-
Bromomethane, also called methyl bromide, is a simple halogenated hydrocarbon with a boiling point of 3.6 °C. Because of its strong fumigation effect and penetration effect, methyl bromide is widely used as a fumigant worldwide, which can effectively kill fungi, bacteria, mites, soil-borne viruses, nematodes, insects and rodents. Methyl bromide has a strong ability to penetrate soil. After the soil has been fumigated, residual gas can quickly be volatilized, and the land can be sown or colonized in a short period of time. Therefore, methyl bromide is one of the most popular soil fumigants for the purpose of pest control, disease prevention and weeding[1]. Moreover, methyl bromide is also used for fumigation of goods to be stored and perishables, and has been widely used in warehouse disinfection, port quarantine and other fields[2].
Although methyl bromide is an excellent fumigant, it is a substance that destroys the ozone layer and causes serious environmental pollution. By 2015, most countries had phased out production and application of methyl bromide in compliance with the Montreal Protocol, with the exception of important applications, such as port quarantine. Since July 1, 2018, China has revoked the pesticide registration certificate of methyl bromide and the agricultural use of methyl bromide has stopped since December 31, 2018. Methyl bromide is also a toxic compound to humans[3]. High dose methyl bromide exposure is harmful to the eyes, skin and lungs, and can cause damage to the respiratory and central nervous system[4,5].
Owing to its high volatility, methyl bromide might be expected to disappear rapidly after fumigation. However, several researchers have reported the persistence of methyl bromide residues in foods such as nuts and seeds, even after processing and airing[6−9]. It is therefore important to develop quantitative methods for the determination of methyl bromide residues in foods. As a highly volatile compound, gas chromatography (GC) is a natural choice for the analysis of methyl bromide. Sample pretreatment methods include solvent extraction[6,10], headspace[8,11,12], and solid phase microextraction[13]. The coupled detectors for GC instruments are flame ionization detector[6], electron capture detector (ECD)[8,14], electron ionization mass spectrometer (EI-MS)[13,15,16] and ion mobility spectrometry[17]. Methyl bromide is an excellent methylating reagent and it can react with active hydrogen-containing organic compounds present in food matrices to generate the corresponding methylation products and result in the residue of inorganic bromine ions in food. Therefore, the content of bromide ions was sometimes used as an indicator of residues from methyl bromide fumigation[18,19]. In the early days, the organic bromide was converted to the inorganic form for measurement by titration, photometry, or other means[18]. Afterwards with the development of instrumental analysis technology, the GC-ECD or GC-MS methods were commonly used to analyze a number of foodstuffs for free methyl bromide residues, such as cereals, vegetables, fruits, etc.[8,13,20]
Tea is one of the three most popular non-alcoholic beverages in the world. Recently, China's national food safety standard-maximum residue limits for pesticides in food (GB 2763-2021) has formulated a temporary limit for methyl bromide in beverages and other foods. However, at present, the residual situation of methyl bromide in tea products in China is not clear. Furthermore, there is no standard or reference for the detection of methyl bromide in tea. While the work is ongoing, a method for detecting methyl bromide residues in tea using GC-ECD was patented by Gao et al.[21]. To the best of our knowledge, there is currently no GC-MS method for the determination of methyl bromide in tea. In this study, critical parameters such as SPME extraction, GC separation and MS detection were all optimized to develop a simple, sensitive and precise SPME/GC-MS method to determine methyl bromide residues in tea.
-
Methyl bromide in methanol (2,000 mg·L−1) was supplied by Aladdin, Shanghai (China). Sodium chloride was analytical reagent grade and purchased from Avantor (Phillipsburg, USA). Ultrapure water (resistivity 18.2 MW cm) was obtained from a Milli-Q system (Millipore, Bedford, MA, USA). SPME fibers (100 μm PDMS, 50/30 μm DVB/CAR/PDMS, 65 μm DVB/PDMS, 60 μm PEG, and 60 μm CarboWAX/PEG) were purchased from Anpel, Shanghai (China). Methyl bromide-free tea samples were donated by Key Laboratory of Tea Quality and Safety & Risk Assessment, Ministry of Agriculture and Rural Affairs, Hangzhou (China). A total of 25 real samples, including 15 green tea, five black tea and five chrysanthemum samples, were purchased from the supermarkets in Hangzhou (China).
GC-MS/MS analysis
-
All determinations were performed using a GC-MS/MS system constituted by a Varian 450 gas chromatograph coupled with a Varian 300 MS mass spectrometer. Chromatographic separation of the analytes was obtained by using a VF-5 MS capillary column (30 m × 0.25 mm × 0.25 μm, Agilent, USA) with helium as carrier gas at a constant 1.0 mL·min−1 flow. The injections were carried out in a splitless mode. The oven was set at the following temperature program: Initial temperature was 35 °C, held for 2 min, ramped at 10 °C min−1 to 220 °C, held at 220 °C for 2 min, ramped at 30 °C min−1 to 300 °C, and held at 300 °C for 10 min. The injector port and transfer line temperatures were set at 250 and 280 °C, respectively. The MS analysis was operated in single ion monitoring (SIM) mode to detect the ions produced by electron ionization (70 eV) at 230 °C ion source temperature. The molecular ions m/z 94 and 96 were selected as the qualifier ions. Molecular ion m/z 94 was used for the quantification.
Optimization of the SPME procedure
-
A volume of 10 μL methyl bromide (2,000 μg·mL−1) was added into 2.0 g methyl bromide-free tea to create a model sample (10 mg·kg−1) for SPME procedure optimization. Five fiber types were tested: 100 μm PDMS, 50/30 μm DVB/CAR/PDMS, 65 μm DVB/PDMS, 60 μm PEG, and 60 μm CarboWAX/PEG. Before extraction of methyl bromide, the fibers were conditioned according to the supplier's instructions in the GC injector at 250 °C for 1 h. A manually operated SPME holder was used throughout these experiments. Tea powder (2.0 g) was weighed into a 50 mL sealed headspace glass vial and 8 mL ultrapure water was added (two or three replicates). After stirring and extraction, the fiber was manually retracted and transferred to the GC injector for desorption.
Method validation
-
Standard solutions of methyl bromide at different concentrations (10, 20, 30, 40, 60, 80, 100, 200, and 400 mg·L−1) were obtained by dilution with methanol. The standard solutions were stored at −18 °C, protected from light and used within 24 h. A volume of 10 μL of each stock solution was added to 2.0 g tea sample to prepare spiked samples. Linearity evaluation was performed by analyzing calibration curves of methyl bromide in spiked samples with six concentrations (0.050, 0.10, 0.20, 0.50, 1.0 and 2.0 mg·kg−1). Three spiked samples (0.15, 0.30, and 1.0 mg·kg−1) were each analyzed three times to assess the repeatability, which is represented by the relative standard deviation (RSD). On the basis of the above added concentrations continuing to dilute and each concentration level being analyzed three times in parallel, the lowest spiked concentration was determined experimentally to be the limit of quantitation (LOQ).
Tea fumigation using methyl bromide
-
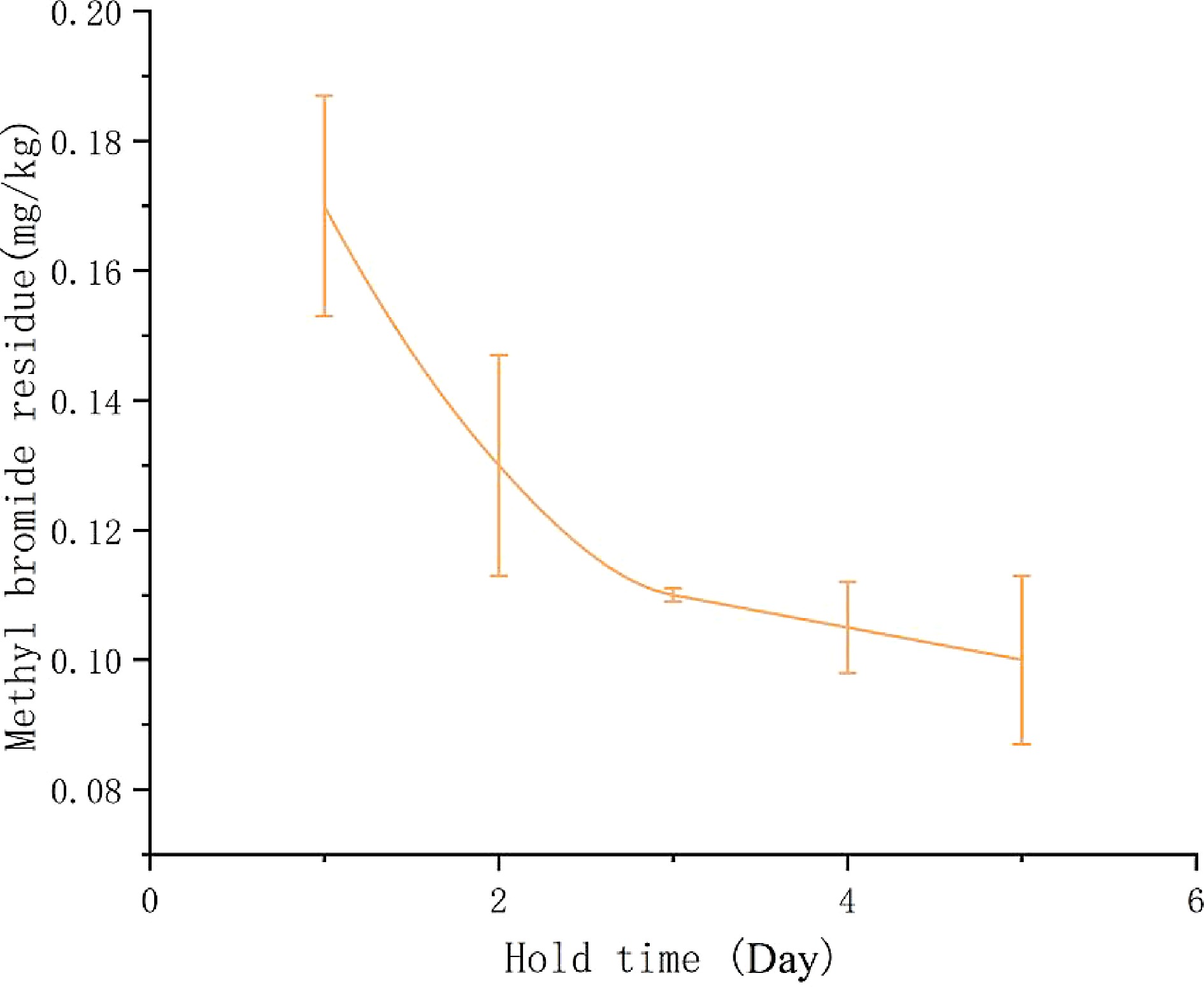
Approximately 200 g of ground green tea was exposed to a concentration of 200 mg·L−1 methyl bromide (10 mL) for an exposure time of 3 h in a fumigation chamber. The chamber was sealed after fumigation. A 100 g tea sample was taken from the chamber and placed open at room temperature and the methyl bromide residues were measured every 2 h. The remaining 100 g tea sample was divided into eight bags and kept in airtight storage after fumigation. Half of them was stored at room temperature, and the other half was placed in the refrigerator. Methyl bromide residues in tea were detected on the 1st, 2nd, 3rd, and 5th days after fumigation.
-
Under the conventional instrument conditions described in the experimental section, methyl bromide can achieve excellent resolution and response. Figure 1a & b show the extracted ion chromatograms for tea samples non-spiked and spiked with methyl bromide at the 1.0 mg·kg−1 level, respectively. The retention time of methyl bromide in the chromatogram is 1.28 min. Several previous methods on the determination of methyl bromide by GC also showed that its retention time was very short, because it is a simple halogenated hydrocarbon with a very low boiling point[12,13]. The SIM mass spectrum of methyl bromide (Fig. 1c) shows two ions at m/z 94 and 96 with almost the same relative abundance that is consistent with the characteristics of monobromoalkanes.
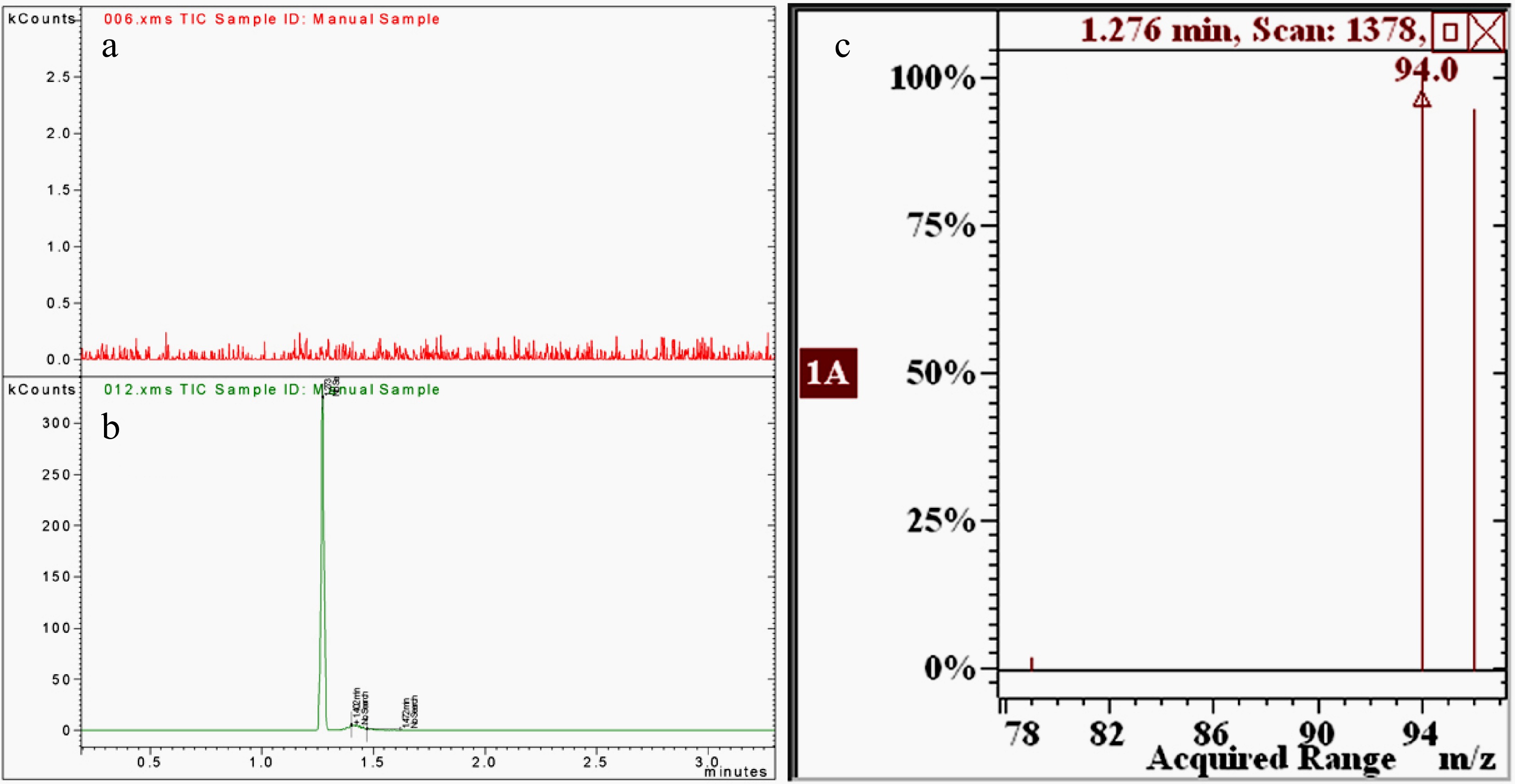
Figure 1.
The extracted ion chromatograms of m/z 94 for (a) non-spiked and (b) spiked tea samples and (c) the SIM mass spectrum of methyl bromide.
Optimization of SPME conditions
-
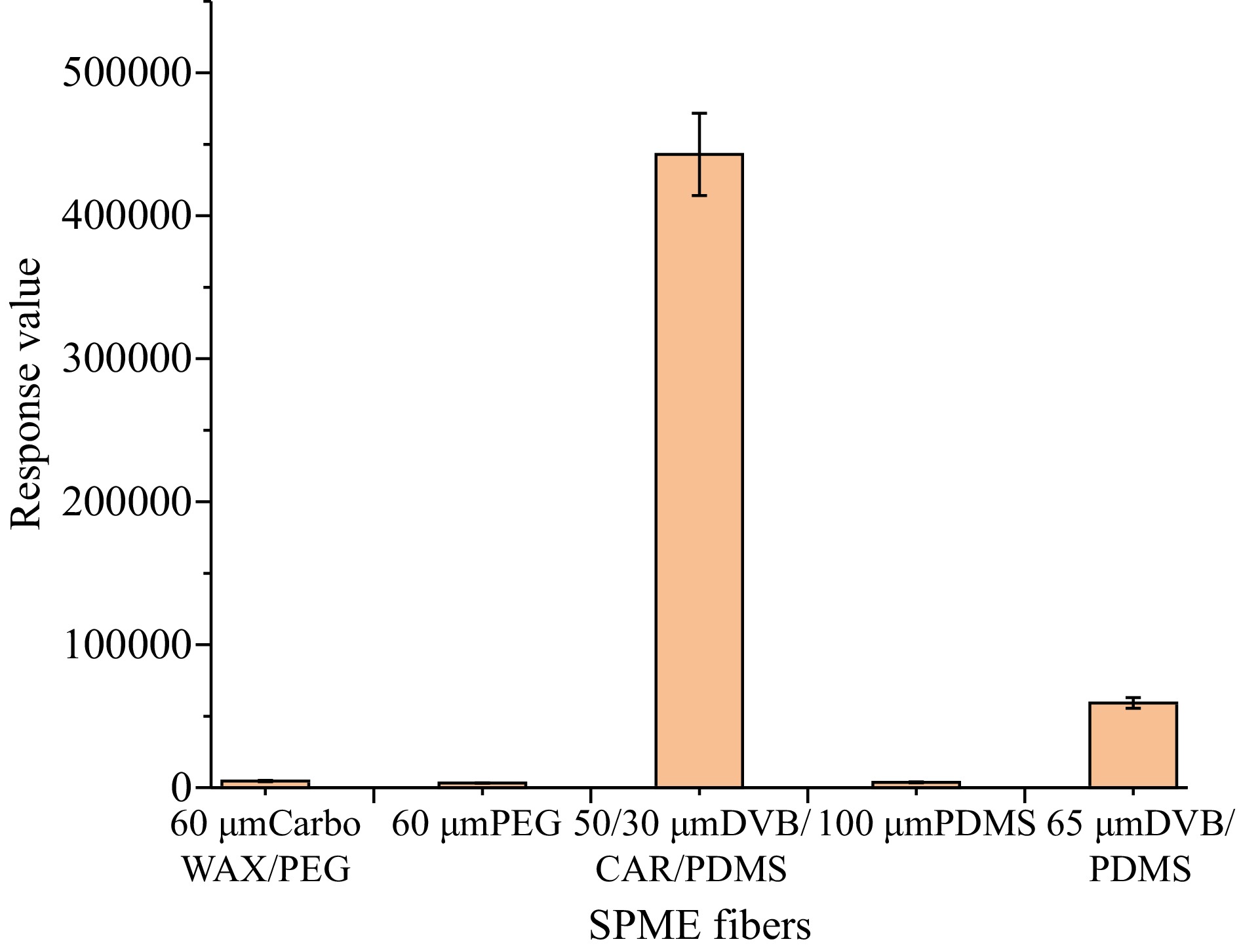
The SPME fiber coating is essentially important for the extraction of analytes[22,23]. Selection of appropriate coating is critically important for the analysis of methyl bromide. To accomplish this, five SPME coating fibers with different polarity (100 μm PDMS, 50/30 μm DVB/CAR/PDMS, 65 μm DVB/PDMS, 60 μm PEG, and 60 μm CarboWAX/PEG) were evaluated at an extraction temperature of 40 °C for 30 min with an agitation speed of 1000 rpm. As shown in Fig. 2, the maximum peak area count of methyl bromide was obtained when the weakly polar fiber 50/30 μm DVB/CAR/PDMS was used, while poor signal was obtained with non-polar fiber 100 μm PDMS and polar fibers 60 μm PEG, and 60 μm CarboWAX/PEG. On the whole, 50/30 μm DVB/CAR/PDMS was selected as the SPME fiber for the subsequent experiments.
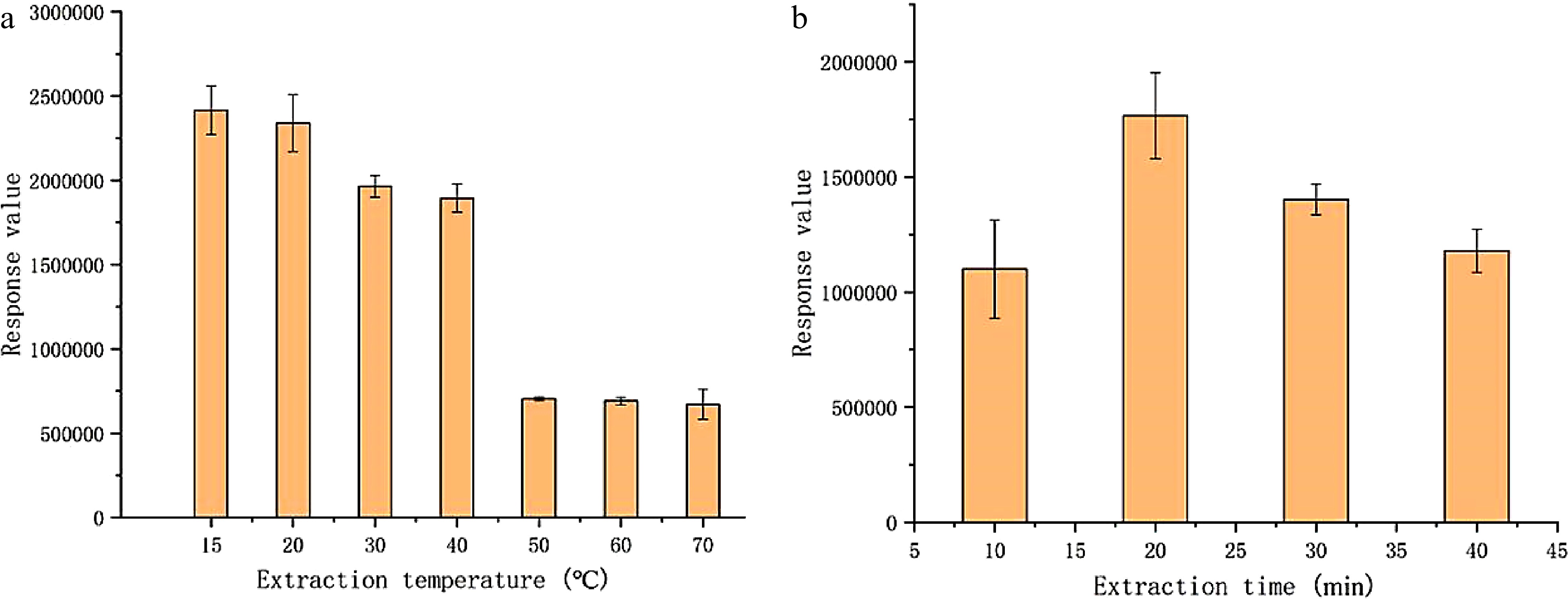
The adsorption temperature and time were optimized to ensure a fast overall analysis time as well as the required sensitivity. Extraction temperature of 15, 20, 30, 40, 50, and 70 °C was tested, respectively, and the results are shown in Fig. 3a. Extraction time of 10, 20, 30, and 40 min was also investigated for the extraction temperature at 20 °C (Fig. 3b). Clearly the extraction efficiency of methyl bromide decreased by raising the temperature. When the extraction temperature was lowered to 15 °C from 20 °C, the peak area of methyl bromide increased slightly. Taking into account the ambient temperature in the laboratory and the convenience of operation, 20 °C was selected for the extraction temperature. Choosing a lower temperature also could reduce as far as possible any interference from volatile components derived from tea to obtain higher resolution (spectra were not presented). At the optimum extraction temperature, it was observed that the peak area of methyl bromide reached the maximum under the extraction time of 20 min. When the equilibrium time was extended or shortened, the extraction efficiency significantly declined. Thus the optimal SPME extraction condition could be established as the extraction time of 20 min and the extraction temperature of 20 °C.
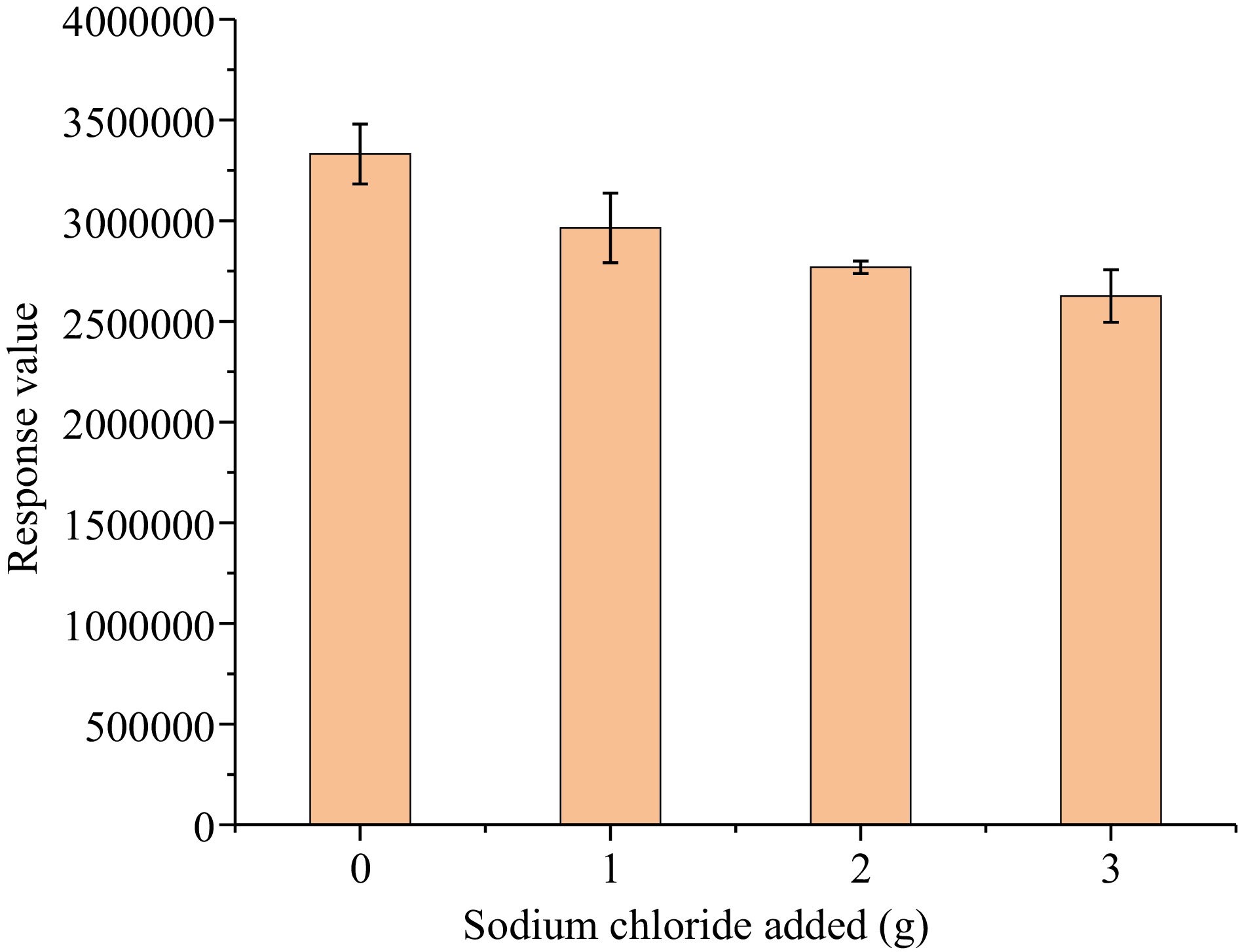
The equilibrium between the fiber and the analyte in the sample is a crucial factor. In HS-SPME, it was usually found that salinity of the sample and stirring allowed the diffusion layer to reach the equilibrium faster and promoted the transfer of target analytes from the solution to the gas phase[24]. It was found that stirring of above 1,200 rpm, tea matrix and soup would splash and contaminate the extraction fiber. The extraction efficiency under 1,000 rpm is slightly better than that under 500 rpm. Therefore, stirring of 1,000 rpm is the best condition. Inorganic salts such as sodium chloride are usually added to the solution to adjust the ionic strength for halogenated hydrocarbon extraction. In the present experiment, four different sodium chloride additions (0, 1.0, 2.0, and 3.0 g) were compared. As shown in Fig. 4, with the increase in the amount of sodium chloride added, the response value of methyl bromide continued to decrease. Better extraction results were obtained without increasing the salinity in this case, so the extraction did not involve salt.
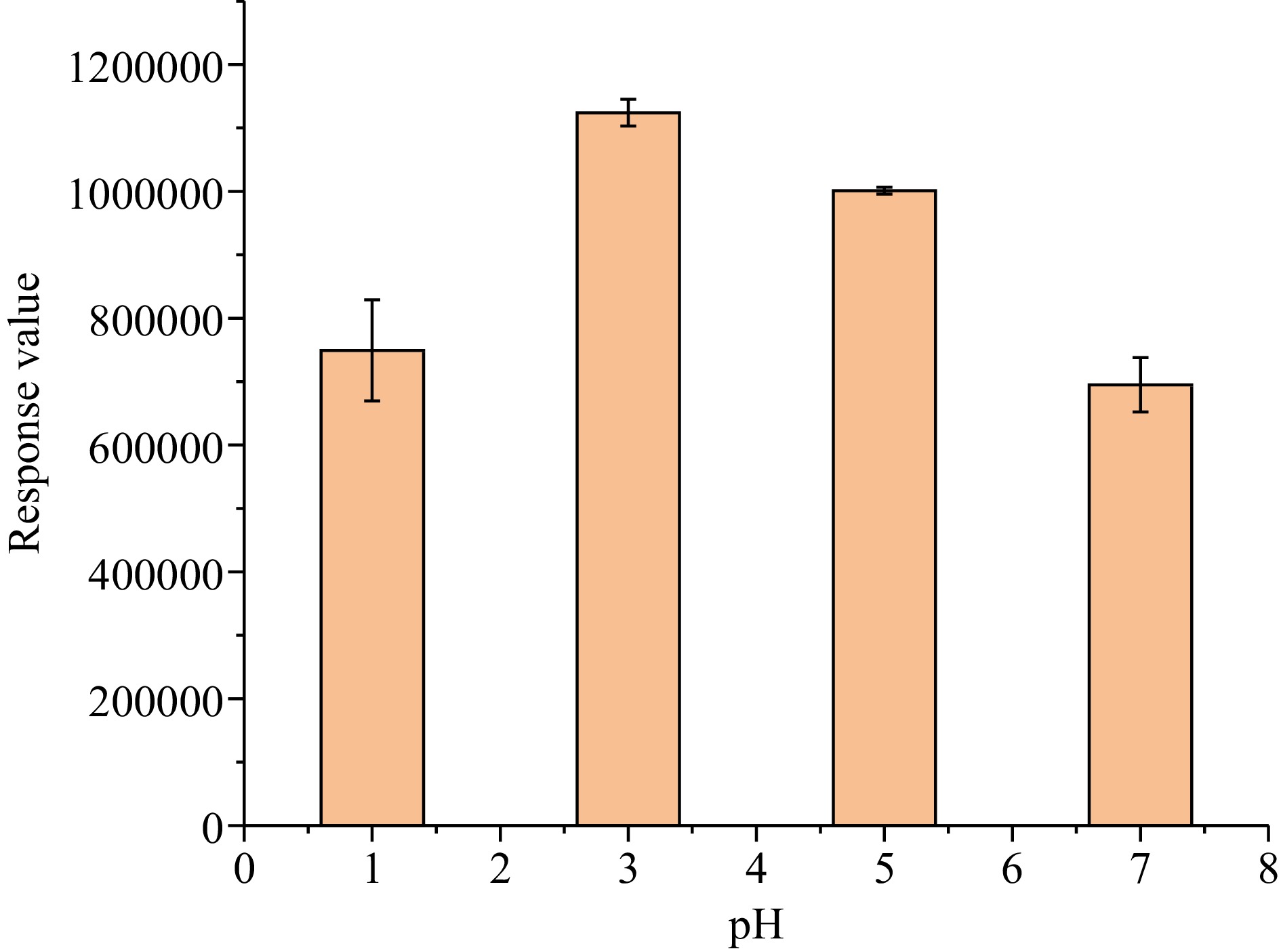
The pH of the solution may also affect the extraction efficiency. Tea soup has a certain buffer capacity and its pH value was measured to be about 5.3. Concentrated hydrochloric acid or concentrated sodium hydroxide was used to adjust the pH of tea-water sample to be 1.0, 3.0 and 7.0, respectively. The results showed that the response value of methyl bromide was the highest at pH 3.0 and the extraction efficiency of methyl bromide was worse under strong acid or neutral conditions (Fig. 5). Although the extraction efficiency was slightly lower at pH 5.3, for the convenience of experimental operation, pH adjustment was not performed in SPME.
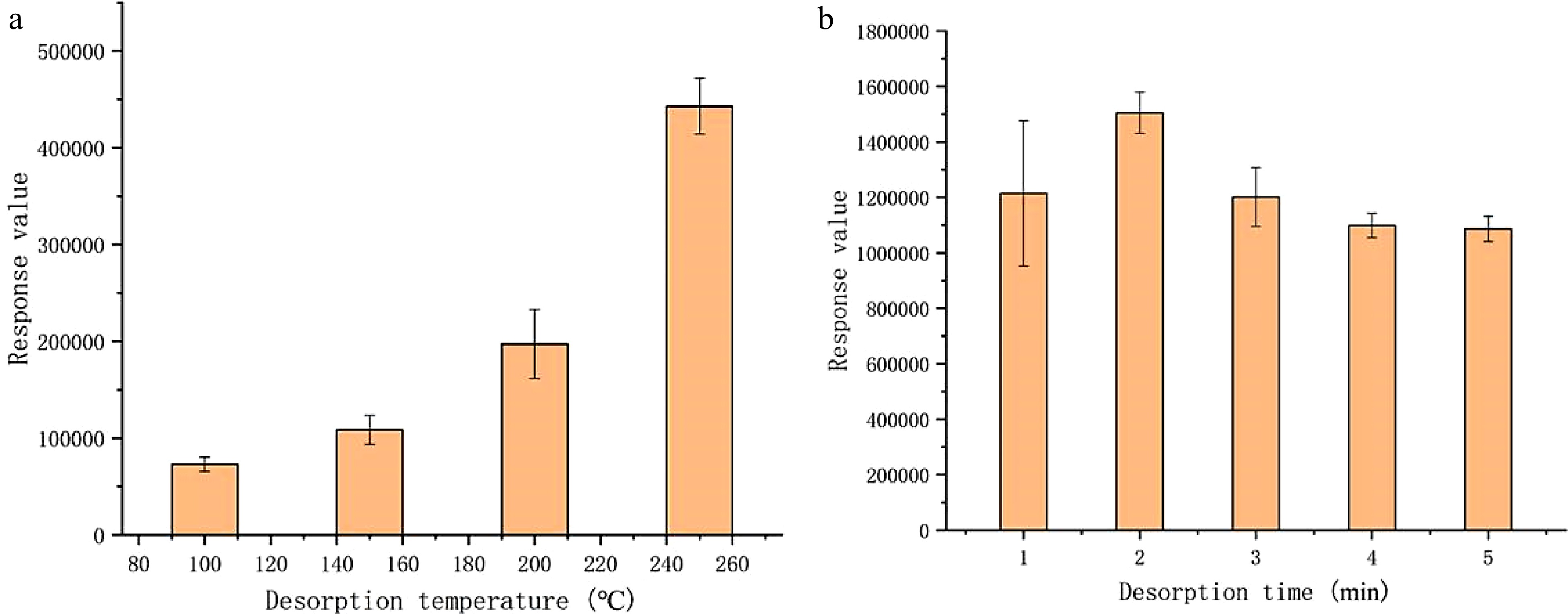
Desorption temperature and time are important SPME condition factors that determine the amount of analytes desorbed from the fibre coating. The response amount of methyl bromide was then investigated under different desorption conditions of temperature and time (Fig. 6). The peak area of methyl bromide increased rapidly with increasing desorption temperature from 100 to 250 °C. At a relatively low desorption temperature, the peak shape of methyl bromide was poor and the interference was serious. The peak shape and resolution were greatly improved with the increase of temperature. For the desorption temperature of 250 °C, optimum response and resolution were obtained. Increasing desorption time from 1 to 5 min had minimal effect on the response amount of methyl bromide and a desorption time of 2 min was the best. The desorption time had no effect on the chromatographic resolution, but a long desorption time shortened the fiber life indicating that an equilibrium time of 2 min in desorption was adequate.
Linearity
-
For evaluation of linearity, methyl bromide-free tea was mixed with 6 concentrations (0.05, 0.1, 0.2, 0.5, 1.0, 2.0 mg·kg−1) of each standard of methyl bromide. Following extraction under the optimized SPME conditions and GC-MS analysis demonstrated that the extracted amount of methyl bromide increased linearly with the concentrations in the ranges of 0.05–2.0 mg·kg−1 and the linear correlation coefficient R2 > 0.99.
Precision and accuracy
-
Precision and accuracy of the SPME/GC-MS analytical method for methyl bromide are shown in Table 1. Three different concentrations (30, 60, and 200 mg·kg−1) of methyl bromide (10 μL) were added in 2.0 g tea (spiked concentrations were 0.15, 0.3, and 1.0 mg·kg−1) to evaluate the precision and accuracy. The precision expressed here as RSD and the accuracy expressed as recovery were determined by using three replicate experiments. The RSD and recovery of the developed method ranged from 0.4% to 6.1% and 95.0% to 107.7%, respectively, which was in general satisfactory and acceptable for an analytical method.
Table 1. Precision and accuracy of methyl bromide residues in tea obtained by SPME/GC-MS.
Compound Spiked levels, (mg·kg−1) 0.15 0.3 1.0 Recovery (%) RSD (%) Recovery (%) RSD (%) Recovery (%) RSD (%) Methyl bromide 102.4 6.1 107.7 3.0 95.0 0.4 Sensitivity
-
In this study, we investigated the recovery and precision, by gradually reducing the spiked concentration, so as to obtain the limit of quantitation (LOQ). The spiked levels in the range of 0.050, 0.10, 0.20, and 0.50 mg·kg−1 were evaluated. The recoveries and relative standard deviations (RSDs) for 0.05 and 0.1 mg·kg−1 were 92.5% and 13.8%, 93.3% and 10.2%, respectively. As the lowest spiked level and satisfied accuracy, LOQ was 0.05 mg·kg−1.
Determination of residual methyl bromide in real samples and fumigated tea
-
The residual methyl bromide in real samples was determined by the method described above under optimal procedures. A total of 25 samples, including 15 green tea, five black tea and five chrysanthemum samples, were collected, and methyl bromide residues were determined. The results showed that methyl bromide residue was not found in all samples.
The developed SPME/GC-MS method was then used to analyze fumigated tea samples. About 80% of methyl bromide volatilized 2 h later and it became undetectable after one day if the fumigated tea was placed open at room temperature. In another controlled experiment, it was found that even under sealed conditions, the residual methyl bromide was below the LOQ after one day at room temperature. As shown in Fig. 7, when the fumigated tea was sealed in the refrigerator (−20 °C), the residual amount of methyl bromide also decreased gradually within 5 d. From the first day to the second day, the volatilization rate of methyl bromide was relatively fast, but after two days, the volatilization rate of methyl bromide slowed down. From the results of exposure and storage temperature experiments, it can be concluded that methyl bromide in tea after fumigation could volatilize rapidly and the volatilization rate at room temperature was significantly faster than that in the frozen state. The obtained results indicated that the probability of occurrence of methyl bromide residue in tea and other plant-origin crops was very low since methyl bromide dissipated very fast. Low boiling point and volatile nature of methyl bromide are responsible for the phenomenon.
-
A method for the determination of methyl bromide residues in tea by HS-SPME/GC-MS was developed. SPME conditions including fiber coating, extraction time and temperature, stirring, salinity, pH, and desorption temperature and time were optimized. The developed method used a 50/30 μm DVB/CAR/PDMS fiber and the tea samples were extracted for 20 min under continuous stirring (1,000 rpm) at 20 °C. The method was validated in terms of linearity, LOQ, precision and accuracy. The validated method has been employed to determine methyl bromide residues in real samples and fumigated tea samples. Methyl bromide residue was not found in all real samples studied. The results showed that no free methyl bromide was detectable 24 h after fumigation when the tea was stored at room temperature. If the fumigated tea was sealed in the refrigerator, the residual amount of methyl bromide reduced by 60% after 5 d. This study indicates the risk of methyl bromide residues in tea is low.
This work has been financially supported by China Agriculture Research System of MOF and MARA (CARS-19), the Agricultural Science and Technology Innovation Project [CAAS-ASTIP-2020], Agricultural Science and Technology Innovation Program of CAAS [CAAS-ZDRW202011].
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhang D, Zhu Q, Li Z, Chai Y, Chen H. 2023. Determination of methyl bromide residues in tea by headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry. Beverage Plant Research 3:2 doi: 10.48130/BPR-2023-0002
Determination of methyl bromide residues in tea by headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry
- Received: 02 November 2022
- Accepted: 03 January 2023
- Published online: 16 January 2023
Abstract: Methyl bromide has been the most widely applied fumigant in the world, but most countries have phased out production and application of methyl bromide due to its toxicity to humans and the ozone. In this study, a specific headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry (HS-SPME/GC-MS) method was developed, optimized and validated for the determination of methyl bromide residues in tea. The established method was evaluated in terms of specificity, sensitivity, linearity, accuracy and precision. Linear plots were obtained in the range of 0.05−2.0 mg·kg−1. The limit of quantification (LOQ) was 0.05 mg·kg−1. Recoveries for the accuracy ranged from 95.0% to 107.7% and the relative standard deviation was in the range of 0.4%−6.1%. This developed method was used to analyze 25 real samples and the results showed that methyl bromide residue was not found in all samples studied. Therefore, the established method can be applied to the determination of methyl bromide residues in tea, and provide a reference for the detection of methyl bromide residues in other plant-based beverages.
-
Key words:
- Methyl bromide /
- Gas chromatography-mass spectrometry /
- Tea /
- Solid phase microextraction