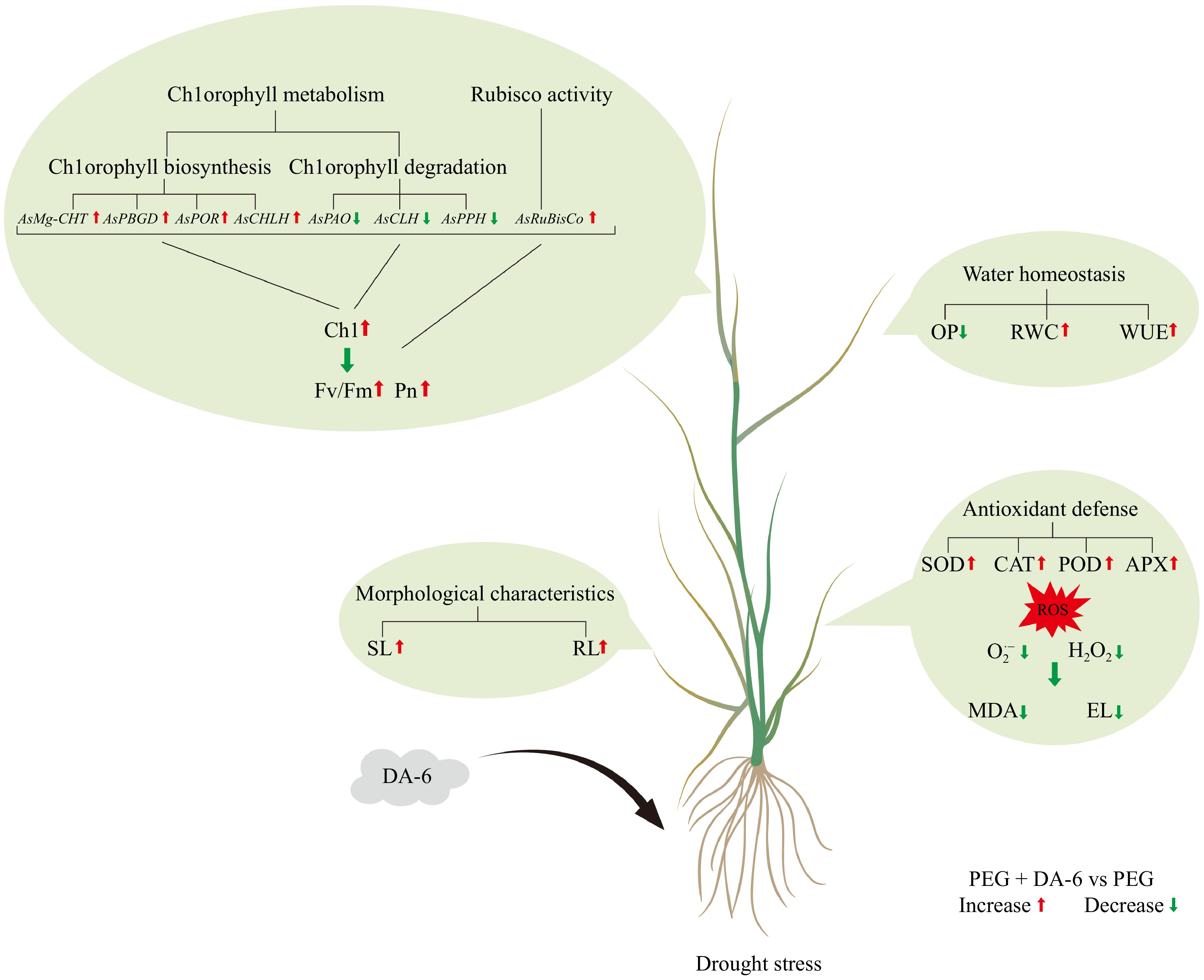
-
Drought is the most drastic environmental hazard hindering plant growth and development, specifically under arid and semi-arid conditions[1,2]. Plants experience various interlinked disturbances of physiological and biochemical functions including stomatal closure, reduced intracellular carbon dioxide assimilation and water use efficiency (WUE), chlorophyll (Chl) degradation, and impaired photosystems associated with decreased crop growth and quality under water stress[1,3,4]. Hence, various approaches have been carried out to mitigate the adverse effects of water shortage when plants suffer from drought stress. One of the most effective, cheap, and eco-friendly approach is the application of plant growth regulators (PGRs)[5,6]. Diethyl aminoethyl hexanoate (DA-6) is a new synthetic PGR exhibiting a similar function to phytokinin and has extensively been applied worldwide to improve yield and stress adaptability of many commercial crops under normal and stressful conditions[7−9]. It has been found that exogenous supplementation of DA-6 strengthened the defense system in plants under chilling stress, high salt, and heavy metal toxicity[10−12]. The DA-6 pretreatment could also significantly ameliorate drought tolerance of white clover (Trifolium repens) through regulating antioxidant defense system, endogenous phytohormone content, photosynthetic rate, and metabolic homeostasis[3,6,13]. Our earlier study reported that foliar application of DA-6 effectively alleviated heat-induced Chl loss, osmotic imbalance, cell membrane damage, and summer bentgrass decline[14]. However, the effect and mechanism of DA-6 associated with drought tolerance of creeping bentgrass (Agrostis stolonifera) remain uninvestigated to date.
Water stress induces a significant decrease in photosynthesis by disrupting Chl metabolism and decreasing rubisco activity[15,16]. Drought stress enhanced enzyme activities and gene expression levels of many key Chl degradation enzymes such as chlorophyllase (CLH), Chl-degrading peroxidase (Chl-PRX), and pheophytinase (PPH) in creeping bentgrass, contributing to stress-stimulated leaf senescence[17]. In addition, exogenous application of 5-aminolevulinic acid significantly mitigated the drought-induced Chl loss via up-regulation of Chl-anabolic genes including PBGD (porphobilinogen deaminase), CHLH (magnesium chetalase H-subunit), and POR (protochlorophyllide oxidoreductase) in grapevine (Vitis labruscana × Vitis vinifera)[18]. Massive production of reactive oxygen species (ROS) including hydroxyl ions (OH−), superoxide radicals (O2·−), and hydrogen peroxide (H2O2) are liable to oxidize chloroplasts, cell membranes, other organelles, and biomacromolecules[19]. Stress-triggered production of ROS is rooted in damaged energy dissipation in the process of Chl fluorescence quenching and electron transport in the photosynthetic electron transfer chain[20]. Therefore, there is a close association between ROS metabolism and photo-oxidation in photosystem II[21]. To counter the drastic effects of ROS, plants have evolved a natural antioxidant defense comprising enzymatic systems such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) as well as non-enzymatic antioxidants including ascorbic acid, glutathione, polyphenols, carotenoids, etc.[22]. It has been found that the DA-6-induced plant tolerance to drought stress, salinity, or high-temperature stress could be associated with enhanced antioxidant defense systems[12−14,23]. However, the DA-6-regulated ROS homeostasis and Chl metabolism contributing to leaf senescence need further investigation in creeping bentgrass under drought stress.
Creeping bentgrass is an important perennial cool-season gramineous turfgrass with a soft, fine texture, hence being widely utilized as an excellent turf in the sports industry worldwide[24]. However, due to its poor tolerance to water scarcity, creeping bentgrass often demands a constant water supply to sustain growth and development, which ultimately results in huge water consumption and management issues[25]. Therefore, the enrichment of drought tolerance is crucial to improve its production, turf quality, and maintenance management. The current study aimed to reveal DA-6-regulated drought tolerance related to alterations in photosynthetic functions, Chl metabolism, osmotic adjustment (OA), and antioxidant defense in creeping bentgrass.
-
Before sowing, seeds of creeping bentgrass cultivar Penncross were sterilized with 75% ethanol for 5 min and washed three times with deionized water. Seeds (0.37 g) were then sown in plastic containers (9 cm depth, 24 cm length, and 18 cm breadth) comprising sterilized quartz sand and deionized water under controlled growth chamber conditions (23/19 °C (day/night), 65% relative humidity, and 650 μmol photon m−2·s–1 PAR). Seeds were first germinated in deionized water for 7 d, and then half-strength Hoagland's solution[26] was utilized as a nutrient source for the next 23 d of cultivation. Plants with similar sizes were selected for hydroponic cultivation. For DA-6 pretreatment, plants were grown in Hoagland's solution containing 0.4 mmol/L DA-6 for 3 d, whereas the unpretreated plants were grown only in Hoagland's solution without the DA-6 for three consecutive days. After this, the DA-6-pretreated and unpretreated plants were exposed to well-watered conditions or drought stress induced by polyethylene glycol (PEG-6000, −0.52 MPa) which was dissolved in Hoagland's solution for 9 d. All solutions were refreshed daily. Four different treatments (four biological replicates for each treatment) were set for this experiment including well-watered control check (CK), well-watered control pretreated by the DA-6 (CK + DA-6), PEG-induced drought stress without the DA-6 pretreatment (PEG), and PEG-induced drought stress with the DA-6 pretreatment (PEG + DA-6). The optimum dose of DA-6 (0.4 mmol·L–1) with the most promising effect on drought tolerance in terms of phenotypic changes was chosen via a preliminary experiment. All treatments were arranged by a completely randomized design. Samples were taken after 9 d of drought stress for various morphological, physiological, and biochemical parameters as well as the analysis of gene expression.
Estimation of growth parameter and leaf water status
-
The shoot length (SL) and root length (RL) of each plant were measured using a ruler, and 2−3 plants were selected randomly from each replication of each treatment. A total of 10 plants were used to detect the SL and RL of each treatment. For relative water content (RWC), the formula RWC (%) = [(FW − DW)/(SW − DW)] × 100 was used, and FW, SW, and DW indicated fresh weight, saturated weight, and dry weight, respectively. Fresh leaves (0.1 g) were sampled and FW was weighed immediately. These leaves were then submerged in deionized water for 1 d to detect SW. DW was weighed after oven drying at 75°C for 3 d[27]. For the measurement of osmotic potential (OP), fresh samples were collected and soaked in deionized water for 8 h. After being blotted to eliminate surface water, saps in leaf samples were expressed. The osmolality (mmol·kg−1) of leaf sap was detected using an osmometer (Wescor), and then OP (MPa) was estimated based on the OP (MPa) = [0.001] × [2.58] × [osmolality][28].
Measurement of chlorophyll content and photosynthetic function
-
To measure the Chl content, 0.1 g of samples were taken and submerged in dimethyl sulphoxide (10 mL) for 2 d under dark conditions. A 200 μL of leaf extract was measured at 663 and 645 nm spectrophotometrically. Later, the contents of Chl a, Chl b, and total Chl were evaluated[29]. Leaf Fv/Fm was estimated with a chlorophyll fluorescence meter (Pocket PEA). Leaves were placed in a dark environment with attached clips for 30 min, and then the Fv/Fm ratio was noted using the chlorophyll fluorescence meter. The WUE and net photosynthetic rate (Pn) were measured with portable photosynthesis apparatus (CIRAS-3) that supplied 800 μmol photon m−2 red and blue light as well as 400 μL·L−1 CO2 in the leaf chamber. A single layer of leaf was placed in the leaf chamber and the estimation of WUE and Pn was performed at 10:30 am.
Measurement of oxidative damage and antioxidant enzyme activity
-
To determine electrolyte leakage (EL), leaf samples (0.1 g) were dipped in 45 mL of distilled water at 4 °C for 1 d. The initial conductivity (Ci) was recorded by using a conductivity meter (DDS-307A). The samples were then autoclaved at 105 °C and the final conductivity (Cf) was detected. The EL was estimated in percent counting method, following the equation EL [%] = Ci/Cf × 100[30]. Assay methods of Elstner & Heupel[31], Velikova et al.[32], and Dhindsa et al.[33] were utilized for the measurement of O2·−, H2O2, and malondialdehyde (MDA) contents, respectively. The reagents and procedures have been clearly mentioned in our previous study[13]. For antioxidant enzymes activities, leaf samples (0.1 g) were put into 4 mL of cold phosphate buffer (50 mM, pH 7.8) and ground mechanically at 4 °C. After the homogenate was centrifuged at 12,000 g for 30 min, the supernatant was collected for further analysis. SOD activity was determined by observing the reduction rate of p-Nitro-Blue tetrazolium chloride at 560 nm spectrophotometrically[34]. The CAT, POD, and APX were also spectrophotometrically measured by noting the variation in absorbance value at a wavelength of 240, 470, and 290 nm, respectively[35,36]. The protein content was estimated using the protocol illustrated by Bradford[37].
Total RNA extraction and qRT-PCR analysis
-
To examine the impact of DA-6 on transcript levels of senescence-associated genes and those genes associated with Chl metabolism and rubisco, a qRT-PCR was used. For total RNA extraction, fresh leaf samples were extracted with RNeasy Mini Kit (Qiagen) following the manufacturer's protocols. Later, the RNA was reverse-transcribed to cDNA with a Revert Aid First Strand cDNA Synthesis Kit (Fermentas). Primer sequences of all genes including reference gene β-actin are presented in Table 1. The PCR conditions (iCycler iQ qRT-PCR detection system with SYBR Green Supermix, Bio-Rad, USA) were as follows: 5 min at 94 °C, denaturation at 95 °C for 30 s (40 repeats), 45 s at 55−58 °C (annealing), and extension from 60 to 95 °C. The transcript level of all genes was computed by using the formula 2−ΔΔCᴛ[38].
Table 1. Primer sequences and relative information of analyzed genes in creeping bentgrass.
Target gene Forward primer (5'-3') Reverse primer (5'-3') Tm (°C) β-actin CCTTTTCCAGCCATCTTTCA GAGGTCCTTCCTGATATCCA 58 AsMg-CHT ACAACGGTTAGGTCATTGGTCG TTATTACTCGGTCTCGCACTTCAA 58 AsPBGD TAGCGCTGCGGATTAGAACT GAAGGATAACGAACCGCTGA 55 AsPOR GCGTCTACTGGAGCTGGAAC GTCACTTCATGCAGGTCACG 58 AsRuBisCo GGCTTCAACAAACGCTCTATCC CTTTAGCAGCGGCTTTAACCAT 58 AsPAO TCATATCAGTTGCTGCAATAGGGA GCGAAAGGCGTGGTTGTAGTC 57 Asl20 GGGTAGACGGCAACGATACT TACTTGGTTGAATCGTCGGA 58 Ash36 TGGGAATGTGTTCAGGGTAA TCACCTCGATGAGGTAGTCG 58 AsCHLH CATCAGGGCGGATAGAGAGA TCTGCCACAATCAGCTTCAG 56 AsCLH GGTCGCATTCCTGAGGTCTA ATCATATTCAACCGGGTCCA 58 AsPPH GAATGTCATTGCCGTCTGAA CAATGAAATGCTGGACCTGA 55 Statistical analysis
-
Data was assessed with two-way ANOVA and Tukey's test by using statistix 8.1 (version, 8.1. Statistix, USA) at p < 0.05. All figures were generated using GraphPad Prism 8.3.0 (538).
-
SL and RL were significantly promoted by the DA-6 under normal conditions (Fig. 1a & b). Drought stress reduced SL and RL, but the DA-6 pretreatment effectively mitigated drought-induced decline in SL and RL (Fig. 1a & b). In response to drought stress, DA-6-pretreated plants maintained a 27% upsurge in RWC than unpretreated plants (Fig. 2a). The OP decreased in all drought-stressed plants compared with well-watered plants. Plants with DA-6 pretreatment demonstrated a 47% lower OP in contrast with untreated plants under water-limited conditions (Fig. 2b). Moreover, exogenous supplementation of DA-6 markedly alleviated the drought-triggered decline in WUE by 36% (Fig. 2c).
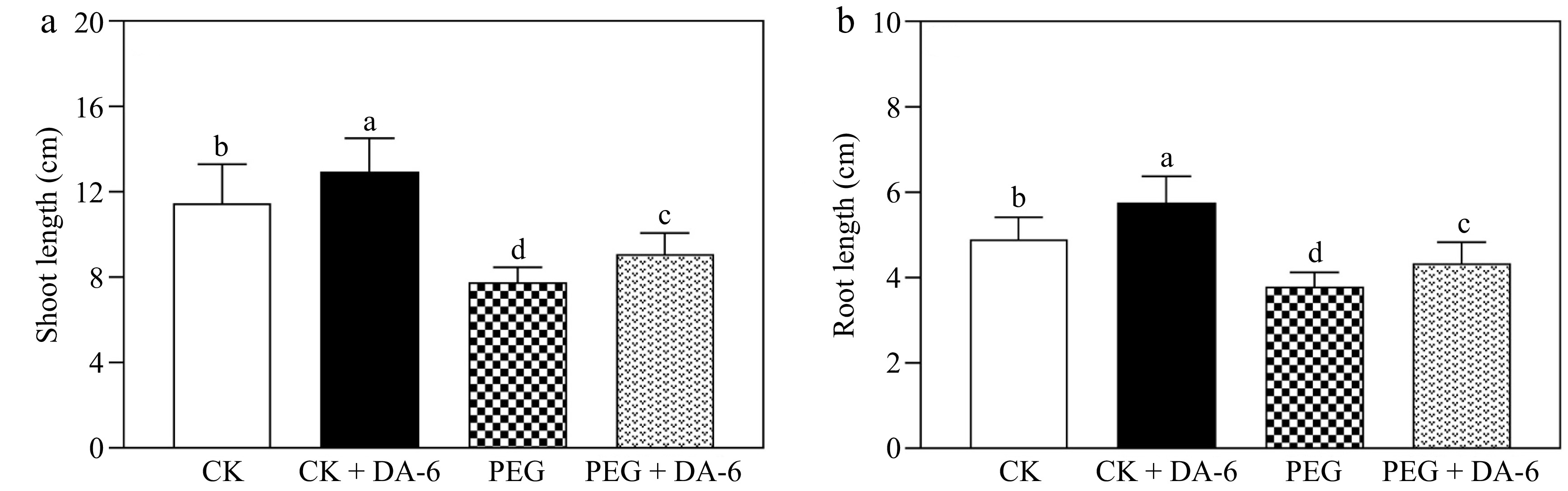
Figure 1.
Impacts of DA-6 pretreatment on changes in (a) shoot length (SL), and (b) root length (RL) of creeping bentgrass under well-watered and drought conditions. Vertical bars indicate standard error (n = 10, and p ≤ 0.05). Different letters above columns represent significant differences among treatments. CK, control check; CK + DA-6, well-watered plants supplemented with DA-6; PEG, drought stress; PEG + DA-6, drought-stressed plants supplemented with DA-6.
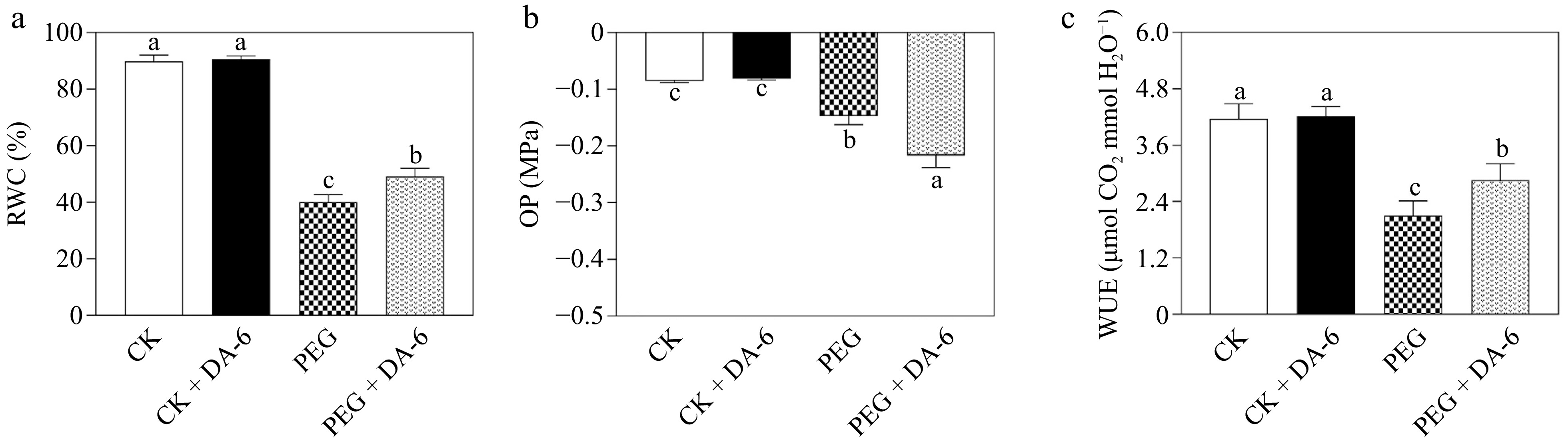
Figure 2.
Impacts of DA-6 pretreatment on changes in (a) relative water content (RWC), (b) osmotic potential (OP), and (c) water use efficiency (WUE) of creeping bentgrass under well-watered and drought conditions. Vertical bars indicate standard error values (n = 4, and p ≤ 0.05). Different letters above or below columns represent significant differences among treatments. CK, control check; CK + DA-6, well-watered plants supplemented with DA-6; PEG, drought stress; PEG + DA-6, drought-stressed plants supplemented with DA-6.
Impact of DA-6 on chlorophyll metabolism and photosynthesis under normal conditions and drought stress
-
Plants with and without DA-6 pretreatment showed no significant differences in the total Chl, Chl a, Chl b, Chl a/b, Fv/Fm, and Pn under normal conditions (Fig. 3a−f). The total Chl, Chl a, and Chl b declined significantly in response to PEG-induced water scarcity (Fig. 3a−c). Exogenous application of DA-6 significantly mitigated declines in the total Chl, Chl a, or Chl b induced by drought stress (Fig. 3a−c). Drought stress and the application of DA-6 did not significantly influence the Chl a/b (Fig. 3d). In addition, drought stress caused significant decline in Fv/Fm and Pn, but DA-6-treated plants maintained significantly higher Fv/Fm and Pn compared with untreated plants under drought stress as shown in Fig. 3e and f.
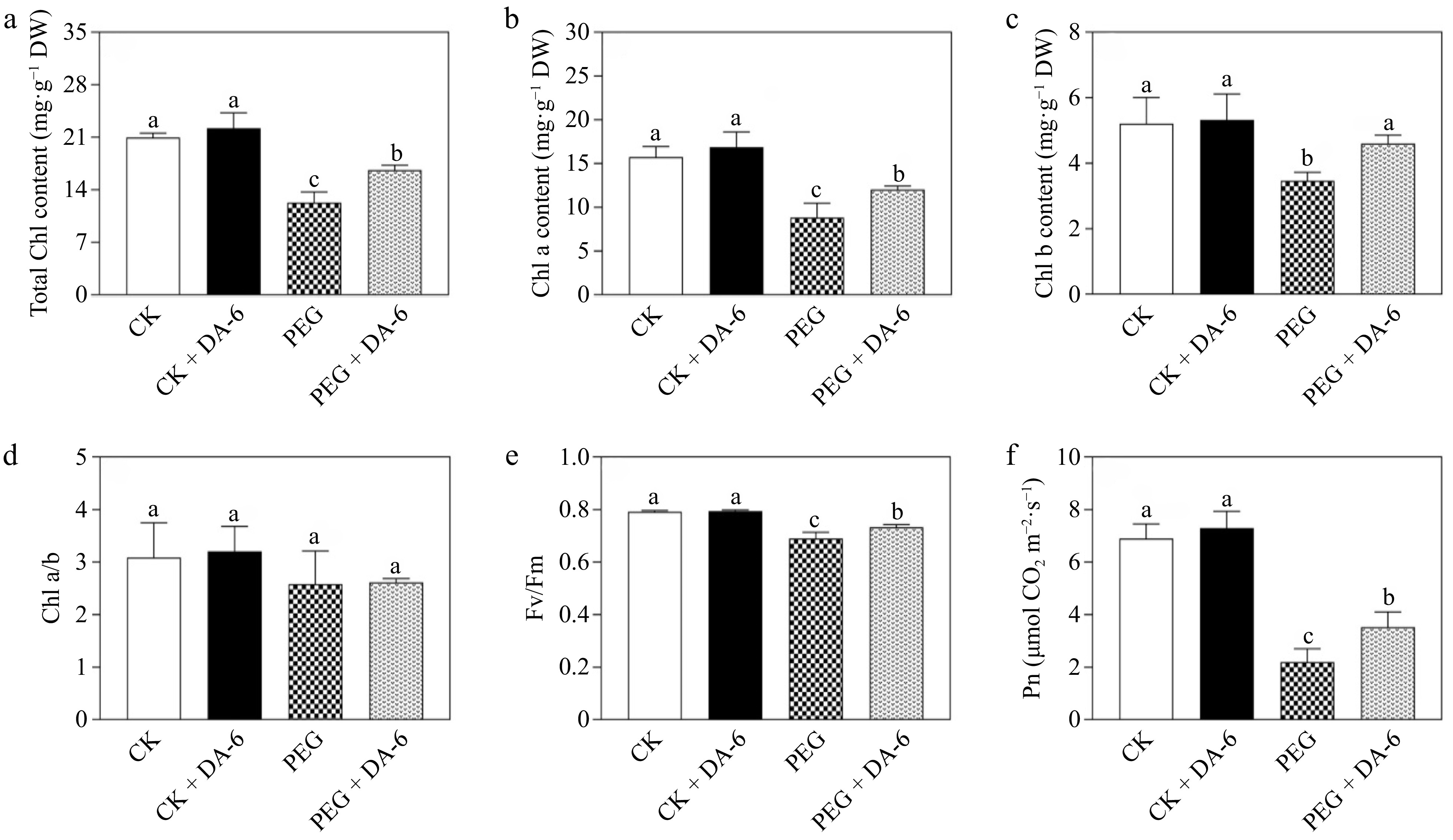
Figure 3.
Impacts of DA-6 pretreatment on changes in (a) total chlorophyll (Chl) content, (b) Chl a content, (c) Chl b content, (d) Chl a/b ratio, (e) photochemical efficiency of PSII (Fv/Fm), and (f) net photosynthetic rate (Pn) of creeping bentgrass under well-watered and drought conditions. Vertical bars indicate standard error (n = 4, and p ≤ 0.05). Different letters above columns represent significant differences among treatments. CK, control check; CK + DA-6, well-watered plants supplemented with DA-6; PEG, drought stress; PEG + DA-6, drought-stressed plants supplemented with DA-6.
In terms of genes encoding rubisco and enzymes involved in Chl metabolism, the DA-6 application significantly up-regulated the transcript levels of AsCHLH, but AsMg-CHT (Asmagnesium-chelatase), AsPBGD, AsPOR, AsRuBisCo, AsPAO, AsCLH, and AsPPH remained unaffected under normal conditions (Fig. 4). The transcript levels of AsMg-CHT, AsPBGD, AsPOR, AsCHLH, and AsRuBisCo were down-regulated, whereas AsPAO, AsCLH, and AsPPH were up-regulated by drought stress. The DA-6-pretreated plants exhibited 2.7, 2.4, 1.9, 3.6, or 6.3 times higher expression level of AsMg-CHT, AsPBGD, AsPOR, AsCHLH, or AsRuBisCo compared with untreated plants when they were exposed to drought stress, respectively (Fig. 4). In addition, the transcript level of AsPAO, AsCLH, or AsPPH was 1.53, 1.50, or 1.32 times higher in drought-stressed plants without the DA-6 application than that in drought-stressed plants with the DA-6 pretreatment. Exogenous application of DA-6 demonstrated no significant effect on transcript levels of senescence-associated genes (Asl20 and Ash36) under normal conditions (Fig. 4). When plants were subjected to drought stress, DA-6-pretreated plants showed a 24% or 31% lower transcript level of Asl20 or Ash36 than untreated plants, respectively (Fig. 4).
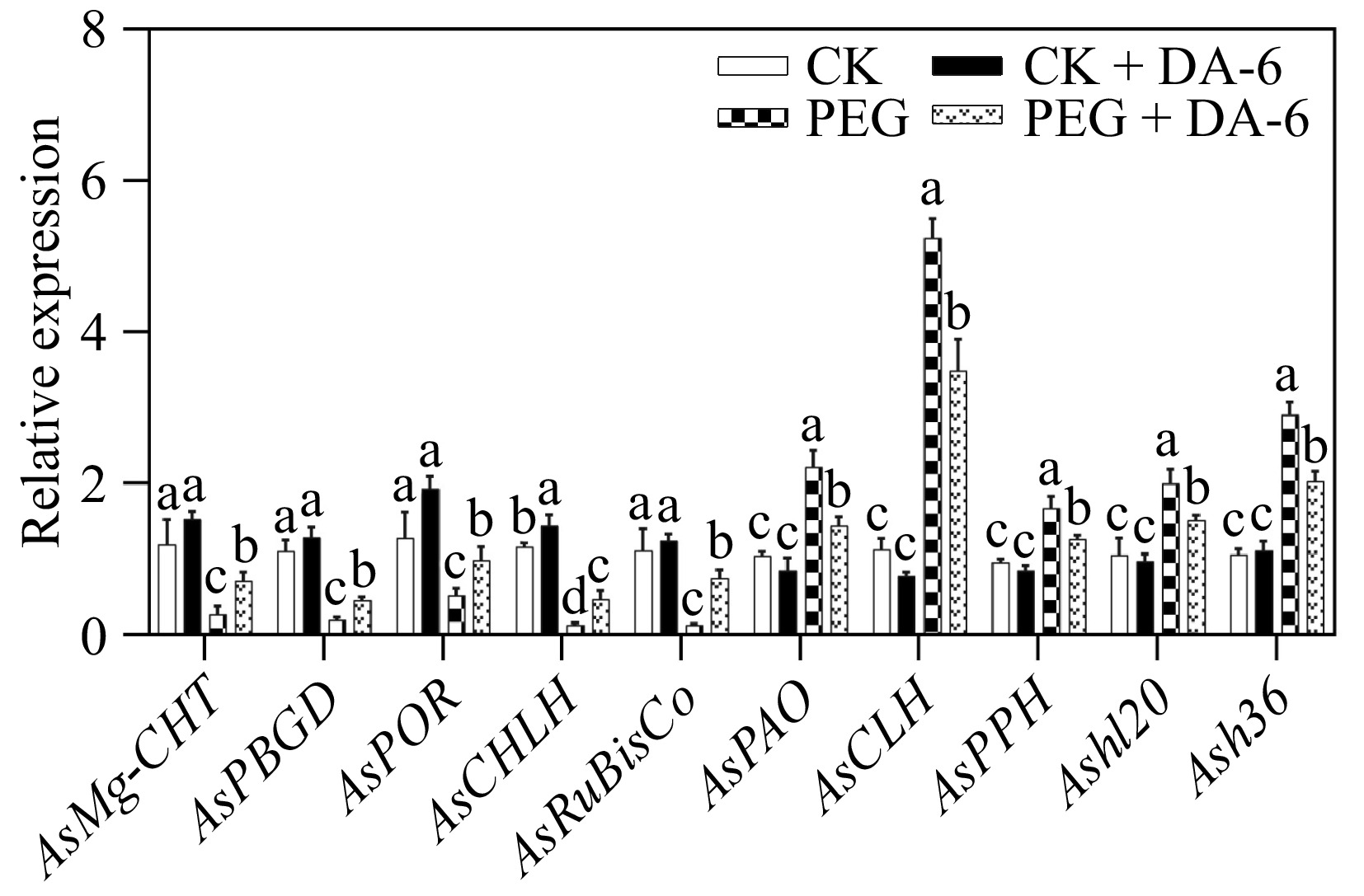
Figure 4.
Impacts of DA-6 pretreatment on relative expression levels of AsMg-CHT, AsPBGD, AsPOR, AsCHLH, AsRuBisCo, AsPAO, AsCLH, AsPPH, Asl20, and Ash36 in leaves of creeping bentgrass under well-watered and drought conditions. Vertical bars indicate standard error (n = 4, and p ≤ 0.05). Different letters above columns represent significant differences among treatments. CK, control check; CK + DA-6, well-watered plants supplemented with DA-6; PEG, drought stress; PEG + DA-6, drought-stressed plants supplemented with DA-6.
Impact of DA-6 on oxidative injury and antioxidant enzyme activity under normal conditions and drought stress
-
The EL, O2·−, H2O2, and MDA contents were not affected by the DA-6 under well-watered conditions (Fig. 5a−d). Drought stress induced significant upsurges in EL, O2·−, H2O2, and MDA contents, but DA-6-treated plants exhibited an 18%, 13%, 29%, or 15% decrease in EL, O2·−, H2O2, or MDA content than plants without DA-6 under drought stress, respectively (Fig. 5a−d). Water deficit caused a significant decline in SOD activity, but promoted POD, CAT, and APX activities in all plants (Fig. 6a−d). The DA-6 pretreatment efficiently mitigated the decline in SOD activity and also further promoted CAT, POD, and APX activities under drought stress (Fig. 6a−d). SOD, CAT, POD, or APX activity increased by 34%, 25%, 17%, or 41% in DA-6-treated plants compared with those plants without DA-6 pretreatment under water-limited conditions, respectively (Fig. 6a−d). Figure 7 shows an integrated diagram depicting the promising role of DA-6 in drought tolerance.

Figure 5.
Impacts of DA-6 pretreatment on changes in (a) electrolyte leakage (EL), (b) superoxide anion (O2·−), (c) hydrogen peroxide (H2O2), and (d) malondialdehyde (MDA) content of creeping bentgrass under well-watered and drought conditions. Vertical bars indicate standard error (n = 4, and p ≤ 0.05). Different letters above columns represent significant differences among treatments. CK, control check; CK + DA-6, well-watered plants supplemented with DA-6; PEG, drought stress; PEG + DA-6, drought-stressed plants supplemented with DA-6.
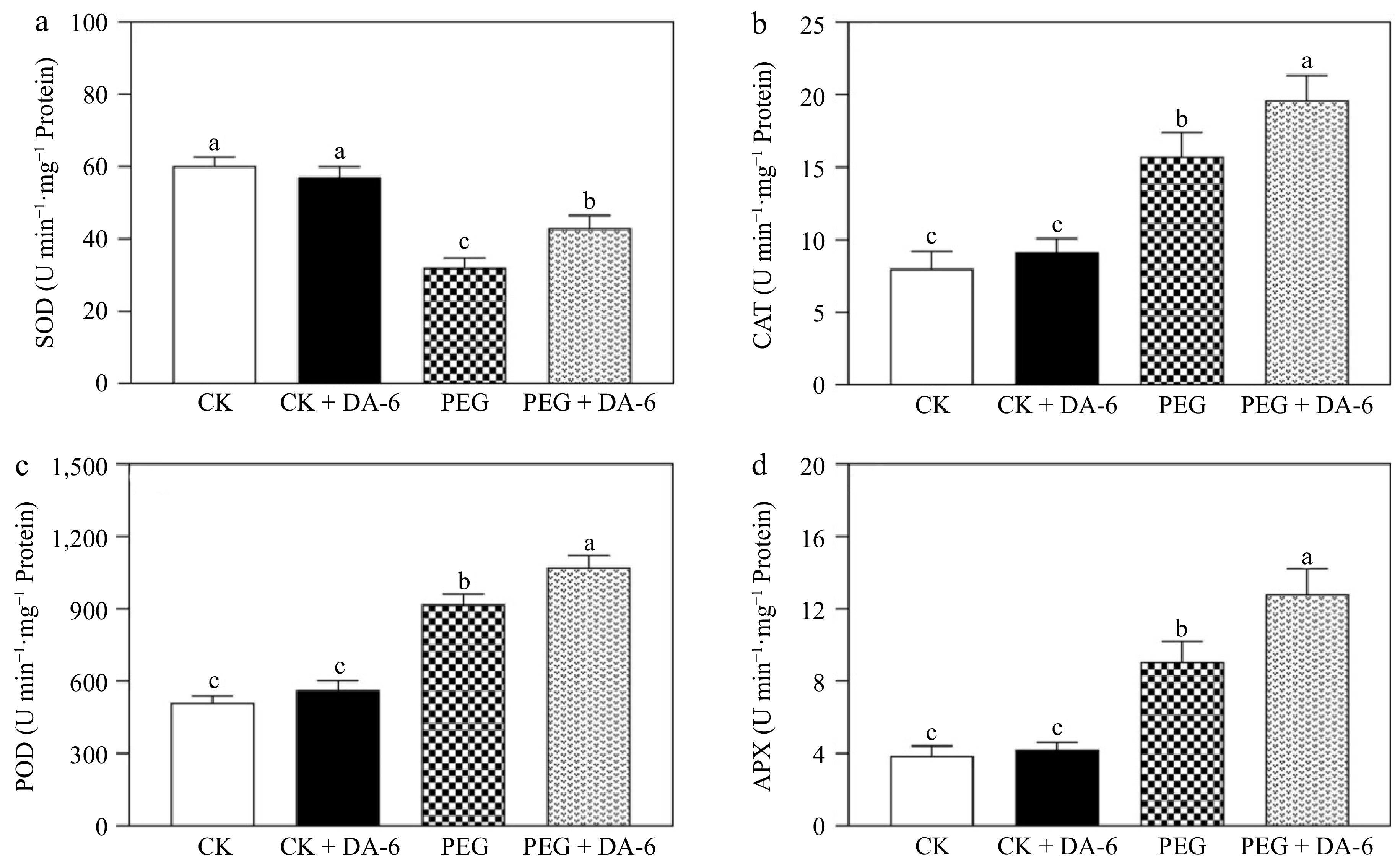
Figure 6.
Impacts of DA-6 pretreatment on changes in (a) superoxide dismutase (SOD), (b) catalase (CAT), (c) peroxidase (POD), and (d) ascorbate peroxidase (APX) activities of creeping bentgrass under well-watered and drought conditions. Vertical bars indicate standard error (n = 4, and p ≤ 0.05). Different letters above columns represent significant differences among treatments. CK, control check; CK + DA-6, well-watered plants supplemented with DA-6; PEG, drought stress; PEG + DA-6, drought-stressed plants supplemented with DA-6.
-
Drought limits the water supply and gas exchange of photosynthetic and respiratory processes, thereby hindering regular plant growth and development[2,3]. Plants normalize water balance in intricate ways, such as water absorption and transport, OA, and WUE when subjected to drought stress[39]. It is a well-known fact that the maintenance of water balance through enhancing OA and WUE is of primary importance for the survival of creeping bentgrass under drought stress[40]. Our previous study found that foliar spray of DA-6 effectively improved OA in white clover associated with the accumulation of multiple organic metabolites under PEG-stimulated water stress[6]. Moreover, exogenous DA-6 also helped to mitigate drought-induced decline in WUE in drought-tolerant and -sensitive white clover cultivars[3]. These studies are in accordance with our current study which demonstrated that drought stress negatively affected the leaf water status as reflected by reduced RWC and WUE in leaves of all creeping bentgrass plants, but DA-6-treated plants maintained significantly higher RWC and WUE as well as lower OP than untreated plants under water-deficient conditions. In addition, drought stress also significantly reduced the shoot and root lengths of creeping bentgrass plants, demonstrating growth restriction. However, the DA-6 pretreatment not only markedly alleviated drought-triggered decreases in shoot and root lengths, but also significantly promoted shoot and root lengths under optimal conditions, suggesting the promising role of DA-6 in plant growth. As a necessary condition for photosynthesis, better water status is propitious to achieve stable photosynthetic carbon assimilation, which provides necessary energy for plant growth[41]. The present results indicated that the DA-6-mediated drought tolerance might be related to better OA and WUE as well as higher photosynthesis in favor of growth of creeping bentgrass under drought stress.
Chl is a crucial pigment for photosynthesis, facilitating the absorption and conversion of light energy[42]. Drought stress induces significant degradation of photosynthetic pigments, hence drastically impairing photosynthesis and plant growth[2]. It has been reported that foliar application of DA-6 ameliorated stress tolerance of white clover via maintenance of better Chl content, photochemical efficiency, and Pn under drought stress[3]. Moreover, accelerated Chl loss and damage to the photosystem induced by salt stress in Cassia obtusifolia could be significantly alleviated by the exogenous application of DA-6[12]. Similar findings were found in the present study which showed that drought stress inhibited Chl biosynthesis by reducing transcript levels of Chl-biosynthetic genes including AsPBGD, AsMg-CHT, AsPOR, and AsCHLH. For Chl biosynthesis, four porphobilinogen subunits are enzymatically combined to form a tetrapyrrole ring by the PBGD, whereas Mg-CHT and CHLH are involved in the insertion of Mg2+ in the tetrapyrrole ring. Subsequently, POR catalyzes the conversion of protochlorophyllide to chlorophyllide[43,44]. Previous studies have reported that drought or abnormal temperature stresses led to significant declines in Mg-CHT, PBGD, POR, and CHLH activities in rice (Oryza sativa), cucumber (Cucumis sativus), and wheat (Triticum aestivum), thus inhibiting Chl biosynthesis[45,46]. An exogenous supply of mannose could significantly alleviate drought-induced Chl loss by maintaining higher expression levels of TrMg-CHT and TrPOR in leaves of white clover[15]. A low dose of Na+-activated expressions of TrPBGD, TrPOR, TrMg-CHT, and TrRubisCo in white clover contributes to better Chl biosynthesis and carbon assimilation in response to a prolonged period of drought stress[47]. In our present study, the DA-6 pretreatment significantly lessened the drought-triggered decline in Chl content, associated with the fact that the DA-6 significantly enhanced expressions of AsMg-CHT, AsPBGD, AsPOR, and AsCHLH. In addition, the DA-6-induced up-regulation of AsRubisCo could be related to better CO2 assimilation in creeping bentgrass under drought stress.
Chl catabolism is positively linked with leaf senescence[48]. CLH is the initial Chl-catabolic enzyme that performs hydrolytic catalysis of ester bonds to produce chlorophyllide and phytol[49]. PPH is responsible for catalyzing the elimination of the phytol chain from pheophytin[50]. Moreover, PAO is involved in the partitioning of the porphyrin ring in the Chl degradation pathway[51,52]. Leaf senescence stimulated by submergence stress was linked with enhanced PPH activity and PPH transcript level in perennial ryegrass (Lolium perenne)[53]. The inhibition of CLH and PPH activities by exogenous application of glutamate or morphactin could significantly mitigate leaf senescence of creeping bentgrass under high temperatures[54,55]. Drought-induced leaf senescence was linked with significant upregulations of TrPAO and TrCLH, and inhibitory expressions of these genes by exogenous application of different PGRs could effectively mitigate leaf senescence under drought stress[15,47]. In creeping bentgrass, Chl-degradation genes (AsCLH and AsPPH) and their enzyme activities were significantly down-regulated by the exogenous application of melatonin contributing to a slowdown in leaf senescence[17]. In addition, the study by Sharma et al.[56] found that elevated CLH transcript levels significantly decreased functional components of photosynthesis in grafted Carya cathayensis plants under drought stress. These results indicated that DA-6-mediated drought tolerance and leaf senescence of creeping bentgrass could be related to higher expression levels of Chl-biosynthesis genes (AsPBGD, AsMg-CHT, AsPOR, and AsCHLH) and lower transcript levels of Chl-degradation (AsCHL, AsPPH, and AsPAO) and senescence-associated genes (Asl20 and Ash36) contributing to stable Chl metabolism, photosynthetic function, and growth under drought stress.
Water deficit promotes an immense amount of ROS which are responsible for oxidative damage to chloroplasts and cell membrane systems, thereby resulting in Chl degradation and severe membrane lipid peroxidation[39]. Antioxidant enzymes are the main constituents of antioxidant defense and perform vital roles in eliminating ROS. Among diverse antioxidant enzymes, SOD is involved in catalyzing O2·− dismutation into H2O2, whereas the POD, APX, and CAT are chiefly associated with H2O2 scavenging[22]. Many studies found that different PGRs such as γ-aminobutyric acid, spermidine, and chitosan could ameliorate the activities of SOD, POD, APX, and CAT or expression levels of genes encoding those antioxidant enzymes in creeping bentgrass, thereby mitigating oxidative damage to cellular membranes and chloroplasts under drought stress[57−59]. It has been reported that an enhanced antioxidant defense system was conducive to the alleviation of leaf senescence, since reduced cellular oxidative damage is propitious to better metabolic homeostasis[15,60]. In addition, our previous study found that the DA-6 priming improved enzymatic antioxidant system to effectively minimize oxidative damage when white clover seeds were germinated under drought stress[13]. Foliar spray of DA-6 also could markedly enhance the drought tolerance of pineapple (Ananas comosus) by strengthening antioxidant defense systems to eliminate ROS[23]. In this study, DA-6 priming greatly improved the activities of SOD, POD, CAT, and APX, which could efficiently reduce ROS-induced oxidative injury when creeping bentgrass plants were exposed to drought stress.
-
DA-6 pretreatment significantly mitigated PEG-induced stress damage to creeping bentgrass including the decrease in plant growth, water deficit, Chl loss, the inhibition of photochemical efficiency and Pn, and membrane lipid peroxidation. In contrast to untreated plants, DA-6-fertigated plants showed significantly higher WUE and lower OP for better water balance under drought stress. Exogenous DA-6 up-regulated transcript levels of genes related to rubisco activity (AsRuBisCo) and Chl biosynthesis (AsMg-CHT, AsPBGD, AsPOR, and AsCHLH), while down-regulated transcript levels of genes for Chl degradation (AsPAO, AsCLH, and AsPPH) and senescence (Asl20, and Ash36), thereby decreasing leaf senescence and ameliorating photosynthetic performance under drought stress. In addition, exogenous DA-6 also significantly enhanced antioxidant enzyme activities (SOD, CAT, POD, and APX), hence efficiently diminishing drought-stimulated oxidative damage and leaf senescence. These findings suggest the importance of DA-6-regulated water balance, antioxidant defense, photosynthetic functions, and Chl metabolism associated with drought tolerance in turfgrass species.
This research work was supported by the Sichuan Students' Innovation Training Program (S202410626051).
-
The authors confirm contribution to the paper as follows: study conception and experiments design: Li Z; experiments performing: Hassan MJ, Najeeb A, Liu S, Ali U, Chandio WA; data analysis: Hassan MJ; manuscript writing: Hassan MJ; manuscript revision: Li Z; manuscript review: Li Z, Li M, Liao Q. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article, and are available from the corresponding author upon reasonable request.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Hassan MJ, Najeeb A, Liu S, Ali U, Chandio WA, et al. 2025. Diethyl aminoethyl hexanoate mitigates drought-stimulated leaf senescence via the regulation of water homeostasis, chlorophyll metabolism, and antioxidant defense in creeping bentgrass. Grass Research 5: e004 doi: 10.48130/grares-0025-0002
Diethyl aminoethyl hexanoate mitigates drought-stimulated leaf senescence via the regulation of water homeostasis, chlorophyll metabolism, and antioxidant defense in creeping bentgrass
- Received: 08 November 2024
- Revised: 25 December 2024
- Accepted: 02 January 2025
- Published online: 11 February 2025
Abstract: Diethyl aminoethyl hexanoate (DA-6) is involved in the regulation of adaptive response of plants to unfavorable environmental conditions. The objective of this experiment was to examine whether the DA-6 pretreatment could effectively alleviate drought-triggered leaf senescence and oxidative injury to creeping bentgrass. Plants were exogenously irrigated with or without DA-6 (0.4 mM·L−1) before being subjected to PEG-induced drought stress for 9 d. Drought stress resulted in significant growth retardation, Chl loss, and decreases in leaf relative water content, water use efficiency, photochemical efficiency, and net photosynthetic rate, but significantly enhanced oxidative damage. Exogenous DA-6 markedly alleviated symptoms of drought stress by improving water homeostasis, ROS scavenging, Chl biosynthesis, and photosynthesis. In contrast to untreated plants, the DA-6-pretreated creeping bentgrass demonstrated significantly higher transcript levels of genes related to rubisco activity (AsRuBisCo), and Chl biosynthesis (AsMg-CHT, AsPBGD, AsPOR, and AsCHLH), but lower transcript levels of Chl degradation-related genes (AsPAO, AsCLH, and AsPPH), and senescence-associated genes (Asl20 and Ash36), thereby decreasing leaf senescence and ameliorating photosynthetic performance under drought stress. Moreover, DA-6 also significantly promoted activities of superoxide dismutase, peroxidase, catalase, and ascorbate peroxidase, hence efficiently diminishing drought-stimulated oxidative damage. The current study supplies important information about DA-6-regulated growth and drought tolerance associated with osmotic adjustment, antioxidant defense, photosynthetic function, and Chl metabolism in cool-season turfgrass species.