-
Generally, type 2 diabetes is a chronic metabolic disorder characterized by insulin resistance and elevated blood glucose levels[1]. The enzyme α-glucosidase plays a pivotal role in starch digestion and subsequent absorption within the digestive tract. The breakdown of dietary starch leads to postprandial hyperglycemia in individuals with diabetes. Consequently, inhibiting α-glucosidase can mitigate and manage postprandial blood glucose levels, offering a practical strategy to alleviate and treat type 2 diabetes mellitus[2,3].
Acarbose, voglibose, and miglitol have been clinically proven as α-glucosidase inhibitors, and they can also be used in combination with antidiabetic medications to act as such inhibitors[4]. However, these compounds are expensive and are associated with digestive side effects such as flatulence, stomach cramps, and diarrhea, along with potential cardiac harm with prolonged use[2]. Given these side effects, there is a growing need for a more effective and safer approach to develop natural products such as tea plants as functional alternatives to mitigate the effects of type 2 diabetes mellitus[5].
The popularity of green tea powder (GTP) is increasing in Indonesia. Green tea powder has several advantages in physical properties, is easy to apply as a food ingredient, and is high in bioactive compounds[6]. Fresh tea leaves are typically subjected to withering by hot steam to obtain green tea powder, followed by drying and size reduction processes[7]. GTP contains highly bioactive compounds with numerous human health benefits[8]. Studies have reported that GTP is used in various food, cosmetic, and pharmaceutical products[9−11]. Food products enriched with GTP exhibit significantly enhanced antioxidant activity, reduced peroxide production during storage, and a lower glycemic index[12].
Tea contains many phenolic compounds, which have been shown to delay glucose absorption by inhibiting carbohydrate hydrolysis enzymes, thereby reducing postprandial blood glucose levels[13,14]. The utilization of GTP to inhibit α-glucosidase presents a promising strategy for treating and preventing type 2 diabetes. Despite the growing interest in the potential health benefits of green tea powder, few studies have investigated the antioxidant activity and α-glucosidase inhibition of GTP derived from Indonesian tea cultivars. Studies have shown that tea can effectively inhibit α-glucosidase activity through hydrogen group binding mechanisms[2,15]. Therefore, the main objective of this study is to investigate the quality of physicochemical properties, antioxidant activity, and α-glucosidase inhibition of green tea powder derived from Indonesian tea cultivars. In this research, four tea cultivars, including 'GMB-3', 'GMB-7', 'GMB-9', and 'GMBS-4', are used to produce green tea powder. These cultivars are widely planted in Indonesian tea plantations due to their high yield productivity and disease resistance. They are generally used to produce Indonesian black or green tea. On the other hand, the potential of these cultivars for producing green tea powder has not been fully explored until now. This research can potentially develop and improve the quality of green tea powder from local cultivars in Indonesia.
-
Bud and three young tea leaves (B + 3) from 'GMB-3', 'GMB-7', 'GMB-9' (Camellia sinensis var. assamica), and 'GMBS-4' (Camellia sinensis var. sinensis) cultivars were manually plucked to produce green tea powder. These fresh leaves were transported to the mini-plant tea facility of the Indonesian Research Institute for Tea and Cinchona (IRITC), Gambung Tea Plantation in West Java, Indonesia.
Processing of green tea powder
-
Fresh tea leaves (10 kg) underwent enzyme inactivation through hot steam (2.5 min at 100 °C). Subsequently, the withered leaves were cooled for 10 min and dried at 90 °C for 60 min, resulting in tea containing less than 2.5% moisture content. These dried tea leaves were then crushed and milled using a millstone grinder machine to produce green tea powder[16]. Finally, GTP was sieved using 100 mesh (140 μm) to obtain uniform size and remove unwanted objects, then packaged, and stored in a low-humidity room before analysis.
Analysis of physical properties of green tea powder
-
The physical properties of GTP were assessed using various methods. The water content was determined according to ISO 1572 standards, while the color of the green tea powder was measured using the Hunter LAB Chromameter method (CR 4000, Konica Minolta, Japan). The morphology of the green tea powder was observed by scanning electron microscopy (FEI, Inspect S50, Japan) at magnifications of 200× and 1,000×. Additionally, particle size analysis of green tea powder was conducted using DelsaTMNano (Beckman Coulter, USA) with a dynamic laser scattering method in which deionized water was used as the dispersion medium. The particle size was assessed based on the relative width (Rw) calculated using the formula:
$ \rm{R}w\ =\ (d_{90}\ -\ d_{10})/d_{50} $ (1) where, d10, d50 and d90 are 10%, 50%, and 90% of the typical particle size distribution.
Preparation of green tea powder extract for analysis
-
Green tea powder (500 mg) was mixed with 20 mL of boiled 70% methanol on a hot plate for 10 min, followed by maceration in a dry oven at 70 °C for 120 min. After maceration, the solution was sonicated for 10 min and then filtered using Whatman paper. The filtrate was then adjusted to a final volume of 25 mL by adding 70% methanol[17]. The GTP extract was stored at 4 °C until further analysis of its total polyphenol, flavonoids, antioxidant activity, and α-glucosidase inhibitor activity.
Determination of the content of total polyphenols and total flavonoids
-
The total polyphenol content was determined according to the Folin-Ciocalteau method, which refers to ISO 14502-1:2005[17]. One mL of diluted tea extract (diluted up to 100 times using distilled water) was added with 5 mL of 10% Folin-Ciocalteau (Sigma-Aldrich) vortex and allowed to stand for 5 min. 7.5% sodium carbonate solution (4 mL) was added, stored in a dark room for an hour, and measured using a UV-vis spectrophotometer (Varian Carry win UV) at 740 nm. Gallic acid (10−100 mg/L concentration) was used as the standard for total polyphenols.
Total flavonoids were determined using aluminum chloride[18]. A total of 1 mL of GTP extract (diluted for 200×) was put into a test tube containing 4 mL of distilled water and reacted with 5% NaNO2 (0.3 mL) solution. After 5 min, add 10% AlCl3 (0.3 mL) and 1 M NaOH (2 mL). Determine the final volume of up to 10 mL with distilled water, homogenize, and continue incubation for 15 min. The absorbance was measured using a UV-vis spectrophotometer (Varian carry win UV) at 415 nm. The total flavonoid content was measured using quercetin with a concentration of 10−100 mg/L to develop the standard curve equation.
Determination of total catechin
-
Total catechin was determined according to Musdja et al. with slight modification[19]. Green tea powder (50 mg) was extracted with boiled water for 15 min and then filtered in hot conditions with filter paper. The extract was partitioned with ethyl acetate (the extract and ethyl acetate are 2:1) and repeated in the water phase until a clear solution was obtained. The ethyl acetate phase was taken, evaporated, washed with cold water, and filtered to obtain crude catechins. The crude catechins were dried in an oven at 70 °C. Green tea powder catechins were determined as the catechin yield of catechins compared to standard (+)-catechins and measured using a UV-vis spectrophotometer UV-VIS at 279 nm.
Determination of caffeine contents
-
Caffeine was determined by previous work with slight modifications[18]. 2.5 g of GTP were added with MgO (5 g) and distilled water in a 500 mL conical flask. The mixture was then heated in the water bath at 40 °C for 2 h. Whatman filter paper (No. 42) was used to filter the solution, and the resulting filtrate was adjusted with distilled water up to 250 mL in a volumetric flask, which constitutes the stock solution.
Subsequently, 150 mL of the stock solution was taken and mixed with 20 mL of 10% H2SO4, followed by heating in a water bath maintained at 90 ± 2 °C until the volume of the mixture was reduced to 50 mL. This solution was then filtered using Whatman filter paper (No. 42). Chloroform (20 mL) was added to the filtrate in a separate funnel, shaken vigorously 20 times, and allowed to stand for 10 min. The chloroform layer (bottom layer) was collected and transferred to a 50 mL conical flask. Different volumes of chloroform (12.5, 10, 7.5, 5.5, and 5 mL) were added to the separation funnel containing the filtrate, and each time, the mixture was washed with 5 mL of 1% KOH. The washed chloroform was collected in a 50 mL conical flask and placed in an oven at 105 °C until a constant weight.
Measurement of the antioxidant activity by DPPH
-
The GTP extract was diluted at different concentrations in 70% methanol. Antioxidant activity was conducted by pipetting 2 mL of different concentrations into a test tube, adding 3 mL of DPPH (0.1 mM), homogenizing, and incubating in a dark room for 30 min. The absorbance of the sample was then measured by UV-vis spectrophotometry (Varian carry win UV) at 515 nm. 70% methanol is used as a blank to replace tea extract[17]. Antioxidant activity was measured as IC50 (mg/L).
Determination of in vitro α-glucosidase inhibition
-
The GTP was extracted as described previously. Furthermore, GTP extract was concentrated using a rotary vacuum evaporator (Buchi Rotavapor, Model R100) at 40 °C to remove solvent and obtain a crude extract for analysis. The inhibitory activity of α-glucosidase was determined according to the protocol described previously[20]. Briefly, 10 μL of the sample (concentration: 500 μg/mL) and 25 μL of the α-glucosidase solution were added to 50 μL of 0.1 M phosphate buffer (pH 7.0), and 25 μL of 0.5 mM 4-nitrophenyl α-D-glucopyranoside, then followed incubation at 37 °C for 30 min. After incubation, 100 μL of 0.2 M sodium carbonate solution was added to end the reaction. The enzyme hydrolysis of the substrate was monitored by the amount of p-nitrophenol released in the reaction mixture at 410 nm using a microplate reader. Acarbose, known as an α-glucosidase inhibitor, was used as a reference standard. All experiments were carried out in triplicate.
Data analysis
-
All data obtained were descriptively analyzed using the average value and standard error. Statistical analysis was conducted using a one-way analysis of variance (ANOVA) with a 95% significance level, followed by the Duncan multiple range test to determine significant differences between groups. Additionally, an analysis of covariance (ANCOVA) was performed, utilizing relative width as a covariate to adjust for the effect of tea cultivars on bioactive compound parameters. All data were tabulated in Microsoft Excel 2019 and analyzed using XLSTAT 2019 software (Addinsoft, New York, USA).
-
This research utilized four local tea plant cultivars to develop green tea powder. These cultivars exhibit different genetic and morphological characteristics, pest and disease resistance, high productivity, and suitability for various tea products (Fig. 1). The GMB cultivars series results from controlled hybridization of first-generation cultivars in Indonesia[21]. The 'GMB-3' cultivar, a crossbreed of 'Cin 143' and 'PS1' cultivars, is characterized by green leaves with a longitudinal shape, conical tips, and medium internodes. It is primarily used to produce high-quality black tea and green tea in Indonesian plantations[22].

Figure 1.
Morphological differences among tea cultivars (a) 'GMB-3', (b) 'GMB-7', (c) 'GMB-9', and (d) 'GMBS-4'. This figure was reproduced with permission from Heri K. Syahrian and M. Khais Prayoga (affiliated with the Indonesia Research Institute for Tea and Cinchona).
The 'GMB 7' cultivar, resulting from hand pollination of the first-generation 'Mal 2' × 'PS1' cultivars, features light green leaves with a thick wax layer on the leaf surface, a high percentage of bud production, and disease resistance[22,23]. This highly productive cultivar is widely used in Indonesian tea plantations and is suitable for white and black tea production. The 'GMB-9' cultivar, originating from hand pollination of 'GP 3' × 'PS1' cultivars, is distinguished by small buds, slightly small oval leaves, small branches, and a high growth rate[22]. In addition, the 'GMBS-4' cultivar belongs to the sinensis variety, characterized by small, elongated oval-shaped leaves with medium green color. The average weight of the bud and third leaves ranges from 0.4 to 0.6 g, with a length of approximately 5.8−9.2 cm[24,25]. This cultivar is particularly suitable for producing green tea products with potential antioxidant activity[18,26].
Physical properties and color of green tea powder
-
Table 1 shows the particle size and color of the Indonesian green tea powder produced from various local cultivars. The particle size of the green tea powder of various cultivars ranged from 1.03 to 1.96 μm at d50 as the median diameter. The relative width of the green tea powder ranged from 0.49 to 0.53, with no significant difference observed between cultivars (p > 0.05). A lower relative width value of green tea powder indicates a smaller and more uniform particle size.
Table 1. Particle size and color of Indonesian green tea powder produced from various tea cultivars.
Parameters Tea cultivars 'GMB-3' 'GMB-7' 'GMB-9' 'GMBS-4' Particle size d10 (μm) 1.67 ± 0.03a 1.17 ± 0.02b 0.92 ± 0.03c 0.86 ± 0.02c d50 (μm) 1.97 ± 0.06a 1.34 ± 0.10b 1.11 ± 0.03c 1.03 ± 0.07c d90 (μm) 2.64 ± 0.17a 1.86 ± 0.12b 1.50 ± 0.19c 1.42 ± 0.10d Relative width (Rw) 0.49 ± 0.07a 0.51 ± 0.03a 0.52 ± 0.13a 0.53 ± 0.04a Color L Hunter 67.78 ± 0.56d 69.4 ± 0.36c 73.38 ± 0.21a 71.12 ± 0.28b a* −2.94 ± 0.03c −1.48 ± 0.02a −1.69 ± 0.04b −1.53 ± 0.02a b* 30.03 ± 0.25a 28.88 ± 0.09c 29.62 ± 0.07b 27.26 ± 0.06d Data are means ± S.E. from the triplicate analysis. d10, d50, and d90 represent 10%, 50%, and 90% of the typical particle size distribution. L* value (brightness), a* value (redness), and b* values (yellowness). Different letters (a−d) within a row indicate significantly different values (p < 0.05). From Table 1, significant differences in the color profile were observed among the Indonesian green tea powder from various cultivars. Green tea powder from the 'GMB-3' cultivar had the lowest L* value among the 'GMB-7', 'GMB-9', 'GMB-9', and 'GMB-4' cultivars (p < 0.05). The L* value indicates the brightness of the green tea powder color. The color parameter with a negative a* value indicates green intensity. The 'GMB-3' cultivar produced green tea powder with a more intense green color than that from 'GMB-7' and 'GMB-9'. A previous study reported that 119 matcha samples have color characteristics L*, a*, and b* values ranging from 50.69 to 65.83, −11.69 to −1.07, and 25.15 to 37.30, respectively[27].
Morphological properties of green tea powder
-
The physical characteristics and particle morphology of the green tea powder were examined using scanning electron microscopy (SEM). Figure 2 shows the particle morphology of green tea powder at different magnifications. The SEM images reveal the particle morphology of green tea powder from different cultivars at two magnifications: 200× and 1,000×. At 200× magnification, the particles appear small and uniform in size across all cultivars, with a relatively smooth surface. At 1,000× magnification, the surface of the green tea powder particles appears smoother and finer, with subtle variations in shape. Despite these variations, the particles still exhibit a high degree of uniformity in size. The small and uniform particle size contributes to the fine and soft texture of the green tea powder. This uniformity is consistent with the particle size analysis results, which ranged from 1.03 to 1.96 μm, as shown in Table 1.

Figure 2.
Scanning electron microscopy observation of Indonesian green tea powder from various tea cultivars at 200× magnification (code 1) and 1,000× magnification (code 2). (a) GTP from 'GMB-3'; (b) GTP from 'GMB-7'; (c) GTP from 'GMB-9'; and (d) GTP from 'GMBS-4' cultivars.
Total polyphenol and total flavonoid content
-
Different tea cultivars can affect green tea powder's total polyphenol and flavonoid content. As shown in Table 2, the GTP from various cultivars contains total polyphenol and flavonoid content ranging from 244.57 to 277.91 mg GAE/g and 156.85 to 223.52 mg QE/g, respectively. Duncan's test revealed no significant differences in the total polyphenol content among the 'GMB-7', 'GMB-9', and 'GMBS-4' cultivars. However, there were significant differences in total flavonoid content between 'GMB-3' and 'GMB-9' cultivars compared to 'GMB-7' and 'GMBS-4' (p < 0.05).
Table 2. Bioactive compounds in Indonesian green tea powder from various tea cultivars.
Parameters Tea cultivars 'GMB-3' 'GMB-7' 'GMB-9' 'GMBS-4' Moisture contents (%, d.b.) 4.27 ± 0.08a 3.58 ± 0.05b 3.36 ± 0.04bc 3.26 ± 0.10c Total polyphenols (mg GAE/g) 244.57 ± 6.80b 276.09 ± 1.54a 273.09 ± 8.18a 277.91 ± 3.12a Total flavonoid (mg QE/g) 156.85 ± 3.13b 208.33 ± 6.02a 164.26 ± 6.15b 223.52 ± 8.00a Caffeine (%. d.b.) 3.58 ± 0.12a 2.33 ± 0.09b 2.50 ± 0.02b 2.37 ± 0.06b Total catechin (%) 4.39 ± 0.03a 3.21 ± 0.01d 2.81 ± 0.05b 3.60 ± 0.07c Data are means ± S.E. from the triplicate analysis. Different letters (a−d) within a row indicate significantly different values (p < 0.05). Correlation analyses was conducted to understand better the relationship between these bioactive compounds and other powder characteristics. Pearson correlation analysis (Fig. 3) showed a positive correlation between total polyphenol and total flavonoid content in green tea powder with R2 0.65 (p < 0.05). Furthermore, total polyphenol content showed a strong negative correlation with particle size distribution for d10, d50, and d90 with R2 −0.83, −0.80, and −0.81, respectively. Similar trends were observed in the correlation between total flavonoid contents, although the results were not statistically significant (Fig. 3).
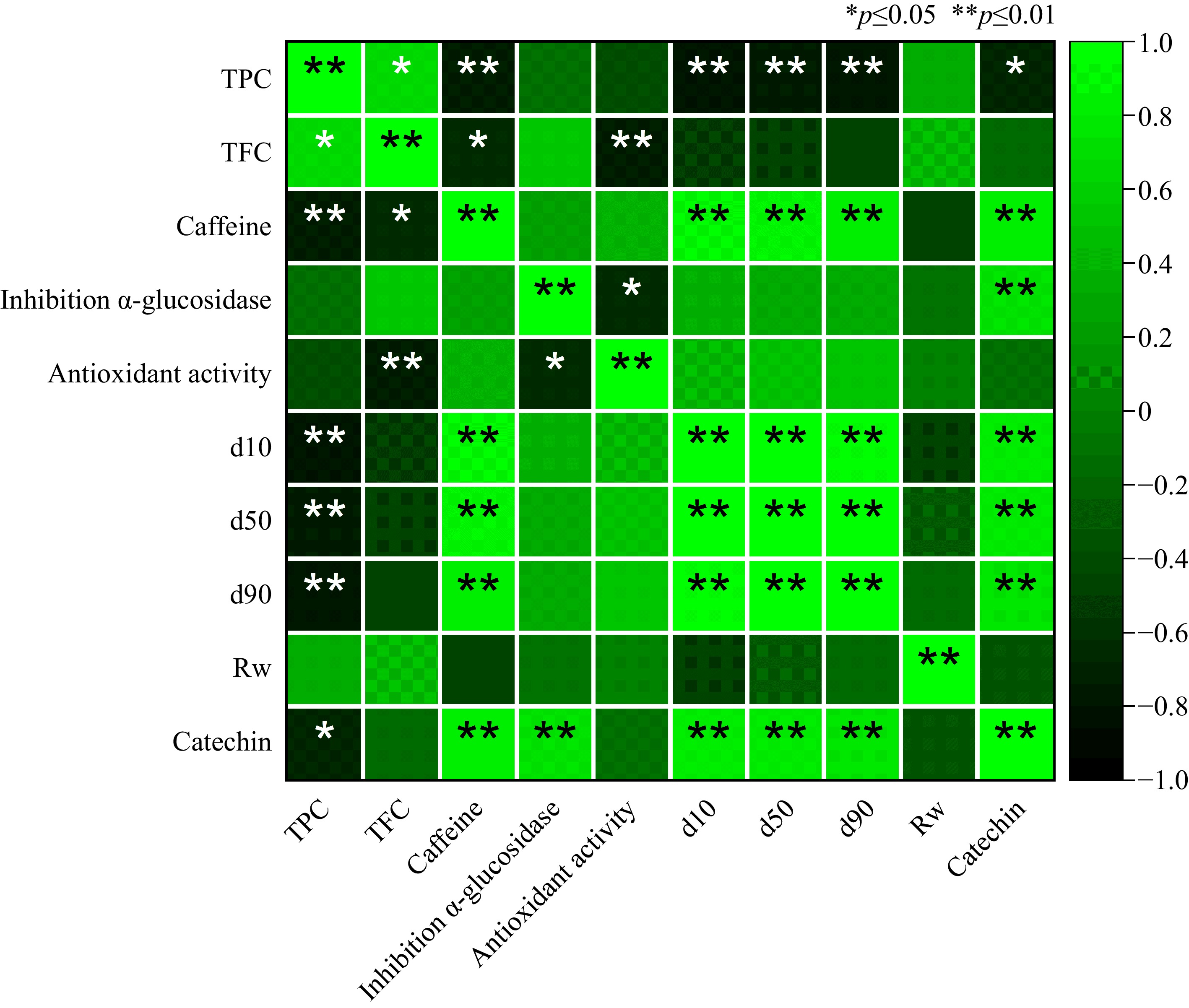
Figure 3.
Pearson correlation analysis of phytochemicals in green tea powder. Abbreviations: TPC, total polyphenol content; TFC, total flavonoid content; d10, d50, and d90 are 10, 50, and 90% of typical particle size distribution; Rw, relative width of particle size; IC50, antioxidant activity at 50% inhibition concentration; Inh. α-glucosidase, inhibition of α-glucosidase activity.
A previous study have reported that green tea powder or matcha from different countries contained total polyphenols ranging from 169−273 mg GAE/g[28]. Another study found that matcha green tea has a total polyphenol content of about 19.84%[29]. A study by Topuz et al. reported that green tea powder produced from Turkish cultivars has a total polyphenol content of 22.0 to 22.73 g GAE/100 g[30]. Additionally, a study by Koláčková et al. showed that the total flavonoid content of green tea powder varied from 99 to 139 mg RE/g[31]. These results are consistent with those obtained by traditional and daily matcha, which contains total flavonoids of 1,222 to 1,460 and 1,379−1,968 mg/L, respectively[28]. Interestingly, the results reveal that the green tea powder from the Indonesian tea cultivar generally has a higher total polyphenol and flavonoid content. It suggests that Indonesian tea cultivars may possess unique characteristics contributing to their higher bioactive compound content.
Caffeine content of green tea powder
-
Table 2 presents the caffeine content (%) of green tea powder from several Indonesian tea cultivars. The results indicate that the caffeine content ranged from 2.33% to 3.58%. Notably, green tea powder from the 'GMB-3' cultivar exhibited the highest caffeine content among cultivars, with a statistically significant difference (p < 0.05). Figure 3 shows that caffeine contents had a significant negative correlation (p < 0.05) with total polyphenol and total flavonoid content in green tea powder. Previous studies have reported that the caffeine content in green tea powder is approximately 300 mg/L[32]. Research has also shown that different particle sizes of green tea powder can affect caffeine content, with ranges from 3.67 to 3.80 g/100 g[33]. Furthermore, a study on optimized spray-dried green tea powder reported a caffeine content of 2.8%[34]. An evaluation of various green tea powders and matcha demonstrated caffeine content ranging between 18.9−44.4 mg/g[31].
Total catechin of green tea powder
-
Table 2 shows the variation in catechin content among green tea powders from different cultivars. Specifically, 'GMB-3' produced green tea powder with higher catechin content (4.39 %) compared to 'GMB-7' (3.21%), 'GMB-9' (2.81%), and 'GMBS-4' (3.60%) cultivars. This variability in catechin content is consistent with findings from a previous study, which reported catechin concentrations ranging from 21.20 to 34.81 mg/g for different brands of green tea powder[35]. Moreover, it is worth noting that the grinding process can influence catechin content, with superfine grinding leading to a decrease in catechin levels[33].
Antioxidant activity by DPPH
-
Table 3 presents the antioxidant activity of Indonesian green tea powder (GTP) from different cultivars, measured by their ability to inhibit DPPH free radicals. 'GMBS-4' exhibited the highest antioxidant activity among the cultivars studied, as indicated by its lowest IC50 value of 12.27 mg/L. In comparison, GTP of 'GMB-3', 'GMB-7', and 'GMB-9' had IC50 values of 16.41, 15.41, and 17.32 mg/L, respectively. Analysis of covariate (ANCOVA) showed a high R2 (0.995) for the corrected parameter relative width (Rw) of green tea powder (Table 4). This indicates that particle size significantly influences the antioxidant activity of green tea powder.
Table 3. Antioxidant and α-glucosidase inhibitory activity of Indonesian green tea powder from various tea cultivars.
Tea cultivars Antioxidant
activity (IC50, mg/L)Inhibition
α-glucosidase* (%)'GMB-3' 16.41 ± 0.48ab 40.93 ± 0.08b 'GMB-7' 15.41 ± 0.23b 39.48 ± 0.16c 'GMB-9' 17.32 ± 0.49a 30.76 ± 0.16d 'GMBS-4' 12.27 ± 0.29c 42.54 ± 0.21a Acarbose** − 87.57 ± 0.38 Data are means ± S.E. from the triplicate analysis. Different letters (a−d) within a column indicate significantly different values (p < 0.05). * Measured at a concentration of 500 μg/mL. ** Inhibition of α-glucosidase activity measured at a 5 μg/mL concentration. Table 4. Mean square summary of covariance (ANCOVA) analysis with relative width (Rw) as a covariate for green tea powder parameters.
Source DF TPC
(mg/g)TFC
(mg/g)Caffeine
(%)Inhibition of
α-glucosidase (%)Antioxidant activity
(IC50, mg/L)Catechin
(%)d10
(μm)d50
(μm)d90
(μm)Rw 1 75.54 79.03 0.001878 0.0531 5.7615* 0.00703* 0.003873 0.097606* 0.52956* Cultivars 3 671.24* 2637.78* 0.792153* 81.8692* 16.5338* 1.20196* 0.298532* 0.50452* 1.06104* Error 7 96.640 115.390 0.023 0.080 0.039 0.000 0.002 0.004 0.004 R² 0.772 0.923 0.953 0.998 0.995 0.9997 0.989 0.984 0.991 DF, degrees of freedom; TPC, total polyphenol content; TFC, Total flavonoid content; Rw, Relative width of particle size; and IC50, 50% antioxidant activity at inhibition concentration; * significant at p < 0.05. The IC50 value represents the concentration of a substance required to scavenge 50% of DPPH radicals, with lower values indicating greater antioxidant activity. Previous research reported that the antioxidant activity of various types of green tea powder ranges from 288 to 346 mg of Trolox equivalent/g[31]. Furthermore, specific types of matcha have been reported to have IC50 values of 29.93 and 27.75 μg/mL[36]. Similarly, another study reported that Turkish green tea powder had an IC50 value of 0.36 mg/mg DPPH[30].
α-glucosidase inhibitory activity
-
Table 3 shows the inhibitory activity of Indonesian green tea powder on the α-glucosidase enzyme. Acarbose, a known α-glucosidase inhibitor, exhibited an inhibition activity of 87.57% at a concentration of 5 μg/mL. In comparison, Indonesian green tea powder showed inhibitory activity ranging from 30% to 42% at a concentration of 500 μg/mL. Notably, green tea powder from the 'GMBS-4' cultivar demonstrated the highest inhibitory activity among the tested cultivars. The higher inhibitory activity of green tea powder from the 'GMBS-4' cultivar can be attributed to the highest total polyphenol and antioxidant activity, as shown in Tables 2 & 3. Analysis of covariate with relative width (Rw) as corrected effect (Table 4) shows that the activity of α-glucosidase inhibitory is mainly influenced by cultivar rather than the particle size of green tea powder. Interestingly, despite its lower total polyphenol and flavonoid content, the green tea powder of the 'GMB-3' cultivar exhibited a higher α-glucosidase inhibitory activity. This may be attributed to the highest catechin content observed in the GTP of 'GMB-3' cultivars compared to others (see Table 2), suggesting that specific catechin profiles may play a crucial role in enzyme inhibition.
-
The particle size and color are key factors influencing the quality of green tea powder products. The optimal particle size for fine green tea powder typically ranges from 1 to 20 μm, with an ideal size range of 13.5 to 20.3 μm at d50[33,37]. Various factors, including the type and duration of the size reduction process, can affect the particle size of green tea powder[7,38]. Smaller particle sizes result in an increased specific surface area, facilitating dispersion when incorporated into food, cosmetic, or pharmaceutical products[37]. On the other hand, the color of green tea powder is influenced by multiple factors such as agronomic cultivation practices, tea cultivar type, grinding techniques, processing methods, and particle size[7,39]. All these factors contribute to the overall quality of appearance and color of green tea powder and should be carefully considered throughout the production process. While physical properties are crucial for product quality, the health-promoting effects of green tea powder are primarily attributed to its bioactive compounds, particularly polyphenols, and flavonoids.
Green tea powder is a rich source of flavonoids and is renowned for its antioxidant and anti-inflammatory activity, which in turn supports the immune system[28]. Among the abundant polyphenolic compounds in tea, flavonoids stand out for their various health benefits. The catechin compounds present in the green tea powder contributes significantly to its health-promoting properties. Notable catechins identified in green tea powder includes EGCG, EGC, EC, and ECG, with EGCG being particularly well studied for its diverse physiological effects, including modulation of inflammation, cell repair, lipid metabolism regulation, and general improvement of health enhancement[29,40]. Catechins exhibit potent antioxidant properties and protect against oxidative stress-induced cognitive decline, especially in older adults[41]. Various factors, including shading levels during cultivation, harvesting period, tea cultivar type, powdering processes, and particle size of green tea powder, can influence the overall content of polyphenols and flavonoids[30,33,38,42].
In this study, green tea powder was produced from young tea leaves with a composition of shoots and three young leaves. Young tea leaves contain more caffeine than old tea leaves[43]. This observation is consistent with findings reported by Prayoga et al., who reported that caffeine content ranges from 3.01% to 4.18% in various tea cultivars in Indonesia[26]. Caffeine is one of the compounds in green tea powder that contributes to the sensation of briskness. Caffeine can increase tea's antioxidant activity when combined with other compounds, such as polyphenols and amino acids[28]. Additionally, caffeine in tea offers several health benefits, serving as an anti-inflammatory agent, increasing alertness and energy, and promoting body rejuvenation[44,45].
As shown in Table 2, the green tea powder from 'GMBS-4' cultivars exhibit higher levels of bioactive compounds compared to other cultivars. Specifically, it demonstrates elevated levels of total polyphenols, flavonoids, and catechins. These compounds significantly influence the antioxidant activity of green tea powder. Hydroxyl groups in phenolic compounds play a crucial role in conferring antioxidant properties[46]. Tea polyphenols, by their antioxidant activity, effectively neutralize free radicals such as reactive oxygen species (ROS) and reactive carbonyl species (RCS) through mechanisms involving hydrogen or electron donation. This process helps mitigate lipid peroxidation and prevents tissue damage[47]. Furthermore, various factors such as grinding techniques, the concentration of green tea powder extract, serving temperature, and particle size play an integral role in determining the DPPH scavenging activity of green tea powder[7,38,48].
In relation to antioxidants and inhibition of α-glucosidase activity, the particle size of green tea powder has a significant effect. The bio-accessibility of polyphenols is enhanced significantly with decreasing particle size of green tea powder[42,49]. Smaller particle sizes of green tea powder are associated with a high surface area that enhances the efficiency of extraction of bioactive compounds to the solvent[50]. The polyphenols and catechins found in various types of tea have demonstrated potential as inhibitors of human α-glucosidase with different sensitivity[51]. Green tea powder contains high flavonoids and exhibits potential as an α-glucosidase inhibitor[52]. Among the main flavonoids in green tea powder, flavan-3-ols predominate, with EGCG (16%), EGC (5.2%), ECG (3.50%), and EC (1.30%) as the primary compound. These compounds have been shown to effectively inhibit both α-glucosidase and glycogen phosphorylase (GP)[53]. The inhibition mechanisms of flavonoids on enzymes typically involve two types of binding mechanisms: (i) direct binding to amino acid residues at the enzyme's active site, thereby excluding the substrate, and (ii) interaction with amino acid residues near the active site, resulting in the closure of the channel leading to the active center[52]. EGCG, for example, binds to starch-degrading enzyme (SCG) through hydrogen bonding and hydrophobic reactions[54]. These interactions highlight the potential of the components of green tea powder as effective inhibitors of α-glucosidase, offering promising possibilities for controlling diseases such as diabetes.
-
This study investigated green tea powders from various cultivars for physical properties, phytochemical compounds, antioxidant activity, and α-glucosidase inhibition. The findings presented in this article highlight the use of 'GMBS-4' cultivars to produce green tea powder with high total flavonoid content, low IC50 values, and potent α-glucosidase inhibitory activity. However, it should be noted that Indonesian green tea powder still requires a relatively high concentration to inhibit the α-glucosidase enzyme compared to the pharmaceutical drug acarbose, particularly at low concentrations. These results promise the potential of Indonesian tea cultivars in developing and producing green tea powder with significant antioxidant activity and α-glucosidase inhibition. By leveraging these characteristics, stakeholders in the Indonesian tea industry can explore opportunities to improve the quality of locally produced green tea powder, contributing to the sustainability and growth of the domestic tea industry. More studies are needed to explore the volatile compounds present in green tea powder and metabolic fingerprints that could offer valuable information to contribute to the quality of green tea powder in Indonesia.
The authors would like to thank Fadhilatul Ula and Isep Sutardi for assisting in the laboratory. We also thank Heri Syahrian and Khais Prayoga for supporting Indonesian tea cultivars. The research support was provided by Politeknik Kesehatan Bandung (Penelitian Kerjasama, Antar Perguruan Tinggi, No.LB.02.01/3.1/2715 B/2020).
-
The authors confirm contribution to the paper as follows: Trinovani E and Prawira-Atmaja MI have equal contributions to this work. Study conception and design: Trinovani E, Prawira-Atmaja MI, Kusmiyati M; data collection: Trinovani E, Prawira-Atmaja MI, Maulana H, Kusmiyati M, Shabri S; analysis and interpretation of results: Trinovani E, Shabri S, Maulana H; draft manuscript preparation: Prawira-Atmaja MI, Trinovani E, Kusmiyati M, Maulana H. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Elvi Trinovani, Mukhammad Iqbal Prawira-Atmaja
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Trinovani E, Prawira-Atmaja MI, Kusmiyati M, Shabri S, Maulana H. 2025. Evaluation of antioxidant and α-glucosidase inhibitory activity of green tea powder from Indonesian tea cultivars. Beverage Plant Research 5: e005 doi: 10.48130/bpr-0024-0036
Evaluation of antioxidant and α-glucosidase inhibitory activity of green tea powder from Indonesian tea cultivars
- Received: 21 April 2024
- Revised: 03 October 2024
- Accepted: 10 October 2024
- Published online: 28 February 2025
Abstract: The enzyme α-glucosidase plays a crucial role in the digestive system by hydrolyzing carbohydrates to produce absorbable glucose. With the recent surge in the popularity of green tea powder (GTP) in Indonesia, this study aims to determine the potential of GTP derived from Indonesian tea cultivars as sources of antioxidants and inhibitors of α-glucosidase activity. Four Indonesian tea cultivars ('GMB-3', 'GMB-7', 'GMB-9', and 'GMBS-4') were utilized to produce green tea powder, following which their physicochemical properties, antioxidant activity, and α-glucosidase inhibition activity were evaluated. The results revealed that GTP has particle size at d50 ranging from 1.03 to 1.96 μm with total polyphenol content of GTP ranging from 244.57 to 277.91 mg GAE/g. Notably, the 'GMBS-4' cultivar produced GTP with the highest total flavonoid content (223.52 mg QE/g), the lowest IC50 (12.27 mg/L), and exhibited the highest α-glucosidase inhibitory activity (42.54%). Furthermore, the GTP derived from the 'GMB-3' cultivar exhibited a higher total catechin content (4.39%) compared to the other cultivars. The inhibition of α-glucosidase activity of GTP ranged from 30% to 42% at a concentration of 500 μg/mL. These findings suggest that Indonesian green tea powder exhibits α-glucosidase inhibitor activity, although at a concentration higher than acarbose. However, Indonesian tea cultivars demonstrate promising potential for producing green tea powder with antioxidant properties and α-glucosidase inhibitors. Therefore, further research and development of Indonesian tea cultivars are expected to improve GTP quality in Indonesia.













