-
The tea plant (Camellia sinensis (L.) O. Kuntze) is a perennial woody plant that is widely known for its high economic, health and cultural value[1,2]. Tea plants have been planted in more than 60 countries around the world as an important economic horticultural crop[3]. Tea plants leaves are generally used to produce a prominent beverage that is the world’s most popular and consumed nonalcoholic beverage after water[4]. There are abundant characteristic compounds in tea that are strongly associated with the quality and health benefits of tea, such as tea polyphenols, theanine, and caffeine[4−6]. It is widely known that tea's metabolite composition and quality are affected by many factors, including some biotic and abiotic factors[7]. Such as drought stress[8−10], low temperature[11−13], heavy metal toxicity[14], insect attack[15,16], and pathogen infection[17−19].
Tea plant diseases are one of the factors reducing tea yield and quality. Meanwhile, foliar disease is one of the most serious diseases of tea plants causing severe tea production losses. It has been reported that a number of foliar blight diseases of tea plants are infected by different fungal pathogens[19]. A disease called tea gray blight is caused by Pseudopestalotiopsis camelliae-sinensis and Pestalotiopsis-like species, resulting in serious losses in tea quality and production[17,20,21]. Anthracnose of the tea plant caused by the Colletotrichum species is also a serious foliar disease of the tea plant, causing severe losses in yield and quality of tea products.[22,23]. At present, many research reports have found that many tea leaf diseases are caused by many new pathogens such as Colletotrichum fructicola[23], Epicoccum layuense[19], Arthrinium arundinis[1], Colletotrichum acutatum[24], Alternaria longipes[25], Epicoccum nigrum[26] and so on. The pathogens that cause these foliar diseases can cause the loss of tea production.
In this study, brown blight disease of tea plants was observed at a tea plantation in Shucheng County, Anhui Province, China. There are reddish brown lesions at the leaf margins of diseased leaves, and the junction of healthy and diseased leaf is obvious (Fig. 1a). One of the fungal isolates was isolated from diseased leaves of tea plants by a traditional fungus separation method, and identified as Muyocopron laterale through a combination of morphology and molecular biology. The Koch hypothesis was successfully fulfilled with the re-isolation of M. laterale from symptomatic plants. This is the first report of leaf disease caused by M. laterale on the tea plant (C. sinensis) in China. The results will provide a basis for controlling the management of tea plant leaf disease in future.
-
In August 2020, a leaf blight disease of tea plant was observed and collected from tea plantations ('Shuchazao', SCZ) located in Shucheng County, in the Anhui Province of China. The symptom of the diseased leaf is reddish brown deadness with irregular margins, and the junction of healthy and diseased leaf is obvious (Fig. 1a). The malt extract agar (MEA), oat agar (OA) and potato dextrose agar (PDA), medium were purchased from HuanKai Microbial (Guangdong, China).
Isolation of fungal pathogens
-
Small tissues (4−6 mm2) were taken from the infected sections of the diseased leaf (junction of healthy and diseased regions) and underwent surface disinfection by 75% ethanol for 45−60 s. After which, the sterile water was rinsed three times. On sterilized filter paper, each piece was blotted dry before being placed on a PDA, which was incubated in the dark at 25 °C for 5 d. In order to obtain a pure isolate, the single-hyphal tip was transferred to a new PDA. A representative isolate (AH-2020-A52) was obtained and deposited in the State key laboratory of tea plant biology and utilization.
Molecular biology identification of fungal pathogens
-
To identify the representative isolate (AH-2020-A52), nucleotide sequence of the small subunit of ribosomal DNA (18S; NS1/NS4), the internal transcribed spacer region of ribosomal DNA (ITS; ITS1/ITS4) and part of translation elongation factor 1-alpha (TEF1-α; EF1-983F/EF1-2281R) were amplified. Analyzing GenBank data with the 18S region, ITS sequence and TEF1-α region to identify, respectively, using BLAST (Basic Local Alignment Search Tool). The phylogenetic tree was generated by Neighbor-joining analysis using MEGA-X with the sequence of 18S, ITS and EF1-α.
Morphological identification of fungal pathogens
-
The pure isolate was cultivated on MEA, OA and PDA medium respectively and the morphological characteristics of the colony were observed. The size and morphology of fungal spores, hyphae and other reproduction organs were observed with a microscope (Olympus BX51, Olympus Corporation, Monolith, Tokyo, Japan).
Pathogenicity tests
-
Pathogenicity tests were conducted on 1-year-old C. sinensis seedlings. Sterile needles were used to generate two wounds per leaf of test tea leaves. Six millimetre mycelial plugs of isolate derived from 10-day-old cultures grown on PDA were inoculated to test leaves. Control leaves were treated with 6-mm agar plugs. The experiments were repeated three times with five biological replicates. All tea seedlings were placed in a black box for 24 h, and then were growth in a greenhouse at 25 °C and 70% relative humidity.
-
The isolate (AH-2020-A52) was inoculated in tea leaves, some strains formed black ascocarp which was observed on the tea tissue after 10−15 d (Fig. 1). Eight-spored asci that are obpyriform, bitunicate, or slightly obpyriform and are pedicellate. (Fig. 1). The ascospore were hyaline, oval to obovoid, with obtuse ends, aseptate, and granular in appearance. The measurements were 7.34 to 12.04 µm × 4.88 to 7.25 µm (mean, 9.60 µm × 6.26 µm; n = 40) (Fig. 1). The morphology of the isolate (AH-2020-A52) was consistent with the description of M. laterale [27].
Further, elevated aerial mycelium appeared on MEA medium at 25 °C after 10 d with white, buff, or pale gray mycelium; reverse umber, paler dark umber toward the periphery. (Fig. 2a & b). At 25 °C after 10 d, an aerial mycelium is scarce, cottony, buff with an apricot center and paler to the periphery on OA medium; reverse buff with brick center and apricot periphery (Fig. 2c & d). The purified colony on PDA after 10 d at 25 °C, floccose with aerial mycelium scarce, white or brown, brown in the center to paler to the periphery, irregular margin; reverse zonate and brown in the center, white to the periphery (Fig. 2e & f). These morphological findings was also similar with M. laterale.
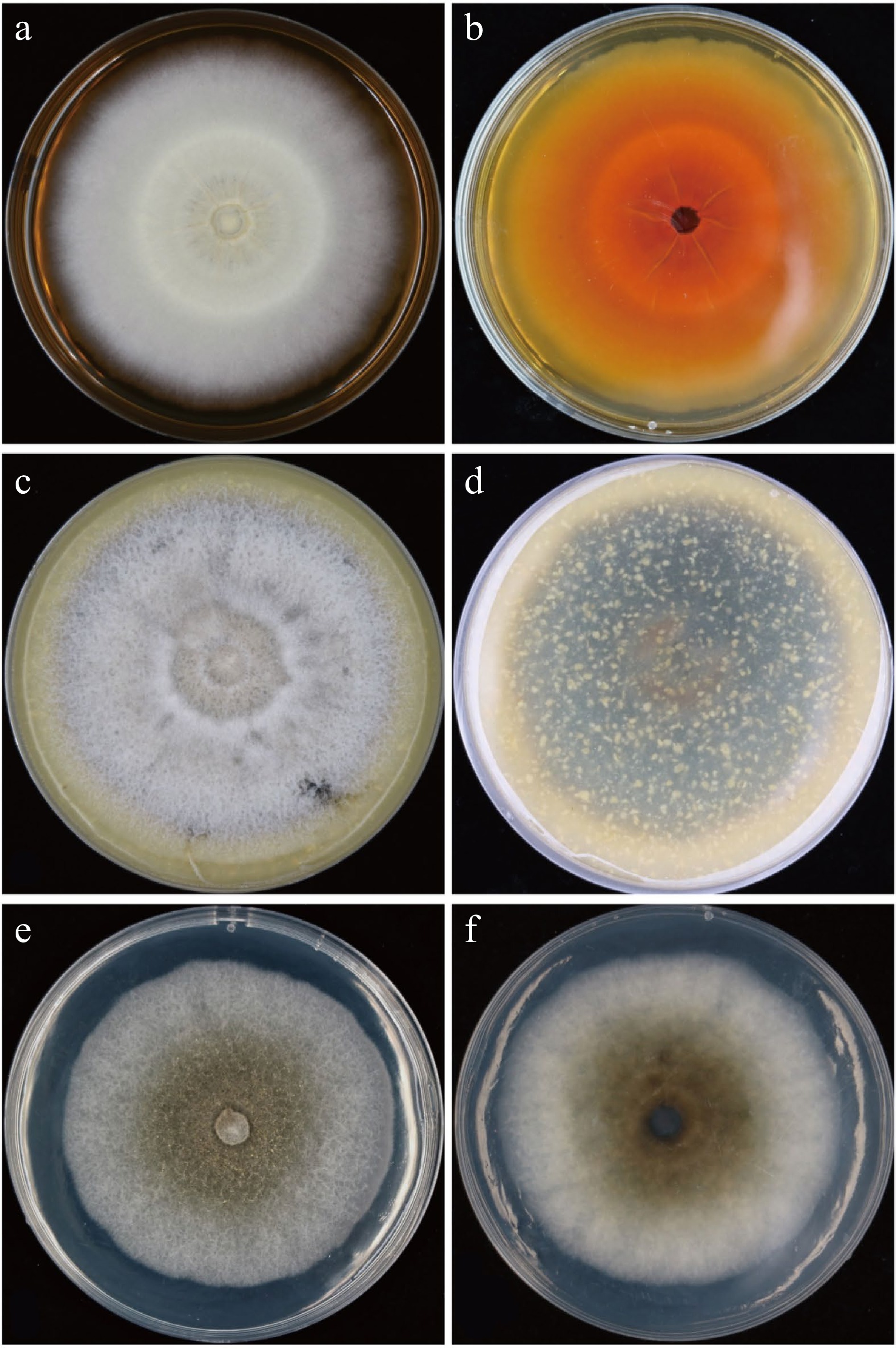
Figure 2.
Growth of Muyocopron laterale (AH-2020-A52) on different culture media. (a) & (b) Culture characteristic on MEA (upper and dorsa) for 10 d. (c) & (d) Culture characteristic on OA (upper and dorsa) for 10 d. (e) & (f) Culture characteristic on PDA (upper and dorsa) for 10 d.
To confirm the identity of this putative pathogen at the molecular level, nucleotide sequence of the small subunit of ribosomal DNA (18S; NS1/NS4), the internal transcribed spacer region of ribosomal DNA (ITS; ITS1/ITS4) and part of translation elongation factor 1-alpha (TEF1-α; EF1-983F/EF1-2281R) were analyzed[28]. The sequences were deposited in GenBank (18S: MW653327, ITS: MW653328, EF1-α: MW661229). Following alignment of the resultant sequences with GenBank via a BLAST analysis, for 18S region showed 99.1% similarity with M. lithocarpi (GenBank accession numbers: MK447740.1, MW079368.1). While, the ITS and TEF1-α region highest nucleotide sequence identity with M. laterale reference sequence (ITS: 99.34%, NR_164055.1; EF1-α: 99.33%, MK495970.1). In order to more accurately identify the molecular level and taxonomic status of the Fungal Pathogens, Phylogram was generated with MEGA-X using bootstrap analysis with 1000 replicates and bootstrap support values equal to or greater than 50% are shown at the nodes. Phylogenetic tree generated by Neighbor-joining analysis using MEGA-X with the sequence of 18S, ITS and EF1-α placed AH-2020-A52 in the clade of M. laterale (Fig. 3). Based upon these morphological and molecular results, this pathogen was identified as M. laterale.
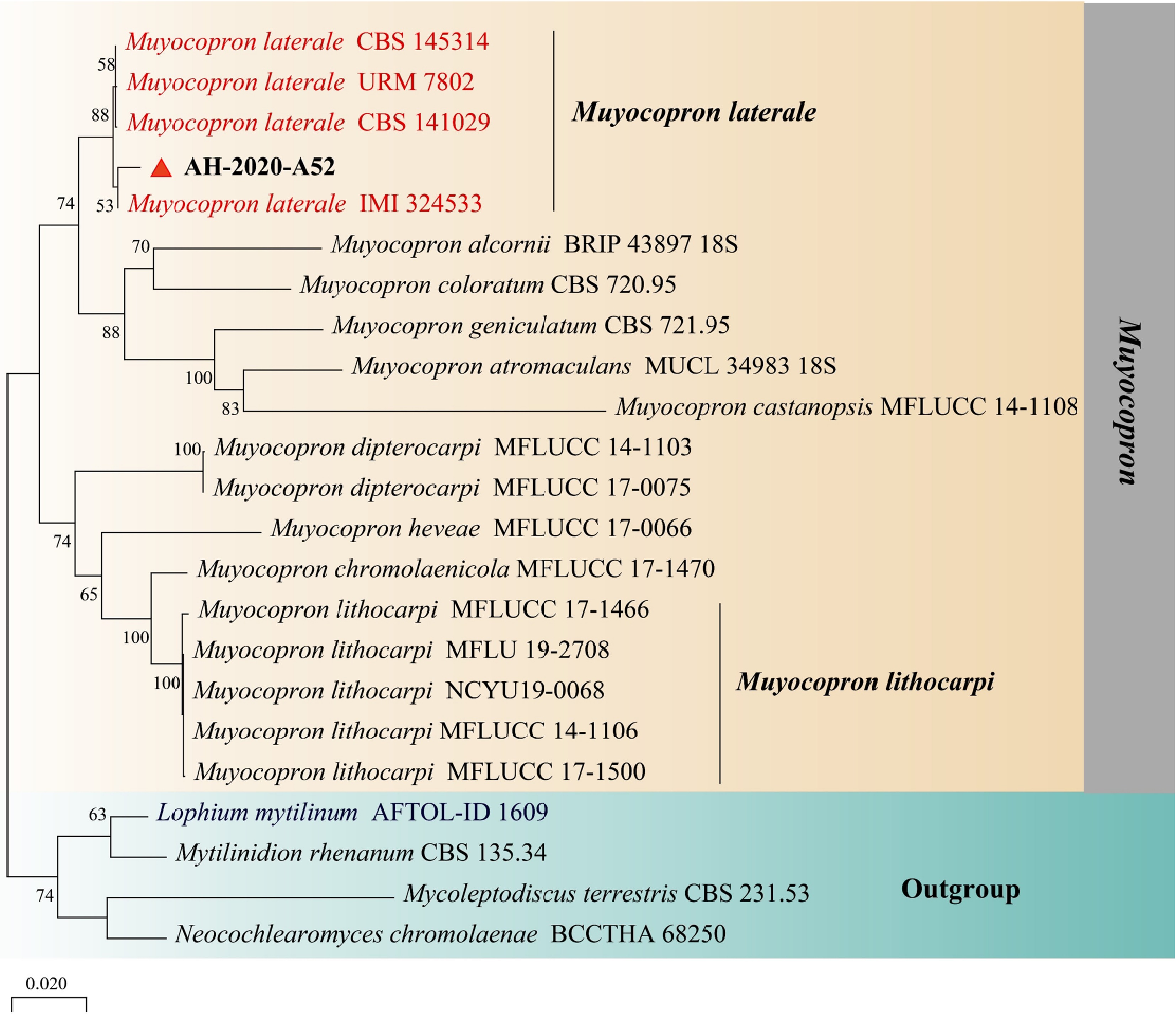
Figure 3.
Phylogenetic tree generated by Neighbor-joining analysis based on combined dataset of 18S, ITS, and TEF1 sequence data. Lophium mytilinum AFTOL-ID 1609, Mytilinidion rhenanum CBS 135.34, Mycoleptodiscus terrestris CBS 231.53 and Neocochlearomyces chromolaenae BCCTHA 68250 were selected as outgroup taxa. Phylogram was generated with MEGA-X using bootstrap analysis with 1000 replicates and bootstrap support values equal to or greater than 50% are shown at the nodes. The M. laterale sequences are in red and M. laterale strain AH-2020-A52 is in bold.
Pathogenicity of M. laterale
-
The mycelium of M. laterale were inoculated on adult leaves and tender leaves of tea seedlings, respectively. The tea leaves were infected by the strain AH-2020-A52, and showed obvious symptoms, reddish-brown spots, in the inoculated leaves after 7 d (Fig. 4a). In contrast, control seedlings remained healthy and asymptomatic (Fig. 4b). We were again able to re-isolate M. laterale from the infected tea seedlings. The re-isolates were ultimately identified as the pathogenic fungal M. laterale based on morphological and molecular analyses, and thus fulfilling Koch's postulate.
-
A fungal pathogen was isolated and identified as M. laterale from diseased leaves of tea plants. As far as we are aware, this is the first report of M. laterale being isolated from leaves of tea plants suffering from leaf blight disease in China. This result will be providing a foundation effort aimed at presenting tea plant diseases caused by this pathogen. Further studies should pay attention to occurrence, spread and control tea plant disease caused by M. laterale in China. This research will provide a basis for controlling the management of tea plant leaf diseases in future.
This research was funded by the National Natural Science Foundation of China (31902075), National Key Research and Development Program of China (2021YFD1601103), Anhui Provincial Department of Education (KJ20198A0196), Anhui Key Research and Development Program (202004e11020006) and Anhui Provincial Postdoctoral Fund (2017B232).
-
The authors declare that they have no conflict of interest.
- Copyright: 2022 by the author(s). Exclusive Licensee Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Jiang H, Zhang M, Zhou Y, Li X, Li J, et al. 2022. First report of Muyocopron laterale causing a new leaf disease of Camellia sinensis in China. Beverage Plant Research 2:10 doi: 10.48130/BPR-2022-0010
First report of Muyocopron laterale causing a new leaf disease of Camellia sinensis in China
- Received: 30 May 2022
- Accepted: 14 June 2022
- Published online: 23 June 2022
Abstract: Tea plant (Camellia sinensis
-
Key words:
- Tea plant /
- Camellia sinensis /
- Muyocopron laterale /
- Disease /
- Pathogens















