, respectively. The respiration rate is increased by treatment of 5% O2 and 5% CO2, which may lead to accelerated fruit senescence. After 28 d, the treatment resulted in a respiration rate that was 1.49 times higher than the control. Therefore, a controlled atmosphere with both 20% O2 and 20% CO2 can be considered a suitable method for prolonging storage life and safeguarding its bioactive components.
Currently, there is a lack of established suitable postharvest storage and fresh-keeping methods for different varieties of blue honeysuckle. In the future, it will be necessary to establish the tolerance of different blue honeysuckle cultivars to oxygen or carbon dioxide at various maturity stages. Furthermore, further investigation is imperative to fully comprehend the comprehensive impact of postharvest treatments on the nutritional value of various blue honeysuckle cultivars.
We gratefully acknowledge the financial support received from the National Key R&D Program Projects of China (2021YFC2101400, 2022YFD1600505-1) and the High-Level Returning Overseas Talents Fund of Ministry of Human Resources and Social Security of China.
-
The authors confirm contribution to the paper as follows: study conception and design: Zhang Y; methodology: Qiao J, Guo L; software: Qiao J; validation: Guo L; formal analysis: Zhang Y; resources: Huo J; draft manuscript preparation: Zhang Y, Qiao J. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors declare that they have no conflict of interest.
- Supplemental Table S1 The quality changes of blue honeysuckle during 28 days of storage.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Qiao J, Guo L, Huo J, Huang D, Zhang Y. 2024. Controlled atmosphere effects on postharvest quality and antioxidant capacity of blue honeysuckle (Lonicera caerulea L.). Food Innovation and Advances 3(2): 155−166 doi: 10.48130/fia-0024-0015
Controlled atmosphere effects on postharvest quality and antioxidant capacity of blue honeysuckle (Lonicera caerulea L.)
- Received: 15 March 2024
- Revised: 22 May 2024
- Accepted: 23 May 2024
- Published online: 19 June 2024
Abstract: Changes in the quality of blue honeysuckle fruit following exposure to air or controlled atmospheres (CA1: 20% O2 and 20% CO2; CA2: 5% O2 and 20% CO2; CA3: 5% O2 and 10% CO2) were investigated. The 'Lanjingling’ blue honeysuckle was stored at a temperature of −1 °C for a duration of 28 d. An elevated concentration of CO2 led to a reduction in fruit weight loss, ethanol content, and respiration rate, while simultaneously increasing the epicuticular wax coverage index, firmness, TSS, ascorbic acid, polyphenols, and antioxidant capacity. Notably, treatment with high levels of carbon dioxide (20% CO2) led to an approximately 150% increase in total anthocyanin content compared to control conditions. Additionally, it was observed that reducing the oxygen content from 20% to 5% had a detrimental effect on the antioxidant capacity of blue honeysuckle during storage. Specifically, there were decreases of 10.4%, 16.8%, and 6.7% in DPPH, ABTS, and FRAP, respectively. The respiration rate is increased by treatment with 5% O2 and 5% CO2, which may result in accelerated senescence of blue honeysuckle. After 28 d, the treatment resulted in a respiration rate that was 1.49 times higher than the control. Hence, it can be deduced that maintaining a controlled atmosphere containing 20% O2 and 20% CO2 can be deemed an effective method of blue honeysuckle for prolonging storage life and safeguarding its bioactive components.













