-
Tea manufactured from leaves of the evergreen shrub Camellia sinensis (L.) O. Kuntze, is one of the most globally popular beverages due to its refreshing taste and health benefits. In 2020 globally, tea production had increased to 5,972 million metric tons, which was a doubling in the past 20 years[1]. Based on different ways of processing, there are six main types of tea, including green tea, black tea, dark tea, oolong tea, white tea and yellow tea[2,3]. To ensure the quality and safety of products, it is very important to monitor the levels of pollutants and define the origin.
Identifying the sources of contaminants, such as pesticide residues, heavy metals and other pollutants such as polycyclic aromatic hydrocarbons (PAHs), is the primary step to control pollution. Direct spraying of synthetic chemicals in tea plantations, as well as air drift caused by operations near tea gardens, are the main source of pesticide residues in tea[4]. Heavy metals can accumulate in tea and lead to toxicity, which are mainly derived from soil, fertilizer and atmosphere[5−7]. As for other pollution appearing unexpectedly in tea, it was quite difficult to identify due to the complex procedures of the production tea chain including plantation, processing, package, storage and transportation. The PAHs in tea came from the deposition of vehicle exhausts and the combustion of fuels used during the processing of tea leaves, such as firewood and coal[8−10].
During the coal and firewood combustion, pollutants such as carbon oxides are formed[11]. As a result, it is susceptible for residues of these above-mentioned pollutants to occur in the processed products, such as grain, smoked stock and cat fish, at high temperature, posing a threat to human health[12,13]. The PAHs caused by combustion are derived from the volatilization of PAHs contained in the fuels itself, the high-temperature decomposition of aromatic compounds and the compound reaction between free radicals[14]. Combustion temperature, time, and oxygen content are important factors that affect the conversion of PAHs. With the increase of temperature, the PAHs content first increased and then decreased, and the peak value occurred at 800 °C; The PAHs content decreased sharply to trace with increasing combustion time when it was below a limit called 'boundary time', with the increase of oxygen content in the combustion air, PAHs emissions reduced significantly, but incomplete oxidation would produce OPAHs and other derivatives[15−17].
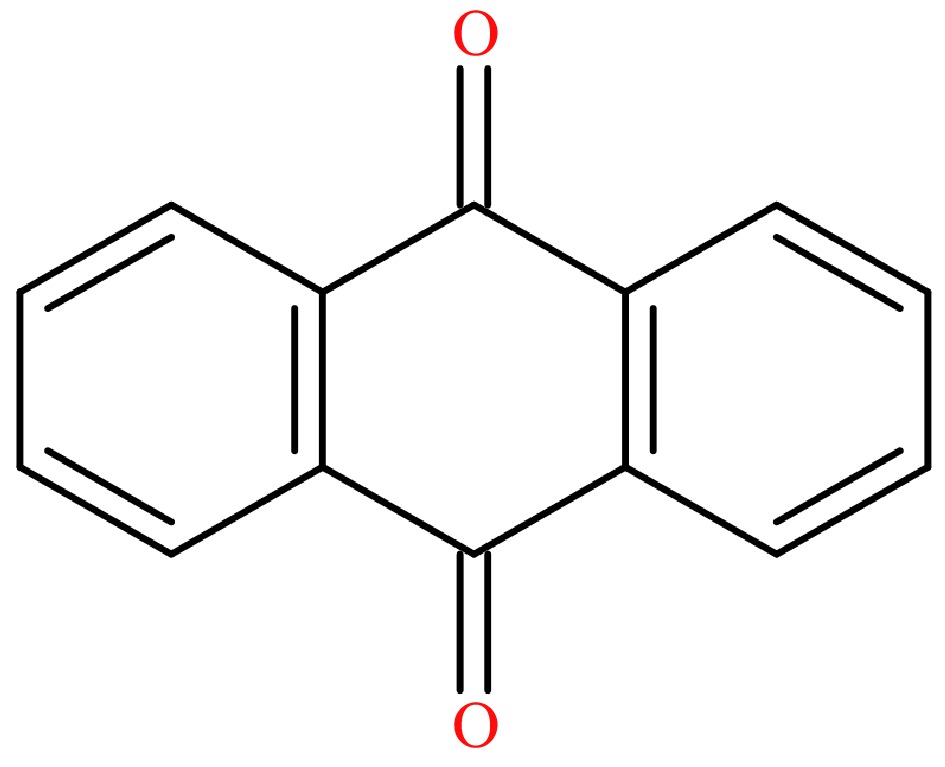
9,10-Anthraquinone (AQ, CAS: 84-65-1, Fig. 1), an oxygen-containing derivative of PAHs[18], consists of three condensed cycles. It was listed as a possible carcinogen (Group 2B) by the International Agency for Research on Cancer in 2014[19]. AQ can poison to topoisomerase II cleavage complex and inhibit the hydrolysis of adenosine triphosphate (ATP) by DNA topoisomerase II, causing DNA double-strand breaks, which means that long-term exposure under AQ-containing environment and direct contact with high level of AQ can lead to DNA damage, mutation and increase the risk of cancer[20]. As negative effects on human health, the AQ maximum residue limit (MRL) of 0.02 mg/kg was set in tea by the European Union. According to our previous studies, the deposits of AQ were suggested as the main source during tea plantation[21]. Also, based on the experimental consequences in Indonesian green and black tea processing, it is obvious that the AQ level changed significantly and the smoke from processing equipment was suggested as one of main reasons[22]. However, the accurate origin of AQ in tea processing remained elusive, although some hypotheses of AQ chemical pathway were suggested[23,24], indicating that it is extremely important to determine the crucial factors affecting the AQ level in tea processing.
Given the research on the formation of AQ during coal combustion and the potential menace of fuels in tea processing, a comparative experiment was carried out to explain the effect of processing heat sources on AQ in tea and air, quantitative analysis on the changes of AQ content at different processing steps, which is helpful to confirm the accurate origin, occurrence pattern and degree of AQ pollution in tea processing.
-
Compared with our previous study[21], a liquid-liquid extraction procedure was combined before injection to GC-MS/MS in order to improve sensitivity and maintain instrumental statements. In Fig 2b, the improved method showed a significant improvement in the purification of the sample, the solvent became lighter in color. In Fig 2a, a full scan spectrum (50−350 m/z) illustrated that after purification, the base line of the MS spectrum reduced obviously and the fewer chromatographic peaks were available, indicating that a large number of interfering compounds were removed after the liquid-liquid extraction.
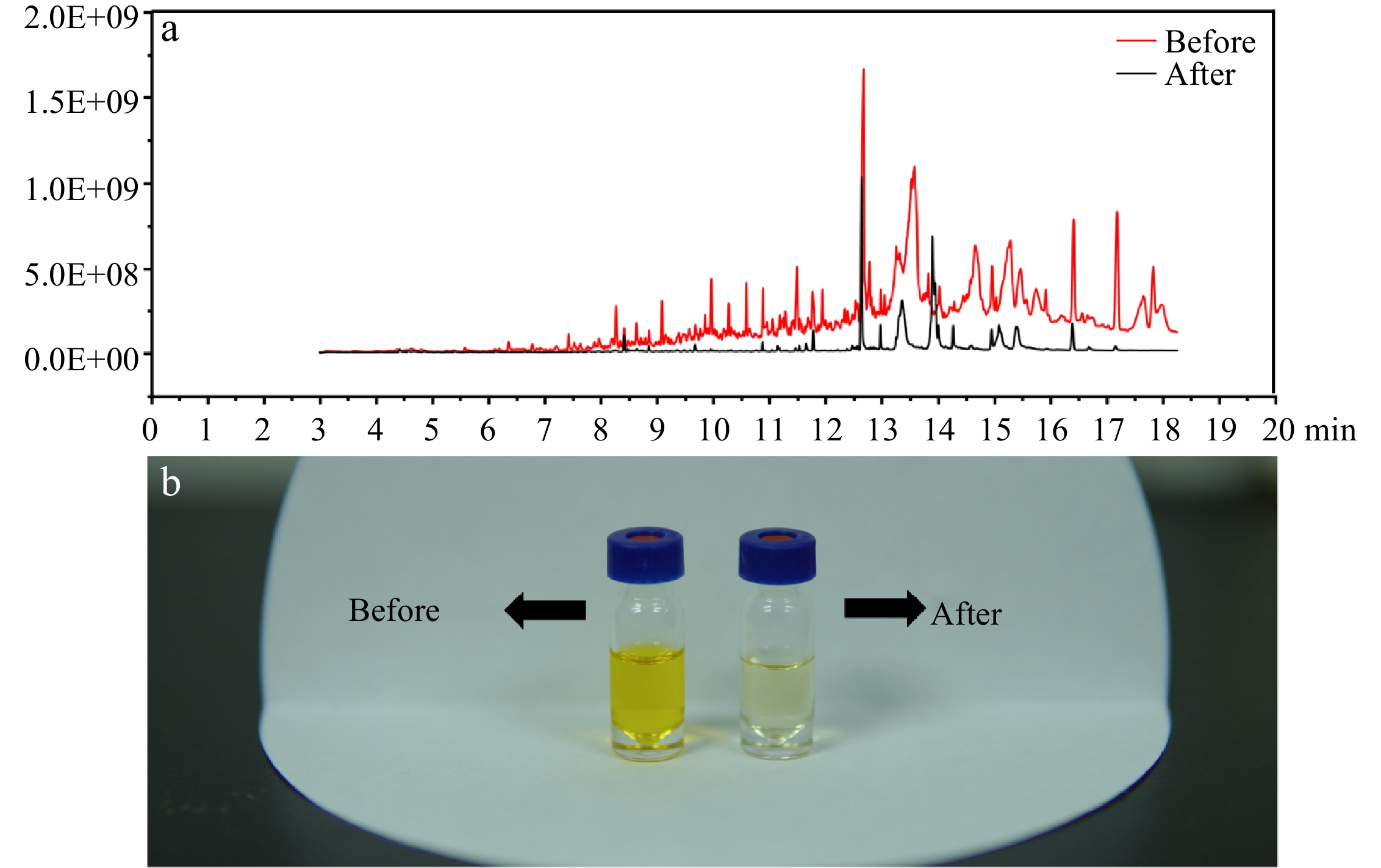
Figure 2.
(a) Full scan spectrum of the sample before and after the purification. (b) The purification effect of the improved method.
Method validation, including linearity, recovery, limit of quantitation (LOQ) and matrix effect (ME), are shown in Table 1. It is satisfactory to obtain the linearity with the coefficient of determination (r2) higher than 0.998, which ranged from 0.005 to 0.2 mg/kg in the tea matrix and acetonitrile solvent, and in the air sample with a range of 0.5 to 8 μg/m3.
Table 1. Validated parameters of AQ in spiked samples.
Martrix Fortified level
(mg/kg)Recovery
(%, SD)RSD
n = 5LOQ
(mg/kg)r2 (range g/L) ME (%) Tea shoots 0.005 125.7 ± 8.6 6.9 0.005 0.999 (5−200) 10.9 0.01 96.5 ± 5.7 5.9 0.02 87.4 ± 6.4 7.3 Dry tea 0.005 77.8 ± 9.7 12.5 0.005 0.998 (5−200) 9.0 0.02 113.0 ± 3.2 3.6 0.005 107.6 ± 1.4 1.5 Air sample 0.5a 117.06 ± 9.4 10.8 0.5a 0.999 (0.5−8) 196.1 1.5 78.47 ± 4.0 5.1 3.0 105.07 ± 14.8 14.1 a: µg/m3 Recovery of AQ was evaluated at three spiked concentrations between measured and actual concentrations in dry tea (0.005, 0.02, 0.05 mg/kg), fresh tea shoots (0.005, 0.01, 0.02 mg/kg) and air sample (0.5, 1.5, 3 μg/m3). Recovery of AQ in tea ranged from 77.78% to 113.02% in dry tea and from 96.52% to 125.69% in tea shoots, with RSD% lower than 15%. Recovery of AQ in air samples ranged from 78.47% to 117.06% with RSD% below 20%. The lowest spiked concentration was identified as LOQ, which were 0.005 mg/kg, 0.005 mg/kg and 0.5 μg/m³ in tea shoots, dry tea and air samples, respectively. As listed in Table 1, the matrix of dry tea and tea shoots slightly increased the AQ response, leading to the ME of 109.0% and 110.9%. As for the matrix of air samples, the ME was 196.1%.
The levels of AQ during green tea processing
-
With the aim of finding out the effects of different heat sources on tea and processing environment, a batch of fresh leaves were divided into two specific groups and processed separately in two processing workshops in the same enterprise. One group was supplied with electricity, and the other with coal.
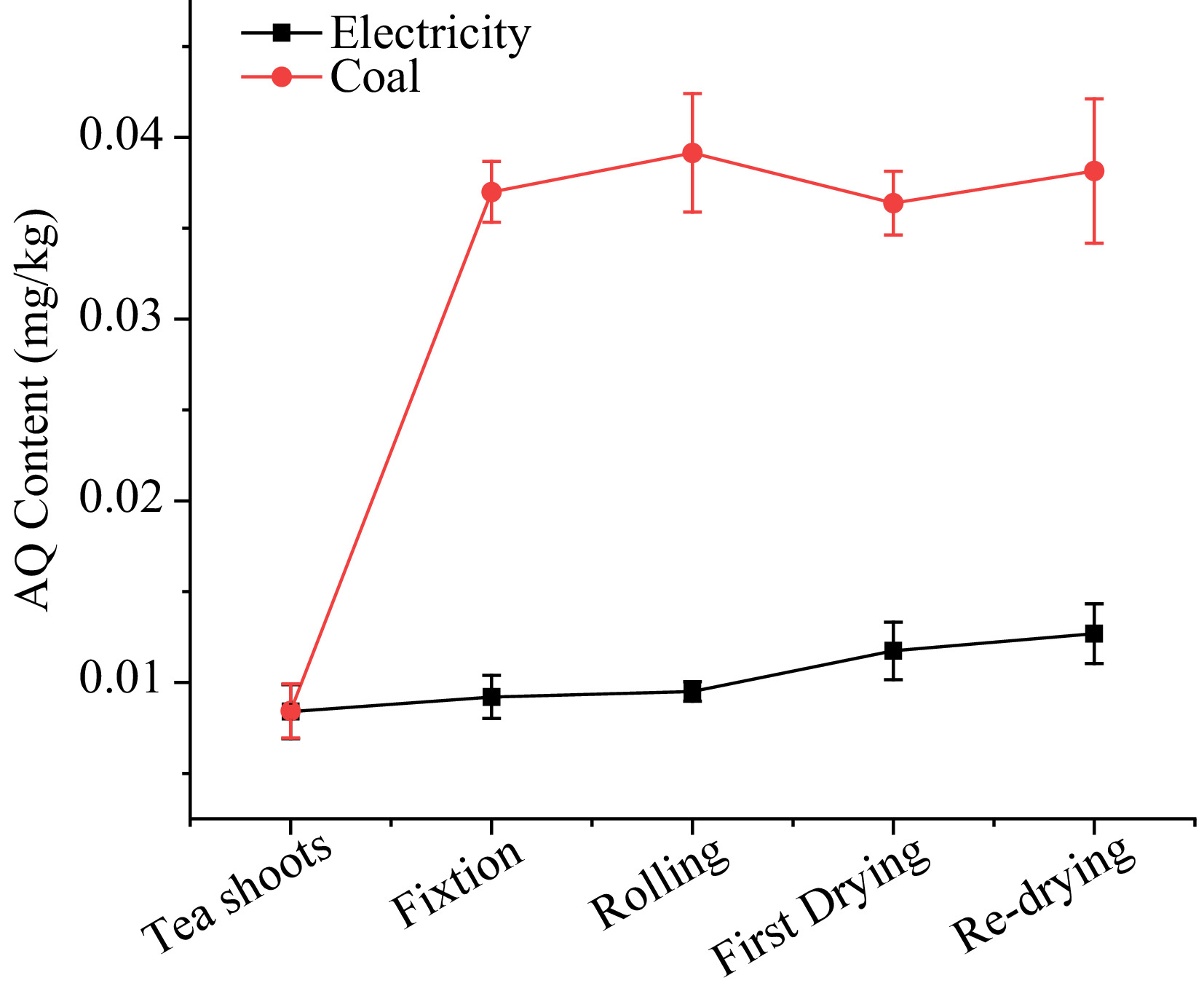
As shown in Fig. 3, the AQ level with electricity as the heat source ranged from 0.008 to 0.013 mg/kg. During the fixation process, the parching of tea leaves caused by processing in a pot with high temperature resulted in a 9.5% increase in AQ. Then, the level of AQ remained during the rolling process despite the loss of juice, suggesting that physical processes may not affect the level of AQ in tea processing. After the first drying steps, AQ level increased slightly from 0.010 to 0.012 mg/kg, then continued to rise to 0.013 mg/kg until the end of re-drying. PFs, which significantly showed the variation in each step, were 1.10, 1.03, 1.24, 1.08 in fixation, rolling, first drying and re-drying, respectively. The results of PFs suggested that processing under electrical energy had a slight effect on the levels of AQ in tea.
In the case of coal as the heat source, the AQ content sharply increased during the tea processing, surging from 0.008 to 0.038 mg/kg. 338.9% AQ were increased in the fixation procedure, reaching 0.037 mg/kg, which far exceeded the MRL of 0.02 mg/kg set by the European Union. During the rolling stage, the level of AQ still increased by 5.8% despite being far from the fixation machine. In first drying and re-drying, the AQ content increased little or decreased slightly. The PFs using coal as the heat source in fixation, rolling first drying and re-drying were 4.39, 1.05, 0.93, and 1.05, respectively.
To further determine the relationship between the coal combustion and AQ pollution, the suspended particulate matters (PMs) in air in the workshops under both heat sources were collected for air assessment, as shown in Fig. 4. The AQ level of PMs with coal as the heat source was 2.98 μg/m3, which was over three times higher than that with electricity 0.91 μg/m3.
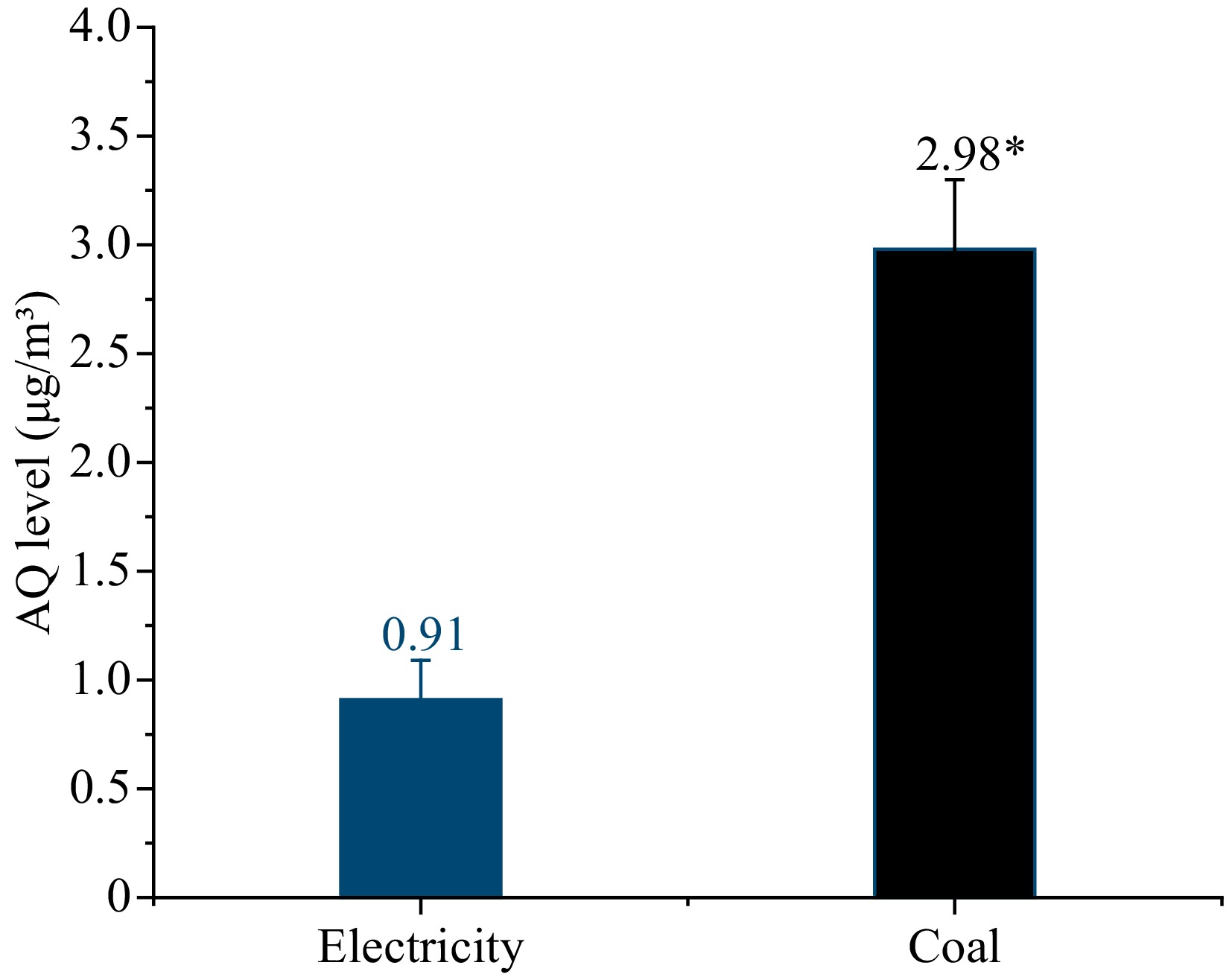
Figure 4.
The levels of AQ in the environment with electricity and coal as heat source. * Indicates significant differences in AQ levels in the samples (p < 0.05).
The levels of AQ during oolong tea processing
-
Oolong tea, mainly produced in Fujian and Taiwan, is a kind of partially fermented tea. To further determine the main steps of increasing AQ level and the effects of different fuels, the same batch of fresh leaves was made into oolong tea with coal and natural gas-electric hybrid as heat sources, simultaneously. The AQ levels in oolong tea processing using different heat sources are shown in Fig. 5. For oolong tea processing with natural gas-electric hybrid, the trend of AQ level was stagnating below 0.005 mg/kg, which was similar to that in green tea with electricity.
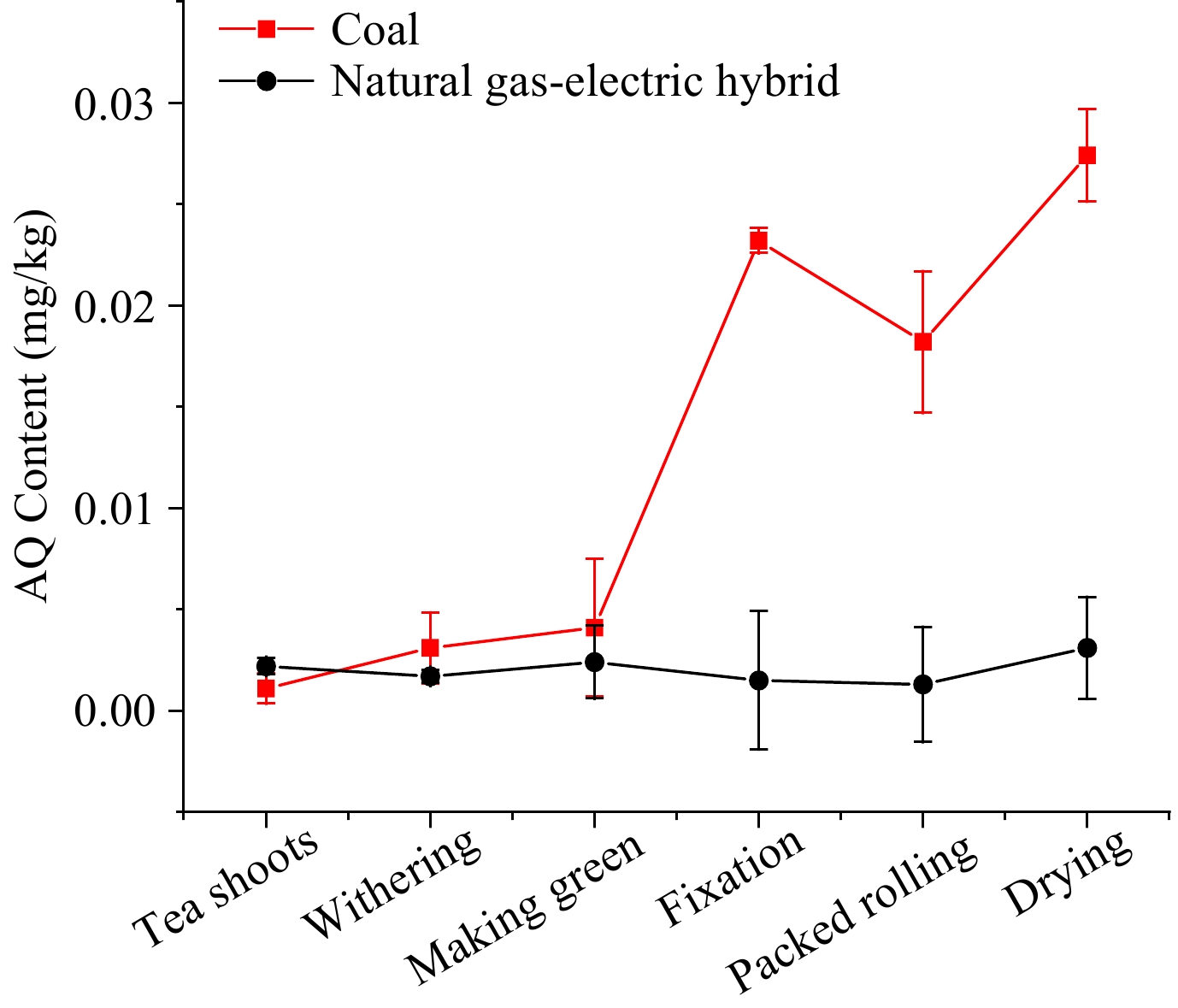
Figure 5.
The AQ level during oolong tea processing with natural gas-electric blend and coal as heat source.
With coal as the heat source, the AQ levels in the first two steps, withering and making green, were essentially the same as with natural gas-electric blend. However, the subsequent procedures until fixation showed the gap widened gradually, at which point the AQ level surged from 0.004 to 0.023 mg/kg. The level in the packed rolling step decreased to 0.018 mg/kg, which may be due to the loss of tea juice carrying away some of AQ contaminants. After the rolling stage, the level in the drying stage increased to 0.027 mg/kg. In withering, making green, fixation, packed rolling and drying, the PFs were 2.81, 1.32, 5.66, 0.78, and 1.50, respectively.
The occurrence of AQ in tea products with different heat sources
-
To determine the effects on AQ content of tea with different heat sources, 40 tea samples from the tea workshops using electricity or coal as heat sources were analyzed, as shown in Table 2. Compared with using electricity as a heat source, coal had the most detective rates (85.0%) with the maximum AQ level of 0.064 mg/kg, indicating that it was easy to cause AQ contaminant by fumes produced by coal combustion, and a rate of 35.0% was observed in samples of coal. Most conspicuously, electricity had the lowest detective and excedance rates of 56.4% and 7.7% respectively, with the maximum content of 0.020 mg/kg.
Table 2. The occurrence for AQ in tea products with different heat sources.
Total samples
analyzedNumber of detective
samplesRates of
detection (%)Number of samples
exceeded MRLRates of
exceedance (%)Min
(mg/kg)Max
(mg/kg)Electricity 20 10 50.0 1 5.0 ND 0.020 Coal 20 17 85.0 7 35.0 ND 0.064 -
Based on the PFs during processing with the two kinds of heat sources, it was clear that fixation was the main step that led to the increase of AQ levels in tea production with coal and processing under electrical energy had a slight effect on the content of AQ in tea. During green tea processing, coal combustion produced a lot of fumes in the fixation process compared with the electric heating process, indicating that maybe fumes were the main source of AQ pollutants from contact with tea shoots instantly in tea processing, similar to the exposure process in the smoked barbecue samples[25]. The slightly increase in AQ content during the rolling stage suggested that the fumes caused by coal combustion not only affected the AQ level during the fixation step, but also in the processing environment due to atmospheric deposition. Coals were also used as the heat source in the first drying and re-drying, but in these two steps the AQ content increased slightly or decreased slightly. This may be explained by the fact that the enclosed hot-wind dryer kept tea away from fumes caused by coal combustion[26]. In order to determine the pollutant source, the AQ levels in the atmosphere were analyzed, resulting in a significant gap between the two workshops. The main reason for this is that the coal used in the fixation, first drying and re-drying stages would generate AQ during incomplete combustion. These AQ were then adsorbed in the small particles of solids after coal combustion and dispersed in the air, elevating the levels of AQ pollution in the workshop environment[15]. Over time, due to the large specific surface area and adsorption capacity of tea, these particulates then settled on the surface of the tea leaves, resulting in the increase of AQ in production. Therefore, coal combustion was thought to be the main route leading to excessive AQ contamination in tea processing, with fumes being the source of pollution.
As for oolong tea processing, AQ were increased under processing with both heat sources, but the difference between the two heat sources was significant. The results also suggested that coal as a heat source played a major role in increasing AQ level, and the fixation was deemed as the main step for increasing AQ contamination in oolong tea processing based on the PFs. During the oolong tea processing with natural gas-electric hybrid as a heat source, the trend of AQ level was stagnating below 0.005 mg/kg, which was similar to that in green tea with electricity, suggesting that clean energy, such as electricity and natural gas, can decrease the risk of producing AQ contaminants from processing.
As for sampling tests, the results showed that the situation of AQ contaminantion was worse when using coal as a heat source rather than electricity, which could be due to the fumes from combustion of coal coming into contact with tea leaves and lingering around the workplace. However, though it was obvious that electricity was the cleanest heat source during tea processing, there were still AQ contaminant in tea products using electricity as the heat source. The situation seems slightly similar to previously published work in which the reaction of 2- alkenals with hydroquinones and benzoquinones was suggested as a potential chemical pathway[23], reasons for this will be investigated in future research.
-
In this work, the possible sources of AQ pollution in green and oolong tea were confirmed by comparative experiments based on improved GC-MS/MS analytical methods. Our findings directly supported that the main pollutant source of high levels of AQ was fume caused by combustion, which not only affected the processing stages but also affected workshop environments. Unlike in the rolling and withering stages, where the changes in the level of AQ were inconspicuous, the stages with direct involvement of coal and firewood, such as fixation, are the main process in which AQ contamination rose due to the amount of contact between tea and fumes during these stages. Therefore, clean fuels such as natural gas and electricity were recommended as the heat source in tea processing. Additionally, the experimental results also showed that in the absence of fumes generated by combustion, there were still other factors contributing to trace AQ during tea processing, while small amounts of AQ were also observed in the workshop with clean fuels, which should be further investigated in future research.
-
Anthraquinone standard (99.0%) was purchased from Dr. Ehrenstorfer GmbH Company (Augsburg, Germany). D8-Anthraquinone internal standard (98.6%) was purchased from C/D/N Isotopes (Quebec, Canada). Anhydrous sodium sulfate (Na2SO4) and magnesium sulfate (MgSO4) (Shanghai, China). Florisil was supplied by Wenzhou Organic Chemical Company (Wenzhou, China). Mircro-glass fiber paper (90 mm) was purchased from Ahlstrom-munksjö company (Helsinki, Finland).
Sample preparation
-
The green tea samples were processed with fixation, rolling, first drying and re-drying (using enclosed equipment), while the oolong tea samples were processed with withering, making green (rocking and standing fresh leaves alternately), fixation, packed rolling, and drying. Samples from each step were collected three times at 100g after thorough mixing. All the samples were stored at −20 °C for further analysis.
Air samples were collected by glass fiber paper (90 mm) using medium volume samplers (PTS-100, Qingdao Laoshan Electronic Instrument Company, Qingdao, China)[27], running at 100 L/min for 4 h.
Fortified samples were spiked with AQ at 0.005 mg/kg, 0.010 mg/kg, 0.020 mg/kg for fresh tea shoots, at 0.005 mg/kg, 0.020 mg/kg, 0.050 mg/kg for dry tea and at 0.012 mg/kg (0.5 µg/m3 for air sample), 0.036 mg/kg (1.5 µg/m3 for air smaple), 0.072 mg/kg (3.0 µg/m3 for air sample) for glass filter paper, respectively. After shaking thoroughly, all the samples were left for 12 h, followed by extraction and clean-up steps.
The moisture content was obtained by taking 20 g of the sample after mixing each step, heating at 105 °C for 1 h, then weighing and repeating three times and taking the average value and dividing it by the weight prior to heating.
Sample extraction and clean-up
-
Tea sample: The extraction and purification of AQ from tea samples was performed based on the published method from Wang et al. with several adaptations[21]. Briefly, 1.5 g of tea samples were first mixed with 30 μL D8-AQ (2 mg/kg) and left to stand for 30 min, then well mixed with 1.5 mL deionized water and left to stand for 30 min. 15 mL 20% acetone in n-hexane was added to the tea samples and sonicated for 15 min. Then the samples were vortexed with 1.0 g MgSO4 for 30 s, and centrifuged for 5 min, at 11,000 rpm. After being moved to 100 mL pear-shaped flasks, 10 mL of the upper organic phase was evaporated to almost dryness under vacuum at 37 °C. 5 mL 2.5% acetone in n-hexane re-dissolved the extract in pear-shaped flasks for purification. The glass column (10 cm × 0.8 cm) consisted from bottom to top of glass wool and 2g florisil, which was between two layers of 2 cm Na2SO4. Then 5 mL of 2.5% acetone in n-hexane prewashed the column. After loading the redissolved solution, AQ was eluted three times with 5 mL, 10 mL, 10 mL of 2.5% acetone in n-hexane. The combined eluates were transferred to pear-shaped flasks and evaporated to almost dryness under vacuum at 37 °C. The dried residue was then reconstituted with 1 mL of 2.5% acetone in hexane followed by filtration through a 0.22 µm pore size filter. Then the reconstituted solution was mixed with acetonitrile at a volume ratio of 1:1. Following the shaking step, the subnatant was used for GC-MS/MS analysis.
Air sample: Half of the fiber paper, dripped with 18 μL d8-AQ (2 mg/kg), was immersed in 15 mL of 20% acetone in n-hexane, then sonicated for 15 min. The organic phase was separated by centrifugation at 11,000 rpm for 5 min and the entire upper layer was removed in a pear-shaped flask. All organic phases were evaporated to almost dryness under vacuum at 37 °C. 5 mL of 2.5% acetone in hexane redissolved the extracts for purification in the same way as in the tea samples.
GC-MS/MS analysis
-
Varian 450 gas chromatograph equipped with Varian 300 tandem mass detector (Varian, Walnut Creek, CA, USA) was used to perform AQ analysis with MS WorkStation version 6.9.3 software. Varian Factor Four capillary column VF-5ms (30 m × 0.25 mm × 0.25 μm) was used for chromatographic separation. The carrier gas, helium (> 99.999%), was set at a constant flow rate of 1.0 mL/min with collision gas of Argon (> 99.999%). The oven temperature started from 80 °C and held for 1 min; increased at 15 °C/min to 240 °C, then reached 260 °C at 20 °C/min and held for 5min. The temperature of the ion source was 210 °C, as well as the transfer line temperature of 280 °C. The injection volume was 1.0 μL. The MRM conditions are shown in Table 3.
Table 3. MRM conditions of 9,10-anthraquinone and D8-anthraquinone.
Equipment Compound TR (min) Parent ion (m/z) Daughter ion (m/z) Collision energy Vairan 450GC-300MS 9,10-anthraquinone 13.02 208 152 22 180 10 D8-anthraquinone 12.99 216 160 20 188 10 Agilent 8890GC-7000DMS 9,10-anthraquinone 12.15 208 152 30 180 10 D8-anthraquinone 12.19 216 160 30 188 10 Agilent 8890 gas chromatograph equipped with Agilent 7000D triple quadrupole mass spectrometer (Agilent, Stevens Creek, CA, USA) was used to analyze the purification effect with MassHunter version 10.1 software. Agilent J&W HP-5ms GC Column (30 m × 0.25 mm × 0.25 μm) was used for chromatographic separation. The carrier gas, Helium (> 99.999%), was set at a constant flow rate of 2.25 mL/min with collision gas of Nitrogen (> 99.999%). The temperature of EI ion source was adjusted at 280 °C, the same as the transfer line temperature. The oven temperature started from 80 °C and was held for 5 min; raised by 15 °C/min to 240 °C, then reached 280 °C at 25 °C/min and maintained for 5 min. The MRM conditions are shown in Table 3.
Statistical analysis
-
The AQ content in fresh leaves was corrected to dry matter content by dividing by moisture content in order to compare and analyze AQ levels during processing.
The changes of AQ in tea samples were evaluated with Microsoft Excel software and IBM SPSS Statistics 20.
Processing factor was used to describe the changes in AQ during tea processing. PF = Rl/Rf , where Rf is the AQ level before the processing step and Rl is the AQ level after the processing step. PF indicates a decrease (PF < 1) or an increase (PF > 1) in AQ residual during a specific processing step.
ME indicates a decrease (ME < 1) or an increase (ME > 1) in response to the analytical instruments, which is based on the ratio of slopes of calibration in the matrix and solvent as follows:
ME = (slopematrix/slopesolvent − 1) × 100%
Where slopematrix is the slope of calibration curve in matrix-matched solvent, slopesolvent is the slope of calibration curve in solvent.
This work was supported by Science and Technology Major Project in Zhejiang Province (2015C12001) and National Science Foundation of China (42007354).
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Jiawei Yu, Li Zhou
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Yu J, Zhou L, Wang X, Yang M, Sun H, et al. 2022. 9,10-Anthraquinone contamination in tea processing using coal as heat source . Beverage Plant Research 2: 8 doi: 10.48130/BPR-2022-0008
9,10-Anthraquinone contamination in tea processing using coal as heat source
- Received: 27 February 2022
- Accepted: 01 April 2022
- Published online: 09 May 2022
Abstract: 9,10-Anthraquinone (AQ) is a contaminant with a potential carcinogenic risk and occurs in tea worldwide. The maximum residue limit (MRL) of AQ in tea set by the European Union (EU) is 0.02 mg/kg. The possible sources of AQ in tea processing and the main stages of its occurrence were investigated based on a modified AQ analytical method and gas chromatography-tandem mass spectrometry (GC-MS/MS) analysis. Compared with electricity as the heat source in green tea processing, AQ increased by 4.3 to 23.9 times in tea processing with coal as the heat source, far exceeding 0.02 mg/kg, while the AQ level in the environment tripled. The same trend was observed in oolong tea processing under coal heat. The steps with direct contact between tea leaves and fumes, such as fixation and drying, are considered as the main steps of AQ production in tea processing. The levels of AQ increased with the rising contact time, suggesting that high levels of AQ pollutant in tea may be derived from the fumes caused by coal and combustion. Fourty samples from different workshops with electricity or coal as heat sources were analyzed, ranged from 50.0%−85.0% and 5.0%−35.0% for detection and exceed rates of AQ. In addition, the maximum AQ content of 0.064 mg/kg was observed in the tea product with coal as the heat source, indicating that the high levels of AQ contamination in tea products are likely to be contributed by coal.
-
Key words:
- 9,10-Anthraquinone /
- Tea processing /
- Coal /
- Contamination source