-
Cancer is a prominent cause of mortality worldwide and represents a significant public health concern. The incidence of this disease is experiencing a rapid rise in Africa, Asia, and Central and South America, collectively representing more than 70% of global cancer-related mortality[1]. The most aggressive form of cancer across the globe in females is breast cancer. Approximately 2.6% of women, which is one in every 38, will develop breast cancer during their lifespan. In America, 12% of women (one in eight) may develop aggressive breast cancer[2]. In India, female breast cancer is the leading cause of cancer incidence and mortality, accounting for 13.5% of new cancer cases and 10% of cancer-related deaths in 2020[3]. Breast cancer is an aggressive tumor that can originate from cells in the breast lobule or glandular milk duct. The degree to which a tumor has started to grow within the luminal surface determines whether it is aggressive or non-aggressive. Lobular carcinomas are those breast cancers that originate from lobules of the mammary gland, whereas ductal carcinomas are those that originate from epithelial cells of the duct of the breasts[4]. The tumorous cells in nonmetastatic (noninvasive) breast cancer are confined to the milk ducts or lobules, whereas in metastatic breast cancer (invasive), the altered cells spread to the surrounding tissues and metastasize to the liver, lungs, brain, and bones[5]. Breast cancer is a complex disease consisting of many functionally diverse components with specific medical and therapeutic ramifications[6]. According to several studies, breast cancers with different anatomical and biological features exhibit distinct characteristics that lead to different clinical results and therefore need to be treated with a wide range of therapies[4]. To maximize clinical results, it is crucial to classify patients with breast carcinoma into therapeutically relevant groups. Using cancer immune-histochemical and gene expression analyses, four distinct subgroups of breast cancer have been classified[7]. Extensive research has been conducted on the development of different synthetic chemical agents for cancer therapies[8]. Chemotherapy is a recognized treatment modality for this particular disease, and continual advancements in anticancer medications have significantly enhanced the quality of patient care. Regrettably, conventional chemical drugs have been found to elicit adverse side effects on normal cells and tissues, including bone marrow, resulting in symptoms such as nausea, vomiting, and alopecia[9]. In contrast, natural antioxidants and various phytochemicals have recently been proposed as adjunctive therapies for cancer because of their anti-proliferative and pro-apoptotic properties[10]. In recent years, significant efforts have been dedicated to the development of potential anticancer drugs. For several decades, more than 200 new chemicals have received approval for the treatment of cancer. Notably, 50% of these compounds are structurally original natural compounds that have undergone modifications to increase their efficacy and safety[11]. Owing to their various structural compositions, compounds such as terpenes, flavonoids, fatty acids, alkaloids, lignans, saponins, vitamins, glycosides, oils, and other secondary metabolites play vital roles in selectively inhibiting cell proliferation and inducing programmed cell death in malignant cells[12]. The development of modified and novel anticancer drugs remains crucial for cancer research. However, a significant portion of research in this area has yielded limited promising results, with relatively minimal advancements made in the development of these prototype drugs. There is a need for the incorporation of novel prototypes and formats in the development of potential chemotherapeutic agents. Notably, numerous natural products offer valuable templates in this regard[13]. Therefore, the ongoing exploration of plant-derived anticancer agents has significant potential for identifying safe drugs and mitigating the adverse effects of chemotherapy. This is due to the many benefits offered by natural herbal medications[14]. Secondary metabolites that are active against breast cancer have been reported from different plants, such as Echinacea, Allium sativum, Curcuma longa, Arctium lappa, Synadenium cupulare, Cimicifuga foetida, Cymbopogon citratus, Zingiber officinale, Rhus coriaria, and Ricinus communis L.[15].
Acorus calamus L. is a perennial monocot belonging to the Acoreacea family. It is primarily distributed in the northern temperate and subtropical regions of Asia, North America, and Europe. The rhizomes of A. calamus have been utilized for the treatment of various medical conditions, including mucus syncope, stroke, epilepsy, amnesia, tinnitus, deafness, dyspepsia-initiating ailments, rheumatic agony, dermatitis, and scabies. Plant-derived extracts and compounds have diverse biological activities, including anti-dementia, antimicrobial, antiepileptic, anti-insecticidal, and antidiabetic effects. Various studies have demonstrated that aqueous and hydroalcoholic concentrates possess lipid scavenging and aerotherapeutic properties[16−18]. Additionally, phytochemical analysis of A. calamus revealed the presence of sesquiterpenes, alkaloids, flavones, fatty acids, glycosides, flavonoids, saponins, tannins, polyphenol compounds, mucilage, and volatile oils[19]. The present study aimed to isolate bioactive compounds from A. Calamus, an herb found in the Kashmir Himalayas, and investigate their potential anticancer activity. To date, no studies have been conducted on this species in this particular area of Kashmir. This lack of information has motivated us to initiate a study to gain a better understanding of the subject. Several chemical compounds were isolated and characterized (1H NMR, 13C NMR, DEPT, etc.) from the rhizome extract of A. calamus. The bioactive secondary metabolites were subsequently investigated for their potential role in anticancer activity. The cytotoxic and anti-proliferative effects of the extracted compounds and some crude extracts were tested in lung carcinoma (A549), colon carcinoma (HCT-116), and breast carcinoma (MDA-MB-231) cell lines.
-
A. calamus L. samples were collected from different habitats of Jammu and Kashmir, India, e.g., Singpora, Ganastan, Kawoosa, Najan Aarath, Kakpora Pulwama, and Tuli Rajouri. The samples were washed with clean water and then brought to the CSIR-IIIM laboratory under proper conditions. The samples were identified at the Center for Biodiversity and Taxonomy (CBT), Department of Botany, University of Kashmir. Supplementary Table S1 shows the unique voucher specimen numbers assigned.
Instruments and reagents
-
MTT (Merck Japan, Tokyo, Japan) was used to assess cell viability. Hexane, chloroform, and methanol (Merck Japan, Tokyo, Japan) (HPLC grade) were used for chromatography. TLC plates (silica gel 60) were purchased from Sigma Aldrich. Biolite 96-well multidish plates (Thermo Fisher Scientific) were used for in vitro assays. An analytical balance (Sartorius) was used for weighing. For cell culture, a BOD incubator (Eppendorf) was used. An Evos M7000 microscope (Thermo Fischer Scientific) was used for imaging. Twenty-four-well transwell inserts with 8 micron-pore sizes (Corning, USA) were used for the transwell invasion assay. A spectrophotometer (Perkin Elmer, USA) was used for the MTT assay.
Preparation of rhizome extracts and thin-layer chromatography (TLC)
-
The washed and cleaned rhizomes were finely chopped, subjected to air drying at 25 °C, and subsequently pulverized to a fine powder via a mechanical grinding apparatus. The powdered rhizome (1.072 kg) was extracted sequentially by maceration with dichloromethane and methanol (1:1) via a cold extraction method at room temperature. The plant material was dipped into 3 L of a dichloromethane and methanol mixture for 24 h before extraction. Three successive washes of the plant material were carried out to obtain the maximum number of constituents extracted. The solvent was removed under vacuum on a rotary evaporator at 45–45 °C to obtain a brownish-colored residue (144.239 g) of the rhizome of A. calamus. The extract was concentrated via a rotary evaporator and stored at a temperature of 4 °C until use.
For TLC, hexane-ethyl acetate (7:3, v/v) was used as a solvent system to balance the polarity and optimize compound separation and resolution. The sample volume (2 to 5 μL) was carefully placed on a TLC plate (5 cm × 10 cm). Loaded TLC plates were then placed in a developing chamber with the solvent solution, ensuring that the solvent level was lower than the sample spots to prevent direct washing. The run or development time normally ranges from 2−3 min.
Isolation of compounds
-
Compared with the other extracts, the Kawoosa wetland sample presented a high percentage yield (13.455%). The extract from the Kawoosa wetland was further processed for compound extraction via column chromatography. One hundred grams of rhizome extract of A. calamus was extracted with different organic solvents of increasing polarity. The column was sequentially eluted with different solvents (100% hexane, hexane : chloroform (1:1), 100% chloroform, 10%, 15%, 30%, 50% methanol and 100% methanol). Each eluate was monitored via TLC. The maximum yield (24.8 g) was obtained when the extract was eluted with 10 and 15% methanol, which resulted in similar TLC patterns, which were fractioned using different combinations of hexane and ethyl acetate.
Analytical techniques
-
The samples were analyzed via the published protocol of Lynn et al., with some modifications[20]. NMR spectra of the isolated compounds were obtained on a 400 MHz Bruker spectrometer in CDCl3 and MeOD with TMS as the internal standard. The experiments involved column chromatography using normal silica gel (60−120 mesh) of Merck grade. Additionally, TLC plates precoated with silica gel 60 F254 (Merck, 0.25 mm) were utilized for monitoring the column chromatography process.
Cell culture, growth conditions, and chemical reagents
-
A549 (human lung cancer), MIAPaCa (human pancreatic cancer), HCT-116 (human colon cancer), MDA-MB-231, and MDA-MB-68 (human breast cancer) cell lines were purchased from American Type Cell Culture (ATCC), USA. The cells were cultured following the standard protocol with some modifications[21]. Briefly, the cells were incubated at 37 °C in a humidified incubator with the appropriate culture media (HAM'S F12, DMEM, and RPMI-Invitrogen) supplemented with 10% heat-inactivated FBS, penicillin (100 units/ml), and streptomycin (100 mg/ml). Penicillin G, streptomycin, trypsin–EDTA, and HBSS were acquired from Invitrogen Corp. 5-diphenyltetrazolium bromide (MTT), doxorubicin, and DAPI stains were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Cells in the log phase were used for experiments, dimethyl sulfoxide (DMSO) was used as a solvent control, and doxorubicin (10 μM) was used as a standard drug.
Cell proliferation assay
-
A549 (lung), MIAPaCa (pancreatic), HCT-116 (colon), MDA-MB-231 and MDA-MB-468 (breast) cell lines were cultivated in 96-well culture plates at a density of 6,000–7,000/200 μl of medium. After 12 h, the cells were treated with various groupings of test compounds for 24 h, ensuring that the final concentration of the DMSO solvent was less than 0.5%. This was followed by termination of the assay with 2.5 mg/ml MTT solution. The formed formazan crystals were dissolved in 150 μl of DMSO per well, and the absorbance was subsequently measured at 570 nm via a microplate reader (Applied Biosystems).
Wound healing assay
-
Cancer cell migration results from various biological processes, with a specific characteristic observed in their coordination. Wound-healing assays are commonly used methods for investigating cell migration[22,23]. At a seeding density of 7 × 105 cells/well, MDA-MB-231 cells were plated in a 6-well plate for 24 h to achieve monolayer confluence. Monolayer cells were scratched with a clean 10 μl pipette tip in a straight horizontal line, washed with Hanks balanced salt solution (HBSS) to remove displaced cells, and photographed (time 0 h). The cells were finally treated for 24 h with various concentrations of the isolated active compound ACS08 (25, 55, and 100 μM/ml) along with a standard (doxorubicin) or a solvent control (DMSO). Using an Olympus C-7070 microscope, the wounded regions were progressively photographed, and the percentage of wound closure was determined via the following equation.
$ \begin{split} & \rm{\text{%}}\; Wound\; closure=1-\dfrac{Wound\ area\ at\ 0\ h}{Wound\ area\ at\ 24\ h}\times 100\text{%}\end{split} $ Transwell invasion assay
-
A transwell invasion assay was performed with Matrigel-coated transwell chambers (pore size of 8.0 μm)[24]. Approximately 1 × 105 cells, together with different concentrations of the isolated active compound and standard drug were resuspended in 200 μl of serum-free RPMI medium and cultivated in the upper compartment of the transwell insert. The lower compartment of the transwell plate contained 800 μl of complete RPMI medium as a chemoattractant for the cells in the upper chamber. The membranes were fixed with paraformaldehyde for 10 min after they were incubated for 24 h at 37 °C in a humidified CO2 incubator.
-
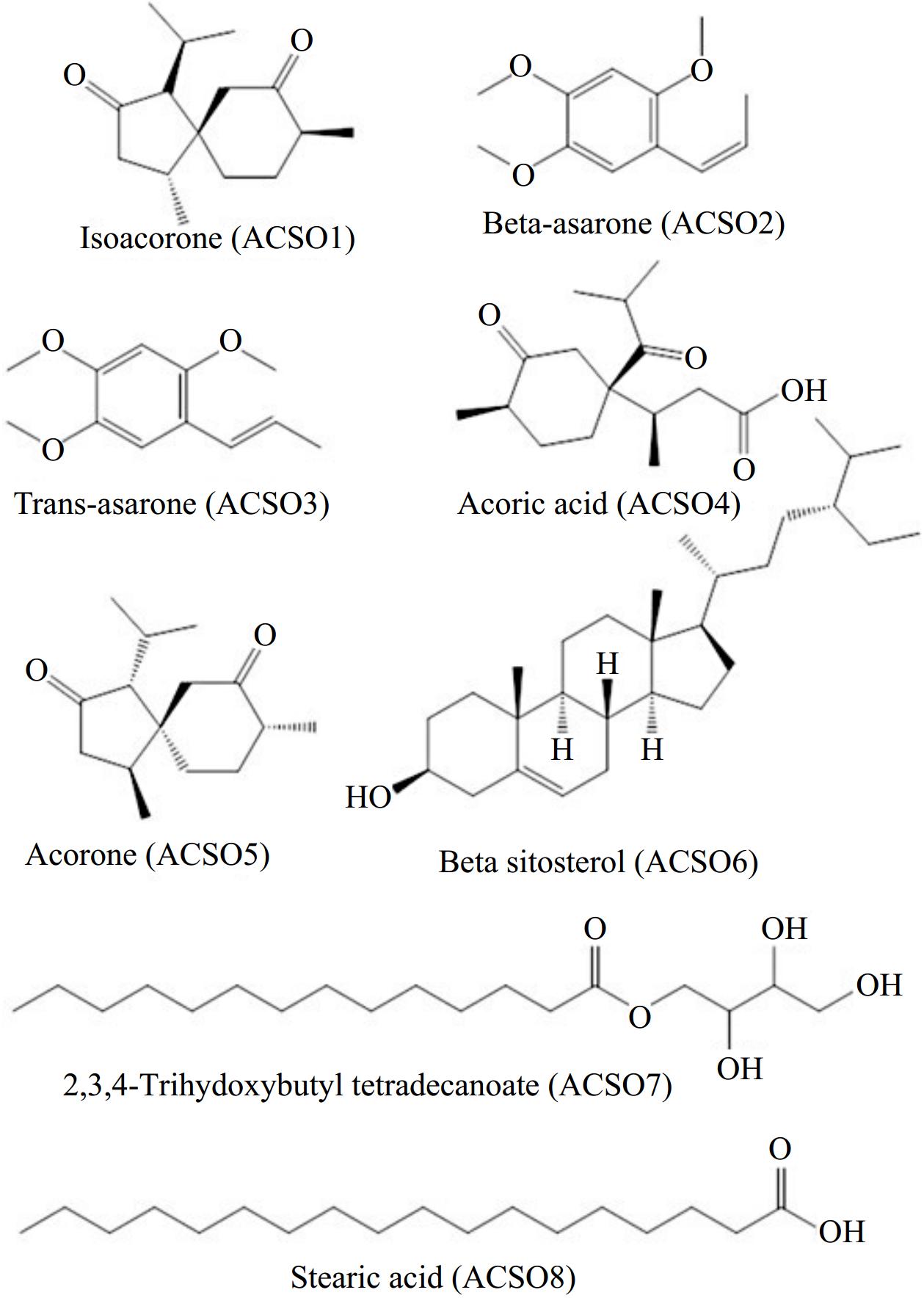
Four fractions, Fr-1, Fr-2, Fr-3, and Fr-4, of the rhizome extract were obtained via the use of pure hexane, hexane–chloroform, and methanol as the eluent with increasing polarity. Purification of Fr-1 yielded two compounds, ACS01 and ACS02. Similarly, ACS03, ACS04, ACS05, and ACS06 were obtained from Fr-2 and Fr-3, respectively. Repeated column chromatography of Fr-4 yielded ACS07 and ACS08 as pure compounds. The structures of the isolated compounds were characterized via spectral data analysis (1H and 13C NMR) in light of the literature (Supplementary Data S1). Thus, the isolated natural products were identified as isoacorone (ACS01), cis-asarone (ACS02), trans-asarone (ACS03), acoric acid (ACS04), acorone (ACS05), β-sitosterol glycoside (ACS06), 2,3,4-trihydoxybutyl tetradecanoate (ACS07), and stearic acid (ACS08) (Fig. 1). All the compounds were isolated for the first time from A. calamus rhizome parts.
Cell growth inhibition and calculation of IC50 values
-
The various extracts were tested for their initial cytotoxicity at a concentration of 100 μg against three different human cancer cell lines: A-549 (lung), HCT 116 (colon), and MDA-MB-231 (breast). This screening aimed to evaluate the possible cytotoxic effects of the extracts via the MTT assay (Table 1). The extract of AC Kakpora Pulwama (referred to as EXKpME) showed enhanced anti-proliferative properties with significant cytotoxicity against the A-549, HCT 116, and MDA-MB-231 cell lines, resulting in inhibition rates of 91%, 88%, and 94%, respectively. A significant proportion of the extracts exhibited an inhibition rate of ≥ 50% against several experimental cell lines.
Table 1. Cytotoxic activity of various extracts at 100 μg/ml concentration.
S. No. Different A. calamus extracts** Percentage inhibition (%)* A549 (Lung) HCT-116 (Colon) MDA-MB- 231 (Breast) 01 ExKwME 57 55 60 02 ExKpME 91 88 94 03 ExGnME 39 50 46 04 ExKwME 28 30 34 05 ExArHX 75 82 90 06 EXArAC 78 85 81 07 EXArEA 65 82 80 08 ExNJME 47 45 50 09 ExNJDC 67 65 80 10 ExRJME 58 55 60 * The values are the average of triplicate experiments. ** ExKwME (Kawoosa Methanolic extract); ExKpME (Kakpora Pulwama Methanolic extract); ExGnME (Ganastan Methanolic extract); ExArHX (Aarath Hexane extract); EXArAC (Aarath Acetone extract); EXArEA (Aarath ethylacitate extract); ExNJME (Najan Methanolic extract); ExNJDC (Najan DCM extract); ExRJME (Tuli Rajouri Methanolic extract). Similarly, an initial assessment was conducted on isolated pure compounds at 100 μM/ml against the selected cancer cell lines. As shown in Table 2, compounds ACS08, ACS06, and ACS02 showed better anticancer activity against all the cancer cell lines with ≥ 50% inhibition.
Table 2. Cytotoxic activity of isolated compounds at 100 μM concentration.
S. No. Different compounds
from A. calamusPercentage inhibition (%)* A549 (Lung) MIAPaCa (Pancreatic) HCT-116 (Colon) MDA-MB-231 (Breast) MDA-MB-468 (Breast) 01 ACS01 27 22 25 34 30 02 ACS02 53 59 72 85 80 03 ACS03 11 13 16 14 12 04 ACS04 25 37 40 54 38 05 ACS05 38 30 45 60 55 06 ACS06 52 45 60 68 72 07 ACS07 36 40 45 44 40 08 ACS08 63 52 74 80 64 * The values are the average of triplicate experiments. Compounds showing significant anti-proliferative effects were further screened at five different concentrations (25, 50, 100, 125, and 150 μM) for determination of their IC50 values (Table 3).
Table 3. IC50 value (μM) of selected compounds against different cancer cell lines.
Compounds A549 (Lung) MIAPaCa (Pancreatic) HCT-116 (Colon) MDA-MB-231 (Breast) MDA-MB-468 (Breast) ACS02 76.4 ± 2.1 80.56 ± 3.5 85.4 ± 2.1 65.4 ± 3.4 70.5 ± 2.3 ACS06 82.7 ± 4.5 75.3 ± 2.0 80.7 ± 1.9 69.5 ± 4.0 73.9 ± 2.1 ACS08 79.7 ± 0.5 65.59 ± 3.9 72.3 ± 2.2 55.89 ± 2.4 65.59 ± 5.0 The values are the average of triplicate experiments. Compared with ACS06 and ACS02, ACS08 displayed more potent cytotoxicity. Among the panel of cancer cell lines, ACS08 was found to be more active against the MDA-MB-231 breast cancer cell line, with an IC50 estimation of 55.89 μM; hence, ACS08 was used for additional examination.
ACS08 inhibits the migration and invasion of MDA-MB-231 cells
-
To investigate the antimetastatic activity of ACS08, experiments were conducted to examine its impact on cell migration and invasion. The wound healing scratch test and transwell invasion assay were used for this purpose. Substantial inhibition of cell migration and invasion was observed across different doses of ACS08 (Fig. 2).
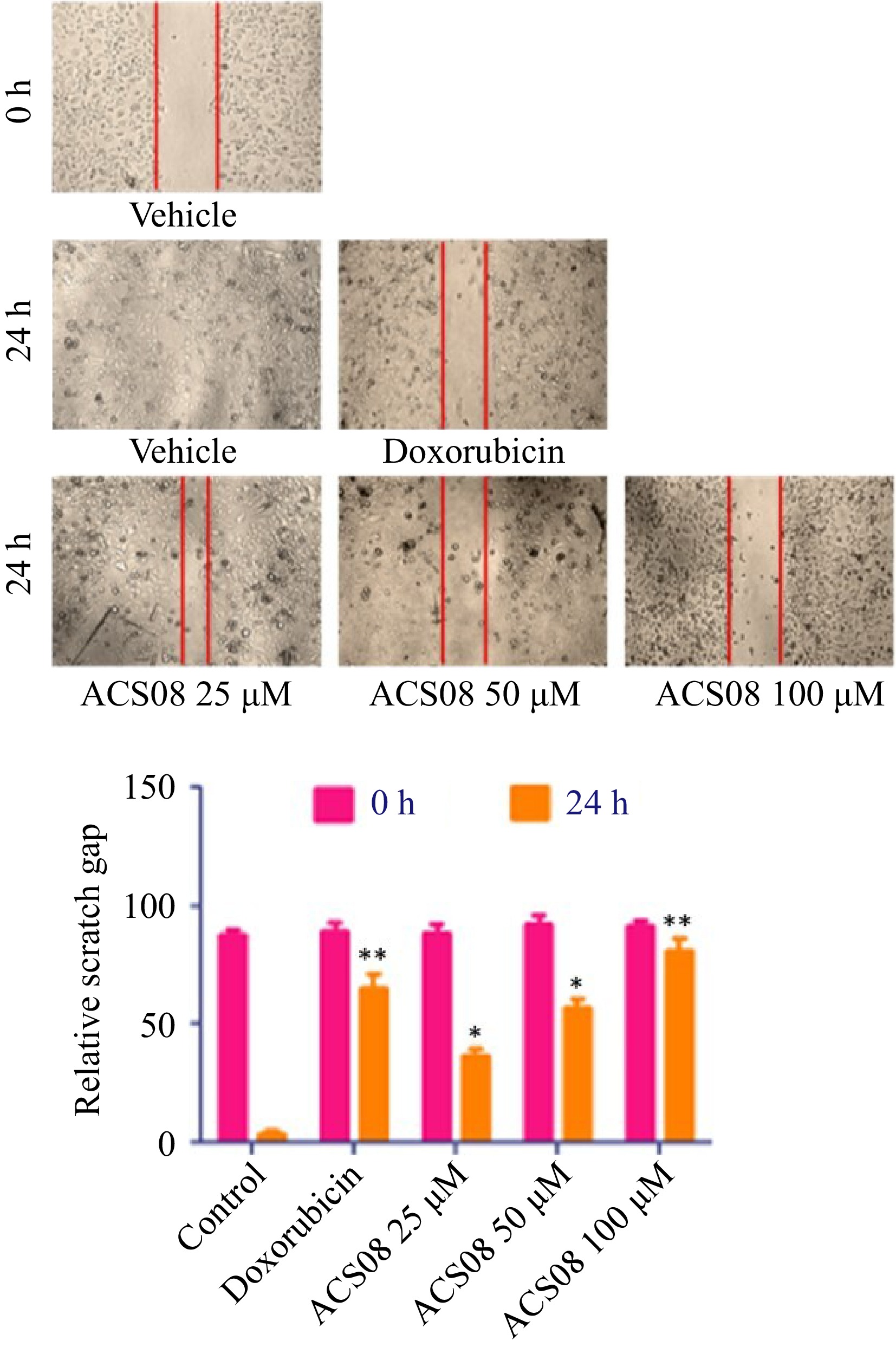
Figure 2.
Wound healing scratch assay experiment of MDA-MB-231 breast cancer cell treated with varying concentrations of ACS08 at different time points. The region of the scratch was estimated at 0 and 24 h time focuses and the migration rate was determined in relation to the corresponding control. Values are expressed as means ± SD representing three independent biological samples, each with three technical replicates. Differences were scored as statistical significance at *** p < 0.0001, ** p < 0.001, and * p < 0.05.
The dimensions of the scratch zone were assessed at both 0- and 24-h time intervals and the rate of migration was calculated relative to the equivalent control. The results revealed that the application of the IC50 dose (55.89 μM) to MDA-MB-231 cells resulted in a significant reduction in wound closure of approximately 50% in comparison with that in the control group. The degree of inhibition was more pronounced when the cells were subjected to elevated doses (100 μM) of ACS08. In the context of the transwell invasion experiment, approximately 140 cells invaded the bottom surface of the insert membrane in the untreated group. Conversely, a reduced number of 80 cells were found to penetrate following treatment with 25 μM ACS08 (Fig. 3).
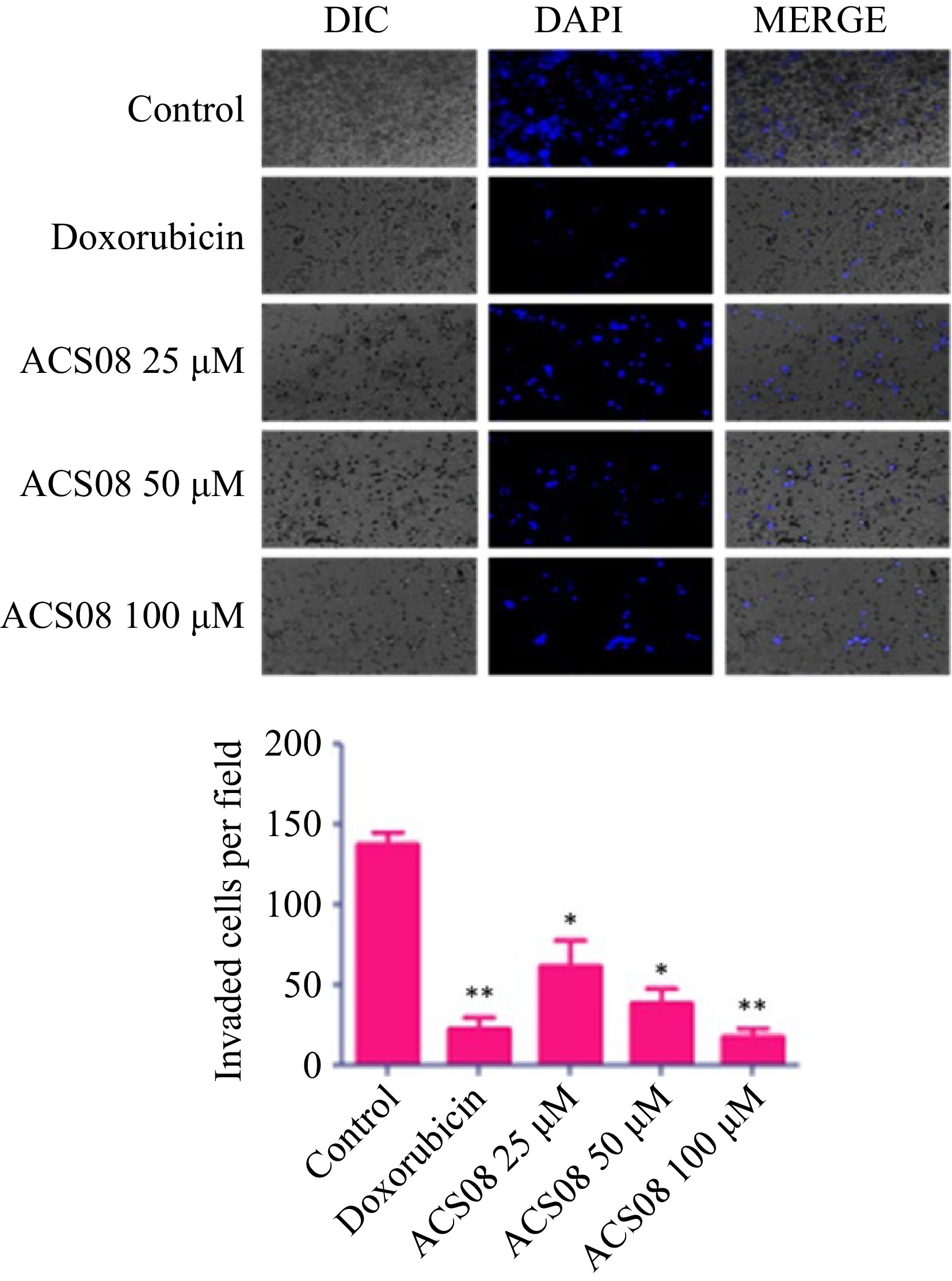
Figure 3.
Histogram and microscopic images showing MDA-MB-231 breast cancer cell migration treated with different concentrations of ACS08. Values are expressed as means ± SD representing three independent biological samples, each with three technical replicates. Differences were scored as statistical significance at *** p < 0.0001, ** p < 0.001, and * p < 0.05.
The number of invading cells decreased to 50 and 25 cells after exposure to ACS08 at concentrations of 50 and 100 μM, respectively. Collectively, our findings showed that ACS08 has significant antimetastatic effects on MDA-MB-231 breast cancer cells.
Cytotoxic activity of various extracts (100 μg/ml) and compounds (100 μM/ml)
-
The application of several extracts derived from the rhizomes of A. calamus to selected cancer cell lines for 48 h resulted in the observed suppression of cell growth. The extracts (ExKwME, ExKpME, ExArHX, EXArAC, EXArEA, ExNJDC, and ExRJME) exhibited notable growth suppression in comparison with the other extracts. Notably, the inhibitory effect on the MDA-MB-231 cell line was substantially greater than that on the other cell lines. We subsequently conducted experiments involving the application of several isolated compounds derived from the same plant to many cancer cell lines, including lung, pancreatic, colon, and breast cancer cells. These compounds were administered at a uniform dosage of 100 μM/ml for 48 h. The results obtained demonstrated a noteworthy reduction in cell growth. Compared with the other isolated compounds, the compounds ACS08, ACS06, and ACS02 exhibited greater activity against the triple-negative breast cancer (MDA-MB 231) cell line.
-
Cancer is a non-communicable ailment that presents considerable obstacles in both emerging and wealthy nations[25]. The therapeutic options available for cancer patients are associated with several limitations, including severe toxicity, the development of drug resistance, and a heightened likelihood of disease recurrence. In recent years, there has been an increasing emphasis on and need for the evaluation of various phytochemicals derived from natural sources, intending to identify superior and more secure alternatives[26,27]. Furthermore, the development of resistance to cancer treatment has compelled researchers to shift their focus toward exploring natural compounds derived from plants and marine sources. Recently, the recognition of the importance of phytochemicals derived from natural sources, such as galantamine, resveratrol, and glycyrrhizin, as noteworthy therapeutic targets in the management of many cancer types has increased[28,29]. A. calamus, together with its two bioactive phytochemicals, alpha (α)-asarone and beta (β)-asarone, are well-recognized substances in traditional medicine. These compounds have been shown to have antitumor and chemopreventive properties, as demonstrated by multiple preclinical investigations conducted both in laboratory settings (in vitro) and in living organisms (in vivo)[30]. The current research aimed to evaluate the cytotoxic potential of extracts from A. calamus via primary cytotoxicity screening against three human malignancy cell lines: A-549 (lung), HCT 116 (colon), and MDA-MB-231 (breast). The extracts were tested at a concentration of 100 μg for fixation via the MTT assay. Cancer has evolved many strategies to evade controlled proliferation and circumvent programmed cell death. Hence, the use of entire plant extracts, which encompass many constituents with diverse potential intracellular targets, may provide a benefit over the utilization of a single isolated plant molecule. The methanolic extract of Kakpora pulwama (EXKPME) had superior anti-proliferative properties and showed significant cytotoxicity against the A-549, HCT 116, and MDA-MB-231 cell lines, resulting in inhibition rates of 91%, 88%, and 94%, respectively. A total of 12 compounds were recovered from the extract of dichloromethane : methanol (1:1) obtained from the subterranean sections of A. calamus. Among the 12 compounds, a total of eight (ACS01-08) were subjected to characterization (Supplemental Data 1). Furthermore, there was a difference in the yield of plant extracts collected from different locations. This variation may be due to varying agroclimatic conditions, such as increased sunshine hours and high temperatures, which led to optimum growth of the plant. The current investigation revealed that the MDA-MB 231 cell line had the highest level of sensitivity toward all the chemicals tested. While the anticancer properties of essential oils derived from A. calamus have been investigated, there is currently a lack of research on the potential anticancer effects of fatty acids isolated from A. calamus. Research has shown the inhibitory impact of stearic acid, a saturated fatty acid often found in dietary sources, on cell proliferation. Research has revealed that stearic acid (ACS08), derived from A. calamus, has inhibitory effects on the migration and invasion of MDA-MB-231 cells. In this study, a wound healing scratch test and a transwell invasion experiment were used to assess the effects of stearic acid on cellular migration and invasion to evaluate its potential as an antimetastatic agent. Inhibitory effects on cell migration and invasion were observed when different doses of stearic acid were applied (Fig. 1).
Collectively, the present findings provide evidence that stearic acid has discernible antimetastatic properties when tested on MDA-MB-231 breast cancer cells. This finding aligns with prior research, which has shown the inhibitory effects of stearic acid on the growth of breast cancer cells and its ability to cause apoptosis in these cells[31,32]. Eicosatetraenoic acid (EPA) and docosahexaenoic acid (DHA), which are long-chain omega-3 fatty acids, are important for the production of bioactive lipid mediators that play crucial roles in the resolution of inflammation[33]. Fatty acids, which are integral constituents of phospholipid membranes and lipid rafts, play crucial roles in the organization and segregation of molecules. Moreover, they have been implicated in cell signaling processes that are believed to influence the development of breast carcinogenesis[34,35]. Plant-based medicine has been shown to facilitate wound healing and tissue regeneration via several methods that are not only cost-effective but also safe.
Nevertheless, before its use, scientific validation, standardization, and safety assessment are crucial[36]. The findings of our investigation indicate that the application of ACS08 at the IC50 (55 μM) resulted in a significant reduction in wound closure by approximately 50% in MDA-MB-231 cells compared with that in the control group. The level of inhibition observed was more pronounced when the cells were subjected to elevated doses (100 μM) of ACS08. Zong et al. reported comparable findings, indicating that fatty acid extracts promote the healing of cutaneous wounds by activating AKT, ERK, and TGF-β/Smad3 signaling, as well as facilitating angiogenesis[37]. Previous studies have investigated the associations between the intake ratios of marine omega-3 fatty acids, specifically eicosatetraenoic acid (EPA) and docosahexaenoic acid (DHA), relative to omega-6 arachidonic acid, and the risk of breast cancer in women. Findings from various case‒control and cohort studies have indicated that women with higher intake ratios of EPA and DHA than arachidonic acid tend to have a decreased risk of breast cancer. However, it is important to note that these associations have not been consistently observed across all studies[38]. Fatty acids have been shown to have potential therapeutic and prophylactic effects against breast cancer in both in vitro and in vivo experimental models[39,40]. The present research evaluated the impact on cell viability and proliferation via an MTT assay. The results indicate that all the tested extracts and compounds substantially reduced cell viability and proliferation across one or more of the examined cell lines. Chemicals may have an anti-proliferative effect by potentially inducing cell death and/or arresting the cell cycle. The findings of this study have expanded the possibility of identifying anticancer chemicals in A. calamus, hence opening new opportunities for the future. Further investigation is needed to elucidate the associations between metabolites and the anticancer properties of plant extracts derived from A. calamus.
-
A. calamus, also known as sweet flag, has a rich historical background and is associated with a wide array of traditional and ethnomedicinal uses. The medicinal efficacy of A. calamus is attributed to the presence of secondary metabolites. The findings of the current investigation demonstrated that extracts and compounds derived from the rhizome of A. calamus had anticancer properties. The results outlined in this paper may serve as a potential resource for researchers seeking further investigations related to A. calamus and its bioactive phytochemical components in the context of cancer chemoprevention.
-
This study involved the use of established human cell lines. The cell lines used in this research were obtained from American Type Cell Culture (ATCC), USA and were used in accordance with institutional and national ethical standards. The cell lines have been previously published or validated, and no new human tissues were used in this study.
-
The authors confirm contribution to the paper as follows: conceptualization: Akhter S, Sultan P, Hassan QP; writing — original draft preparation: Hassan QP, Manzoor MM, Akhter S; experimental work: Akhter S, Mir SA, Manzoor MM, Khaliq T; facilities: Hassan QP, Sultan P. All authors have read and agreed to the final version of the manuscript.
-
All the data generated or analyzed during this study are included in this published article and its Supplemental information files.
Sabiyah Akhter and Tahirah Khaliq acknowledge the University Grants Commission (UGC) India for the Maulana Azad National Fellowship (MANF) received during this study. Malik Muzafar Manzoor acknowledges the Indian Council of Medical Research (ICMR) India for a Research Associateship.
-
The authors declare that they have no conflict of interest.
- Supplementary Table S1 Voucher specimen numbers of collected plant material submitted at KASH Herbarium, Centre for Biodiversity and Taxonomy, University of Kashmir, India.
- Supplemental Date S1 1H NMR and 13C spectral data of the isolated compounds.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Akhter S, Manzoor MM, Mir SA, Khaliq T, Sultan P, et al. 2024. Extracts and isolated compounds from the Acorus calamus L. (sweet flag) rhizome showed distinct antimetastatic activity against MDA-MB-231 breast cancer cells. Medicinal Plant Biology 3: e021 doi: 10.48130/mpb-0024-0021
Extracts and isolated compounds from the Acorus calamus L. (sweet flag) rhizome showed distinct antimetastatic activity against MDA-MB-231 breast cancer cells
- Received: 03 June 2024
- Revised: 02 September 2024
- Accepted: 04 September 2024
- Published online: 18 October 2024
Abstract: The present work aims to investigate and validate the medicinal potential of Acorus calamus, a plant known for its high therapeutic potential. The focus was identifying biologically active extracts and compounds that exhibit promising anticancer properties. The cytotoxicity of the extracts and compounds extracted from the rhizomes of A. calamus was assessed in three different cancer cell lines: lung carcinoma (A549), colon carcinoma (HCT-116), and breast carcinoma (MDA-MB-231). The application of extracts derived from the rhizome of A. calamus significantly inhibited cell proliferation in various cancer cell lines, including lung, colon, and breast cancer cells. Furthermore, the isolated compounds ACS08 (stearic acid), ACS06 (beta-sitosterol), and ACS02 (β-asarone) exhibited superior anticancer activity against all the tested cancer cell lines, with an inhibition rate of ≥ 50%. Compared with ACS06 and ACS02, ACS08 exhibited greater cytotoxicity. Among the panel of cancer cell lines utilized, compound ACS08 exhibited greater efficacy against the MDA-MB-231 breast cancer cell line, as evidenced by an IC50 estimation of 55.89 μM.
-
Key words:
- Acorus calamus /
- Compounds /
- Anticancer /
- MTT assay /
- MDA-MB-231