-
Auxin, the first plant hormone discovered, is widely distributed in various plant tissues and plays an essential role in regulating plant growth and development[1,2]. Changes in auxin levels, whether an increase or decrease, have a remarkable effect on the normal growth of the root system[3,4]. At the same time, the directional transport of auxin between the root endodermis and the pericycle cells promotes the formation of lateral roots[5]. In the auxin signaling pathway, the disruption of AUXIN RESPONSE FACTOR (ARF) function results in aberrant root development. For example, arf7 arf19 double mutants have a reduced number of lateral roots compared to wild type[6−8]. Auxin also induces somatic embryogenesis in plant tissue culture[9,10]. Auxin has dual properties, with low concentrations stimulating plant growth and high concentrations inhibiting it. In addition, dicotyledonous plants are more sensitive to auxin than monocotyledonous plants[11]. As a result, auxin analogs are widely used for weed control in crops, particularly for broadleaf weed management[11,12].
The Herbicide Resistance Action Committee (HRAC) categorizes auxinic herbicides into two types: Class O and Class P[12,13]. Class O compounds are auxin analogs that modulate auxin signaling by regulating the expression of the auxin receptor TIR1 (TRANSPORT INHIBITOR RESPONSE 1)/AFB (AUXIN-SIGNALING F-BOX)[13,14], exemplified by 2,4-dichlorophenoxyacetic acid (2,4-D). 2,4-D, an auxin-derived synthetic herbicide, resists degradation by plants and exerts its effects by altering cell wall plasticity and upregulating genes associated with ethylene and abscisic acid (ABA) synthesis. These processes induce plant senescence, ultimately leading to plant death[15,16]. In addition, 2,4-D triggers the production of reactive oxygen species (ROS) in plants, the accumulation of which induces cytotoxicity, leading to weed death[15]. Class P compounds, such as naphthylphthalamic acid (NPA), disrupt polar auxin transport (PAT) by competing with endogenous IAA for the PIN auxin efflux carrier proteins, thereby controlling weeds[13,17].
In addition to auxinic herbicides, various sterilant herbicides, such as paraquat (PQ), are widely used in agriculture. PQ, a bipyridyl non-selective herbicide, has been popular in agricultural production due to its cost-effectiveness and efficacy in weed control[18]. PQ primarily targets Photosystem I (PS I) in plant chloroplasts, disrupting the electron transfer process by competing for electrons and redirecting them to oxygen molecules. As a result, this disruption generates excess ROS, triggering an imbalance in the plant’s redox response and ultimately leading to plant death[19,20]. Bai et al. observed inhibited electron transfer in the photosystems of both Pseudomonas aeruginosa and Chlorella following PQ treatment, accompanied by inhibited growth[21]. Furthermore, PQ treatment significantly increased ROS production in both organisms, with Pseudomonas aeruginosa showing higher ROS levels compared to Chlorella. This suggests that the application of PQ affects the photosystem of both prokaryotic and eukaryotic organisms, leading to the accumulation of ROS.
PQ plays an important role in agricultural weed control, but widespread use can lead to the development of weed resistance[22,23]. Through transcriptome sequencing of susceptible and resistant goosegrass (Eleusine indica L.) seedlings before and after PQ treatment, An et al. observed a significant upregulation of genes involved in ROS scavenging, polyamine (PA) metabolism, and intracellular transport after PQ treatment, suggesting their involvement in goosegrass resistance[22]. In addition, inhibition of PQ transport may enhance plant resistance. Yu et al. applied C14-labelled PQ to ryegrass leaves and found normal PQ uptake, but inhibited PQ transport in resistant ryegrass leaves[24]. Given the structural similarity between PQ and PA, it is likely that PQ shares a plant transporter with PA, suggesting a competitive relationship between the two[25]. Jóri et al. studied PQ-resistant Verbena Canadensis and demonstrated the role of polyamines in inhibiting PQ transport in both the vacuole and cytoplasm[26]. In Arabidopsis, PQ transport is mediated by RESISTANCE TO METHYL VIOLOGEN 1 (RMV1), an L-type amino acid transporter. The rmv1 mutant shows blocked PQ transport, reduced PQ uptake, and increased tolerance, while RMV1 overexpression renders plants hypersensitive to PQ[25]. These findings suggest that PQ-resistant plants may enhance resistance through ROS scavenging and inhibition of PQ transport.
In recent years, weeds have developed resistance to herbicides due to the overuse of similar herbicide types and improper application methods, significantly reducing their effectiveness. Consequently, there is an urgent need to explore alternative herbicide application strategies. It has been found that auxinic herbicides in tank-mixture with PQ provided more effective control of hairy fleabane, a weed in Brazil’s soybean crop[27]. However, the underlying mechanism remains unknown. This study examines the combined application of auxin and PQ to assess their effects on Arabidopsis seedling phenotype, chlorophyll content, root auxin response signals, and the expression of genes related to PAT and lateral root development. The aim is to develop new methods for herbicide application. The results will provide insights into agricultural weed control.
-
PQ was synthesized by conventional methods and its purity was determined by NMR to be over 95%. IAA-Na was purchased from Bio Basic Inc. (Shanghai, China) and its purity is over 99%. Both PQ and IAA-Na were dissolved in distilled water.
Plant materials and culture conditions
-
Arabidopsis thaliana (Arabidopsis) Columbia-0 (Col-0) and transgenic lines expressing the auxin response reporter DR5:GUS were used as experimental material. Seeds were first surface sterilized with 70% ethanol for 5 min, followed by treatment with 1% sodium hypochlorite for 8 min, and then rinsed five times with sterile water. After stratification at 4 °C for 3 d, the seeds were sown on Murashige & Skoog (MS) medium supplemented with 1% sucrose and 0.8% plant agar (pH 5.8). The MS plates were then placed vertically in an incubator and grown at 22 °C under a 16 h light/8 h dark photoperiod for 5 or 7 d before further use.
Treatment of cotyledons with IAA and PQ
-
7-day-old seedlings with uniform growth were selected for treatment. IAA-Na and PQ were dissolved in distilled water and then six concentration gradients were prepared ranging from 10 nM to 1 mM of IAA and PQ alone and in combination. The solution (0.2 μL) were pipetted onto each of the two cotyledons as shown in Fig. 1a, and distilled water was used as a control. After the cotyledons had absorbed the solutions, the seedlings were cultured in an incubator at 22 °C with a 16 h light/8 h dark photoperiod. After 24 h of culture, root elongation, hypocotyl length, number of lateral roots, and cotyledon albinism rate were assessed.
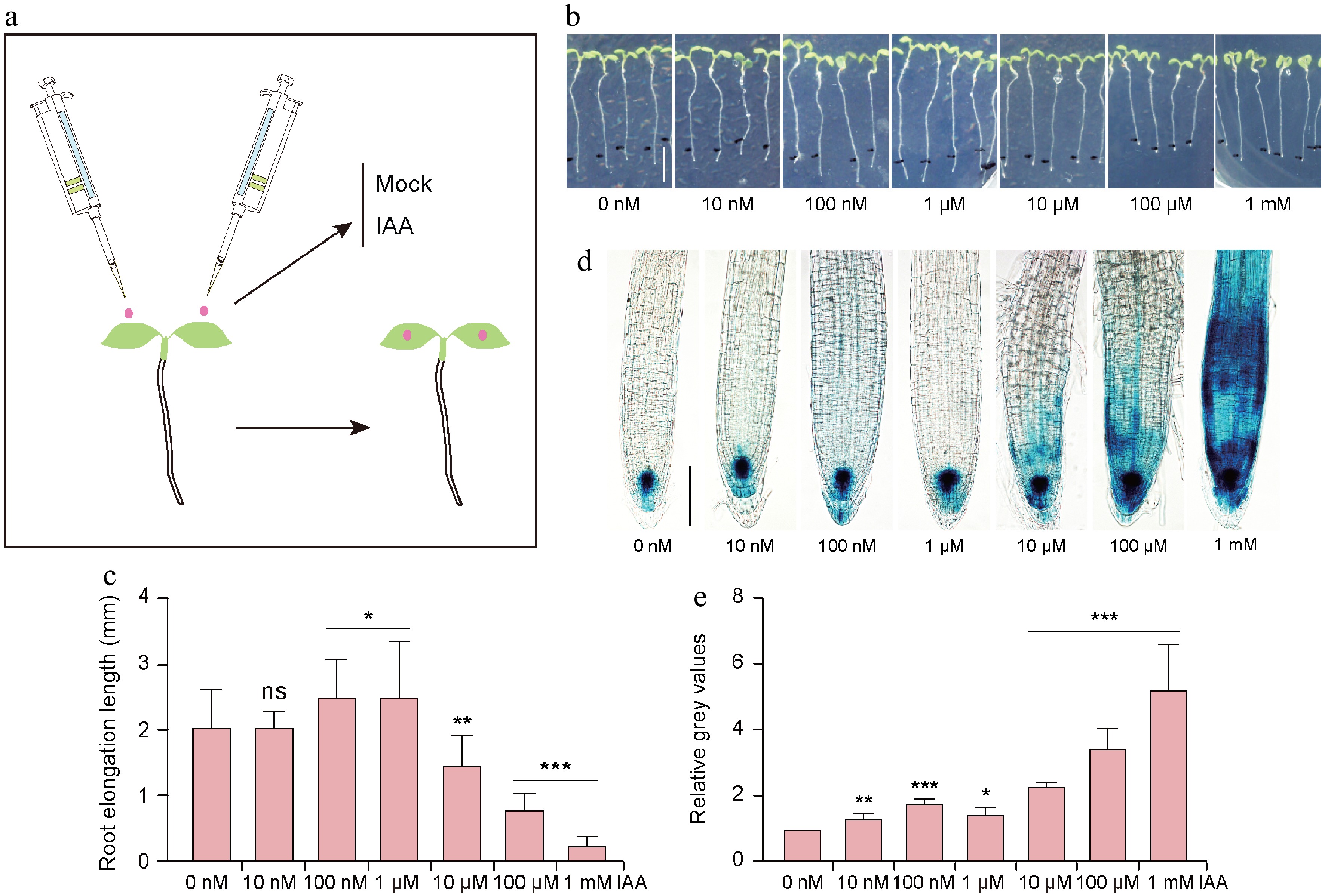
Figure 1.
Effects of exogenous application of IAA on Arabidopsis cotyledons and root growth and auxin response signals in the root.
(a) Schematic illustration of IAA application to the cotyledons of Arabidopsis seedlings. (b) 5-day-old Arabidopsis seedlings after application of different concentrations of IAA to the cotyledons for 24 h. (c) Root elongation length after 24 h of IAA treatment (n = 13−19 seedlings). (d) GUS staining results at the root tip of 7-day-old DR5:GUS seedlings after application of different concentrations of IAA to the cotyledons for 24 h. (e) Relative grey value of GUS staining (n = 3 roots). Values in (c) and (e) represent the mean ± SD. Significant differences were analyzed by Student's t-test (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ns = not significant). Scale bars = (a) 0.5 cm and (c) 100 μm.Extraction and determination of chlorophyll content
-
Chlorophyll extraction was performed according to the ethanol-acetone method with slight modifications. Arabidopsis leaves (0.1 g) were weighed, and 2 mL of extraction buffer (ethanol : acetone : H2O = 4.5:4.5:1, v:v:v) was added. The supernatant was extracted for 12 h at 4 °C in the dark. The absorbance values at wavelengths 645 and 663 were measured using a spectrophotometer, and the contents of chlorophyll a, b, and total chlorophyll were calculated using the following formula: Chlorophyll a (μg/g) = (12.72 × A663 − 2.59 × A645) × v/w × 1000; Chlorophyll b (μg/g) = (22.88 × A645 − 4.67 × A663) × v/w × 1000; Total chlorophyll (μg/g) = (20.29 × A645 + 8.05 × A663) × v/w × 1000. Where, v is the volume of the extract (mL) and w is the weight of the seedlings (g).
GUS histochemical staining
-
7-day-old Arabidopsis DR5:GUS seedlings treated with mock, IAA, PQ, and IAA + PQ for 24 h were immersed in GUS staining solution (0.5 mg·mL−1 5-bromo-4-chloro-3-indolyl-β-D-glucuronic acid, 50 mM sodium phosphate buffer, pH 7.0, 0.1% Triton X-100, 0.5 mM potassium ferricyanide, and 0.5 mM potassium ferrocyanide) and incubated for 1 h at 37 °C. After staining, the seedlings were transferred to a fixative solution (ethanol : acetic acid = 3:1, v:v) and fixed for 6 h. The fixative was then replaced with a clearing solution (chloral hydrate : distilled water : glycerol = 8:3:1, w:v:v) for 24 h. The GUS staining signal was visualized using a Leica microscope (Leica, Wetzlar, Germany).
Real-time fluorescence quantitative PCR (RT-qPCR)
-
Seven-day-old Arabidopsis Col-0 seedlings were treated with 100 μM IAA, 100 μM PQ, or 100 μM IAA + 100 μM PQ in liquid MS medium for 6 h. Subsequently, 200 mg of fresh seedlings were ground into powder using liquid nitrogen. Total RNA was extracted using TRIzol reagent (TransGen Biotech, Beijing, China), and 1 μg of total RNA was reverse transcribed into cDNA using the EasyScript First-Strand cDNA Synthesis SuperMix kit (TransGen Biotech, Beijing, China). Next, 1 μL of cDNA was used as a template for the PCR amplification. The reaction mixture consisted of 1 μL cDNA, 0.5 μL forward primer, 0.5 μL reverse primer, 10 μL 2× qPCR Mix (containing SYBR Green, Taq enzyme, buffer, and dNTPs) and 8 μL ddH2O, making a total volume of 20 μL. The TIP41 gene was used as an internal reference. The relative expression level of genes was calculated using the 2−ΔΔCᴛ (Ct, cycle threshold) value. The primers used are listed in Supplementary Table S1.
Statistical analysis
-
Root length, hypocotyl length, and root elongation were quantified using ImageJ software (version 1.40). The number of lateral roots was calculated by dividing the total number of lateral roots by the number of seedlings. The albino rate of cotyledons was determined by dividing the number of albino cotyledons by the total number of cotyledons of all seedlings. All data were obtained from three independent biological experiments. Statistical analysis was performed using Student's t-test with significance levels as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001. Multi-sample significance analysis was performed using One-way ANOVA.
-
To investigate the effect of foliar application of auxin on leaf and root growth in Arabidopsis seedlings, different concentrations of IAA were applied to the cotyledons of 5-day-old Arabidopsis seedlings (Fig. 1a). The results showed that when the IAA concentration was below 1 mM, it had negligible effects on cotyledon growth, whereas at 1 mM it induced epinastic growth of the cotyledons (Fig. 1b). Conversely, lower concentrations of IAA (100 nM and 1 μM) significantly promoted root elongation (p < 0.05), whereas higher concentrations (10 μM, 100 μM, and 1 mM) inhibited root elongation. (p < 0.01) (Fig. 1b & c). For example, the average root elongation after 24 h of mock treatment was about 2.05 mm, whereas it increased to about 2.52 mm with 1 μM IAA treatment but decreased to about 0.25 mm with 1 mM IAA treatment (Fig. 1b & c). The expression level of the Arabidopsis auxin response reporter DR5:GUS indirectly reflects auxin levels[28]. To investigate whether foliar application of IAA affects auxin levels in the root, cotyledons of DR5:GUS seedlings were treated with different concentrations of IAA. The results showed that after 24 h of treatment, the GUS signal at the root tip of seedlings increased significantly with increasing IAA concentration (Fig. 1d & e). Under 10 nM to 1 μM IAA treatment, GUS signals were mainly observed in the quiescent center (QC) and columella cells of the root tip; under 10 μM IAA treatment, DR5:GUS expression was slightly detected in the root apical meristem; under 100 μM IAA treatment, DR5:GUS was expressed in both the meristematic and elongation zones of the roots, whereas under 1 mM IAA treatment, DR5:GUS expression was significantly enhanced in the root apical meristem and elongation zones (Fig. 1d & e). This suggests that IAA applied to the cotyledons is transported to the root tip, thereby enhancing the expression of the auxin-responsive reporter DR5:GUS at the root tip.
PQ inhibits root elongation and induces cotyledon bleaching in Arabidopsis seedlings
-
To investigate the effect of PQ on the growth of Arabidopsis seedlings, different concentrations of PQ were applied dropwise to the cotyledons of 7-day-old Arabidopsis seedlings. It was observed that root elongation of Arabidopsis seedlings was significantly inhibited with increasing PQ concentration (Fig. 2a & c). When seedlings were treated with 10 nM to 10 μM PQ, there was no significant difference in root elongation compared to the control (CK). However, under 100 μM and 1 mM PQ treatments, the root elongation was reduced by approximately 21.1% and 68.9%, respectively, compared to CK (Fig. 2a & c). In addition, the cotyledons of the seedlings exhibited bleaching, with albino rates of 21.8% and 79.5%, respectively (Fig. 2b & d). This indicates that PQ inhibits seedling root elongation and causes cotyledon bleaching. Notably, root elongation inhibition and cotyledon bleaching are more pronounced under 1 mM PQ treatment.
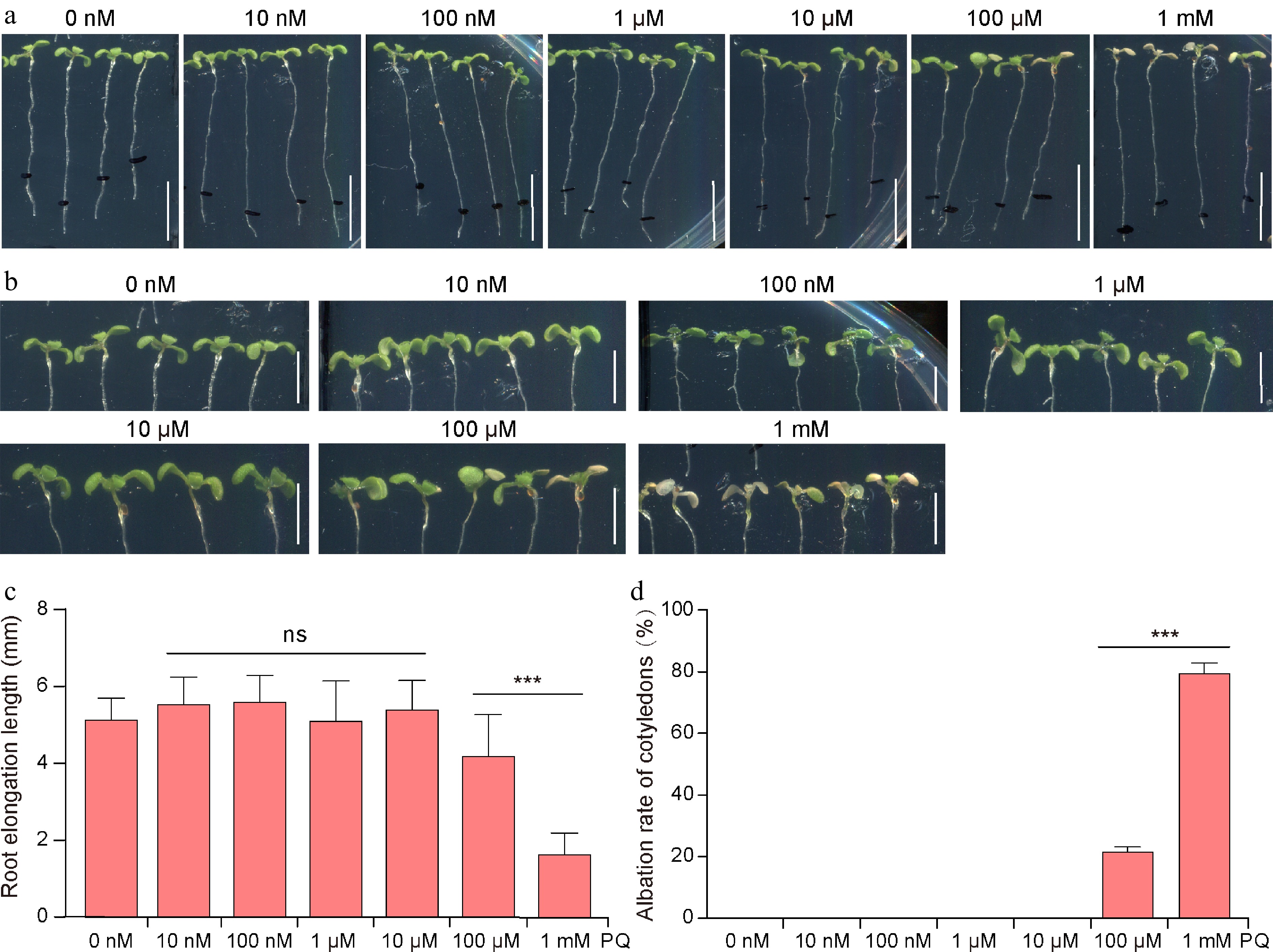
Figure 2.
Effects of PQ on Arabidopsis seedlings.
(a) 7-day-old Arabidopsis seedlings after application of different concentrations of PQ to the cotyledons for 24 h. (b) Enlarged view of the seedling shoot. The (c) elongation length of the seedling roots, and (d) cotyledon bleaching rate after 24 h of treatment with PQ (n = 13−23 seedlings). Values in (c) and (d) represent the mean ± SD. Significant differences were analyzed using Student's t-test (***, p < 0.001; ns = not significant). Scale bars = (a) 1 cm and (b) 5 mm.Effects of combined application of IAA and PQ on Arabidopsis seedling roots
-
Both IAA and PQ affect the growth of Arabidopsis seedlings. To investigate whether the simultaneous application of IAA and PQ would have an additive inhibitory effect, the cotyledons of 5-day-old Arabidopsis seedlings were treated with 1 mM IAA and 1 mm PQ, either alone or in combination. The results showed that both treatments individually inhibited root elongation of Arabidopsis seedlings, with IAA showing a higher degree of inhibition. The average root elongation length after 24 h of mock treatment was about 3.71 mm, while that of PQ treatment was about 2.2 mm. In contrast, the elongation length under IAA treatment was 0 mm and the combined treatment with IAA and PQ also resulted in 0 mm elongation length (Fig. 3a & b). Therefore, it remains inconclusive whether simultaneous treatment with PQ and IAA has additive inhibitory effects. Under IAA treatment, seedling roots became thicker, whereas under PQ treatment they became thinner. However, under the combined treatment of IAA and PQ, the root diameter was between those of the two individual treatments, indicating an antagonistic effect between them (Fig. 3c & d). The effect of the combined application of IAA and PQ on DR5:GUS expression was also investigated. The results showed that under IAA treatment, DR5:GUS expression in seedling roots was significantly higher than in the mock, whereas under PQ treatment, DR5:GUS expression in seedling roots was slightly weaker than in the mock. Under the mixed treatment of 1 mM IAA and 1 mM PQ, the DR5:GUS signal in seedling roots was lower than when treated with IAA alone (Fig. 3e). These results suggest that PQ inhibits auxin signaling. Similarly, IAA and PQ had antagonistic effects on lateral root development (Fig. 3f & g).
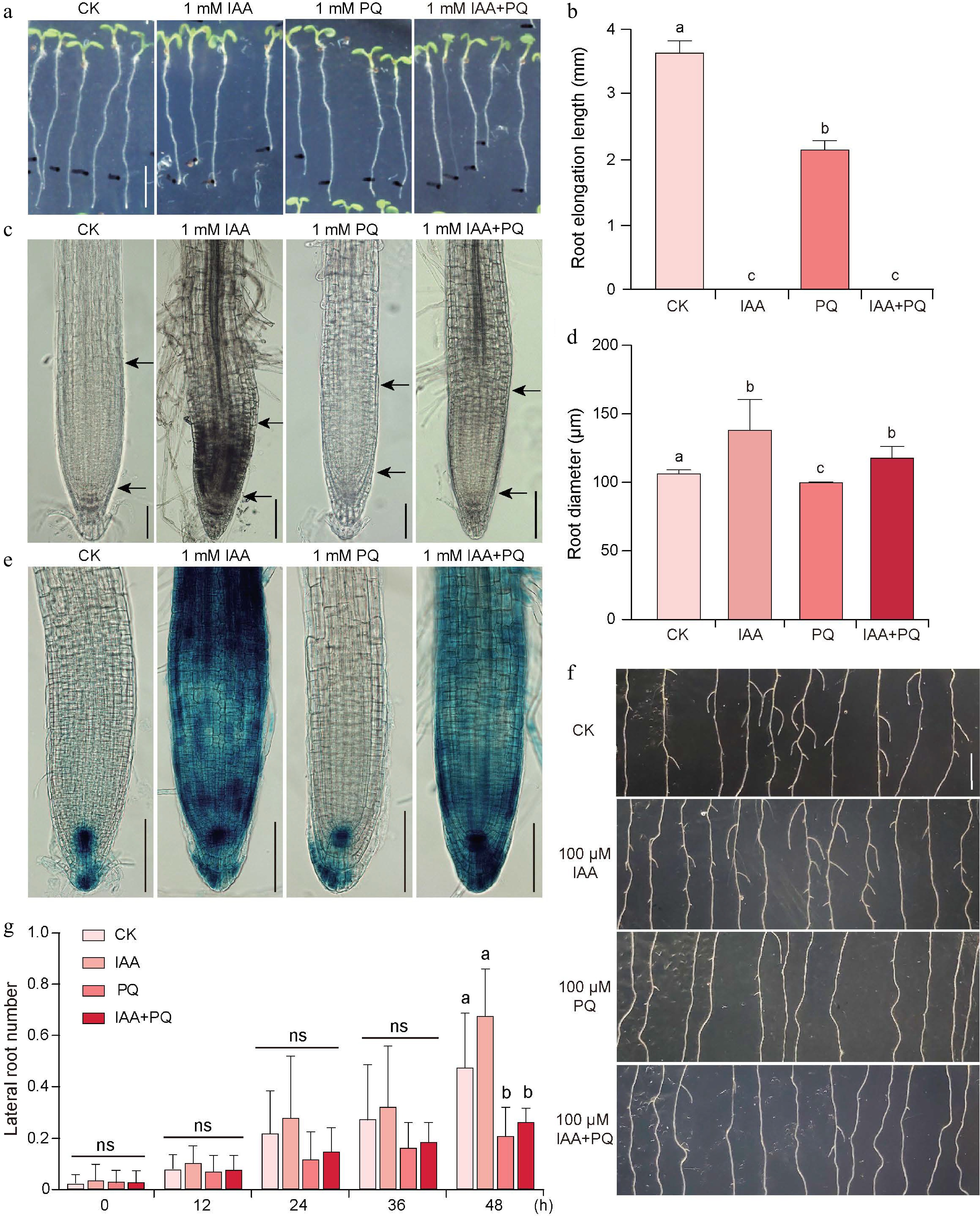
Figure 3.
Effects of IAA and PQ on Arabidopsis roots.
(a), (b) Root elongation of 5-day-old Arabidopsis seedlings 24 h after application of 0.2 μL 1 mM IAA, PQ, and IAA + PQ to the cotyledons. (c) The root tip meristem zone, and (d) the root diameter. (e) GUS staining of the root tips of 5-day-old DR5:GUS seedlings after application of 0.2 μL 1 mM IAA and 1 mM PQ solution to the cotyledons for 24 h. (f), (g) Lateral root growth and lateral root number of 7-day-old Arabidopsis seedlings after application of 0.2 μL 100 μM IAA, PQ, and IAA + PQ to the cotyledons for 48 h. Values in (b), (d), and (f) represent the mean ± SD of three independent experiments. Significant differences were analyzed by Student's t-test. Different lowercase letters indicate significant differences (p < 0.05); ns = not significant. Scale bars = (a) 0.5 cm, (c) 50 μm, (e) 100 μm, and (f) 0.5 cm.Effects of the combined application of IAA and PQ on the shoot of Arabidopsis seedlings
-
We then examined the effects of the combined application of IAA and PQ on the shoot of Arabidopsis. The results showed that the combined application of 100 μM IAA and 100 μM PQ promoted cotyledon bleaching. When treated for 24 h, the cotyledons of seedlings treated with mock and 100 μM IAA alone did not turn white. The cotyledons of seedlings treated with 100 μM PQ alone had a whitening rate of approximately 35.04%. In contrast, the cotyledons of seedlings treated with 100 μM IAA and 100 μM PQ showed a bleaching rate of approximately 58.53%, which is significantly higher than that of PQ alone (p < 0.05) (Fig. 4a & b). After 36 and 48 h of treatment, the cotyledon whitening rate caused by PQ treatment alone and the mixed treatment of IAA and PQ increased. Although the cotyledon whitening rate caused by the mixed treatment of the two was still higher than that of PQ alone, the difference between the two was not significant. Consistent with this, the measurement results for chlorophyll content showed that under the combined treatment of IAA and PQ, the levels of chlorophyll a and total chlorophyll in Arabidopsis cotyledons were lower than when IAA or PQ were applied individually. However, there was no significant difference in the chlorophyll b content (Fig. 4c). These results show that the combined application of IAA and PQ enhances cotyledon bleaching in Arabidopsis seedlings and mainly accelerates the degradation of chlorophyll a. Application of IAA promotes hypocotyl elongation in Arabidopsis seedlings. When treated with a mixture of IAA and PQ, the hypocotyl length of Arabidopsis seedlings was shorter than that of seedlings treated with IAA alone (Fig. 4d & e). The average length of the hypocotyls after the mock treatment for 24 h was about 1.52 mm, while for the IAA treatment, it was about 2.15 mm. For the PQ treatment, the average length was about 1.58 mm and for the combined IAA and PQ treatment, the average hypocotyl length was about 1.68 mm. In addition, treatment with 1 mM IAA caused epinastic growth of cotyledons, whereas cotyledons treated with a combination of 1 mM IAA and PQ did not exhibit an epinastic growth phenotype (Fig. 4d). These results indicate that exogenous application of PQ can inhibit hypocotyl elongation and cotyledon epinastic growth induced by excess auxin.
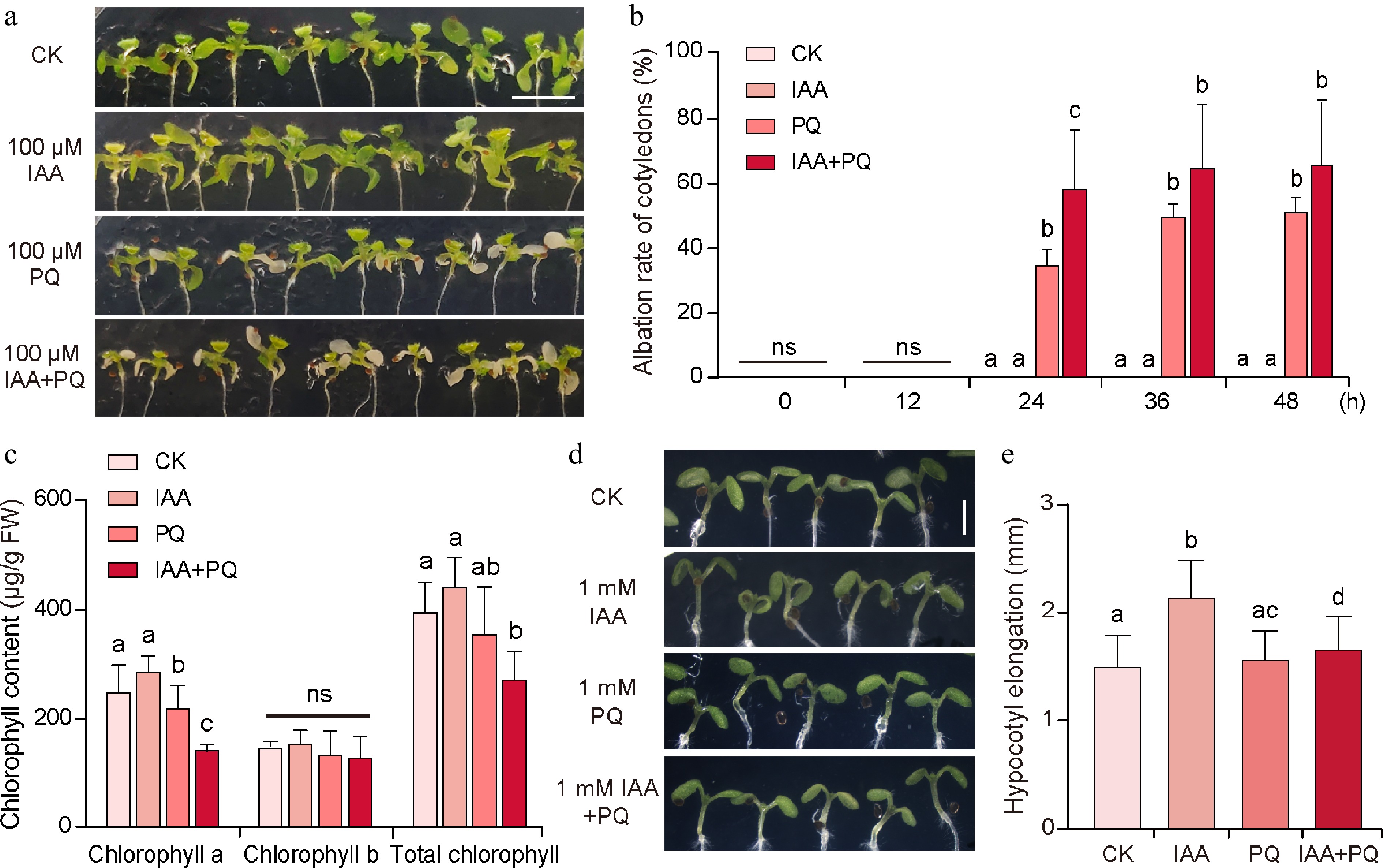
Figure 4.
Effects of combined application of IAA and PQ on the shoot of Arabidopsis seedlings.
(a) Shoot phenotype and (b) cotyledon albation rate of 7-day-old Arabidopsis seedlings after application of 0.2 μL 100 μM IAA, PQ, and IAA + PQ to the cotyledons for 48 h. (c) The levels of chlorophyll a and b and total chlorophyll in the seedlings measured after treatment for 24 h. Shoots of (d) 5-day-old Arabidopsis seedlings and (e) hypocotyl length after 24 h of treatment. Values in (b), (c), and (e) represent the mean ± SD of three independent experiments. Significant differences were analyzed by Student's t-test. Different small letters indicate significant differences (p < 0.05); ns = not significant. Scale bar = (a) 0.5 cm and (d) 2.5 mm.Combined application of IAA and PQ affects the expression of genes involved in PAT and lateral root development
-
Application of IAA and PQ affects the auxin response and the number of lateral roots in Arabidopsis seedlings. To explore the molecular mechanism of PQ antagonizing auxin action, we used RT-qPCR to detect the effects of IAA, PQ, and IAA + PQ treatment on the expression of auxin polar transport genes (including AUX1, LAX1, LAX2, PIN1, PIN2, and PIN4) and lateral root development-related auxin signaling genes (including ARF7, ARF19, IAA19, LBD16, SAUR22, and SAUR24). The results showed that IAA application upregulated the expression of most of these genes, except for PIN2, compared with the mock treatment, while PQ treatment downregulated the expression of most of these genes, except AUX1, PIN2, ARF7, and LBD16 (Fig. 5). Consistently, the expression levels of all genes were lower under the combined treatment of IAA and PQ than under IAA alone, except for LAX1, LAX2, PIN2, ARF19, and LBD16 (Fig. 5). These results indicate that PQ antagonizes the role of auxin by inhibiting the expression of PAT and signaling pathway genes.
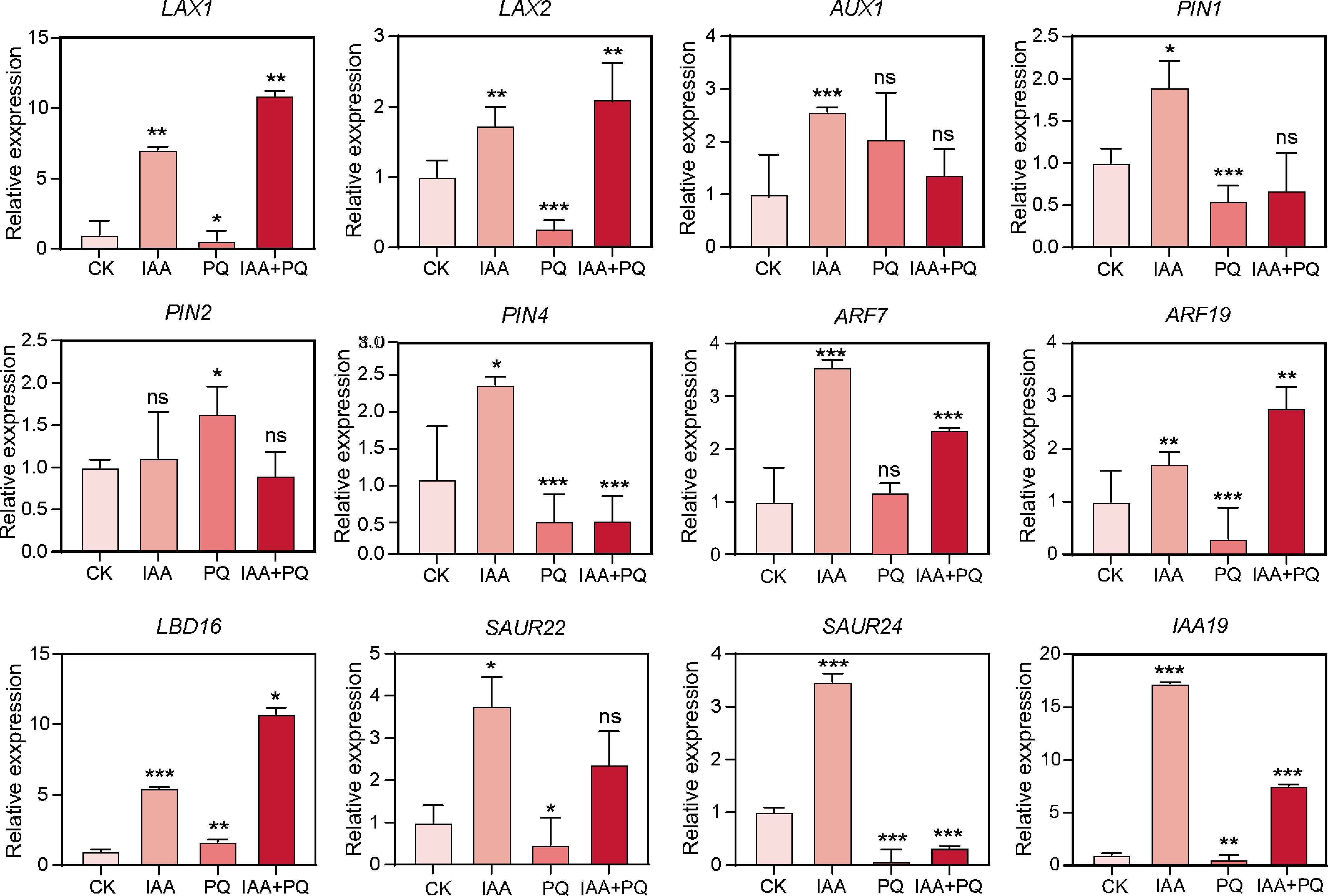
Figure 5.
Effects of combined application of IAA and PQ on the expression of PAT and lateral root formation-related genes.
7-day-old Arabidopsis seedlings were treated with liquid MS medium containing 100 μM IAA, PQ, and IAA plus PQ for 6 h, and RT-qPCR was performed to detect the relative transcript levels of PAT and lateral root formation-related genes. The TIP41 gene was used as an internal reference. -
Auxin plays an essential role in both vegetative and reproductive growth of plants[4,29−31]. Low concentrations of IAA promoted root elongation in maize, while high concentrations inhibited root growth[32]. In addition, excessive accumulation of endogenous auxin in the roots also inhibited root growth. Similarly, this study applied IAA to the cotyledons of Arabidopsis seedlings and found that low concentrations of IAA promoted root elongation, whereas high concentrations of IAA inhibited root elongation. In addition, IAA treatment enhanced root branching. In contrast, cotyledons are less sensitive to IAA than roots and only exhibit an epinastic growth phenotype when treated with very high concentrations of IAA (1 mM). The auxin-responsive reporter DR5:GUS was used to show that the application of IAA to cotyledons can induce an increase in the GUS signal at the root tip. The intensity of the GUS signal increases with the concentration of IAA, indicating that IAA applied to cotyledons can be transported over long distances to the roots. The AUX1/LAX and PIN family proteins mediate PAT and affect lateral root growth[33,34]. The AUX1/LAX family proteins are auxin influx carriers that mediate auxin transport into the cell, whereas the PIN proteins are efflux carriers that transport auxin out of the cell. We found that exogenous application of IAA induced the expression of AUX1, LAX1, LAX2, PIN1, and PIN4, suggesting that auxin has a positive feedback on its transport.
PQ is a biocidal herbicide that primarily targets green tissues with active photosynthesis and has shown beneficial effects in agricultural weed control[18]. Benina et al. found that spraying 25 μM PQ on Arabidopsis rosette leaves for 7 h reduced plant fresh weight and chlorophyll content, increased ROS levels, and the NADP/NADPH ratio[35]. This indicates that the redox balance of the chloroplast is disturbed. Further molecular biological experiments showed that the expression of most ROS-responsive genes was significantly upregulated after 7 h of PQ treatment[35]. In this study, PQ was applied to the cotyledons of Arabidopsis seedlings and was found not only to cause cotyledon bleaching but also to inhibit root elongation. We also found that PQ inhibited the expression of genes involved in auxin transport such as LAX1, LAX2, PIN1, PIN4, and genes of the auxin signaling pathway such as SAUR22, SAUR24, ARF19, and IAA19. Consistently, PQ inhibited IAA induced DR5:GUS expression, root thickening, and branching. These results suggest that PQ may inhibit root growth by interfering with the polar transport and signaling pathways of auxin. However, we cannot rule out that PQ caused a reduction in photosynthesis and limited the nutrient supply for the root growth.
Research on auxin and PQ herbicides has primarily focused on weed resistance, with less attention given to their combined application. The present study investigated the effects of single and combined treatments of IAA and PQ on the growth of Arabidopsis seedlings. It was found that treatment with high concentrations of IAA-induced epinastic growth of Arabidopsis cotyledons, hypocotyl elongation, root thickening, and an increase in the number of lateral roots, whereas PQ had an antagonistic effect. PQ was also found to inhibit the expression of PAT and auxin signaling pathway genes. Studies have shown that increased ROS levels downregulate the expression of genes involved in auxin synthesis and transport[36]. In addition, PQ treatment causes an increase in ROS levels[35]. Perhaps PQ downregulates the expression of auxin-related genes and antagonizes the effect of auxin via the induced ROS.
-
In this study, we observed that the cotyledon bleaching rate of Arabidopsis seedlings under the combined treatment of IAA and PQ was significantly higher than with PQ treatment alone. This suggests that the simultaneous application of IAA and PQ may result in an enhanced herbicidal effect. These findings offer a theoretical foundation for further exploration of IAA and PQ combination treatments and present a novel approach for herbicide application in agricultural practices.
This research was funded by the National Natural Science Foundation of China (32070281 and 32370271 to Shuzhen Men).
-
The authors confirm contribution to the paper as follows: project conception and research plans: Men S, Bian Q, Zhang YM; experiments design: Men S; experiments conducted (leading): Li Z; Experiments conducted (assisting): Song J, Li S, Arif M; data analysis: Li Z, Song J, Li S, Men S; draft manuscript preparation: Men S, Song J, Shao D, Li S. All authors reviewed the results and approved the final version of the manuscript.
-
All relevant data can be found within the manuscript and its supporting materials. All other data are available from the corresponding author upon reasonable request.
-
The authors declare that they have no conflict of interest.
-
#Authors contributed equally: Jia Song, Zitong Li
- Supplementary Table S1 List of primers used in this research.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of Yunnan Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Song J, Li Z, Li S, Tan C, Shao D, et al. 2025. The combination of auxin and paraquat produces an additive effect on leaf bleaching but an antagonistic effect on root branching. Agrobiodiversity 2(1): 1−8 doi: 10.48130/abd-0025-0001
The combination of auxin and paraquat produces an additive effect on leaf bleaching but an antagonistic effect on root branching
- Received: 30 September 2024
- Revised: 27 November 2024
- Accepted: 31 December 2024
- Published online: 22 January 2025
Abstract: Auxin is a pivotal hormone that regulates plant growth and development; however, excessive levels can inhibit growth, making auxin analogs valuable as herbicides. Paraquat, a synthetic organic herbicide, has been widely used in agricultural weed control. While previous studies have largely examined the individual effects of auxinic herbicides or paraquat on weeds, the combined application of auxin and paraquat remains unexplored. This study investigates the combined effects of auxin and paraquat on the growth of Arabidopsis thaliana seedlings. The results reveal that paraquat and auxin have antagonistic effects on hypocotyl elongation, root thickening, and lateral root formation in Arabidopsis seedlings, while they have additive effects on cotyledon bleaching. Further analyses indicate that paraquat inhibits the expression of polar auxin transport and auxin signaling pathway genes. These findings provide a basis for elucidating the interaction mechanism between auxin and paraquat, which may help to optimize weed management strategies in agriculture.
-
Key words:
- Auxin /
- Paraquat /
- Arabidopsis /
- Herbicide













