-
Aluminum (Al) is the most abundant metallic element in the earth's crust. It occurs in the form of insoluble aluminosilicate or oxides at near-neutral or alkaline pH levels, which are not absorbed by plants. When the soil pH drops below 5.5, Al is released as soluble Al ions, including Al3+, Al(OH)2+,
${\text{Al(OH)}^+_2} $ Unlike most plant species, tea plant grows well on acidic soils. Some hydroponic experiments showed that tea plant can withstand at least 1,000 micromolar Al without a negative effect on its normal growth[3, 4]. Not only that, Al can dramatically stimulate root growth at low concentrations (0.2−1 mM), enhancing nutrient uptake, photosynthesis and antioxidant defense, implying Al is beneficial or even essential for tea plant growth[5−7]. In addition, tea plant is an Al hyperaccumulator. Matsumoto et al.[8] reported that the Al content in old leaves could reach up to 30,000 mg/Kg, dozens of times higher than that in herbs. These results indicate that tea plant has developed some adaptive mechanisms to cope with Al toxicity.
The physiological and biochemical mechanisms of Al tolerance in tea plant have been extensively studied and can be generally grouped into external exclusion mechanisms and internal detoxification mechanisms. In external exclusion mechanisms, tea plant secrets organic acids from the root tips to chelate Al in the rhizosphere, forming nontoxic complexes that do not enter the root. Oxalate is the key compound for Al exclusion. Other compounds, including citrate, malate, glycolic acid, and quinic acid are also secreted by Al addition and may be responsible for external Al detoxification[9, 10]. The internal detoxification mechanisms work in a variety of ways, such as redistribution of Al in the less active tissues, sequestration of Al in the cell wall or vacuoles, chelation of Al in the cytoplasm, and induction of the antioxidant defense system. Al is mainly accumulated in old and mature leaves, that are less sensitive to Al stress. At the subcellular level, Al is preferentially fixed in cell walls, once Al enters into cytoplasm, it is chelated as nontoxic forms by organic acids or phenolic compounds and subsequently sequestered into the vacuole[4, 10−12]. These strategies reduce the interference of Al ion with those active organelles and tissues. Additionally, tea plant enhances the activity of several antioxidant enzymes to reduce the excessive accumulation of reactive oxygen species (ROS) in cells caused by Al toxicity[6, 13].
Tea is one of the most popular non-alcoholic beverages worldwide. However, the high levels of Al in tea leaves may pose a potential threat to human health since Al has been linked to a possible risk factor for Alzheimer's Disease[14]. Additionally, the abundant absorption and accumulation of Al by tea plant may cause soil acidification in tea plantations, leading to reduced nutrient uptake and, consequently, lower tea yield and quality[15, 16]. Therefore, it is crucial to limit the uptake and accumulation of Al by tea plant. Approximately 50% of the world's arable land is acidic[17]. However, due to natural and human causes such as acid rain, industrial discharges, and excessive use of nitrogenous fertilizer, more and more soils are becoming acidic[18, 19]. Thus, the threat of Al stress to plant growth and human food security is further increased. Therefore, developing crops that are tolerant to Al is essential for sustainable agricultural production and global food security. The tea plant can tolerate and accumulate a high concentration of Al. Hence, understanding the mechanisms of Al tolerance and accumulation in tea plant not only helps to improve the tea quality but also provides valuable knowledge for crop improvement to adapt to acidic soils. Recently, the biochemical mechanism of Al tolerance in tea plant has been reviewed in detail by Ding et al.[3]. In this review, we focus on the research progress on the uptake, transport, and accumulation of Al in tea plant. We summarize information obtained from Arabidopisis and crops, and then review the relevant research on tea plant. Furthermore, we highlight recent studies on Al in tea plant based on omics approaches, including transcriptomics, proteomics, metabolomics, ionomics and microbiomics, which will advance our understanding of the mechanisms underlying Al tolerance and accumulation in tea plant.
-
Plants absorb Al ions mainly through their roots. Physiological experiments have revealed that Al uptake is a passive process because respiratory inhibitors have no effect on its absorption[20, 21]. The chemical form of Al transported to the roots of tea and other plants remains poorly understood due to the lack of a suitable Al isotope and the complex forms of Al in the solution, which are susceptible to pH changes. In buckwheat and Brassica rapape kinensis, Al is possibly absorbed in the form of Al3+ and Al-malate complex, respectively[20, 22]. Previous studies have found that Al may be taken up by the roots of tea plant in the form of Al-fluoride (Al-F) complexes (such as AlF2+ and AlF2+) and/or Al-phosphate complexes from the soil solution (Fig. 1)[23−25].
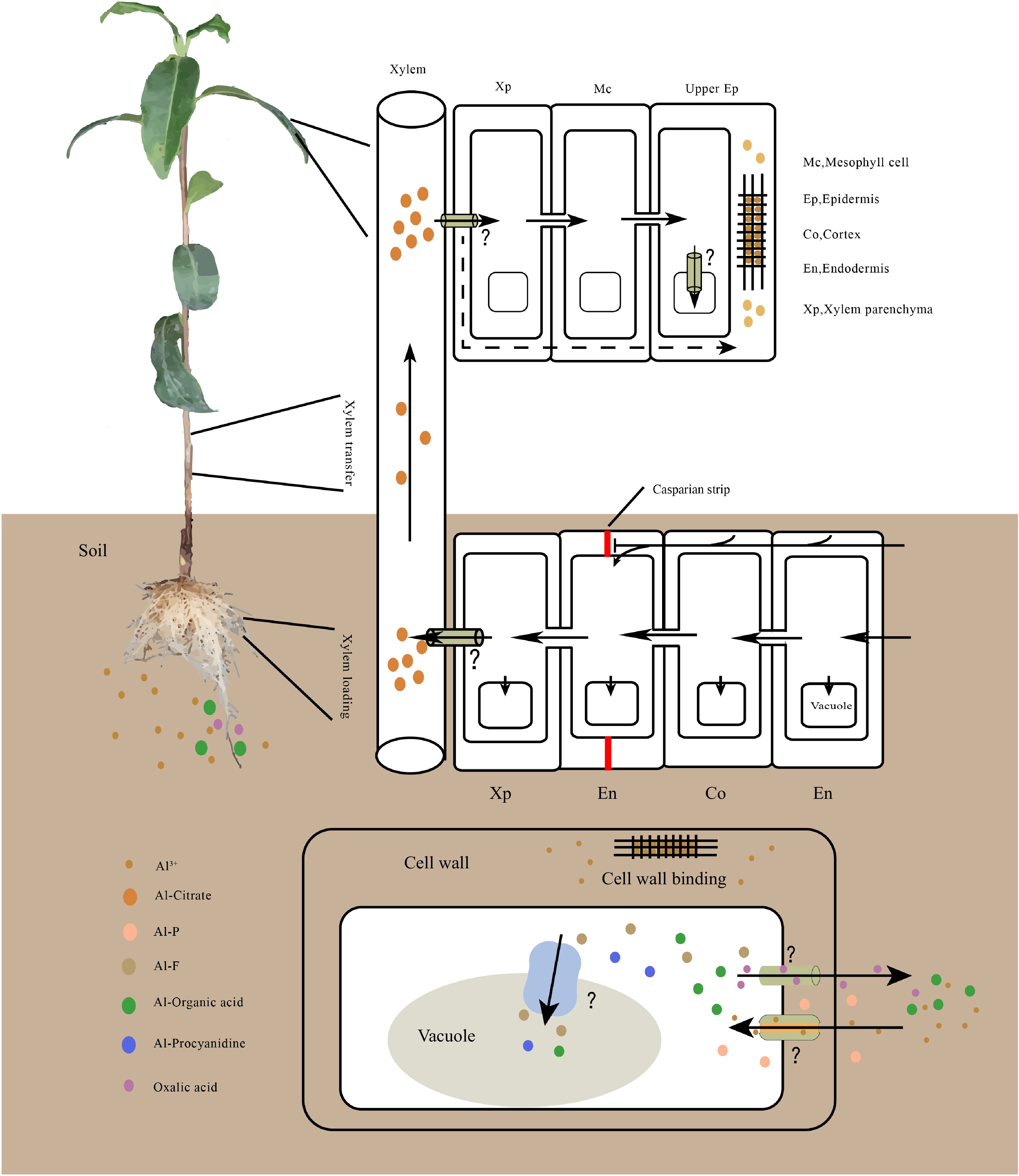
Figure 1.
Model for Al uptake, transport, accumulation in tea plant. Al is primarily absorbed by root cells, then most of the Al moves to the xylem parenchyma through symplastic and apoplastic transport. It is subsequently loaded into the xylem for root-to-shoot translocation. On leaves, Al is unloaded and transported to the upper epidermis for storage. In cells, Al is preferentially accumulated in the cell wall; once Al enters in the cytoplasm, it is chelated by organic acids or phenolic compounds and is subsequently sequestered into the vacuole. The transmembrane transport of Al requires transporters, however, the Al transporter in tea plant remains unknown. Al speciation varies in different tissues, Al-oxalate, Al-F and Al- proanthocyanidin have been detected in roots. Al-citrate is the predominant Al species in the xylem, and Al-catechin and Al-flavonol complexes have been detected in leaves.
After being taken up, most of the Al is chelated and accumulated in the roots of numerous plant species. However, a few plant species, such as tea plant, buckwheat, and hydrangea, are capable of translocating large amounts of Al from the roots to the shoots. These plants, which are also known as Al hyperaccumulators, can accumulate over 1,000 mg/Kg of Al in dry matter in their leaves[2]. The process of Al root-to-shoot translocation involves the removal of Al from the root cell wall into the symplasm of root cells, Al efflux across the plasma membrane of xylem parenchyma cells, loading into the xylem vessels, and subsequent upward translocation of Al toward the shoots through the xylem transpiration stream[26]. The form of Al for translocation from roots to shoots has been detected in several Al hyperaccumulators, such as buckwheat, tartary, and tea plant. In these plants, Al is most likely bound to citrate rather than oxalate (as found in their root cytosol) in the xylem sap, indicating that a ligand exchange occurs during the xylem loading process[27−29]. In contrast, Arabidopsis appears to transport Al to the shoots as Al-malate complex[26].
In plants, several transporters have been characterized that are involved in Al uptake and transport. In rice, the plasma membrane-localized transporter Nrat1 specifically transports trivalent Al ions and may work in tandem with the tonoplast-localized transporter ALS1 to remove Al from the cell wall and sequester it in the root vacuole[30, 31]. In hydrangeas, two aquaporin genes, HmPALT1 and HmVALT1, encode plasma membrane and tonoplast Al transporters, respectively[32]. In Arabidopsis, the nodulin 26-like intrinsic protein NIP1;2 facilitates the transport of Al-malate from the root cell wall into the symplasm of root cells and its subsequent translocation from roots to shoots[26]. Another plasma-localized transporter, AtALS3, is speculated to transport Al to the less sensitive tissue[33]. Recently, Hao et al.[34] identified CsALS8, the homologous gene of AtALS3 in tea plant, which confers increased Al tolerance in the transgenic yeast cells. The expression of CsALS8 in the roots of tea plant was upregulated under high Al treatment. Moreover, the Al content remaining in the growth media of yeast expressing CsALS8 was higher than that of the control, suggesting that CsALS8 may prevent Al from entering cells or be involved in its efflux. In tea plant, CsALS8 is substantially expressed in mature and old leaves, whereas its expression is reduced in roots, stems and young leaves, similar to the pattern of Al accumulation in tea plant. Taken together, we suggest that CsALS8 may play an important role in Al accumulation and tolerance in tea plant. Additional research, including tissue and subcellular localization and substrate specificity analysis, is required to determine its exact involvement in Al transport, accumulation and tolerance mechanisms in tea plant.
In addition to Al transporters, several other types of transporters have been shown to affect Al uptake and transport in plants. Currently, the Al-activated secretion of organic acids is the best characterized Al resistance mechanism in plants. Under Al stress, plants release large amounts of organic acids (mainly malate, citrate, oxalate) from their root tips to form non-phytotoxic compounds in the rhizosphere that do not enter the root. Some members of the ALMT (Al-activated malate transporter) and MATE (multi-drug and toxic compound extrusion) families have been identified as responsible for secreting of malate and citrate from root tips, respectively, and conferring Al resistance. TaALMT1, the first identified Al resistance gene in plants, encodes a plasma-localized malate efflux transporter and underlies a major wheat Al resistance locus[2]. Overexpression of TaALMT1 in transgenic barely plant and cultured tobacco cells resulted in increased Al-activated malate efflux and decreased Al accumulation in roots, which consequently enhanced Al resistance[35, 36]. Subsequently, the homologous gene of TaALMT1 has been identified and characterized in many plant species, but only a subset of these genes mediated Al-activated malate efflux and were involved in Al resistance, including AtALMT1, BnALMT1/2 (Brassica napus), ScALMT1(Secale cereale), GmALMT1(Glycine max), MsALMT1 (Medicago sativa), and HlALMT1(Holcus lanatus)[37]. Recently, CsALMT1, the homologous gene of TaALMT1 in tea plant, has been cloned[34]. Heterologous expression of CsALMT1 in yeast cells increased its tolerance to Al treatment, suggesting CsALMT1 may play a role in Al tolerance in tea plant. Another organic acid efflux transporter family known as MATEs has been identified in several plants, including barley (HvAACT1), sorghum (SbMATE1), wheat (TaMATE1), Arabidopsis (AtMATE1), rye (ScFRDL2), maize (ZmMATE1), and rice (OsFRDL2 and OsFRDL4)[37]. These transporters function as plasma membrane efflux transporters and are responsible for the Al-activated citrate exudation. In addition to citrate and malate, the Al-activated oxalate secretion has been found to play a key role in Al tolerance in many plant species, such as buckwheat, alfalfa, and tea plant. However, the transporter responsible for the oxalate transport remains unclear. Recently, Lv et al.[38] reported that two aquaporins genes, MsPIP2;1 and MsTIP1;1, are likely involved in the secretion of oxalic acid from root tips of alfalfa. MsPIP2;1 and MsTIP1;1 expressed in the roots, stems, and leaves of alfalfa, with their expression significantly upregulated by Al stress in both roots and leaves. Overexpression of either MsPIP2;1 or MsTIP1;1 in transgenic alfalfa hairy roots confers a significant increase in root oxalate exudation but a decrease in Al accumulation in root tips, compared to the control vector lines under Al stress. However, whether these two transporters have the capability to transport oxalate remains to be investigated via analysis in a heterologous gene expression system, such as yeast or Xenopus oocytes. Moreover, it is worthwhile to investigate the functions of their homologous genes in tea plant.
Al accumulation in tea plant
-
Plants with Al contents exceeding 1,000 mg/Kg dry mass of their leave tissues are usually considered as Al hyperaccumulators. In most herbaceous plants, the Al content of leaves are less than 200 mg/Kg of dry matter, whereas tea plant can accumulate as much as 30,000 mg/Kg of Al in its leaves, making it a typical Al hyperaccumulator[39]. The distribution of Al in the plant is not uniform. In tea plant, Al is preferentially accumulated at the tips of lateral or absorbing roots, and in the upper epidermis of leaves[40]. Old leaves exhibit the highest concentration of Al, followed by roots, stems, and young leaves[41]. More Al is accumulated in old leaves than in young leaves, because it is difficult to mobilize once Al has been accumulated in the leaves. Moreover, several studies showed that most of the Al is bound to catechins in the leaves, which is different from the form of Al in the roots and xylem[12, 42]. At the subcellular level, Al is mainly accumulated in the cell wall, and only a small amount can enter the cytoplasm. Gao et al.[11] reported that approximately 69.8% and 75.2% of the total Al was detected in root and shoot cell walls, respectively, and 88.3% of the Al measured in protoplasts of tea leaves was compartmentalized in the vacuoles. While in buckwheat, 80% of total Al in the leaves is present in the vacuoles, indicating different internal Al detoxification mechanisms between these two Al hyperaccumulators[43]. The plant cell wall is primarily composed of pectin, cellulose, and hemicellulose. Several studies have revealed that over 60% of the Al accumulated in the cell wall is located in pectin fractions[44, 45]. The degree of methylation of pectin, which is catalyzed by pectin methylesterases (PMEs), has been found to play a role in both Al accumulation and tolerance in plants[2]. For example, rice genotypes that are resistant to Al exhibit greater levels of pectin methylation and less Al accumulation than Al-sensitive cultivars[46]. In tea plant, Li et al.[9] reported that low-methylester pectin was increased in a dose-dependent manner following exposure to increasing concentrations of Al treatment. Furthermore, the expression patterns of PME family genes have been investigated under various Al treatment levels, but the function of these genes in Al accumulation and tolerance in tea plant requires further study[47]. In addition, Al accumulation also differs among varieties within a species. Zheng[48] reported that the Al content of young shoots (one bud and two leaves) ranged from 77.63 to 564.68 mg/Kg across 93 tea varieties, with the coefficient of variation of 43.82%. However, the genetic basis underlying the variation of Al accumulation in aboveground tissues in the tea plant as well as other plants species remains unknown.
Al uptake and accumulation are affected by environmental factors
-
Apart from genetic factors, the uptake and accumulation of Al in tea plant are modulated by various environmental factors. Al absorption is positively correlated with the soil's available Al concentration but negatively correlated with its pH. Tea plant prefers ammonium (
$\text{NH}_4^+ $ $\text{NO}_3^- $ $\text{NH}_4^+ $ $\text{NO}_3^- $ $\text{NH}_4^+ $ $\text{NO}_3^- $ $\text{NH}_4^+ $ -
Omics technologies provide feasible approaches for characterizing cell response at the genome, transcript, protein, and metabolite levels, which can advance our understanding of plant tolerance mechanisms and can eventually be explored for crop improvement. And the advances of omics technology have facilitated the studies on responses of tea plant to Al stress. In this section, the Al-induced alterations in the plant biological process of tea plant is summarized in terms of transcriptomics, proteomics, metabolomics, ionomics and microbiomics (Table 1).
Table 1. Summary of applications of omics in Al related studies in tea plant.
Approaches Treatment time Samples Reference Transcriptomics 3 h Roots [64] Transcriptomics 7 d Leaves [68] Transcriptomics 7 d Roots [74] Transcriptomics 1, 3, 7, 14, 21, 28, 43 d Stem segment of nodal cutting [72] Transcriptomics and ionomics 6 weeks Roots [73] Transcriptomics and ionomics 0, 12, 24, 48 h Roots [34] Transcriptomics and metabolomics 0, 12, 24, 48 h Roots and leaves [82] Proteomics 12 weeks Roots and leaves [77] Metabolomics 45 d Roots and leaves of two cultivars [86] Metabolomics 7 months Tender leaves (one bud and two leaves), mature leaves, tender roots [83] Metabolomics and ionomics 8 weeks Leaves [90] Microbiomics Half a year Roots [100] Transcriptome alteration during Al exposure
-
As the representative of whole RNA transcripts in particular cells or tissues, transcriptome can record the present gene expression profile of plants, which is useful for mining functional genes and exploring the molecular mechanism and regulatory network of plant resistance. In recent studies, RNA-seq has been utilized to examine the dynamic gene expression in some tissues of tea plant exposed to Al (Fig. 2). These investigations have given us a preliminary insight into the molecular mechanisms of Al tolerance and Al's beneficial effect in tea plant.
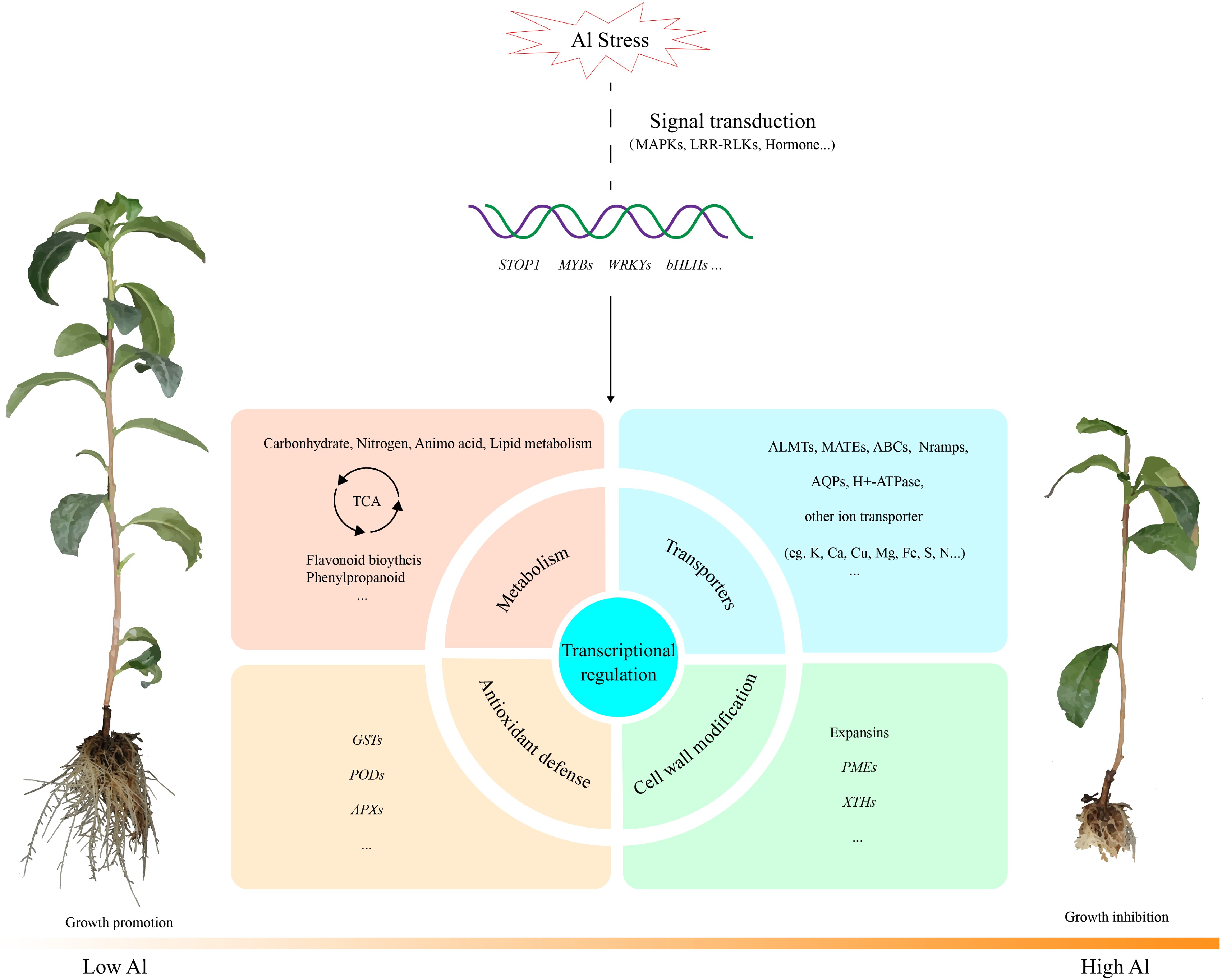
Figure 2.
General model of the major transcriptional changes in tea plant under Al stress according to transcriptomic analysis. Al alters the expression of genes involved in signal transduction, transcription factors, metabolic pathways, antioxidant defense system, transporters, cell wall modifications, etc. Abbreviations: MAPKs, mitogenactivated protein kinases; LRR-RLKs, leucine-rich repeat receptor-like protein kinases; ALMTs, Al-activated malate transporters; MATEs, multidrug and toxic compound extrusions; Nramps, natural resistanceassociated macrophage proteins; AQPs, aquaporins; GSTs, glutathione-S-transferases; PODs, peroxidases; APXs, ascorbate peroxidases; PMEs, pectin methylesterases; XTHs, xyloglucan endotransglucosylase/hydrolases.
Li et al.[64] used RNA-seq to investigate the early response of roots to Al stress. Based on de novo transcriptome analysis, they found that exposure to high levels of Al altered the expression of many genes related to transporters, transcription factors, cytochrome P450, heat shock proteins, ubiquitin ligase and organic acid biosynthesis. The transcription factor STOP1/ART1 regulates multiple genes in Al tolerance in Arabidopsis and rice[65, 66]. Li et al.'s transcriptome analysis showed that several homologs of STOP1/ART1-regulated genes including CsMATE, CsALMT, CsALS3 and CsFRDL2 were also upregulated in tea plant, indicating some common Al tolerance mechanisms across different plant species. However, tea plant also exhibits distinct Al tolerance mechanisms, as the majority of homologous genes regulated by STOP/ART1 were unaffected by Al stress, and a few were even found to be downregulated. Recently, the homologous gene of AtSTOP1 in tea plant, CsSTOP1, was cloned. Overexpression of CsSTOP1 in Arabidopsis increased the resistance of transgenic lines to Al toxicity, suggesting that CsSTOP1 might function conservatively as AtSTOP1 in Arabidopsis by modulating the expression of other crucial genes involved in Al tolerance[67].
A time series transcriptome study performed by Hao et al. highlighted the expression change of transporters induced by Al[34]. The study showed that exposure to Al resulted in differential expression of over 1000 transporter genes in tea roots, compared to control samples that received no Al treatment. Notably, under high Al conditions, the expression levels of several homologous transporter genes associated with Al transport and sequestration, cell wall modification, and organic acid exclusion in other plant species, such as AtMATE, AtALMT1, and OsNrat1, were found to be upregulated in tea roots, suggesting their potential involvement in conferring Al tolerance in tea plant. Furthermore, numerous cation transporter genes responsible for the uptake and transportation of Ca, Mg and Fe demonstrated differentially expression under Al stress, which could potentially contribute to nutrient imbalances caused by Al toxicity.
As an Al hyperaccumulator, tea plant can tolerate and detoxify Al not only in its roots but also in its leaves. A transcriptome analysis of the tea plant leaves reported that genes related to cellular transport, signal transduction, detoxification of ROS, polysaccharide and cell wall metabolism were involved in the response to Al stress[68]. Compared with the result of Hao et al.[34], a few genes share the same expression pattern in roots and leaves under Al stress. For instance, several genes encoding glutathione S-transferase and peroxidase were significantly upregulated in both roots and leaves. In plants, glutathione S-transferase and peroxidase are responsible for ROS scavenging and play a crucial role in Al detoxification and other stress tolerance processes[69]. These findings suggest that there are some common Al tolerance mechanisms between roots and shoots in tea plant. Remarkably, Al stress overwhelmingly upregulated many genes involved in phenolics biosynthesis especially in catechin biosynthesis in tea leaves, such as 4-coumarate-CoA ligase 4 (4CL4), anthocyanidin reductase, flavanone 3-hydroxylase, leucoanthocyanidin reductase, and phenylalanine ammonia lyase. Research has found that the mutation in 4CL4 (LOC_Os06g44620) in rice leads to increased accumulation of 4-coumaric acid and ferulic acid in roots, which can reduce Al binding to the hemicellulose component of the cell wall and thus enhance Al resistance[70]. Additionally, a recent study has shown that the transcription factor MYB741 activates the expression of phenylalanine ammonia-lyase 1 (MsPAL1) and chalcone isomerase (MsCHI) to increase flavonoid accumulation in roots and secretion from roots, leading to enhanced alfalfa resistance to Al stress[71]. These findings provide molecular evidence for the role of phenolic metabolism in the mechanism of Al tolerance in plants. Moreover, it has been reported that Al-catechin complex constitutes the main chemical form of Al in tea leaves[12]. Taken together, we speculate that genes related to catechin biosynthesis may play an important role in the mechanisms of Al internal detoxification in tea leaves.
At a benefit dose, the addition of Al can dramatically increase the number of new roots and root biomass. Several transcriptomic studies have revealed that some nutrient element transporters are upregulated under Al supply, which may enhance nutrient uptake and therefore promote root growth[34, 72, 73]. Based on transcriptome analysis, Fan et al.[73] identified a set of differentially expressed genes (DEGs) involved in cell growth and division. They found that these DEGs were uniquely upregulated under a proper concentration of Al compared to non-Al or high Al treatments. In line with the results of Fan et al., Cheng et al.[72] have reported that several transcription factors and enzyme-coding genes related to cell cycle and root development were significantly induced by a 0.3 mM Al supply. They also inferred that the changes in the expression of genes involved in the biosynthesis of auxin, ethylene, and jasmonate and their signal transduction pathways may participate in the perception and transmission of Al stress signals, leading to growth promotion. Recently, Ding et al.[3] proposed that non-toxic levels of Al may directly affect cellular hormonal signaling, thus stimulating cell division and elongation. This hypothesis warrants serious consideration, and further study is necessary to identify the critical genes that regulate the Al-induced growth stimulation.
Proteome alteration during Al exposure
-
It is common knowledge that much of the biological processes are ultimately determined by proteins rather than nucleic acids. The proteomics study seeks to completely characterize the entire protein complement present in a cell, tissue or organism, providing a useful tool to comprehend gene function and cellular process at the protein level[75]. In the past few decades, proteomics approaches have been extensively applied to explore the mechanisms of Al toxicity and Al tolerance in Arabidopsis, maize, tomato, etc.[76]. However, only one study has been conducted on the Al-responsive proteome of tea plant[77]. In this research, the moderate Al treatment was found to enhance the abundance of many proteins involved in antioxidant defense in tea roots and photosynthesis in tea leaves, potentially explaining the beneficial effect of Al on tea plant growth. Additionally, several enzymes involved in glycolysis and phenylpropanoid pathways were upregulated in roots but downregulated in leaves, indicating that the roots and shoots of tea plant may employ different strategies to resist Al toxicity. The tricarboxylic acid (TCA) cycle regulates the biosynthesis of various organic acids, such as malate, oxalate, and citrate, playing a critical role in alleviating Al toxicity in bacteria, yeast and plants[78−80]. Under Al exposure, the abundance of citrate synthase increased in tea roots, while most of the enzymes in the TCA cycle showed no significant changes. Given that the Al-citrate complex is the main chemical form during the translocation of Al from roots to shoots, we suggest that the accumulation of citrate synthase in tea roots would accelerate citrate biosynthesis and provide more ligands to form the Al-citrate complex for long-distance Al transport, thus playing a key role in Al detoxification in tea plant.
Metabolome alteration during Al exposure
-
Metabolomics is an ideal platform to study the mechanisms of plant stress through the qualitative and quantitative analysis of various small molecule metabolites by high-throughput techniques. Li et al.[9] found that the concentration of organic acids, including oxalate, citrate, malate, and glycolic acid in tea roots increased with increasing external Al concentration (from 0 to 2 mM), but significantly decreased following treatment with 4 mM Al. Furthermore, the study revealed that oxalate was the predominant organic acid at all levels of Al treatment, providing further evidence that oxalate serves as the primary contributor to detoxify Al in both the rhizosphere and tea roots. Phenolics have been considered as important chelating agents for Al detoxification in tea plant. Al occurs mainly as Al-catechin complexes in tea leaves, and proanthocyanidins (PAs)-Al and EGCG-Al complexes have been detected in roots and old leaves, respectively[12, 42]. Chen et al.[81] observed that the content of total phenols and total catechins in tea leaves rose when external Al concentrations increased. Moreover, Peng et al.[82] found that the high Al treatment significantly increased the contents of catechin and PA but decreased the content of epicatechin gallate in tea roots, while the same treatment slightly increased the contents of epigallocatechin and epicatechin in tea leaves. Recently, flavonols have been reported to exhibit a greater binding capacity to Al than EGCG and PAs. A significant positive correlation has also been discovered between Al accumulation and flavonols content in tea plant[83]. Moreover, the study showed that Al treatment has a different impact on the accumulation of polyphenol compounds in various tea varieties. Future research is necessary to determine whether these differences are associated with the Al tolerance of different tea varieties.
Chelation of metal ions by amino acids and their derivatives confers metal tolerance in plants[84]. Hajiboland et al.[85] discovered that the free proline content in leaves and roots of tea plant significantly increased under Al supply. Peng et al.[82] observed a significant increase in the content of theanine in both the roots and leaves of tea plant under Al treatment, while it decreased under F treatment. A comparative metabolomic study reported that amino acid synthesis was more active in an Al-tolerant tea cultivar under high Al treatment[86]. In addition, Al treatment increased the phosphate content in the roots and leaves of tea plant. The Al-phosphate compound was also detected in old leaves[59]. These findings show that phosphate may possibly contribute to the Al tolerance in tea plant.
Al is beneficial for tea plant growth at low concentrations. With the appropriate amount of Al supply, an increase in carbohydrates has been observed, which may in turn improve the growth of tea plant[85, 87]. Moreover, Morita et al.[10] reported that Al exposure enhanced caffeine secretion from the roots of tea plant, and they proposed that this may promote root growth by inhibiting callose deposition. Additionally, the beneficial concentration of Al resulted in a significant reduction of phenolics and lignin contents in the cell walls of tea leaves and roots[6, 85]. This reduction may lead to increased cell wall extensibility and cell elongation, which further promotes plant growth and development.
Ionome alteration during Al exposure
-
Maintaining ion homeostasis is essential for plant growth and development. In tea plant, ionomic studies have revealed that Al can interfere with the uptake or transport of various nutrients. Al supply lowered Mg and Ca levels in roots, possibly because Al can compete with them for membrane transporter or the binding sites on the cell wall[34, 73]. Similarly, Al also affected the accumulation of P and K in roots, with an increase under moderate Al addition and a reduction under high Al treatment[73]. At a beneficial dose, Al facilitated the accumulation of boron (B) and F in both roots and leaves[61, 88]. Fung et al.[6] showed that Al supply decreased Fe accumulation in tea roots. A similar result was found by Hajiboland et al.[89] in tea leaves and he proposed that the inhibitory effect of Al on Fe uptake might be a reason for the Al-induced growth stimulation. Conversely, Hao et al.[34] found that Al treatment led to a dose-dependent increase in the accumulation of Fe in tea roots. The discrepancy in these findings might be attributed to the differences in pH values and the ionic composition in nutrient solution. It is important to note that Al supply also affects the distribution of elements in different parts of the tea plant. For example, Al addition enhanced the Ca concentration in young leaves but reduced it in old leaves[90].
Microbiome and Al-tolerant microorganisms
-
Plant growth is affected by microorganisms in the environment. It has been revealed that certain microorganisms which colonize the root system of plants can reduce both biotic as well as abiotic stress on the plants and promote their growth. These microorganisms are commonly referred to as plant growth-promoting rhizobacteria (PGPR). In plants, several PGPRs have been found to alleviate Al toxicity in acidic soils. For example, inoculation of Bacillus toyonensis Bt04 reduced Al accumulation and lipid peroxidation in maize roots under Al stress, therefore promoting maize growth and root development[91]. Additionally, Farh et al.[92] reported that inoculation of three mixed Al-resistant bacteria strains (Pseudomonas simiae N3, Chryseobacterium polytrichastri N10, Burkholderia ginsengiterrae N11-2) was able to support Arabidopsis growth under Al stress, leading to higher expression levels of several Al-stress related genes, such as AtALMT1, AtALS3 and AtAIP (Al induced protein). This finding indicates that certain microorganisms might enhance the Al tolerance of plant hosts by affecting the expression of Al-tolerant genes in these plants.
As a typical Al-tolerant plant, the tea plant's Al tolerance mechanism and its association with endogenous and external microorganisms have attracted extensive attention. Li et al.[93] reported that the structure and function of fungal community were evidently interrelated to exchangeable Al in tea garden soils. Recently, a number of Al-tolerance yeast, fungi as well as bacteria have been isolated from tea garden soils. These microorganisms can tolerate Al salts at concentrations up to 100 mM[94−97]. Additionally, several Al-tolerant endophytic bacteria have been screened and identified. For instance, Zhao et al.[98] isolated 53 Al-resistant endophytic bacteria from tea plant. Among these strains, the Burkholderia cepacia G3 exhibited the ability to adsorb Al ion and facilitate seed germination and seedling growth of wheat. Moreover, Zhang et al.[99] identified an Al-tolerance endophytic fungi, Rhodotorula glutinis A3, which was able to grow in the presence of Al3+ at concentrations as high as 250 mM. However, additional research is required to determine if and how these Al-tolerant bacteria influence Al tolerance of the tea plant.
Recently, Jiang et al.[100] conducted a study on the endophytic bacteria present in tea roots under different Al treatments, providing new insights into the mechanism by which Al promotes tea plant growth. The addition of Al significantly promoted the growth of tea roots in both hydroponic and filed systems. Meanwhile, meta-16S rDNA analysis revealed significant differences in the diversity and abundance of endophytic bacteria in tea roots depending on the Al treatment concentration, with Burkholderia and Actinobacteria being enriched after Al treatment. Furthermore, the authors isolated 16 growth-promoting endophytic bacteria from tea roots. Among these strains, Leifsonia shinshuensis A1 exhibited remarkable tolerance to Al, as it was able to survive in the LB liquid medium containing 200 mg/L of Al. The inoculation of tea cuttings with L. shinshuensis A1 resulted in a significant increase in the number and weight of roots and shoots. Moreover, inoculation of L. shinshuensis A1 reduced Al accumulation in wheat and mitigated the inhibitory effects of Al on wheat growth. These results led to the hypothesis that Al may indirectly promote tea plant growth by promoting the distribution of Al-tolerant and growth-promoting endophytic bacteria.
-
The cell wall is the primary site of Al accumulation in tea plant. Several studies have investigated the distribution of Al in cell wall fractions, as well as the expression patterns of genes related to cell wall modification in response to Al stress. However, the role of cell walls in Al tolerance in tea plant and its underlying physiological and molecular mechanisms remains to be further investigated in detail. It is crucial to explore the relationship between Al tolerance and cell wall properties of different tea varieties. The accumulation of Al increases with leaf age; hence, examining the characteristics of Al accumulation in cell walls at various leaf ages would be interesting and valuable. Moreover, identifying genes that can regulate the ability of cell walls to bind with Al will provide significant insights into understanding the mechanisms of Al tolerance in tea plant.
Recent research on rice and alfalfa has provided molecular evidence that phenolic compounds play an important role in plant tolerance to Al. The polyphenolic compounds, accounting for approximately 18%−36% of the dry weight of tea leaves, have been suggested to play an important role in Al tolerance mechanism of tea plant. The 27Al NMR indicated that most of Al was bound to catechins in the leaves, and Al-EGCG, Al-flavonol complexes have been detected in old leaves[12, 42, 83]. Notably, Al accumulation in young shoots of tea plant was positively correlated with their flavonol content. Moreover, both transcriptome and metabolome studies have revealed alterations in polyphenol metabolism in response to Al stress[74, 81]. However, further research is needed to investigate the direct relationship between polyphenol metabolism and Al tolerance in tea plant, such as the role of genes involved in polyphenol metabolism in Al tolerance of tea plant.
Current reports indicate that transporters are crucial in the tolerance and accumulation of Al in plants. Therefore, identifying the transporters responsible for Al tolerance and accumulation in tea plant is vital for developing low Al-accumulating tea cultivars and Al-tolerant crops. Using the yeast system could be an efficient way to identify transporters that mediate Al tolerance or accumulation. Furthermore, the recent significant breakthrough in the establishment of a genetic transformation system in tea plant will greatly accelerate the elucidation of the molecular mechanisms underlying Al tolerance and accumulation in this species[101]. The transcriptome analyses of tea plant subjected to Al stress have provided a set of candidate transporter genes. Further study is necessary to verify their functions in Al tolerance and Al accumulation. In addition, investigating the modulation of the transcriptome and proteome of organelles, such as vacuoles, in response to Al stress would enable the identification of specific transporters. This strategy has been successfully employed to uncover several Al transporters in hydrangea and buckwheat[32, 102].
In recent years, a number of tea genomes have been assembled and subsequently released[103−108], providing an excellent opportunity to study the molecular mechanism of Al tolerance and accumulation in the tea plant. For example, several gene families with members that play important roles in Al tolerance in plants, such as PME, ALMT, and NRAMP, have been identified throughout the tea genome, laying the foundation for further exploration of their functions in Al tolerance in tea plant[47, 109, 110]. Over the past few decades, QTL mapping and GWAS approaches have been employed to identify various Al resistance genes and other ion transporters[111−113]. In the future, these approaches can be further applied and will provide new insights into the genetic basis of Al tolerance and accumulation in the tea plant. The different cell types mediate Al uptake, long-distance transportation and its accumulation in leaves. The state-of-the-art single-cell sequencing technologies reveal the intracellular dynamics of individual cells[114]; hence, the application of single-cell sequencing to the study of Al in the tea plant will expedite our understanding of the mechanisms underlying Al tolerance and hyperaccumulation in tea plant.
The research was financially supported by the National Key R&D Program of China (2022YFF1003103) and National Natural Science Foundation of China (3211101118) NSFC-DFG collaborative project.
-
The authors declare that they have no conflict of interest. Weiwei Wen is the Editorial Board member of Beverage Plant Research who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and her research groups.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhang X, Liu L, Luo S, Ye X, Wen W. 2023. Research advances in aluminum tolerance and accumulation in tea plant (Camellia sinensis). Beverage Plant Research 3:18 doi: 10.48130/BPR-2023-0018
Research advances in aluminum tolerance and accumulation in tea plant (Camellia sinensis)
- Received: 29 April 2023
- Revised: 05 July 2023
- Accepted: 06 July 2023
- Published online: 31 July 2023
Abstract: Tea plant (Camellia sinensis) is widely planted on acidic soils where aluminum (Al) toxicity is considered as a primary factor limiting plant growth. Unlike most plant species, tea plant is Al tolerant and accumulates high levels of Al. Understanding the mechanisms underlying Al tolerance and accumulation in tea plant may contribute to the improvement of tea plant cultivation and development of Al-tolerant crops. In this review, we summarize recent advances in the uptake, transport and accumulation of Al in tea plant and the genetic and environmental factors that affect these processes. We further highlight recent studies of Al in tea plant based on omics approaches, including transcriptomics, proteomics, metabolomics, ionomics and microbiomics. We present perspectives for future research that will be helpful to decipher the mechanisms underlying Al tolerance and accumulation in tea plant.
-
Key words:
- Tea plant /
- Aluminum /
- Accumulation /
- Tolerance /
- Omics study













