-
Alkaloids are heterocyclic nitrogen-containing compounds produced by higher plants and some microorganisms. They usually have remarkable biological activity and pharmacological effects, and are widely distributed in more than 2,000 kinds of plants in more than 100 families[1]. Tea, coffee and cocoa are the three most popular non-alcoholic beverages in the world. They all contain purine alkaloids such as caffeine, and theobromine. Some tea plants also contain theacrine, another purine alkaloid with a different function. Purine alkaloids not only affect the taste and quality of tea, but also have health benefits. The content profiles of main purine alkaloids in Chinese tea germplasm, the molecular mechanism of purine alkaloid metabolism in some hypernormal tea germplasms, the potential of DNA markers in marker-assisted selection and genetic engineering in the low caffeine/high theacrine tea breeding were comprehensively reviewed. In order to better understand the content profile of purine alkaloids in tea germplasms, the function of key genes in the purine alkaloid biosynthesis pathway, as well as providing a roadmap for special tea cultivar's genetic improvement in the future.
-
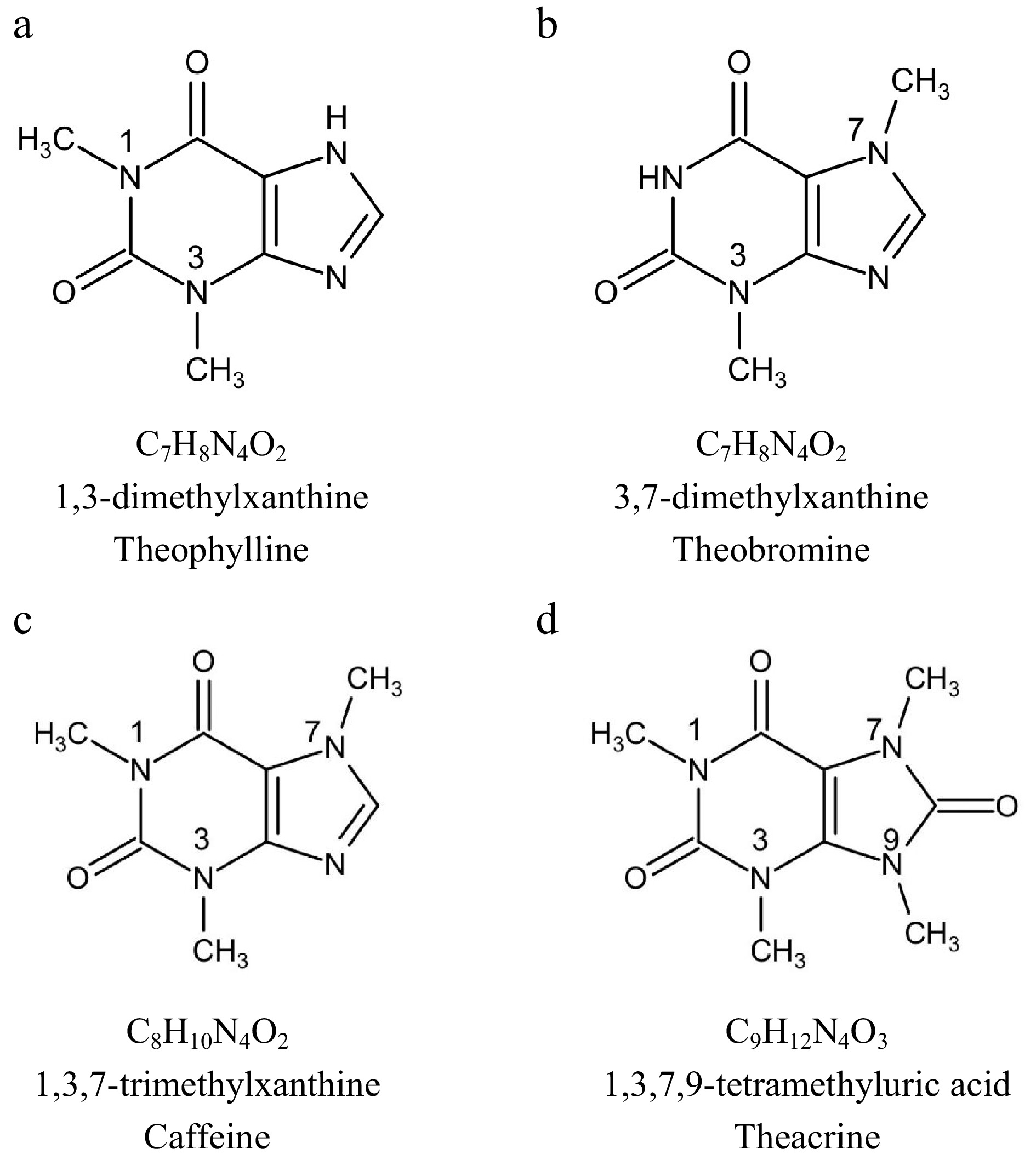
The alkaloids in tea plants are mainly purine alkaloids, with a small amount of pyrimidine base. Purine alkaloids are the secondary metabolite of purine nucleotide[2], including methylxanthine and methyluric acid. Their chemical structures are based on the skeleton of xanthine and uric acid[3], differing by the number and position of methyl groups on the purine ring. The three main purine alkaloids in tea plants are caffeine, theobromine and theacrine.
Caffeine
-
1,3,7-trimethylxanthine, its chemical formula is C8H10N4O2 (Fig. 1c). Caffeine is the most abundant purine alkaloid in tea, generally accounting for 2%−5% of the dry matter content[4]. It is one of the most important quality and functional components in tea. Chen & Zhou[5] analyzed the caffeine content of 596 accession tea germplasms preserved in China National Germplasm Hangzhou Tea Repository (CNGTR), which basically represent the genetic diversity of Chinese tea plants. The caffeine content varied from 1.2% to 5.9%, averaging 4.2%.
Theobromine
-
3,7-dimethylxanthine, the chemical formula is C7H8N4O2 (Fig. 1b). Theobromine, a precursor for caffeine synthesis, lacks a methyl group at the N-1 position compared to caffeine[6]. Theobromine is the main alkaloid of the cocoa plant. Generally, theobromine content is less in tea plants, and the theobromine content in young leaves is 0.05%−0.80%[7]. Among the tea plants in Sect. Thea (L.) Dyer Camellia L., C. ptilophylla Chang discovered in the 1980s is a new tea plant resource with high theobromine (4.7%) and no caffeine[8]. Additionally, C. gymnogyna Chang[9], C. irrawadiensis P. K. Barua, C. crassicolumna Chang, C. purpurea Chang et Chen[10], Hongyacha[11] are all naturally rich in theobromine, no or low caffeine tea genetic resources, respectively.
Theacrine
-
1,3,7,9-tetramethyluric acid, the chemical formula is C9H12N4O3 (Fig. 1d), the structure is similar to caffeine, the difference is that the C8 position is increased by a carbonyl group and the N9 position is increased by a methyl group[12]. Theacrine is a special purine alkaloid contained in Kucha, a special tasting bitter tea plant. Kucha once was classified into C. kucha (Chang et Wang) Chang[13], C. sinensis var. kucha and C. assamica var. kucha, but actually it has no significant morphological difference with C. sinensis. Ye et al. isolated and identified a high content of 1,3,7,9-tetramethyluric acid (1.29%) in the young shoots of Kucha for the first time, and they found a new distribution pattern of purine alkaloids in tea plants[14]. The theacrine content in the tender shoots with 'two and a bud' of Kucha was 1.3%−3.4%. The pure theacrine could be obtained from Kucha leaf by high-speed countercurrent chromatography[15].
In addition, the purine alkaloids found in tea also include theophylline (1,3-dimethylxanthine) (Fig. 1a), which is the isomer of theobromine, and the content in tea is much lower, generally about 0.05%. It occurs naturally in trace amounts in coffee and cocoa (Table 1).
Table 1. Purine alkaloids in tea, coffee and cacao (%).
Purine alkaloids Tea Coffee Cacao Arabica Robusta Caffeine 2.0−5.0 1−1.5 2−2.7 1.5 Theobromine 0.06−1.0 Trace Trace 1.8 Theacrine 0.5−3.6 (rare) / / / Theophylline 0.05 Trace Trace Trace Physiological function
-
Purine alkaloids not only affect the flavor of tea, but also have important biological activities and physiological functions. Although several major purine alkaloids in tea have similar structures, their functions are quite different.
Caffeine
-
At present, the role of purine alkaloids in plants has not been fully determined, there are two hypotheses to explain the physiological role of caffeine in plants, the 'chemical defense' and 'allelopathic function' theories[16]. The 'chemical defense' proposed that high concentrations of caffeine in young tissues and fruits of Coffea arabica and Camellia sinensis have a defensive effect, protecting young shoots from pathogens and reducing herbivore feeding. And the 'allelopathic theory' proposes that the release of caffeine in the seed coat and fallen leaves into the soil can inhibit the growth of competitive plants and regulate the plant spacing[17]. Caffeine in plants has long been thought to have a defensive effect, acting as a natural pesticide and antifeedant. But a recent study found that caffeine, which is naturally present in nectar of plants such as Citrus and Coffea, has no repellent effect on bees. Instead, it may affect the behavior of pollinators to enhance pollination and protect the normal reproduction of plants[18].
Caffeine occurs naturally in many foods and beverages, and most caffeine is consumed as coffee and tea. In long-term consumption, it has been found that caffeine has significant effects on cognitive, physical and ocupational performance[19]. Current research suggests that low to moderate (approximately 40−300 mg or ~0.5−4.0 mg kg−1) doses of caffeine improve cognition, which is mainly manifested in improving alertness, learning and memory[19]. Caffeine is structurally similar to adenosine, and exerts central nervous system (CNS) effects by antagonizing adenosine receptors (ARs)[20], affecting brain function. Epidemiological studies have shown that caffeine, as a non-selective adenosine receptors antagonist[21], has the potential to treat cognitive disorders, including Alzheimer's disease, Parkinson's disease, depression, and schizophrenia[20]. It has been confirmed in many studies involving animals and humans[22]. By studying a transgenic mouse model for Alzheimer's disease (AD), it was found that caffeine can rapidly reduce the level of abnormal protein (amyloid-β; Aβ) in the plasma of AD mice and humans (the core of AD pathogenesis)[23], and reverse AD-induced cognitive impairment in aged mice[24]. Decreased levels of Aβ protein may be the mechanism of action of caffeine to improve cognition[25]. In addition, studies have shown that caffeine also has pharmacological effects such as anti-obesity[26], analgesia[27], diuresis[28], reducing the risk of type II diabetes[29] and cardiovascular mortality[30].
Low doses of caffeine had positive effects on cognition, while higher doses improved exercise performance. Doses > 200 mg (~3 mg kg−1) of caffeine showed ergogenic properties on endurance, muscle strength, and high-intensity exercise[19]. Special occupational groups, especially athletes, should control the time and dosage of caffeine intake.
Theobromine
-
Compared to caffeine, theobromine's effects are not well studied. Caffeine is a central nervous system stimulant, while theobromine is a smooth muscle stimulant, promoting vasodilation[31]. The hypotensive effect of cocoa are well established[32], theobromine seems to be partially responsible for this effect. A randomized, double-blind crossover trial demonstrated that high-dose theobromine lowers central systolic blood pressure and is beneficial for cardiovascular health in humans[33]. In addition, theobromine may have hypoglycemic effects[34]. In many cases, theobromine is considered a natural compound with therapeutic properties. An in vitro study have shown that theobromine inhibits uric acid crystallization and has high clinical potential in the treatment of uric acid nephrolithiasis[35]. Usmani et al. demonstrated for the first time that theobromine suppresses cough without CNS side effects. It can be used clinically to treat acute and chronic cough[36]. Similar to caffeine, theobromine has potential obesity therapeutic[37] and neuroprotective effects[38]. Notably, theobromine is less stimulatory to the CNS and may have a higher potential than caffeine. In addition, theobromine also has special effects of protecting tooth enamel[39], immune regulation[40], and anti-inflammation.
Theacrine
-
Sheng et al.[41] comprehensively reviewed the beneficial health effects of theacrine, including antioxidant effect, anti-inflammatory effect, reducing fatigue effects, improving cognitive effect and hypnotic effect. Theacrine has been identified as non-toxic by toxicological tests, and a clinical study in which subjects took theacrine supplementation for 8 weeks also concluded that theacrine is safe for humans[42]. Its potential medicinal functions have been studied in recent years. A behavioral test showed that oral theacrine significantly reversed central fatigue-induced learning and memory impairments[43]. There are many studies proving that theacrine can prevent Parkinson's disease by directly activating the Sirt3 signaling pathway, restoring mitochondrial function and inhibiting the apoptosis of dopaminergic neurons[44]. Unlike caffeine, theacrine does not stimulate central excitation and is now considered a new antidepressant purine alkaloid drug[45].
Theophylline
-
Theophylline occurs naturally in tea and cocoa beans. Although the content is extremely low, it has a high medicinal value and is an important alkaloid in tea plants. In 1985, theophylline was first extracted from tea and chemically synthesized. It was found that theophylline has diuretic and bronchodilating effects. Recent studies have shown that low-dose theophylline helps treat asthma and chronic obstructive pulmonary disease (COPD), with multiple anti-inflammatory effects. Clinically, it can be administered intravenously or orally. It is worth noting that high-dose theophylline has side effects, such as headache, vomiting and arrhythmia, clinical use should pay attention to the recommended dose[46].
-
Purine alkaloids are secondary metabolites of purine nucleotides. Studies have shown that adenine nucleotide are the most effective presumed precursors in caffeine biosynthesis[47]. Tea alkaloids are methylxanthine derivatives, xanthine is considered to be the most important precursor in caffeine synthesis, and SAM (S-adenosyl methionine) is an active methyl donor in caffeine biosynthesis[48]. Koshiishi et al.[49] proposed the main pathway for SAM to xanthosine (SAM → SAH (S-adenosyl-homocysteine) → adenosine → adenine → AMP → IMP → XMP → xanthosine), and believed that the purine ring in caffeine also came from SAM. In addition to the SAM pathway, xanthine can also be synthesized through the AMP pathway, the GMP pathway and the de novo pathway. Currently, it is believed that tea plants in Sect. Thea (L.) Dyer Camellia L. mainly synthesize xanthine through the SAM pathway and the AMP supplementation pathway (adenosine → AMP), while Coffea arabica mainly synthesize xanthine through the AMP pathway[50] (Fig. 2).
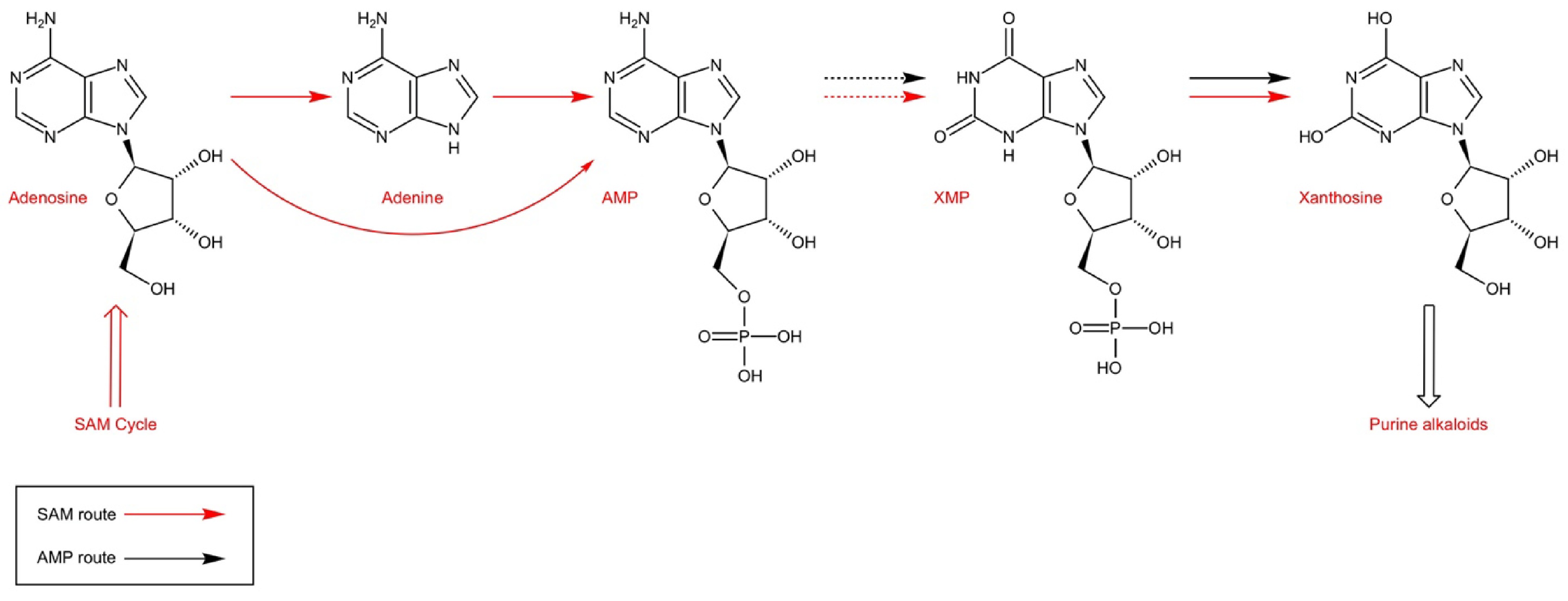
Figure 2.
Purine alkaloids are synthesized from xanthosine which is produced via two main routes in tea and coffee: SAM route and AMP route (adapted from Ashihara et al.[16]).
Ashihara et al.[16] reviewed the main caffeine biosynthetic pathway, which is the four-step reaction of caffeine synthesis from xanthine, including one nucleosidase step and three methylation steps: xanthosine → 7-methylxanthosine → 7-methylxanthine → theobromine → caffeine (Fig. 3) . Among them, the tea caffeine synthase (TCS), which catalyzes N-3 and N-1 methylation, is considered to be the most critical enzyme in the caffeine biosynthetic pathway of tea plants.
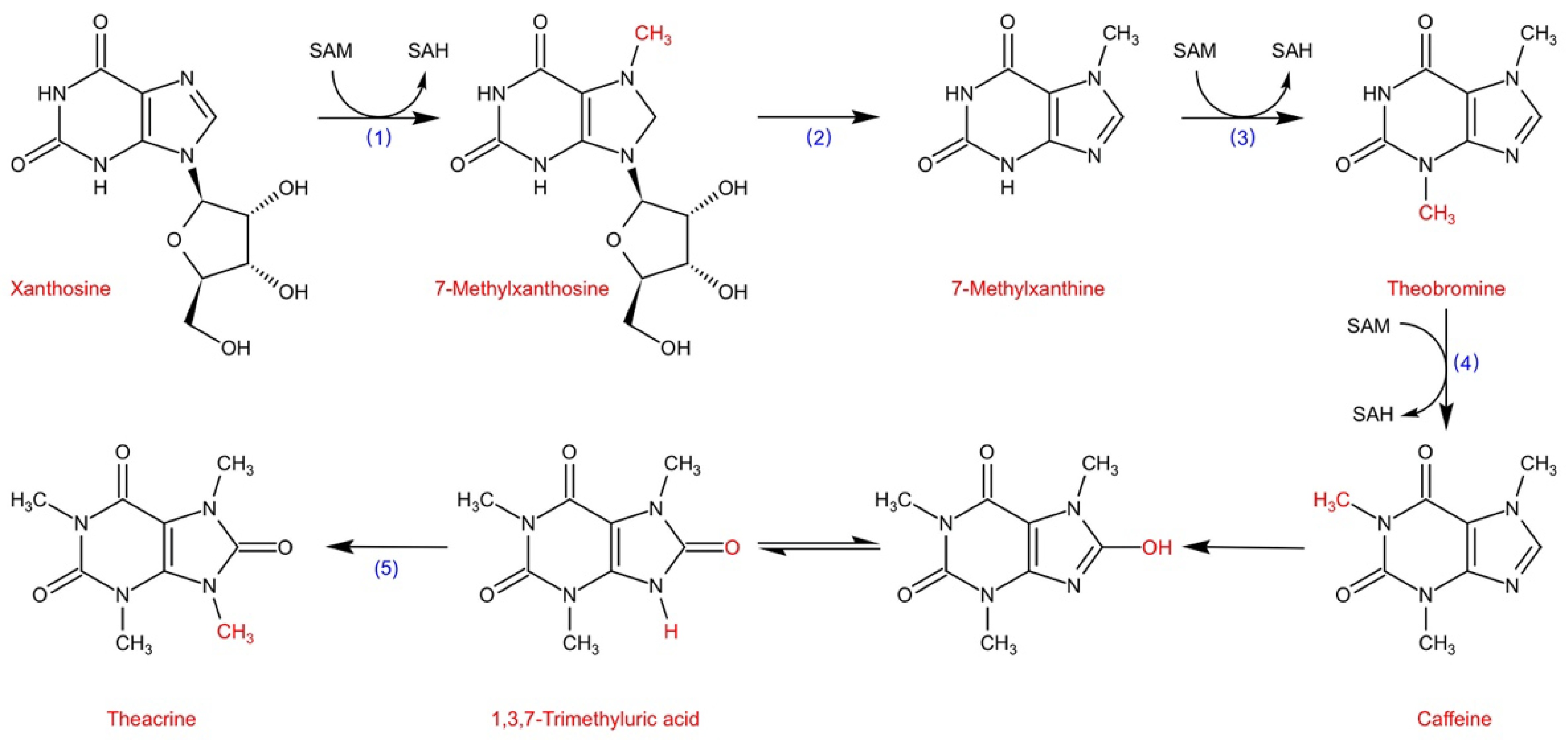
Figure 3.
The main purine alkaloids biosynthesis pathway in tea plants (Adapted from Ashihara et al.[16]). (1) xanthosine 7-N-methyltransferase; (2) N-methylnucleosidase; (3) monomethylxanthine N-methyltransferase; (4) 3,7-dimethylxanthine N-methyltransferase TCS1; (5) 1,3,7-trimethyluric acid N-methyltransferase (CkTcS).
Although the caffeine biosynthetic pathway has been extensively studied in tea plants and other caffeine-rich plants, the regulatory mechanism of caffeine synthesis remains unclear. In a recent study, metabolite profiling and transcriptome profiling provided a comprehensive understanding of the transcriptional regulatory mechanisms of caffeine biosynthesis and metabolism in tea and coffee. The analysis concluded that the regulatory mechanisms of caffeine biosynthesis in tea and coffee plants are partially conserved[51]. In addition, transcription factors related to the caffeine biosynthesis pathway were identified, and it was determined that CsMYB184 positively regulates caffeine biosynthesis in tea plants[52].
Screening and identification of TCS1 alleles
-
In 2000, the tea caffeine synthase (TCS) was first cloned[53], it belongs to N-methyltransferase (NMT), and studies have shown that high caffeine biosynthesis in tea plant shoots mainly depends on the expression of TCS1 gene[16]. The full-length DNA genome of 6 NMTs from 'Baiye 1' (TCS1-TCS6) were cloned and found that TCS1 has the activity of catalyzing N-3 and N-1 methylation, while TCS2 does not have N-methylation transferase activity; the expression levels of TCS3-TCS5 are very low in tea shoots, and TCS6 is a pseudogene. It is believed that in the NMT gene family, the function of TCS1 is the most important for the synthesis and regulation of caffeine[54]. Further research found that TCS1 has seven types of allelic variations, namely TCS1a-TCS1g, and these genes were all composed by four exons and three introns through analysis of gene structure. Among them, TCS1a is the predominant allele, and TCS1b-g is the rare allele, which only appears in a few wild germplasms. The natural variation of TCS1 change the transcription level. TCS1 alleles with low transcription levels or its encoded proteins with only TS activities (TCS1b, TCS1c, TCS1g), and TCS1 alleles with high transcription levels or its encoded proteins with both TS and CS activities (TCS1a, TCS1d, TCS1e, TCS1f), suggesting that the allelic variation of TCS1 plays an important role in caffeine biosynthesis[55]. The TCS1a expression protein present in regular tea plant resources converts theobromine accumulated in tea plants into caffeine, so caffeine becomes the purine alkaloid with the highest content in regular tea plants; and the allelic variation with only TS activity is an excellent allelic variation that can be exploited during the breeding of low-caffeine tea cultivars[55,56].
In addition, the 225th and 269th amino acids of TCS1 were identified as key active sites by site-directed mutagenesis. The 225th amino acid residue was arginine or histidine, which determined whether TCS1 had caffeine synthase activity[55]. By resequencing the TCS1 locus of 44 accessions tea genetic resources, 87 SNPs were identified. And two polymorphic sites, SNP4318 and SNP6252, were selected to develop CAPS markers[57]. Through association analysis, SNP4318 was determined to be associated with caffeine content in four environments, explaining 4.0%–7.7% of the phenotypic variance[57].
QTL mapping for caffeine and theobromine
-
A F1 pseudo-testcross population which was generated by crossing between C. sinensis var. sinensis 'Yingshuang' and C. sinensis var. pubilimba 'Beiyue Danzhu' was used for purine alkaloids QTL (quantitative trait locus) analysis[58]. Ten QTLs were identified for four measurement years in total: 1 controlled caffeine content (CAF), 4 controlled theobromine content (TBR), 2 controlled sum of caffeine and theobromine (SCT), 3 controlled caffeine-to-theobromine ratio (CTR)[58]. The major QTL that controls caffeine content (qCAF1) was mapped onto Linkage Group (LG) 01, explaining an average of 20.1% of the phenotypic variance, helping to elucidate the molecular mechanisms of theobromine and caffeine accumulation for genetic improvement of tea plants.
Theacrine biosynthesis in tea plants
-
Theacrine has special health-care effects, and it has, in recent years, gradually become well known to the public, but the molecular mechanism of its metabolism is still unclear. Zheng et al.[59] used the 14C isotope tracer method to demonstrate for the first time that theacrine in Kucha leaves is synthesized from caffeine, which may produce the intermediate 1,3,7-trimethyluric acid and is further methylated to generate 1,3,7,9-tetramethyluric acid. Wang et al.[60] analyzed the transcriptome of Kucha and found that the NMT encoded by TEA024443 may catalyze N-9 methylation. Zhang et al.[61] identified the theacrine synthase (CkTcS) from Kucha, which has N9-methyltransferase activity and can only catalyze the methylation of 1,3,7-trimethyluric acid to form theacrine, confirming that C8 oxidation takes place prior to N9-methylation, but currently the mechanism of C8 oxidation is unclear (Fig. 2). The above findings are helpful to analyze the synthesis mechanism of theacrine and provide new approaches for the production of decaffeinated beverages.
Catabolism
-
Kalberer[62] first reported the catabolism of caffeine in Coffea arabica using 14C-labeled tracer experiment, and believed that caffeine could be completely degraded in coffee leaves. Ashihara et al.[63] reported the catabolism of purine alkaloids in Camellia sinensis, and indicated that the catabolism of caffeine in tea leaves is very slow, which is the main reason for the accumulation of caffeine in tea plants. The conversion of caffeine to theophylline is the major rate-limiting step of caffeine catabolism. At present, it is believed that caffeine catabolism begins with its conversion to theophylline catalyzed by N7-demethylase, and demonstrate that the major catabolic pathway is caffeine → theophylline → 3-methylxanthine → xanthine, and xanthine is further degraded by the conventional purine catabolism pathway to CO2 and NH3[16]. Theophylline and 3-methylxanthine produced during metabolism can be reused to synthesize caffeine in young leaves[16].
Some Camellia species accumulate other purine alkaloid instead of caffeine. Theobromine is the major purine alkaloid in C. ptilophylla, and studies have shown that theobromine is a precursor of caffeine, as opposed to a catabolite, of caffeine. Theobromine is finally degraded to CO2 and NH3 via 3-methylxanthine, xanthine[64]. In C. sinensis, caffeine can also be converted to theobromine to xanthine, but it is not the main catabolic pathway of caffeine (Fig. 4).
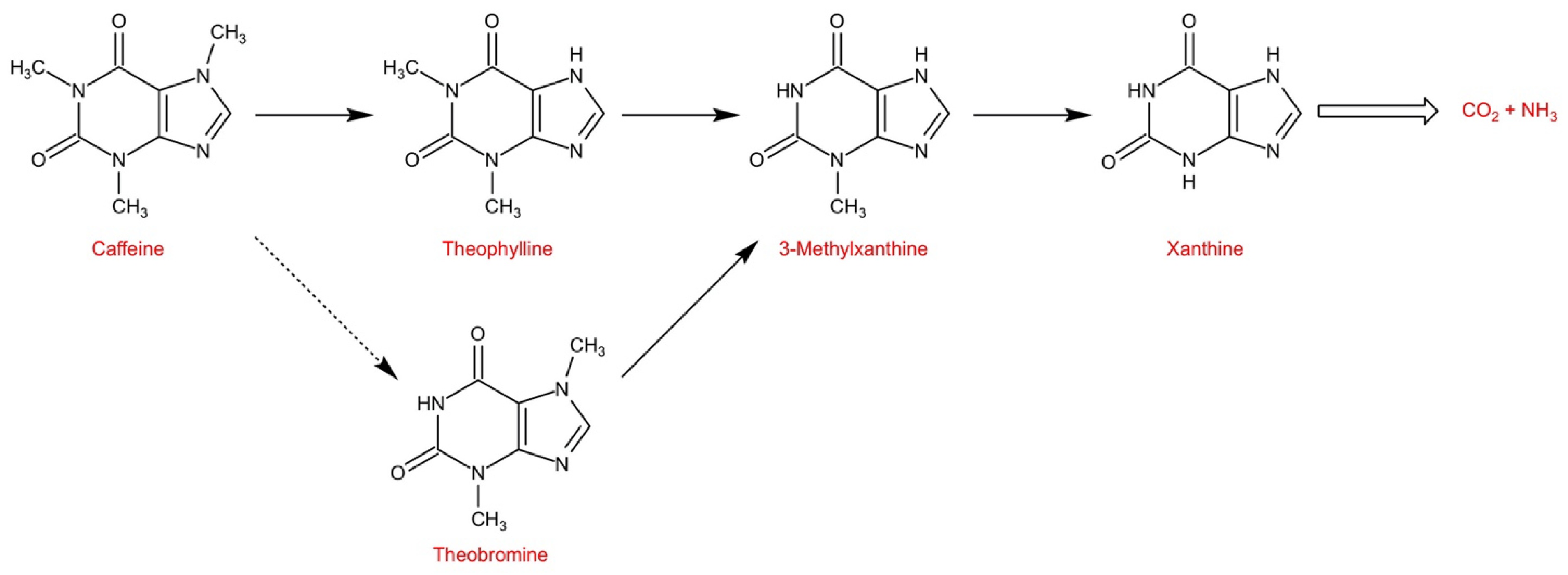
Figure 4.
The main purine alkaloids catabolic pathway in tea plants (Adapted from Ashihara et al.[16]).
-
The China National Germplasm Tea Repository (CNGTR) holds more than 3,000 accessions of tea germplasm from tea growing provinces of China and around the world. It is the most abundant field genebank for preserving tea germplasms in the world. Until 2019, 2,296 accessions were preserved in the China National Germplasm Hangzhou Tea Repository (CNGHTR)[65]. The rich and diverse tea genetic resources provide the material basis for tea plant genetic improvement. However, it is still difficult to identify, evaluate and utilize all resources. Therefore, it is necessary to establish the core collections of tea plants in China, hoping to represent the diversity of the entire genetic resources to the greatest extent with the minimum number of resources and the minimum genetic repeatability[66]. The identification and evaluation of the core collections will help to discover excellent genes of tea plants, and screen out a batch of tea germplasms with specific purine alkaloid components, and promote tea plant improvement and the breeding of new varieties.
According to the Chinese Agriculture Industry Standard 'Evaluating standards for elite and rare germplasm resources-Tea plant (Camellia sinensis (L.) O. Kuntze) (NY/T 2031−2011)', accessions with caffeine content ≥ 5% or ≤ 1.5% are cataloged as rare germplasms. Chen & Zhou tested the caffeine content of 596 accessions tea germplasm collected from tea producing provinces in China and found that more than 60% of the resources were higher than 4.1%[5]. Among them, 30 accessions were high caffeine (≥ 5.0%) rare germplasms, and most of the valuable hypernormal germplasms were found to come from Yunnan and Fujian.
The profiling of caffeine and theobromine content for selected 403 accessions Chinese tea germplasm core collection were identified using High Performance Liquid Chromatography (HPLC). The results showed that caffeine content was generally stable between years and seasons, ranging from 0.23% to 5.34% in spring and 0.11% to 6.0% in autumn, and more than 93% of the resources varied from 2.5% to 4.5%[67]. Theobromine content varied significantly in spring, and about 80% of the resources varied from 0.2% to 0.6%[67]. The representative 403 accessions genetic resources were collected from 18 tea producing provinces in China, from which three high caffeine, two low caffeine and high theobromine, and one low caffeine and high theacrine accessions, a total of six specific tea accessions were screened, all from Yunnan and Guangdong. It is believed that Yunnan and Guangdong had higher coefficient of variation and diversity indices.
About 500 accessions of tea germplasm preserved in the China National Germplasm Menghai Tea Repository Branch (CNGMTRB) were comprehensively identified and evaluated. Nine accessions with super caffeine content were screened out, including seven high caffeine and two low caffeine accessions[68]. Among the tea plant in Sect. Thea (L.) Dyer Camellia L., natural low-caffeine resources are scarce, which is of great significance for the breeding of low-caffeine tea plant cultivars. In addition, the biochemical components of 66 Yunnan tea genetic resources were analyzed, and the results showed that coefficient of variation of purine alkaloids was the total amount of purine alkaloids < caffeine < theobromine[69], which was the same as the results of Jin et al.[67]. Moreover, four rare germplasms of high theacrine were screened out.
Japanese and South Korea collections
-
In addition to China, Japan, India, Kenya and other tea producing countries have also preserved a large number of tea plant germplasms. According to reports, Japan has been planning to introduce a large number of tea plant seeds from China, South Korea, India, Indonesia, Sri Lanka and other tea growing countries, and has accumulated more than 6,800 tea genetic resource accessions[70]. Takeda[71] analyzed the tannin and caffeine content of 1,500 accessions of tea germplasm, the caffeine content ranged from 1.64% to 5.46%, similar to Chinese tea germplasms[67]. The caffeine content of the two varieties of C. sinensis (L.) O. Kuntze was significantly different. Compared with C. sinensis var. sinensis, the variation in caffeine content of the C. sinensis var. assamica was larger, and the average content was higher (4.1%). And then, nine high caffeine content accessions were screened out from the assamica variety. The results of the Chinese core collection also showed that the caffeine content of the three varieties of C. sinensis (L.) O. Kuntze was significantly different, while the caffeine content of the assamica variety increased in autumn, and the content changed significantly between seasons[67].
Ogino et al.[72] first reported the detection of caffeine-less plants in the offspring of cultivated tea. In the natural hybrid progeny of the hybrid 'Cha Chuukanbohon Nou 6' (C. taliensis × C. sinensis), two low-caffeine and high-theobromine tea resources were found, named 'CafLess1' and 'CafLess2', respectively[72]. According to reports, the National Institute of Vegetable and Tea Science in Japan has preserved about 3,600 accessions of tea germplasms[72]. Before that, no caffeine-less (≤ 0.2%) germplasm was found in any resource. The discovery of the two rare germplasms will be applied to the breeding of caffeine-less tea varieties. Analysis using simple sequence repeat (SSR) marker suggested that caffeine-less traits may be recessively inherited. This not only facilitates the analysis of the mechanism of caffeine synthesis, but also obtains DNA markers closely related to caffeine-free trait for molecular marker-assisted selection (MAS) in tea breeding programs.
In South Korea, a set of 462 tea accessions was used for the core collection construction[73]. The average caffeine content in two years was 1.67% (0.04−3.25). It is low compared with the Chinese and Japanese collections.
Some newly discovered hypernormal tea germplasms
-
Since the discovery of cocoa tea (C. ptilophylla Chang) by Professor Chang Hong-ta in Guangdong, China in the 1980s, people have realized that the tea germplasm with specific purine alkaloids may have special pharmacological effects, and thus began to pay attention to the collection, screening and utilization of natural low-caffeine tea germplasms. Wild tea genetic resources are abundant in Southwest China. The wild tea plants were screened with caffeine content as an indicator, and a number of high-caffeine tea germplasms were screened in Yunnan, but only two natural low-caffeine accessions were found: 'Jinchang Dashucha' (0.06%) and 'Daba Dashucha' (0.07%)[68]. Taxonomically, both belong to C. crassicolumna Chang. Compared with the main cultivars in Yunnan, their economic characters and sensory qualities are poor[74]. Two high-caffeine accessions were screened in Sichuan-Chongqing region: 'Nanchuan 1' (6.25%) and 'Nanchuan 2' (5.87%), and one natural low-caffeine accession: 'Huangshan Kucha' (0.323%) and its progeny plant (0.36%). It is believed that the caffeine content of tea plants may be determined by the characteristics of the variety, and 'Huangshan Kucha' can be used as breeding material for low-caffeine tea plants[75]. In Guangxi, a germplasm (C. gymnogyna Chang) containing theobromine, caffeine and theacrine was screened, which provided new materials for the development of functional components of tea plants[76]. Recently, two new tea germplasms with specific purine alkaloid components were discovered in Fujian, which have high research value and will be introduced separately below.
'Hongyacha' (HYC) is a naturally caffeine-less/free wild tea plant[77]. It was first reported in Fujian Province, China in 2018, which is significantly different from cocoa tea in morphological characteristics and biochemical components. The young leaves are purple or light purple, contain abundant theobromine, trans-catechins, gallocatechin-(4→8)-gallocatechin gallate (GC-(4→8)-GCG), 1,3,4,6-tetra-O-galloyl-β-D-glucopyranose(1,3,4,6-GA-glc), and (−)-gallocatechin-3,5-di-O-gallate (GC-3,5-diGA), which are not detected in regular tea. In addition, the study found that the recombinant protein of caffeine synthase gene (TCS1) in HYC only has theobromine synthase activity, and it is considered that 'Hongyacha' is a new type of caffeine-less tea plant, which can be used for scientific research and efficient utilization in the future.
'Baiyacha' (BYC) is a wild tea plant naturally rich in theacrine[78]. Most recently, Jin et al.[78] reported that 'Baiyacha' was also found in Fujian Province, China, which has similar morphological characteristics to C. gymnogyna Chang. Some individual plants rich in catechins, 3"-methyl-epigallocatechin gallate (EGCG3"Me) and theacrine. The study found that with increasing leaf maturity, the content of caffeine decreased significantly, and the content of theacrine and EGCG3"Me increased. EGCG3"Me is an O-methylated EGCG, which has obvious pharmacological effects like theacrine. 'Baiyacha' is the first discovered tea plant resource rich in both theacrine and EGCG3"Me, which is of great value for breeding new varieties with functional components and conducting scientific research in the future.
-
Purine alkaloids are a class of substances that exist in large quantities in non-alcoholic beverages, of which caffeine is the most important component. Beverages containing caffeine, such as coffee, tea, and functional drinks, have a large global consumption. Appropriate intake of caffeine is beneficial to human health, but excessive intake of caffeine or special populations' intake of caffeine may cause negative effects of anxiety and insomnia. Some consumers are naturally sensitive to caffeine intake. At present, the market demand for low-caffeine or decaffeinated products is increasing rapidly. To decaffeinate tea, manufacturers often treat it with supercritical carbon dioxide or hot water treatments. However, these methods can affect the flavor of the brew and destroy compounds in the tea associated with health benefits. Since Kato et al. successfully cloned the caffeine synthase gene in 2000, scientists began to explore the possibility of obtaining natural low-caffeine coffee or tea through genetic engineering. Using the identified gene encoding caffeine synthase, transgenic plants with reduced caffeine content can be produced by RNA interference. It has been reported that coffee seedlings with a 70% reduction in caffeine content have been obtained through genetic engineering[64], but there has been no relevant reports on tea.
Mohanpuria et al.[79] tried to use RNAi technology to produce low-caffeine content tea plants. Transgenic plants showed a significant suppression of Caffeine Synthesis transcript expression and also showed a reduction of 44%−61% in caffeine and 46%−67% in theobromine content as compared to controls[79]. These results suggest that the RNAi construct developed using a single partial fragment of CS gene reduced the expression of the targeted endogenous gene significantly[79]. It might be a potential molecular technique for generating low-caffeine tea plants.
Application of low caffeine TCS1 alleles
-
Allelic variations of TCS1 play an important role in caffeine biosynthesis of tea plants. There are two molecular mechanisms controlling the caffeine biosynthesis in low-caffeine-accumulating tea germplasms. TCS1 natural variant reduces transcript level or its encoded protein has only theobromine synthase activity. Allelic variations of TCS1 can be used for the selection of low-caffeine tea plant varieties. Low caffeine individuals might be selected from progenies resulting from crosses between specific accessions containing TCS1b, TCS1c, or TCS1f, TCS1g with low caffeine biosynthetic activity[55]. Ogino et al. believed that the caffeine-free phenotype was closely related to the TCS1, and used a three-nucleotide insertion TTC in TCS1, developed a DNA molecular marker 'CafLess-TCS1', and confirmed that 'CafLess-TCS1' is an effective selection marker for breeding of caffeine-less tea cultivars[80].
Application of cocoa tea and Kucha
-
Among hypernormal tea germplasms, although the health benefits of cocoa tea and Kucha have long been known, relevant scientific study is still very limited. In recent years, scientists have paid more attention to the discovery and utilization of natural tea germplasm rich in theobromine or theacrine. Hypernormal germplasms not only have different morphological and biochemical characteristics, but may also have unique regulatory mechanisms and candidate genes. Ye et al. screened out a batch of low-caffeine, high-theobromine tea germplasms from the wild population, and established the first natural caffeine-free tea germplasm base in China. In the later stage, the screening of excellent individuals of wild cocoa tea will be strengthened to further determine the pharmacological and physiological functions of cocoa tea. Several recent studies on Kucha have identified highly expressed TCS genes (TEA010054, TEA022559) at the transcriptional level[81], which are helpful to reveal the metabolic mechanism of purine alkaloids at the molecular level. This will help in identification and tea plant breeding rich in theobromine or theacrine.
This work was supported by the National Natural Science Foundation of China (U19A200592 & 32072631), the Chinese Academy of Agricultural Sciences through the Agricultural Science and Technology Innovation Program (CAASASTIP-2017-TRICAAS), Earmarked Fund for China Agriculture Research System of MOF and MARA (CARS-19) to L Chen.
-
The authors declare that they have no conflict of interest.
- Copyright: 2022 by the author(s). Exclusive Licensee Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhang S, Jin J, Chen J, Ercisli S, Chen L. 2022. Purine alkaloids in tea plants: component, biosynthetic mechanism and genetic variation. Beverage Plant Research 2:13 doi: 10.48130/BPR-2022-0013
Purine alkaloids in tea plants: component, biosynthetic mechanism and genetic variation
- Received: 04 July 2022
- Accepted: 29 July 2022
- Published online: 18 August 2022
Abstract: The purine alkaloids, including caffeine, theobromine, and theacrine, are one of the most important quality and functional components of tea plants and commercial teas. In this paper, we review the component, biosynthetic mechanism and genetic variation of purine alkaloids in tea plants. The content of caffeine and theobromine in 403 accessions Chinese tea germplasms core collection preserved in the National Germplasm Hangzhou Tea Repository were analyzed using High Performance Liquid Chromatography (HPLC). The purine alkaloid profiles of different tea varieties, germplasm types, geographical origin, seasons, were highlighted. Some naturally low caffeine or caffeine-free, high theobromine or high theacrine germplasms were identified and the possible biosynthetic mechanism of hypernormal purine alkaloid content in tea plants were partially revealed. Some Cleaved Amplified Polymorphic Sequences (CAPS) DNA markers were developed for the identification of purine alkaloid content. The potential application of genetic engineering and DNA markers developed based on the low caffeine TCS1 (tea caffeine synthesis gene) alleles for marker assisted selection (MAS), and cocoa tea and kucha in low caffeine/high theacrine tea cultivar breeding programs were also reviewed.
-
Key words:
- Alleles /
- Genetic variation /
- Purine alkaloids /
- Tea caffeine synthesis gene /
- Tea plants