-
Type 2 diabetes mellitus has become a global disease that seriously affects human health and manifests as insulin resistance and hyperglycemia, with the liver as the major target organ[1]. The liver regulates systemic glucose metabolism to carefully maintain blood glucose levels[2]. High blood levels of glucose lead to conversion into glycogen which in turn is stored in the liver. When blood glucose levels are low, glycogen can be converted to glucose to meet energy needs[3]. The liver plays a primary role in maintaining glucose homeostasis by balancing gluconeogenesis and glycogen synthesis. During this process, increased glucose production and decreased glycogen content in the liver can lead to the occurrence of insulin resistance (IR) and eventually hyperglycemia[4]. Therefore, it is necessary to identify effective ways to regulate glucose homeostasis as a possible strategy for diabetes therapy.
The side effects and resistance of existing drugs make research into new natural agents for diabetes therapy urgent. Dendrobin is a natural sesquiterpene alkaloid abundant in a well-known traditional Chinese medicinal herb and one of the major active constituents of Dendrobium nobile Lindl. Recently, dendrobin has been shown to possess several important properties including antioxidant, lower blood sugar, and anti-inflammatory properties[5−7]. It was found that dendrobine improved insulin sensitivity and lowered blood glucose levels by reducing the secretion of inflammatory cytokines. It was suggested that dendrobine can suppress the number of pulmonary metastatic nodules by inhibiting the insulin resistance signaling pathway[8]. However, the possible mechanism underlying dendrobines ability to regulate glucose homeostasis have not been fully elucidated.
N6-methyladenosine (m6A) modification has recently been shown to play an essential role in modulating gene expression during diabetic progression. m6A modification, which accounts for nearly 60% of all RNA chemical modifications, is an important form of transcriptional regulation[9,10]. m6A methylation modification is a reversible modification mediated by an interplay between methyltransferases such as methyltransferases 3 (METTL3), demethylases, and reading proteins[11−14]. m6A RNA methylation exerts a pivotal function in different cellular processes, for example, lipid accumulation, cell differentiation, and glucose metabolism[15,16]. Importantly, m6A has a crucial effect in nutritional physiology and metabolism, e.g., in glucose metabolism, lipid accumulation, and lipogenesis[17]. Alteration of the m6A modification pattern can lead to anomalies in gene expression as well as an imbalance in intracellular homeostasis, potentially resulting in metabolic diseases[18]. There is increasing evidence that nutritional factors such as betaine[19], resveratrol[20], and curcumin[21] may regulate m6A RNA methylation.
Key enzymes involved in gluconeogenesis, such as phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), play significant roles in glucose production and are critical targets in this study. PEPCK and G6Pase are essential for gluconeogenesis, the process of producing glucose from non-carbohydrate sources, which is often dysregulated in diabetes. Additionally, the expression of genes like glycogen synthase kinase 3 beta (GSK3β), glycogen synthase (GS), and protein kinase B (Akt) was examined in this study. These genes are involved in glycogen metabolism and insulin signaling pathways, making them relevant for understanding how dendrobine affects glucose homeostasis. GSK3β and GS are crucial for glycogen synthesis, while AKT is a key player in insulin signaling and glucose uptake.
There is currently limited research on the protective effect of dendrobine on glucose metabolism disruption and its underlying molecular mechanisms. In this study, the aim was to elucidate the effects of dendrobine on both glycogenesis and gluconeogenesis, and the associated changes of m6A methylation using human hepatocellular carcinoma cells (LO2). Specifically, the present study provides evidence that dendrobine has an effect on glucose metabolism in part via the m6A methyltransferase METTL3.
-
Human hepatocellular carcinoma cells (LO2, BeNa Culture Collection company) were chosen as the experimental model for this study due to their relevance in mimicking human liver function and metabolic processes. These cells provide a valuable system for studying hepatic glucose metabolism and insulin resistance, as they retain many of the characteristics of normal liver cells while being easier to manipulate in vitro. LO2 cells were cultured in RPMI-1640 + 10%FBS + 1%P/S in a humidified environment (5% CO2, 37 °C). In glucosamine assay, cells were treated with 18 mM glucosamine (Invitrogen) for 18 h in a serum-free medium[22], and then incubated with different concentrations of dendrobine (1, 2.5, and 5 μg·mL−1 , MedChemExpress) for another 24 h.
MTT assays
-
To examine viability, cells were treated with different concentrations of dendrobine (0, 0.5, 1, 2.5, 5, 7.5, 10, 15, and 20 μg·mL−1), and then tested for viability using an MTT assay[23]. Briefly, cells were seeded at 1 × 104 per ml density in a 96-well plate and then incubated with glucosamine and different concentrations of dendrobine for 24 h. MTT reagent at a concentration of 0.5 mg·mL−1 was injected into each well for 4 h at 37 °C and then dissolved in 100 μL of DMSO for another 30 min on a gyro shaker. The plates were analyzed in a microplate reader at 570 nm (BioRad, CA, USA).
Colony formation
-
Cells growing in the logarithmic phase were taken and the seed plates counted. A 6-well plate is inoculated with 1,000 cells per well in a culture system of 100 μL, and the culture is maintained for 1−2 weeks. When the cell mass is observed by the naked eye, the culture is immediately terminated. The original culture medium is discarded, and the plate is washed twice with PBS. Gentle operation is ensured to avoid the loss of adherent cells formed by cloning. An appropriate amount of 4% polymethyl alcohol is added to each well for fixation for 20 min, after which the fixing solution is poured out. Crystal violet staining solution (0.1% concentration) is added to each well, with a volume of 500 μL per well, for dyeing. The staining duration is approximately 15−30 min. The faucet is slightly unscrewed to create a slow flow of water, and the culture plate is placed under running water to gently wash out the staining solution. The plate is left to air dry, and photos are then taken to determine the number of cloned cells.
Cell transfection
-
Cells were cultured at a density of 2 × 105 per mL in six-well plates. A METTL3 siRNA (50 nM) and control siRNA (50 nM) were purchased from GenePharma (Shanghai, China), and then transfected with Lipofectamine 2000 according to the manufacturer's instructions. After 24 h of transfection, the cells were further incubated with glucosamine and 5 μg·mL−1 dendrobine.
Glucose production assay
-
Treated cells were cultured in medium containing 2 mM sodium pyruvate and 20 mM sodium lactate for 3 h. Glucose production was determined using a glucose oxidase-peroxidase assay kit.
Glycogen quantification
-
A glycogen assay kit (BioVision) was used for glycogen quantification. Briefly, treated cells were homogenized in ice-cold buffer, centrifuged at 15,000× g for 5 min, and the supernatant was collected and used for the assays.
qPCR
-
Total RNA was isolated (NucleoZol reagent, MACHEREY-NAGEL, Germany) and converted to cDNA. Real-time quantitative PCR was performed using an ABI 7500 system (Applied Biosystems, Inc., Foster City, CA, USA) according to the manufacturer's instructions. The primers used are shown in Supplementary Table S1.
RIP-PCR
-
Total RNA is extracted from the cells or tissues using a standard RNA isolation method. The quality and quantity of RNA are assessed using a spectrophotometer or bioanalyzer. The isolated RNA is fragmented to a manageable size (usually 100–200 nucleotides) by heating or using specific fragmentation buffers. This step ensures that the immunoprecipitation (IP) is more efficient. The fragmented RNA is incubated with an m6A-specific antibody (usually anti-m6A antibody) to bind the RNA fragments containing m6A modifications. This mixture is then incubated with protein A or G magnetic beads that have been pre-coated with the m6A antibody. After binding, the RNA-antibody-bead complex is washed multiple times to remove non-specifically bound RNA. The m6A-modified RNA fragments are eluted from the beads using a specific elution buffer that releases the RNA while retaining the m6A modifications. The eluted RNA is reverse-transcribed into complementary DNA (cDNA) using reverse transcriptase and specific or random primers, allowing for further amplification and analysis. Specific primers targeting the genes of interest are used to amplify the cDNA regions that were immunoprecipitated. PCR or quantitative PCR (qPCR) is performed to detect and quantify the levels of m6A-modified RNA. The relative enrichment of m6A-modified RNA is calculated by comparing the PCR signal from the m6A-immunoprecipitated sample to that of an input RNA sample (total RNA before IP) or a negative control. The results are typically normalized to a housekeeping gene or total RNA input to account for variations in sample processing.
Western blot assay
-
Total LO2 cells protein were extracted using lysis buffer supplemented with 0.1 mM PMSF. Equal amounts of protein samples were loaded on an SDS-PAGE gel and transferred to PVDF membranes. Membranes were blocked with milk and incubated in 1:800 diluted primary antibodies (GS (cat#4818), phosphor-GS (Ser641) (cat#3891), GSK3 (cat#9338), phosphor-GSK3 (Ser9) (cat#5558), AKT (cat#9272), phospho-AKT (Ser473) (cat#9271), METTL3 (cat#86132), beta-actin (cat#4967) (Cell signaling technology), G6Pase (ab243319)) and PEPCK(ab239714) (Abcam) at 4 °C for 12 h. Membranes were then incubated in 1:1000 diluted secondary antibodies and proteins detected using a Pierce ECL western blotting kit.
Statistical analyses
-
All experiments were three times independent experiments. Data were reported as mean ± SEM. All groups were examined using one-way ANOVA. p < 0.05 was considered statistically significant.
-
Dendrobine is one of the main active ingredients of Dendrobium, and its structure is shown in Fig. 1a. An MTT assay was used to determine the viability of LO2 cells in the presence of different concentrations of dendrobine. As shown in Fig. 1b, the viability was gradually reduced with increasing dendrobin concentration. When the concentration of dendrobine reached 10 μg·mL−1, the viability decreased significantly to about 60% (Fig. 1b). A clone formation experiment revealed that, compared to controls, dendrobin inhibited the number of clones formed in a dose-dependent manner (Fig. 1c). Therefore, 5 μg·mL−1 dendrobine was selected, a concentration that did not show obvious cytotoxicity, for subsequent experiments.

Figure 1.
Effects of dendrobine in hepatic cells. (a) Structure of dendrobine, drawn using ChemDraw; (b) cell viability of LO2 cells, treated with different concentrated of dendrobine (0, 0.5, 1, 2.5, 5, 7.5, 10, 15, and 20 μg·mL−1); (c) colony formation of LO2 cells with different concentrated of dendrobine (0, 1, 5, and 10 μg·mL−1). * p < 0.05, ** p < 0.01 vs control group, n = 3. Dend: dendrobine.
Effect of dendrobine on the viability, glucose production, and RNA m6A in glucosamine-treated LO2 cells
-
Previous studies have shown that in LO2 cells glucosamine induces IR and its typical elevated levels of glucose[22,24]. It was found that in LO2 cells, viability was significantly decreased (p < 0.01) after glucosamine administration. Cells were then treated with different concentrations of dendrobin (1, 2.5, and 5 μg·mL−1), and dendrobin concentrations of 2.5 and 5 μg·mL−1 showed effective protection against glucosamine-induced cell death (Fig. 2a). A 5 μg/ml dendrobin concentration was chosen for further experiments. Glucose production in LO2 cells was also investigated. The results showed that dendrobine treatment significantly corrected the increased glucose production in glucosamine-treated cells (Fig. 2b). The effect of dendrobin on the expression of PEPCK and G6Pase proteins was similar in glucosamine- and dendrobin-treated LO2 cells (Fig. 2c–e). The mRNA expression levels of PEPCK and G6Pase showed a similar trend to protein expression (Fig. 2f). The m6A RNA modification levels of PEPCK and G6Pase were then verified by RIP-PCR and were found to behave similarly to the mRNA and protein levels (Fig. 2g). These results indicate that dendrobin promotes cell viability and decreases glucose production in glucosamine-treated cells.
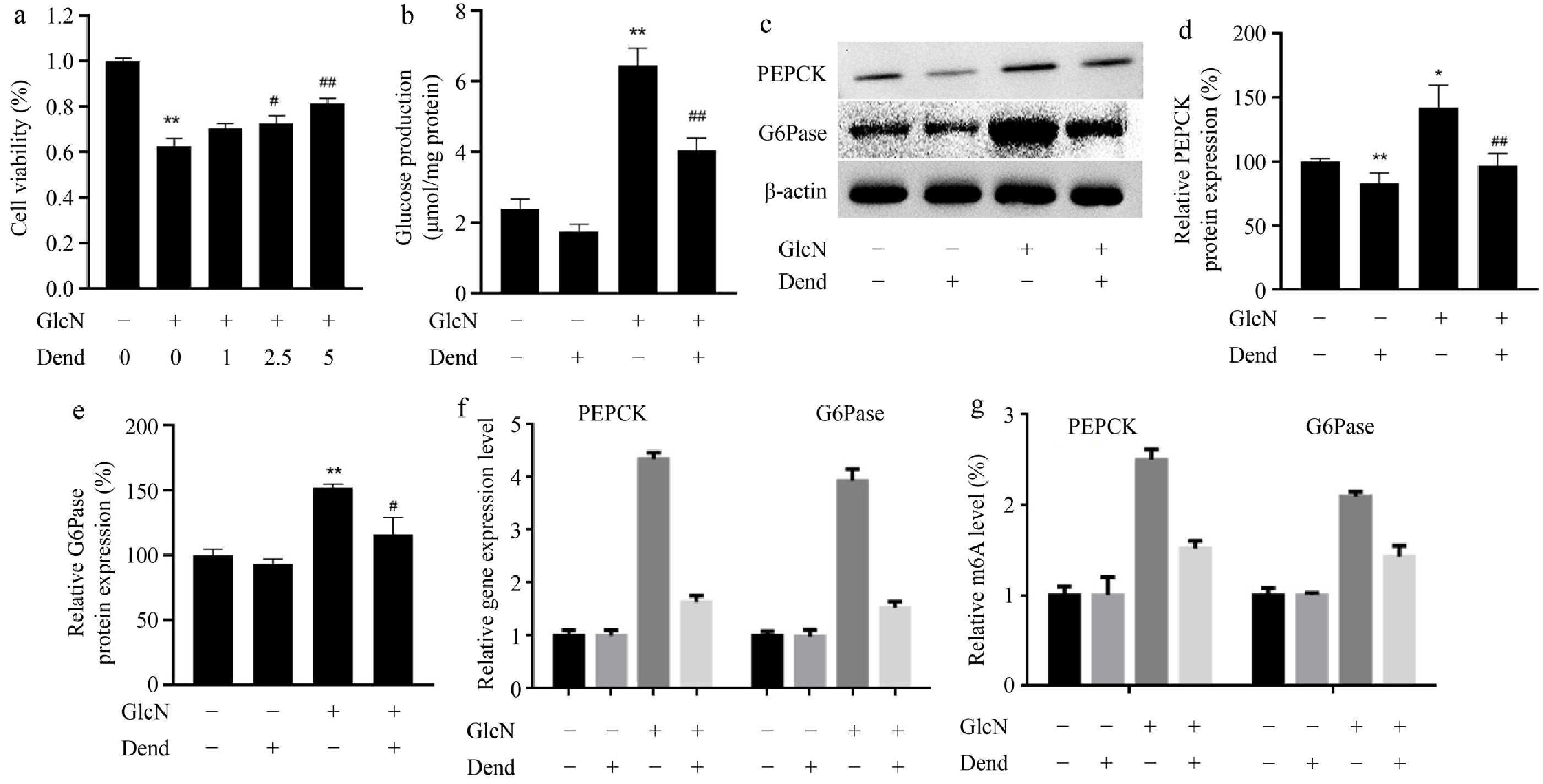
Figure 2.
Effects of dendrobine on glucose production and the key enzymes involved into gluconeogenesis in glucosamine-treated LO2 cells. (a) Viability of LO2 cells, treated with 0, 1, 2.5, and 5 μg·mL−1 dendrobine and 18 mM glucosamine; (b) glucose production of LO2 cells; (c) representative western blot images showing PEPCK and G6Pase; (d), (e) relative values of PEPCK and G6Pase proteins; (f) relative gene expression level; (g) relative values of RNA m6A levels. **, #, ## respectively indicate p < 0.01, p < 0.05, and p < 0.01, n = 3. GlcN: glucosamine; Dend: dendrobine.
Effect of dendrobine on glycogen synthesis in glucosamine-treated LO2 cells
-
Another important feature of IR is the inhibition of hepatic glycogen synthesis. In this study, glucosamine administration inhibited glycogen levels in LO2 cells, but this effect was prevented by dendrobine administration (Fig. 3a). In addition, glucosamine administration promoted the expression of phosphorylated GS and suppressed the expression of phosphorylated GSK3β and AKT, suggesting that dendrobine was efficient in counteracting the effects of glucosamine in LO2 cells (Fig. 3b–e). These results provide evidence that dendrobine ameliorates decreased glycogen synthesis in vitro.
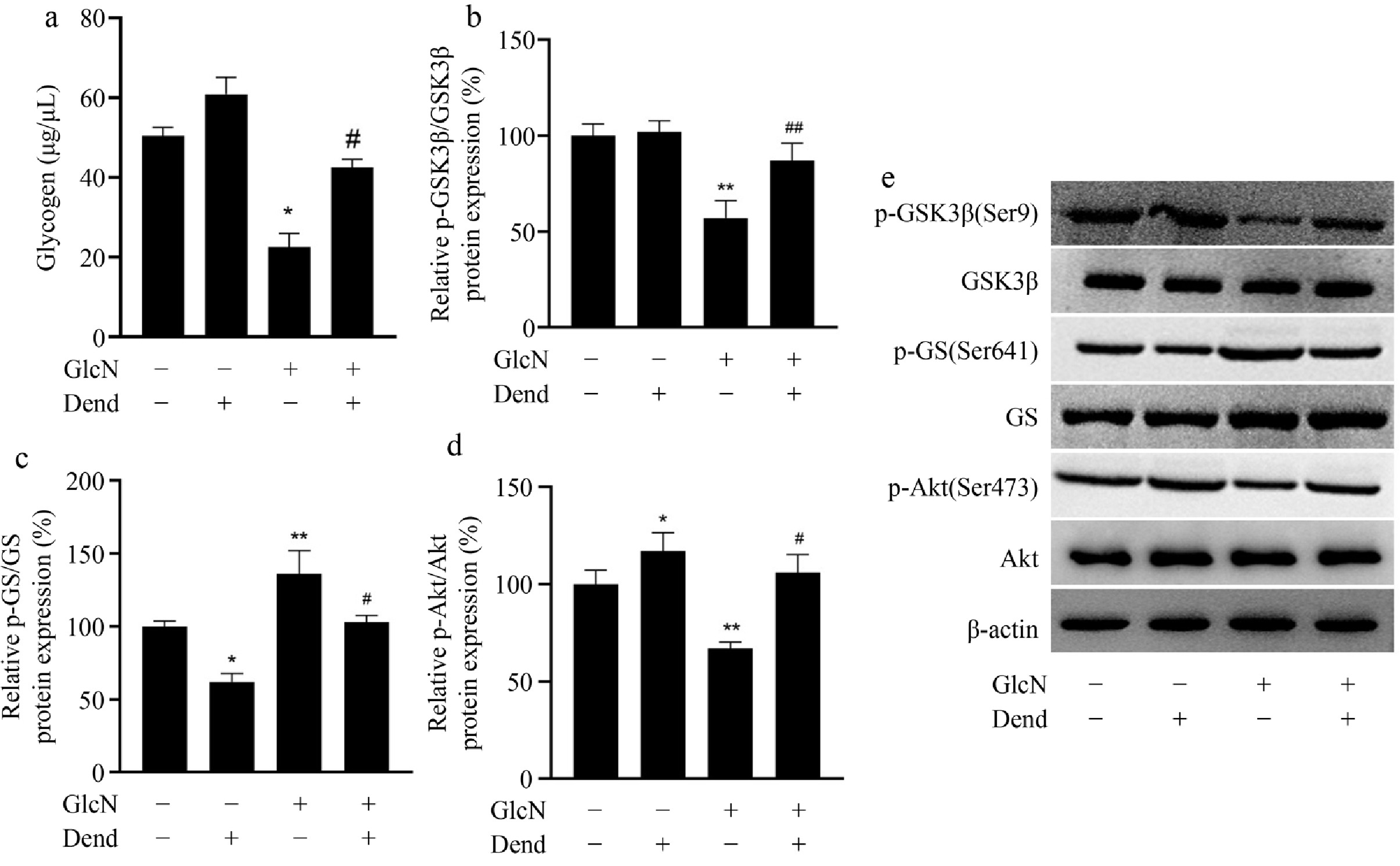
Figure 3.
Regulator function of dendrobine on glycogen content in glucosamine-treated LO2 cells. (a) Glycogen level; (b) phosphorylated GSK3; (c) phosphorylated GS; (d) phosphorylated AKT; (e) western blot image of GSK3, GS, and AKT proteins. **, #, ## respectively indicate p < 0.01, p < 0.05, and p < 0.01, n = 3. GlcN: glucosamine; Dend: dendrobine.
Effects of dendrobine on RNA m6A methylation in glucosamine-treated LO2 cells
-
To determine the possible role of dendrobine on m6A RNA methylation, the levels of m6A modifications and the levels of proteins involved in m6A modifications have been examined. The relative mRNA expression of some critical methyltransferases (METTL3 and METTL14), demethylases (FTO) and reader proteins (YTHDF1, YTHDF2, and YTHDF3) were examined. Compared to controls, glucosamine remarkably increased the mRNA levels of METTL3 and METTL14, while it suppressed the mRNA levels of FTO (Fig. 4a). The level of m6A RNA methylation was significantly increased in glucosamine-treated LO2 cells compared with the control. However, dendrobin effectively reduced this increased level of m6A-methylated RNA (Fig. 4b). Moreover, in glucosamine-treated LO2 cells, dendrobine significantly decreased the mRNA level of METTL3 to the control level and increased the mRNA level of FTO. Furthermore, METTL3 protein expression was also reduced, consistent with METTL3 mRNA level (Fig. 4c & d). These results indicate that dendrobine may be involved in the regulation of m6A methylation, and that METTL3 is a critical factor in this regulation.
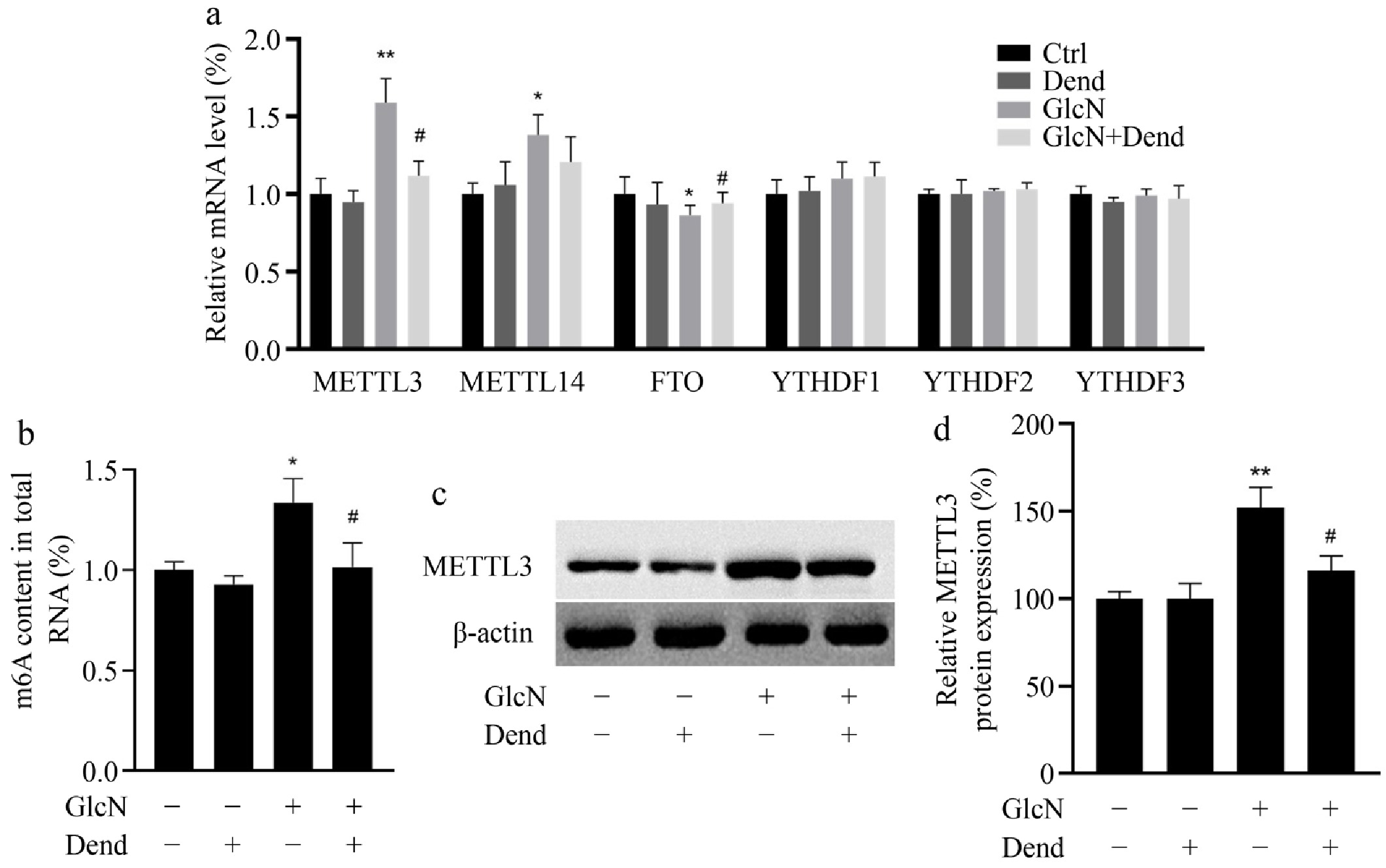
Figure 4.
Effect of dendrobine on the level of m6A modification and its methyltransferase METTL3 in glucosamine-treated LO2 cells. (a) relative level of critical methyltransferase (METTL3 and METTL14), demethylase (FTO), reading proteins (YTHDF1, YTHDF2, and YTHDF3); (b) Level of m6A methylation; (c) western blot image of METTL3; (d) relative value of METTL3 protein. **, #, ## respectively indicate p < 0.01, p < 0.05 and p < 0.01, n = 3. GlcN: glucosamine; Dend: dendrobine.
Effects of dendrobine on RNA m6A modification of PEPCK and G6Pase in glucosamine-treated LO2 cells
-
To determine whether the role of dendrobine in IR is associated with decreased METTL3 levels, METTL3 was downregulated in LO2 cells. Silencing of METTL3 led to remarkably decreased METTL3 expression levels, suggesting that METTL3 was efficiently downregulated (Fig. 5a & b). In addition, two key enzymes involved in gluconeogenesis were examined. In LO2 cells treated with control siRNA, PEPCK, and G6Pase protein levels were increased 1.23-fold and 1.07-fold, respectively, after administration of dendrobin and glucosamine compared with glucosamine alone. However, in METTL3 knockdown LO2 cells, PEPCK and G6Pase levels decreased 1.10-fold and 1.04-fold, respectively, after administration of dendrobin and glucosamine compared with glucosamine alone. (Fig. 5c–e). m6A methylation levels of PEPCK and G6Pase RNA were also measured showing that this modification has a trend similar to that of protein levels (Fig. 5f). This suggests that in glucosamine-treated LO2 cells, dendrobine decreases insulin resistance in part by suppressing METTL3 expression.
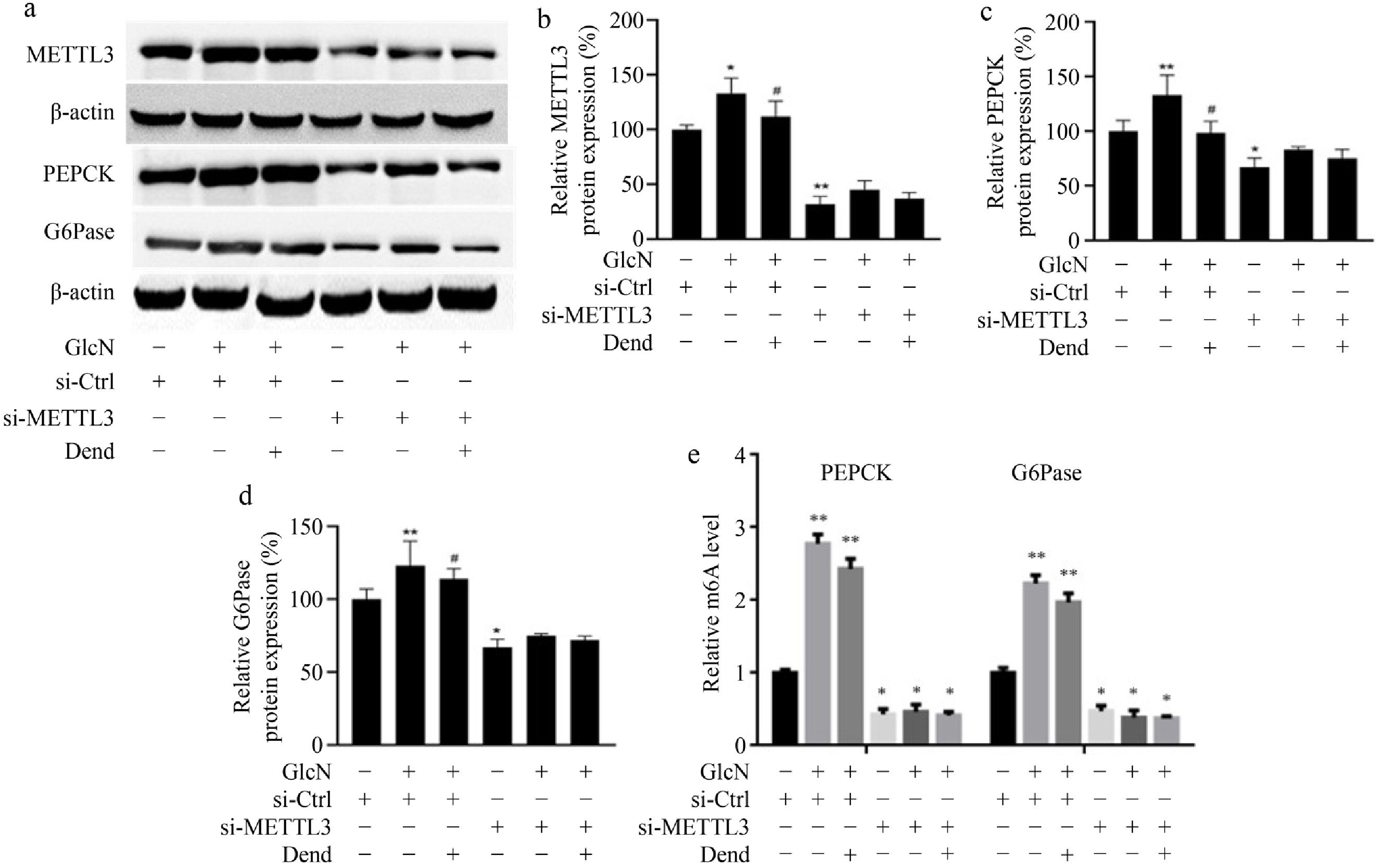
Figure 5.
Effects of downregulated-METTL3 and dendrobine on key enzymes involved into gluconeogenesis in glucosamine-treated LO2 cells. (a) Western blot image of METTL3 , PEPCK, and G6Pase; (b) relative values of METTL3 protein; (c) and (d) relative values of PEPCK and G6Pase proteins. (e) relative values of RNA m6A level. * p < 0.05, ** p < 0.01; # p < 0.05 vs GlcN and si-METTL3 group, n = 3. GlcN: glucosamine; Dend: dendrobine.
Effects of dendrobine on AKT, GS, and GSK3β in glucosamine-treated LO2 cells
-
To explore the involvement of METTL3 in glycogen synthesis, the protein expression levels of AKT, GS, and GSK3β were examined. In LO2 cells treated with control siRNA, the phosphorylation levels of AKT and GSK3β was increased to 1.97-fold and 2.22-fold, respectively, after administration of dendrobine and glucosamine compared with glucosamine alone. In METTL3 knockdown LO2 cells, the phosphorylation levels of AKT and GSK3β was increased only 1.18-fold and 1.92-fold, respectively, after administration of dendrobine and glucosamine compared with glucosamine alone (Fig. 6a & b). Moreover, the phosphorylation levels of GS was downregulated by 1.37-fold after administration of dendrobine and glucosamine compared with glucosamine alone in si-control. However, in METTL3 knockdown LO2 cells, the expression of phosphorylated GS was increased only 1.05-fold after administration of dendrobine and glucosamine compared with glucosamine alone (Fig. 6c & d). Taken together, these results strongly suggest that dendrobine improves insulin resistance in part by suppressing the expression of METTL3 in glucosamine-treated LO2 cells.
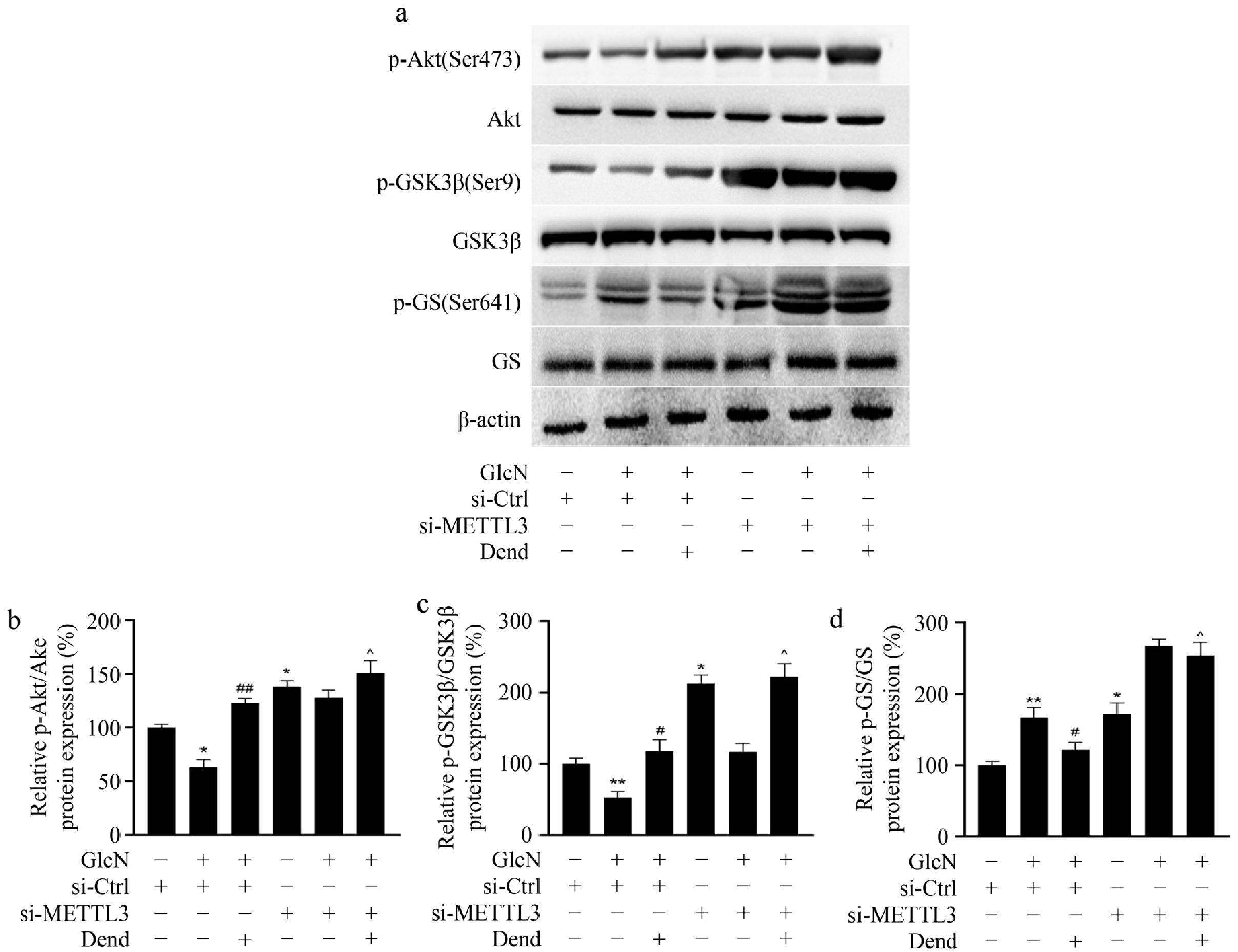
Figure 6.
Effects of downregulated-METTL3 and dendrobine on the key enzymes involved in glycogenesis in glucosamine-treated LO2 cells. (a) Level of GSK3, GS, and AKT proteins; (b)–(d) relative level of GSK3, GS, and AKT proteins. * p < 0.05, ** p < 0.01; # p < 0.05, ## p < 0.01; ^ p < 0.01, n = 3. GlcN: glucosamine; Dend: dendrobine.
-
Blood glucose levels are maintained mainly by the liver which balances the uptake and storage of glucose via glycogenesis and the release of glucose via glycogenolysis and gluconeogenesis[2]. Increased production of endogenous glucose and dysfunction of glycogen storage lead to metabolic abnormalities, and excessive glucose in the liver is the main cause of rapid hyperglycemia in diabetes[25]. IR is closely related to the progression of diabetes and is considered an important factor for elevated fasting blood glucose. Glucose metabolism abnormalities contribute to hyperglycemia and lead to further insulin resistance[26]. The liver could function as an integrative tissue for the absorption of excessive glucose and thus maintain metabolic homeostasis in diabetes[27]. In the present study, dendrobine was shown to be effective on glucose regulation. Dendrobine is used in traditional Chinese medicine and has numerous biological activities. Several recent studies have reported the protective properties of dendrobine in diabetes mellitus. Nevertheless, there is insufficient information on the mechanism underlying its effectiveness. In the present study, dendrobine was found to improve glucose production and glycogen content via METTL3. This is consistent with previous studies showing that dendrobine suppresses glucose production and gluconeogenesis, while promoting glycogen synthesis in LO2 cells with insulin resistance. These results suggest that dendrobine may be a promising candidate for diabetes therapy.
Hepatic glucose metabolism is mainly influenced by gluconeogenesis and glycogen synthesis. Hepatic gluconeogenesis is mediated by PEPCK and G6Pase[28]. When the expression of these two enzymes is increased, hepatic gluconeogenesis is enhanced and promotes the synthesis of cholesterol and protein. Glycogen synthesis is primarily regulated by GSK3 and GS. Increased expression of phosphorylated GSK3 in the liver promotes hepatic glycogen synthesis and reduces glucose production, an effect that could improve IR in diabetes[29]. Insulin can promote phosphorylation of GSK3 increasing GS expression and leading to the conversion of glucose to glycogen. Decreased glucose production can thus be considered a possible target of diabetes therapy[30,31]. In this study, dendrobine was shown to inhibit PEPCK and G6Pase levels and promote the expression of GS in glucosamine-induced LO2 cells. Moreover, AKT as an indicator of the IR pathway is considered a key factor in conjunction with insulin for gluconeogenesis and glycogen synthesis[32]. On the one hand, AKT activation suppressed the expression of PEPCK and G6Pase, resulting in the inhibition of hepatic gluconeogenesis[33,34]. On the other hand, AKT activation inhibited the expression of GSK3β and the subsequent activity of GS, leading to increased hepatic glycogen synthesis[33,34]. This result also suggests that dendrobine promotes the phosphorylation of AKT in glucosamine-induced LO2 cells. Overall, dendrobine suppressed hepatic gluconeogenesis through PEPCK and G6Pase and promoted glycogen synthesis via GS activation.
Increasing evidence suggests that m6A RNA methylation is related to glucose metabolism. It was shown that METTL3 downregulation suppresses m6A levels and expression of fatty acid synthase (FAS), and further suppresses fatty acid metabolism[35]. Moreover, high expression of FAS was mediated by adeno-associated virus in METTL3 knockout mice, inhibiting insulin sensitivity and the decrease of FAS, suggesting that METTL3 inhibits liver insulin sensitivity through m6A methylation of FAS, promotes fatty acid metabolism, and eventually leads to the progression of type 2 diabetes. It was shown that β-cell-specific METTL14 knock-out mice characterized by reduced m6A levels, exhibit cell-cycle arrest, and impaired insulin secretion via a reduction in AKT phosphorylation and PDX1 protein levels, leading to early onset and development of type 2 diabetes[14]. It was shown that METTL3/14 is critical for governing neonatal β-cell functional maturation and identity maintenance and directly regulates essential transcription factor MafA expression by modulating its mRNA stability. Indeed, the loss of METTL3/14 led to overt neonatal diabetes due to the inability to establish adequate functional β-cell mass after birth[36]. It is evident that the function of m6A methylase in regulating metabolic homeostasis, insulin sensitivity, and β-islet cell survival and function influences the progression of diabetes. Importantly, recent research has shown that m6A methylation is involved in nutritional physiology and metabolism. However, there is still a lack of relevant studies on the role of dendrobine in altering mRNA methylation levels and the underlying mechanism. In this study, dendrobine treatment of glucosamine-induced LO2 cells resulted in changes in m6A RNA modification, which were regulated at least in part by the methylase METTL3. This suggests that effect of dendrobine in controlling glucose metabolism is in part due to the alteration of m6A RNA modifications by METTL3, which regulates gluconeogenesis and glucose synthesis. However, further studies are needed to investigate the precise interaction between regulation of glucose homeostasis by dendrobine and m6A RNA modifications.
In conclusion, this study shows that dendrobin showed effective protection against glucosamine-induced cell death and is effective in preventing hepatic IR in glucosamine-induced LO2 cells. Specifically, dendrobin could ameliorate diabetes by acting on METTL3-mediated gluconeogenesis and glucose metabolism in glucosamine-induced LO2 cells. This suggests that the effect of dendrobin in regulating glucose metabolism is in part related to m6A RNA modification.
-
This study (protocol number: 2020-(2016)) was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University under the reference number 2020-(2016). Due to the nature of the research and following an informed consent waiver granted by the ethics committee (reference number: 2020-(2016)), written informed consent from the participants was not required for their inclusion in the study.
This study was funded by Joint Funds for the Innovation of Science and Technology, Fujian province (2020Y9136), and Fujian Provincial Natural Science Fund (2020J0112).
-
The authors confirm contribution to the paper as follows: studys conception, experimental design, and data analysis (contributed equally): Chen S, Zheng X; data collection, curation, and result interpretation: Lin Q; project supervision, writing - draft manuscript preparation: Wang X; writing - manuscript revision: Wang X, Liu Z; methodology support, results validation: Liu Z. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article and its supplementary information files.
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Shouzhen Chen, Xiaohua Zheng
- Supplementary Table S1 Primers used in this study.
- © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Chen S, Zheng X, Lin Q, Wang X, Liu Z. 2024. Dendrobine inhibits hepatic gluconeogenesis and promotes glycogen synthesis through control of m6A RNA modification of PEPCK/G6Pase by METTL3 in hepatocytes. Epigenetics Insights 17: e005 doi: 10.48130/epi-0024-0004
Dendrobine inhibits hepatic gluconeogenesis and promotes glycogen synthesis through control of m6A RNA modification of PEPCK/G6Pase by METTL3 in hepatocytes
- Received: 07 August 2024
- Revised: 15 September 2024
- Accepted: 25 September 2024
- Published online: 13 December 2024
Abstract: Disruption of glucose homeostasis in type 2 diabetes mellitus is characterized by increased glucose production and decreased glycogen content. Hepatic glucose metabolism is crucial in this disruption, with key enzymes like phosphoenolpyruvate carboxykinasen (PEPCK) and glucose-6-phosphatase (G6Pase) involved in gluconeogenesis and glycogen breakdown. m6A RNA modification, mediated by methyltransferase-like 3 (METTL3), regulates the expression of these enzymes and plays a significant role in glucose metabolism. Dendrobine, a natural sesquiterpene alkaloid, has various biological functions, but its impact on these processes remains unclear. This study aims to investigate the protective effects and mechanisms of dendrobine on hepatic glucose metabolism, particularly focusing on its modulation of PEPCK and G6Pase through m6A modification and METTL3 activity. The present findings reveal that dendrobine suppresses gluconeogenesis, promotes glycogen production, and modulates m6A modification levels of PEPCK and G6Pase RNAs in insulin-resistant hepatocytes. Additionally, dendrobine inhibits the glucosamine-induced upregulation of METTL3. Notably, the beneficial effects of dendrobine are partially reversed by METTL3 downregulation and changes in m6A modification levels of PEPCK and G6Pase RNAs. These results suggest that dendrobine mitigates aberrant glucose metabolism by modulating m6A modifications and METTL3 activity, highlighting its potential as a therapeutic candidate for insulin resistance in diabetes.
-
Key words:
- Dendrobine /
- RNA m6A modification /
- METTL3 /
- Glucose homeostasis /
- Insulin resistance













