-
Mayonnaise is a good example of oil-in-water emulsions with a semi-solid structure emulsified by egg yolk. Egg yolk plays a critical role in mayonnaise with an oil volume fraction greater than 0.74[1−3]. The combination of yolk lipoproteins and phospholipids contributes to the formation and stabilization of mayonnaise[4]. Because of the health concerns of increased cholesterol, many attempts have been made to develop low-cholesterol or cholesterol-free sauces with similar characteristics to 'real' mayonnaise, such as use of wheat gluten[1], apple pomace particles[5], and gums[6] as replacements for the egg yolk. Besides the use of non-egg ingredients, the granular fraction of yolk with enhanced solubility and functionality might provide a possible solution to enable the production of mayonnaise with increased protein and reduced cholesterol content.
Egg yolk plasma, rich in low-density lipoprotein (LDL), and granular fraction, rich in high-density lipoprotein (HDL), are produced as co-products during the isolation of the yolk immunoglobulins, IgY. Creating high value applications for these co-products are needed. The plasma and granular fractions have been shown to have emulsifying properties and could be potentially used in food applications such as mayonnaise[7]. The granules contribute to about 22% of yolk dry matter with about 50% of yolk proteins and 7% of yolk lipids, whereas plasma represents about 78% of the dry matter accounting for about 50% of yolk proteins and 90% of yolk lipids[4]. When isolating IgY, the insoluble fraction of egg granules (70% HDL, 16% phosvitin and 12% LDL) is removed by centrifugation, while plasma (85% LDL and 15% livetins) is further separated into water-soluble livetins which contains IgY and the insoluble polysaccharide-LDL complex[8]. Carboxymethyl cellulose (CMC), a common polysaccharide used as a food stabilizer, could be used for LDL removal by forming a unique LDL product, i.e., LDL-CMC fat pad that floats on top after centrifugation[9]. This unique LDL product may have an improved emulsifying property due to the presence of CMC compared to LDL alone, and can be used as an emulsifying agent. However, the emulsification property of LDL-CMC complex in mayonnaise has not been examined. This research intends to address this knowledge gap. In addition, a reduced fat LDL-CMC was examined in this study to evaluate the effect of defatting on its emulsifying property when preparing mayonnaise.
Due to its low solubility and aggregated structure, the egg granular fraction had shown greater challenges in its use in food emulsions[10]. Hence, conditions and novel methods for effectively solubilizing the granular fraction are of interest for this investigation. Phosphocalcic bridges are known to account for the poor solubility of granules. Breakage of the phosphocalcic bridge via calcium removal may help increase the solubility of the granular fraction leading to an enhanced emulsifying property[11]. To the best of our knowledge, such a novel concept of a de-calcium granular product and its application as an emulsifier have not been reported in the literature.
In this study, egg yolk was fractionated to obtain plasma and granules followed by modifications to produce two unique products, LDL-CMC and de-calcium granular fraction (DC-Gran). These products were used to prepare mayonnaise, and the effect of the CMC and de-calcium treatment on the emulsifying properties of the granules was evaluated by characterizing the properties of mayonnaise. Textural and rheological properties, heat stability, and oil droplet size were assessed and compared to the mayonnaise control made with fresh yolk. We hypothesized that modified granules with enhanced solubility would lead to a mayonnaise with improved emulsion characteristics. We also hypothesized the presence of CMC would result in further enhanced emulsion stability of mayonnaise.
-
Figure 1 demonstrates a general schematic for fractionating egg yolk into different products. To remove excess egg white, the yolk was carefully separated from the white and rolled on a paper towel. The yolk membrane was then pierced, and the contents of the yolk were poured into a beaker. Deionized (DI) water was added to the beaker in a 1:1 (w/w) ratio to the yolk. The mixture was stirred slowly until homogenous and then poured into 50 mL centrifuge tubes. The tubes were centrifuged for 45 min at 10,000 ×g at 4 °C. The plasma (supernatant) from each tube was poured off into a new beaker, and the granule (pellet) was removed and 200 ppm of sodium azide (NaN3) was added for preservation. CMC solution (1% w/w) was added into the plasma at 0.2:1 (w/w) ratio and the pH of the mixture was adjusted to 5.0 using 1 N HCl. CMC-plasma mixture was incubated at room temperature for 30 min prior to centrifugation at 10,000 ×g for 45 min at 4 °C. After centrifugation, livetin fraction (subnatant) was separated from the LDL-CMC (fat-pad). For comparison and evaluating the effect of CMC, LDL was also prepared using the protocol established by Anton et al.[12] with modifications. In brief, ammonium sulfate (40%) was added to the plasma followed by constant stirring at 4 °C for 1 h and centrifuged at 10,000 ×g for 30 min at 4 °C. The precipitated LDL fraction was dialyzed in deionized water for 3 d under refrigerated temperature to remove the residual ammonium salt.

Figure 1.
Fractionation schematic of fresh egg yolk to obtain the yolk fractions (red) used in this study.
Defatting of LDL-CMC complex
-
A fresh LDL-CMC fat pad was dispersed in acetone in 1:10 solid to solvent ratio (w/v). After the pellet was in contact with acetone, homogenization was carried out at 10,000 rpm for 2 min followed by incubation at room temperature (23 °C) for 12 h. After incubation, the solubilized neutral lipid was filtered and removed from the residual pellet fraction, resulting in samples with reduced neutral lipids. The acetone was rotary evaporated to collect the neutral lipid fraction for lipid removal quantification.
Calcium removal from the granules by precipitation
-
Fresh granule pellets were dispersed in DI water at 2% (w/w) solid content. The sample was stirred slowly and the pH of the dispersion was adjusted to 11 using 2 N NaOH until the granular component was completely solubilized in water. Sodium carbonate (Na2CO3) was added to the solution in the 2:1 molar ratio to the calcium in the solubilized granules followed by constant stirring at room temperature for 5 hr and continuous stirring at 4 ºC for 3 d. Granule solution was then centrifuged twice at 15,000 g for 15 min at 4 °C to remove the precipitated calcium carbonate. The supernatant was collected, and the pH of the solution was adjusted to 7.0 followed by centrifugation at 15,000 ×g for 10 min at 4 °C to recover the de-calcium granules. The recovered granules were dialysis at 4 °C for 2 d to remove the salt.
Calcium content quantification
-
Egg granules and de-calcium granules were freeze-dried prior to calcium analysis as described by Barickman et al.[13] and Masson & Dalix[14] with some modifications. In brief, 10 mL of 70% HNO3 was added to 0.5 g dried samples followed by digestion at 95 °C overnight. Calcium content was measured using an inductively coupled plasma mass spectrometer (ICP-MS; Agilent Technologies, Inc., Wilmington, DE, USA). Calcium content is expressed on a dry weight basis.
Microstructure of native and de-calcium granular fraction by using confocal laser scanning microscopy (CLSM)
-
The microstructure of native and de-calcium granular fraction was observed by CLSM (Leica SP8, Leica Microsystems Inc., Buffalo, IL, USA), as described by Gallier et al.[15]. Fresh native and de-calcium granules, 0.2 g, were dispersed in 2 mL of deionized water. Then the lipid and protein of the sample were fluorescently stained by adding 100 µL of the fluorescent dyes (1 mg/mL) Fast Green FCF for protein and Nile Red for lipid. All the samples were observed under a 40× oil-immersion objective. The Nile Red probe was excited at 488 nm and the filters were set to collect the emitted light between 550−620 nm. The Fast Green FCF probe was excited at 633 nm and emitted light was collected between 650 and 750 nm.
Protein solubility of native and de-calcium granular fraction under various pH
-
The protein solubility of native and the modified granular products was measured as described by Anton et al.[16] with some modifications. In brief, samples were diluted in a DI water to an approximate protein concentration of 4% (w/w). The pH of diluted samples was adjusted from 2.0 to 12.0. Samples were then equilibrated at room temperature for 2 h under constant stirring followed by centrifugation at 10,000 ×g for 20 min at 10 °C. Prior to centrifugation, a sample (1 mL) was taken for the determination of initial protein content. Concentration of proteins in the supernatant was determined as the solubilized protein. The protein solubility was calculated as:
${\rm Protein \;solubility} {\text{%}} = \frac{S olubilized \; protein\left(mg\right)}{Initial\;protein\;content\left(mg\right)} \times 100{\text%} $ Protein concentration was measured using the Lowry method[17,18].
Preparation of mayonnaise
-
Mayonnaise containing different fractionated egg yolk products (fresh yolk (FY), de-calcium egg granules (DC-Gran), native egg granules (Gran), LDL-CMC, defatted LDL-CMC (DF-LDL-CMC), LDL, combination of Gran and LDL-CMC (Gran-LDL-CMC)) were prepared in duplicate based on the formulation used by Kim et al.[19]. The detailed formula of each mayonnaise is shown in Table 1. Fractionated egg yolk products were applied with a same solid content as 4% (w/w) as using the fresh yolk control. Vinegar, salt, sugar, water and egg products were mixed using KitchenAid® mixer at speed 4 for 3 min. The mixing speed was increased to level 8 while 10 mL of oil was added dropwise using a titration burette within 10 min. Then, another 70 mL oil was added within 10 min. The remaining oil (154 mL) was added using a disposable pipette and the mixing speed was increased to 10 for 5 min for all samples except Gran and DC-Gran mayonnaises. For these samples, the remaining oil was added and mixed at speed level 8 for 5 min to maintain the structure of the mayonnaise. The high-speed mixing at level 10 would cause a breakdown of the emulsion. All mayonnaise samples were stored in capped containers at 4 °C. All analyses were performed after equilibrating at room temperature on storage day 0, 2, 7, 14 and 28 for storage stability evaluation.
Table 1. Ingredients used for mayonnaise preparation.
Sample Egg products (g) Water (g) Oil (g) Vinegar (g) Salt (g) Sugar (g) FY 24.0 25.5 234.0 9.0 4.5 3.0 LDL 47.1 2.4 LDL-CMC 26.3 23.2 Gran 27.7 21.8 DC-Gran 31.0 18.5 Gran-LDL-CMC Gran: 6.1
LDL-CMC: 20.522.9 Defatted LDL 13.5 36.0 FY: Fresh yolk; Gran: native granular fraction; DC-Gran: de-calcium granular fraction; LDL: low-density lipoprotein; CMC: carboxymethyl cellulose. Characterization of rheological properties
-
Rheological measurements of mayonnaise were performed using a Discovery Hybrid rheometer (TA Instruments, New Castle, DE) with a serrated parallel plate (diameter 40 mm) at a gap distance of 1 mm and normal force maintained at 0.5 ± 0.2 N. Before starting the measurements, all samples were allowed to rest for 10 min after loading to allow temperature equilibration and sample relaxation. The static viscoelasticity of mayonnaise was analyzed. Furthermore, the dynamic viscoelasticity of mayonnaise was measured by oscillatory tests.
Flow behavior of mayonnaise
-
Shear rate was increased logarithmically from 0.01 to 100 s−1 in 100 s, then decreased to 0.01 s−1 in 100 s. This test was conducted at 20 °C to simulate how mayonnaise is often used at room temperature. The data curve was fitted using the Herschel-Bulkley model, and the model parameters of dynamic yield stress (
$\iota_{\rm o} $ $\rm \tau = \tau_{0} + K\gamma^{\rm n} $ where τ is the shear stress, γ is the shear rate, τ0 is the yield stress, K is the consistency coefficient, and n is the flow index. All R2 values of the model fitting were higher than 0.98.
Viscoelasticity of mayonnaise
-
To obtain dynamic viscoelastic measurements, the linear viscoelastic range (LVR) was determined using strain sweep (0.01−300%) at a fixed frequency of 1.0 Hz at 20 °C. The storage modulus (G') was recorded as the mean G' within the LVR and it is often correlated with the sample firmness. The static yield stress was obtained as the stress where a 10% reduction in the average G' from the LVR region was observed[20]. The loss modulus (G'') was also recorded as the mean G'' within the LVR.
Dynamic frequency sweep was conducted over a frequency range of 0.1−100 Hz at a constant strain of 0.1%, which was within the LVR to determine the stability of samples over time[20,21]. The ratio of G'' and G' was recorded as tan δ. The experimental data of all frequency sweep tests were correlated to the Power Law equation:
$\rm G^* = Aw^{1/z} $ G* is the complex modulus of the sample (G* = (G'2 + G''2)0.5), w is the applied angular frequency, and z is the coordination number. All R2 values are higher than 0.98.
Texture analysis of mayonnaise
-
Tests were carried out using a TA.XT-Plus Texture Analyzer (Stable Micro Systems, UK) with a load cell of 50 kg. Compression test was performed using a cylindrical probe (3.8 cm diameter). Forty grams of mayonnaise samples were carefully scooped into 60 mL polystyrene jars. One cycle compression was applied at a constant 1 mm/s, to a sample depth of 5 mm, and returned to the start position. The positive peak was taken as a measurement of firmness[22].
Heat stability
-
The heat stability of mayonnaise was evaluated using the method modified from Ghazaei et al.[23]. In brief, 5 g samples were heated at 80 °C for 30 min, then centrifuged at 3,000 g for 15 min at 4 °C. The separated oil was carefully withdrawn and discarded. The heat stability of the mayonnaise was calculated as:
$\begin{split}&{\rm{ Mayonnaise \;heat\; stability}} ({\text{%}}) = \\&\quad \frac{weight\;o f\;mayonnaise\;a f ter\;oil\;separation\left(g\right)}{initial\;weight\;o f\;mayonnaise\left(g\right)} \times 100 \end{split}$ Light microscopy for particle size analysis
-
Mayonnaise structure was observed under a microscope as described by Primacella et al.[20]. A drop of diluted mayonnaise sample (1:2 sample to water w/w) was placed on a slide. The sample was covered with a cover glass and observed at 40× magnification at room temperature for oil droplet size measurement. Particle size analysis was carried using ImageJ[24] followed by statistical analysis to obtain size distribution and mean particle size. The polydispersity index (PDI), indicating the spread of the particle size or size distribution, was calculated as follow:
$\rm PDI = (\delta/d)^{2} $ where PDI is a dimensionless value and δ and d are the standard deviation and mean droplet diameter[25].
Statistical analysis
-
All experiments and measurements were performed in duplicate. Statistical analysis was performed with JMP Pro 13, statistical software from Statistical Analysis System (SAS) Institute Inc. (Cary, NC, USA). One-way analysis of variance (ANOVA) was conducted, and significance of difference (P < 0.05) was calculated using Tukey's HSD (honest significant difference) test.
-
Various mayonnaises were made through emulsification using different types of egg yolk products including FY, DC-Gran, Gran, LDL-CMC, DF-LDL-CMC, LDL and the combination of Gran and LDL-CMC at a ratio of 22:78 (Gran-LDL-CMC, representing the same proportion as in fresh yolks). After de-calcium treatment, a 55.0% calcium reduction (of the total calcium) was achieved in DC-Gran products as measured by ICP-MS calcium analysis. A semi-solid and viscous mayonnaise was successfully formed using all types of fractionated egg yolk products except DF-LDL-CMC.
After acetone extraction which removed, about 11% (w/w) of total lipids from LDL-CMC, the DF-LDL-CMC product was not able to facilitate the formation of the semi-solid mayonnaise, but rather a liquid oil-in-water emulsion. The loss of functionality of DF-LDL-CMC is likely due to the denaturation of LDL apoproteins during solvent extraction which reduced the emulsification property of the products[26]. Thus, DF-LDL-CMC was not further evaluated in the following experiments, and only the properties of the other six types of mayonnaise were discussed.
Particle size of mayonnaises
-
The size and uniformity of various mayonnaise samples are demonstrated in Fig. 2. Particle size is an essential parameter for emulsion systems which affects the stability and rheological properties of emulsions[27]. The characteristics of mayonnaises and their stabilities are associated with the mean particle size and particle-size distribution of the oil droplets. Smaller droplet size leads to a higher droplet surface area per gram of sample and a more stable emulsion system.
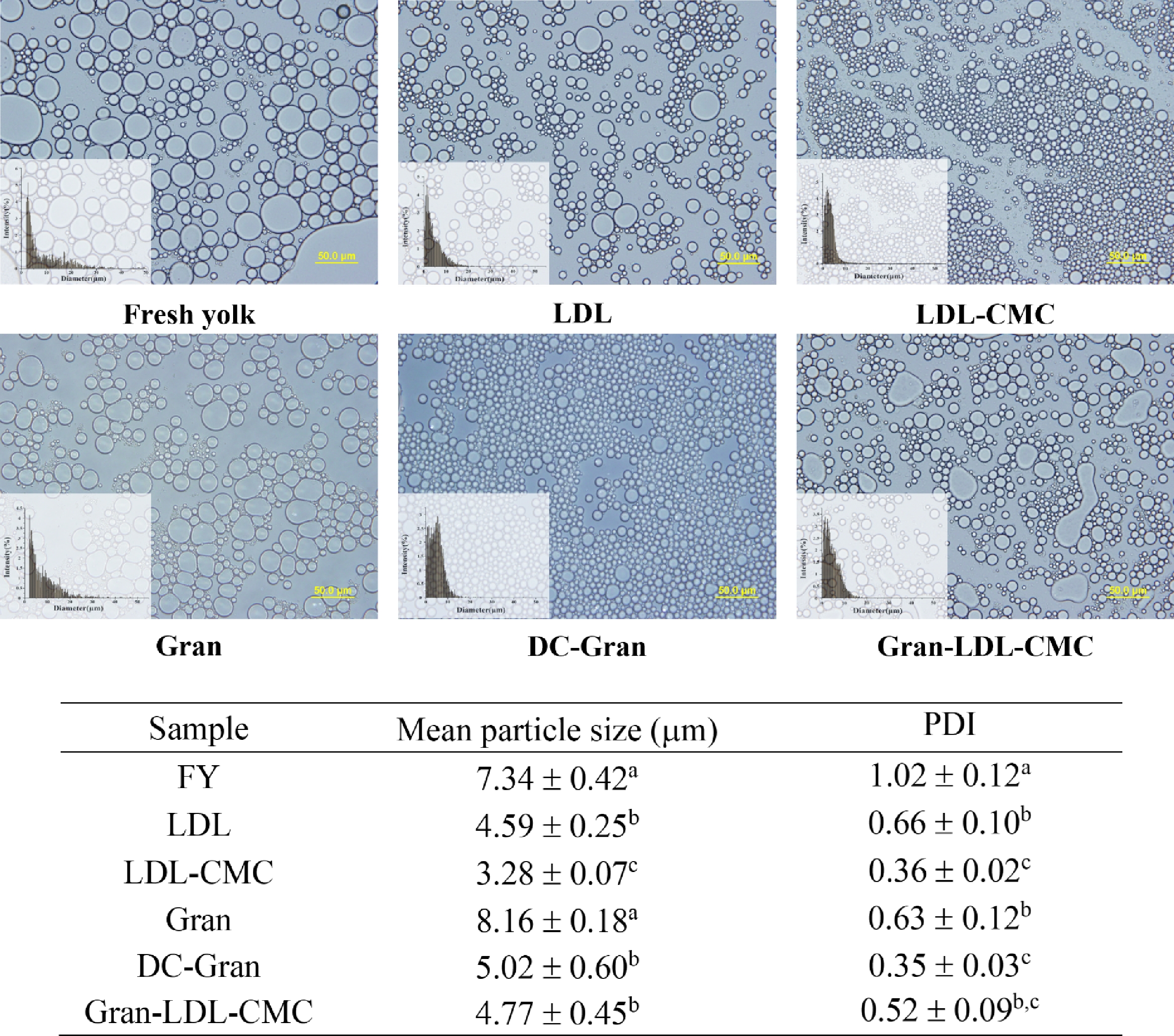
Figure 2.
Bright field optical microscopic images of mayonnaises at 40× magnification, mean particle size of mayonnaise droplets, and polydispersity index (PDI). Scale bar = 50 μm. Values with different superscripts are significantly different within the same column (P < 0.05).
As shown in Fig. 2, the mean particle sizes of LDL containing mayonnaises were significantly lower than samples made from Gran and FY (P < 0.05). Mayonnaise prepared by LDL-CMC had the smallest particle size of 3.28 µm indicating that the complexing of CMC with LDL, at a level of 1.2% CMC (w/w, dry weight basis) in LDL, was able to enhance the emulsification property of LDL. It was reported that the functional properties of protein are generally improved by complexation with other polysaccharides[28]. Studies have shown that the addition of CMC increased the viscosity of the dispersion and electrostatic repulsion among protein particles, thus, leading to a long-term stability of acidified skimmed milk drinks containing 0.04% CMC[29] and whey protein isolate stabilized oil-in-water emulsion with 0.08% CMC[28].
The addition of CMC also led to a more uniform mayonnaise emulsion with the lowest polydispersity index (PDI) of 0.36. FY mayonnaise had the highest value of PDI followed by LDL, Gran, Gran-LDL-CMC, LDL-CMC and DC-Gran. No significant difference was observed between the PDI value of LDL-CMC and DC-Gran (P > 0.05). PDI represents the distribution of particle size populations where an increased PDI indicates a wider range of particle size population[25,30]. A PDI value less than 0.1 indicates monodisperse particles and a value higher than 0.1 implies polydisperse particle size distribution[25]. All six mayonnaise samples evaluated in this study had polydisperse particles (PDI > 0.1) while FY demonstrated the broadest particle size distribution indicating the least homogeneity. The addition of Gran in LDL-CMC (Gran-LDL-CMC, reconstituted yolk system) increased the polydispersity (PDI) compared to LDL-CMC alone. This is due to the presence of the insoluble and aggregated granular particles leading to a reduced emulsification activity.
Mayonnaise with LDL alone had the second smallest particle size of 4.59 µm among the six products examined. LDL is known to be the most important contributor to the emulsifying properties of egg yolk, showing a better emulsion stabilizing ability than egg yolk and the granular fraction in water at neutral pH[31]. As shown in Table 2, LDL fraction had the highest lipid content of 88% (w/w, dry weight basis) followed by the combination of Gran and LDL-CMC, FY and Gran. Gran had the lowest lipid content and highest protein content of 64% (w/w, dry weight basis). The high lipid content of LDL can result in a higher content of phospholipids compared to FY and Gran products. The phospholipid-protein complexes could contribute to the superior emulsifying properties of LDL products[32,33]. As a zwitterionic surfactant, phospholipids are one of the most effective natural emulsifiers which can reduce the interfacial tension of emulsions[34], where the hydrophobic interaction plays a critical role in maintaining the protein-phospholipids interaction by incorporating proteins into phospholipids micelles[35]. In addition, electrostatic and hydrophobic interactions between phospholipids and protein can lead to desirable conformational changes of protein resulting in an improved emulsifying property[36]. Thus, the abundance of phospholipid-protein complexes in LDL products is responsible for their enhanced emulsifying property compared to Gran and FY products.
Table 2. Composition of egg yolk ingredients relative to the total solid content.
Sample Protein
(%, w/w) (d.b.)Lipid
(%, w/w) (d.b.)Phospholipids
(%, w/w) (d.b.)FY 33.0 62.5 20.6 Gran 64.0 31.0 11.1 LDL 10.0 88.0 23.8 Gran-LDL-CMC 21.4 76.0 21.1 d.b.: dry weight basis. Composition was calculated based on yolk, granule and plasma composition summarized by Anton (1997[11]; 2006[37]; 2007[38]) and solid content of each product. This study most notably demonstrates an enhanced emulsifying property of DC-Gran after disruption of the native granule aggregation. DC-Gran samples demonstrated a significant smaller particle size of 5.02 µm and more uniform mayonnaise emulsion, with a PDI value of 0.35, compared to the native Gran samples as shown in Fig. 2 (P < 0.05), indicating a greatly enhanced emulsification property of DC-Gran. In addition, there was no significant difference in particle sizes among samples made with LDL, DC-Gran and Gran-LDL-CMC (P > 0.05).
Under low ionic strength and acidic pH, the egg granules mainly contain the non-soluble HDL-phosvitin complexes linked by phosphocalcic bridges in an aggregated state leading to its poor solubility[7,39]. During the de-calcium process, aggregated egg granules were first solubilized under alkaline conditions. Calcium was then removed by the formation of insoluble calcium carbonate. The replacement of the divalent calcium by monovalent sodium is able to destabilize the compact structure of granules[40]. The destabilization of egg granules had increased the accessibility of the proteins during the emulsification of mayonnaise leading to the formation of smaller and more uniform oil droplets compared to the one emulsified by the native granules. Figure 3 demonstrates the microstructure of native and de-calcium granular fractions dispersed in deionized water. Aggregated clusters were observed in the native granular fraction, whereas smaller and more dispersed particles were found in the de-calcium treated granular fraction. This confirms that de-calcium treatment is able to successfully break the phosphocalcic bridge resulting in smaller granular particles which provides an enhanced functional property. Anton & Gandemer[11] also observed an increased solubility of granules in sodium chloride solution (> 0.3 M) due to the disruption of the phosphocalcic bridges by monovalent sodium.

Figure 3.
Confocal microscopy images of native and de-calcium granular fractions stained with Nile Red (lipid) and Fast Green FCF (protein). Scale bar = 20 µm.
Rheological properties of mayonnaise
-
Mayonnaise is an oil-in-water emulsion which contains a high oil content of up to 80% (w/w), giving a semi-solid and viscoelastic behavior. The rheological behavior is an imperative property of mayonnaise which strongly affects its sensory properties as well as its perceived textures. Difference in emulsification ingredients can change the rheological properties of mayonnaise. Hence, to evaluate the potential utilization of fractionated egg yolk ingredients in preparation of mayonnaise, rheological behavior of mayonnaise was evaluated and compared. Flow behavior was assessed to demonstrate the relationship between shear stress and shear rate, while dynamic viscoelasticity was measured in the linear viscoelastic range (LVR) to obtain storage and loss modulus and sample behaviors upon various frequencies applied.
Flow behavior
-
Table 3 represents the flow behavior of mayonnaises with the yield stress (
$\iota_{\rm o} $ Table 3. Herschel-Bulkley model parameters of mayonnaise samples measured at 20 °C.
Sample $\iota $ (yield stress) (Pa) K (Pa.s) n FY 17.5 ± 2.8c 45.1 ± 5.3b 0.24 LDL 23.6 ± 6.9c 100.2 ± 11.8a 0.19 LDL-CMC 44.4 ± 2.3c 118.1 ± 8.8a 0.23 Gran 98.6 ± 15.5b 117.9 ± 25.1a 0.18 DC-Gran 204.2 ± 36.6a 120.9 ± 32.0a 0.24 Gran-LDL-CMC 46.6 ± 5.9c 116.5 ± 3.6a 0.19 Within the same column, values with different superscripts are significantly different (P < 0.05). Yield stress is an important parameter to consider during food manufacturing. The yield stress values, as shown in Table 3, represent a dynamic yield stress in contrary to the static yield stress obtained by the viscoelasticity measurement. Dynamic yield stress is the minimum stress required to maintain the flow of the material[41]. Compared to FY mayonnaise, Gran and DC-Gran had a significant difference (P < 0.05) in the yield stress. The high yield stress of Gran containing mayonnaise likely results from the high protein content of the granular fraction leading to a thicker emulsion formation compared to mayonnaise emulsified by LDL products[11]. Moreover, DC-Gran had an even higher yield stress than the native Gran (P < 0.05) which was due to the increased dispersion and accessibility of proteins and enhanced emulsification. Although not statistically different due to the high variation of the DC-Gran and Gran mayonnaises, FY had the lowest yield stress followed by LDL, LDL-CMC, and Gran-LDL-CMC. The yield stress of LDL-CMC was significantly higher than of LDL (P < 0.05) which was due to the thickening effect of the CMC addition. The dynamic yield stress values of the six types of mayonnaises ranged from 17.5 to 204.2 Pa, which in general were close and within the range reported in previous studies: 23−305 Pa[42]. Various factors, such as particle volume fraction, particle size, and magnitude of interparticle forces, could also lead to differences in dynamic yield stresses[43].
The consistency coefficient (K) is often used as an indication of fluid viscosity. As shown in Table 3, the calculated K values ranged from 45.1 to 120.9 Pa s, where only FY mayonnaise had a consistency coefficient value within the range previously reported by Juszczak et al.[44] on Polish commercial mayonnaise: 11.86−67.91 Pa s. The four other types of mayonnaises exhibited a K value greater than 100 Pa s, where the highest coefficient was observed for DC-Gran mayonnaise followed by LDL-CMC, Gran, Gran-LDL-CMC, LDL, and FY. These differences in consistency coefficient could be caused by the balance of interparticle colloidal forces which includes attractive (mainly van der Waals and hydrophobic) and repulsive (mainly electrostatic and steric) forces[27]. Since an increased viscosity of emulsion could slow droplet movement, thus retard creaming and coalescence, a higher viscosity of mayonnaise can be an indicator for a better emulsion stability[27].
The flow index (n) values of all mayonnaises at 20 °C were lower than 1.0 indicating the pseudo-plastic fluid behavior[45]. Overall, the flow index of the six types of mayonnaise was within the range reported previously at ambient temperature (20 °C)[44,46]. The flow index varied widely from 0.13 to 0.91 for some commercial or self-prepared mayonnaises likely resulting from the different evaluation methods applied such as capillary viscometer, cone-plate viscometer, and different shear rate applied[42].
Viscoelasticity
-
As shown in Fig. 4a, all samples had a viscoelastic behavior where the storage modulus (G') is greater than the loss modulus (G''), which is typical for concentrated emulsions indicating a more solid-like behavior[47]. The storage modulus represents the recoverable energy when a material is subjected to deformation. When shear stress continues to increase and reaches the threshold, the storage modulus will decrease resulting in a less solid-like behavior. As shown in Fig. 4a, FY and LDL mayonnaise had the lowest G' followed by LDL-CMC, Gran-LDL-CMC, Gran and DC-Gran mayonnaise which was the firmest. The high G' of the DC-Gran mayonnaise could have resulted from the high interaction strength (A) as mentioned in the later discussion. Compared to the dynamic yield stress, the static yield stress is more applicable during food manufacturing which relates to the need to initiate a flow of a material normally by pumping. As shown in Fig. 4b, FY mayonnaise required the lowest stress to flow and DC-Gran the highest.
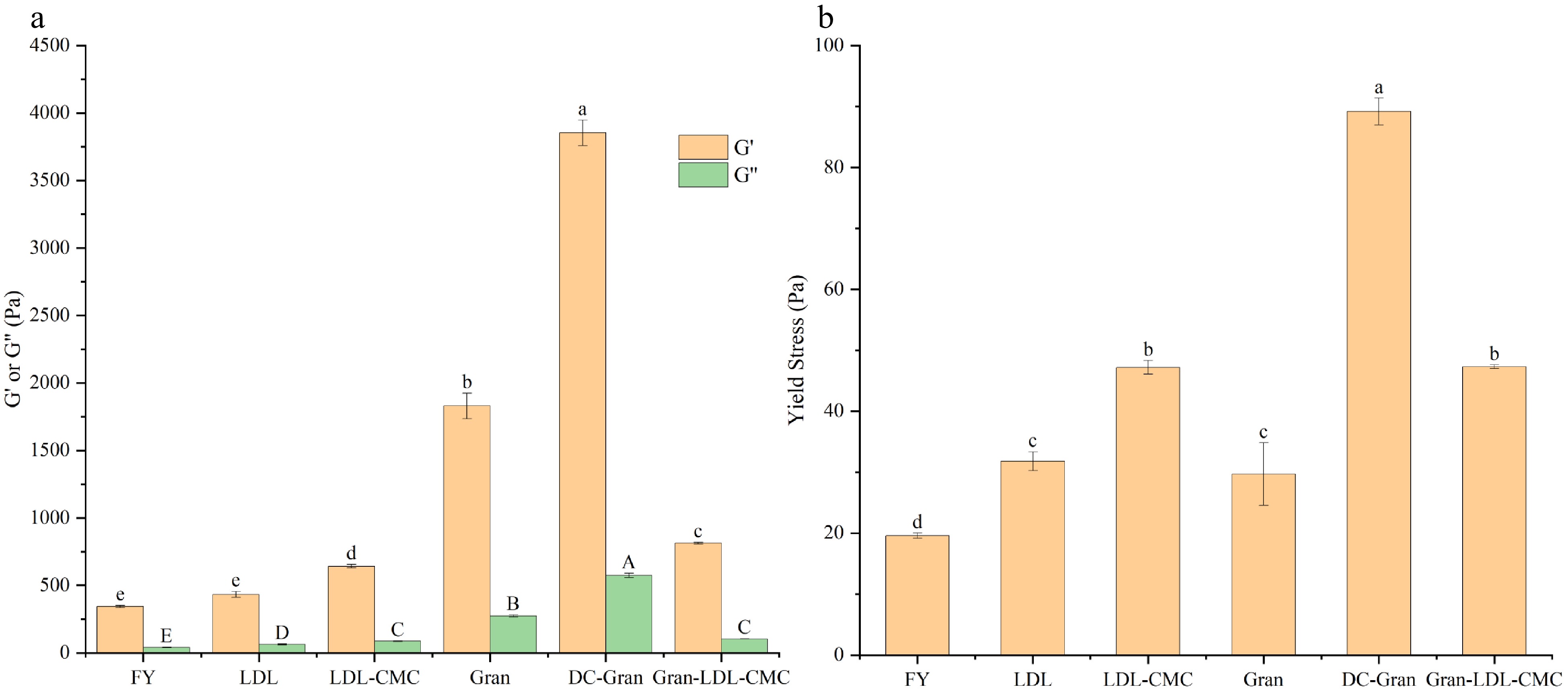
Figure 4.
Viscoelasticity of mayonnaises. (a) Average storage modulus (G') and loss modulus (G'') within the linear viscoelastic region (LVR). (b) Static yield stress. Bars with different letter are statistically different (P < 0.05) within the same chart and parameter.
Coordination number (z) and the coefficient A can be used to distinguish the rheological characteristics among mayonnaises made from different fractionated egg yolk products[48]. Based on the weak gel model proposed by Gabriele et al.[49] and Bohlin's theory[50], the rheological structure of mayonnaise can be viewed as a three-dimensional network where weak and strong interactions are used to link the droplet particles or units. The coordination number z is the number of the rheological units correlated with one another in the three-dimensional structure, while the coefficient A is the strength of the interaction between those units[49]. Higher value of A and z indicates an increased emulsion stability[48]. As shown in Table 4, granule containing mayonnaises (Gran, DC-Gran, Gran-LDL-CMC) exhibited a significantly (P < 0.05) higher coefficient A compared to FY, LDL and LDL-CMC mayonnaises. This indicates a stronger interaction between oil droplets in granule containing samples as compared to the other three mayonnaises. Moreover, DC-Gran had the highest coefficient A indicating the increased emulsifying properties by the de-calcium treatment. The z values obtained in this study are similar to those reported by previous researchers[48,51]. Table 4 shows that Gran, DC-Gran and LDL mayonnaises had a significantly (P < 0.05) lower z value compared to FY, LDL-CMC, and Gran-LDL-CMC indicating smaller number of droplet particles were linked by the interactions.
Table 4. Power law parameters for mayonnaise samples.
Sample A z FY 292.3 ± 17.8e 11.8 ± 0.7a LDL 378.1 ± 3.4d,e 10.4 ± 0.0b LDL-CMC 540.9 ± 16.0c,d 11.5 ± 0.2a Gran 1,370.6 ± 93.3b 9.7 ± 0.4b,c DC-Gran 3,427.4 ± 170.9a 9.3 ± 0.20c Gran-LDL-CMC 707.0 ± 50.7c 12.3 ± 0.3a Within the same columns, values with different superscripts are significantly different (P < 0.05). Texture
-
Texture analysis was performed to measure the firmness of the mayonnaise samples at large strain deformation by the compression test. As shown in Fig. 5, DC-Gran mayonnaise demonstrated the highest firmness followed by Gran, Gran-LDL-CMC, LDL-CMC, LDL and FY. The high firmness of Gran and DC-Gran mayonnaise could be resulting from the high protein content of Gran product which can lead to a firm and thick multilayer formed by granular proteins[11]. The firmness data obtained using the texture analyzer followed the trend of the storage modulus (G') obtained from the rheological analysis, a similar trend was observed by Primacella et al.[20].
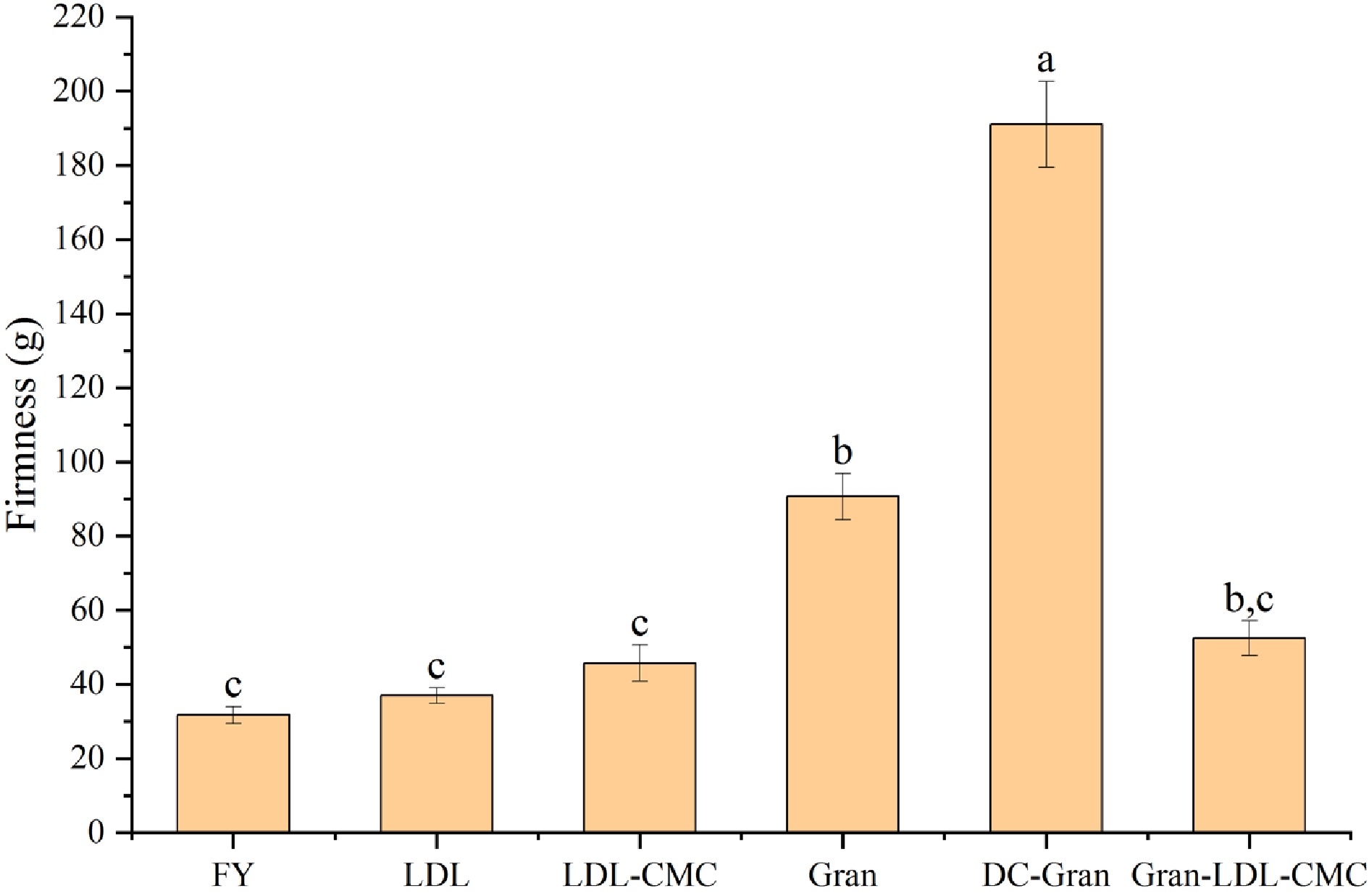
Figure 5.
Firmness values of mayonnaises determined by penetration test. Bars with different letters are statistically different (P < 0.05).
Heat stability
-
At elevated temperatures, emulsion can break down due to the coalescence or separation of the water and oil phase[52] and merging of oil droplets[53]. Heat stability evaluation is often used as an accelerated stability test of samples during storage to monitor droplet coalescence, flocculation, and creaming. Egg yolk protein starts to denature at temperatures higher than 64 °C[54], and we chose a higher temperature to evaluate protein stability in the mayonnaise system. As shown in Fig. 6, after heating at 80 °C for 30 min, minimal oil separation was observed in DC-Gran, LDL and LDL-CMC mayonnaise indicating a high temperature resistance of these samples. DC-Gran mayonnaise was found to be significantly more heat stable compared to Gran sample (P < 0.05). The high temperature resistance of DC-Gran could be resulting from the high interaction strength (A) between the connection units. LDL and LDL-CMC also had a fair heat resistance, which maybe due to the small particle size of the emulsion droplets[55,56].
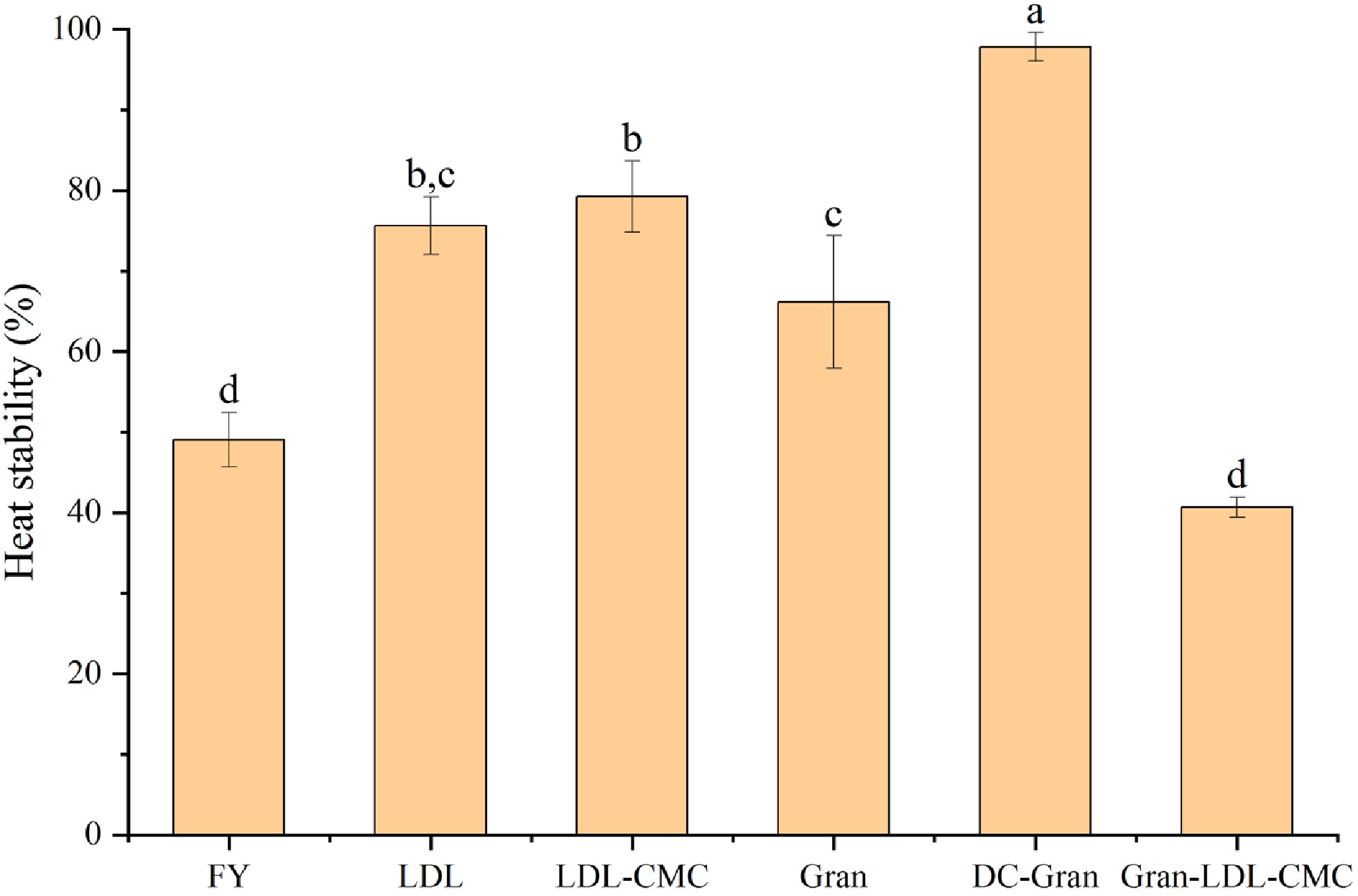
Figure 6.
Heat stability of mayonnaises prepared with different fractionated egg yolk components. Bars with different letters are statistically different (P < 0.05).
Effect of modification on protein solubility of granular products
-
To evaluate the effect of calcium removal on the granular fraction, protein solubility of DC-Gran and the native Gran was assessed at pH 2-12. As shown in Fig. 7, the removal of calcium enhanced the solubility of the granular fraction at pH < 5, which can increase the accessibility of DC-Gran proteins during emulsification at acidic conditions. Mayonnaises normally have a pH less than 4.1[57,58]. Despite the de-calcium treatment, both Gran and DC-Gran had the lowest solubility at pH 6.0 resulting from the isoelectric point (pI) of lipoprotein which is around 6.0 for egg yolk proteins[59].
The 55.0% calcium reduction of the total calcium improved the emulsification property of Gran, leading to the formation of a more stable emulsion under acidic environments. Since the emulsifying activity is related to the capacity of surface-active molecules (proteins or phospholipids), it is important to have a high protein dispersibility under the application conditions[31]. At pH 4.0, DC-Gran had a solubility of 42.9% compared to the 4.2% of Gran. This increased solubility of DC-Gran allows higher accessibility of DC-Gran proteins during emulsification resulting in a finer and more homogenous emulsion compared to that from Gran. Several studies have compared emulsifying properties of native granules and granules after disruption with NaCl, which showed that disrupted granules was more effective to form and stabilize oil-in-water emulsions[7,60], however, the dissociation of granules by calcium removal has not been reported.
Effect of storage time on mayonnaise properties
-
Figure 8 summarizes the changes in mayonnaise properties over a storage period of 28 d in refrigeration temperature. As shown in Fig. 8a, there were no drastic changes of G´ over the 28 days with only a slow decrease over time. Changes of yield stress are shown in Fig. 8b, where an increased yield stress was observed within the first 2 days for samples except FY, Gran and Gran-LDL-CMC. The yield stress of FY decreased significantly over time (P < 0.05) with an initial yield stress of 19.6 Pa and 10.8 Pa after 28 d refrigeration storage. The yield stress of Gran and Gran-LDL-CMC also gradually decreased with time, but Gran-LDL-CMC was more stable with a higher yield stress compared to Gran. The addition of Gran (Gran-LDL-CMC) significantly decreased the yield stress of mayonnaise compared to LDL-CMC sample (P < 0.05). The yield stress of the LDL, LDL-CMC, and DC-Gran samples stayed stable until the end of the 28 d storage. Except Gran and DC-Gran mayonnaises, the firmness of samples (Fig. 8c) significantly (P < 0.05) increased within the first 2 d and stays stable over the remaining days of storage. This is likely resulting from the kinetic equilibration of the emulsion system[52].
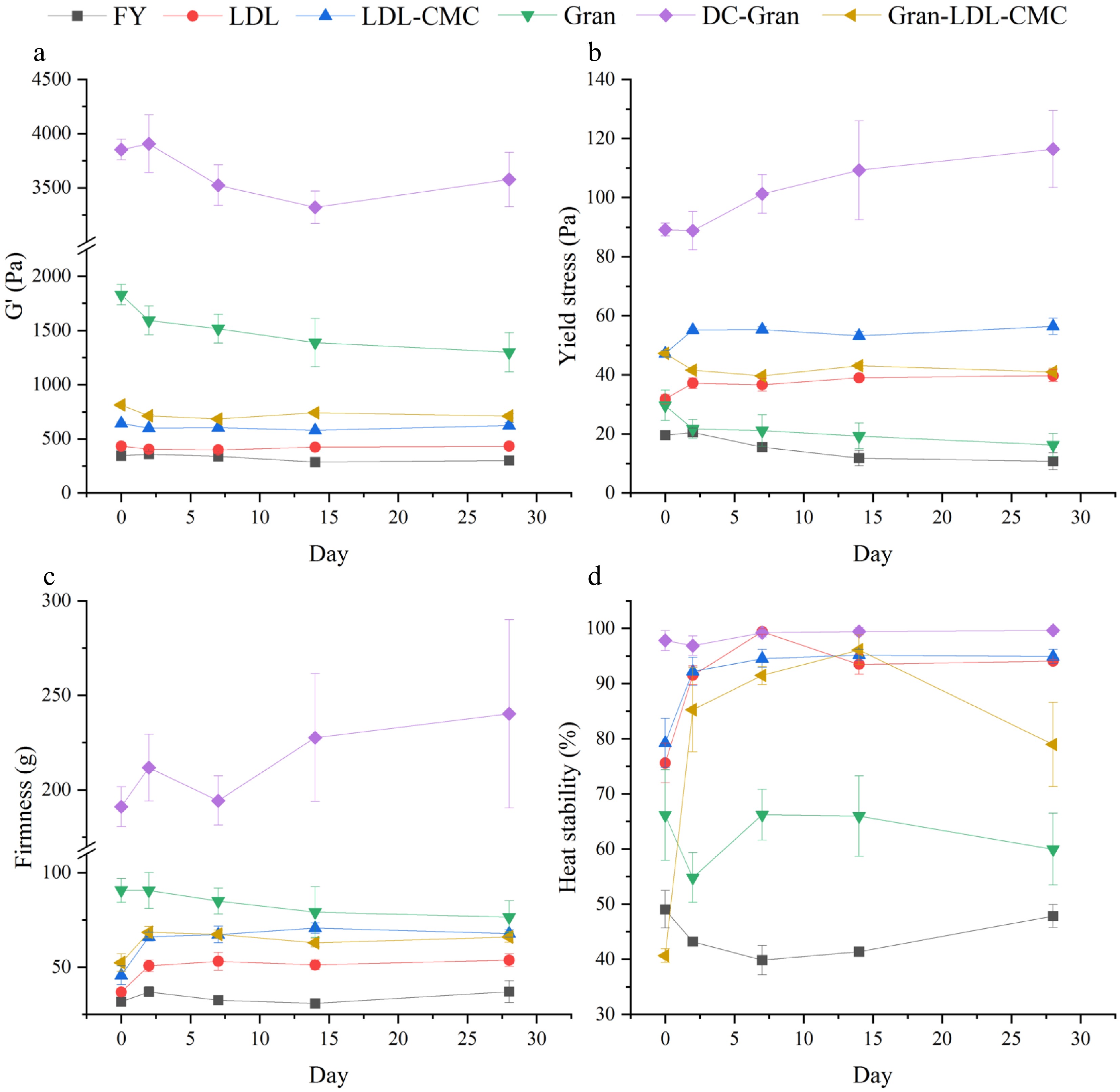
Figure 8.
Changes in mayonnaise properties upon storage at 4 °C. (a) Storage modulus (G'). (b) Static yield stress. (c) Firmness. (d) Heat stability.
Figure 8d demonstrates the changes in heat stability over time. Except FY, Gran and DC-Gran, there was a drastic increase in heat stability of samples within the first 2 d of storage. After 2 d, the heat stability of LDL and LDL-CMC remained constant, and they exhibited the best heat stability along with DC-Gran at the end of 28-d storage compared to the other samples. No significant changes in heat stability were observed for Gran and DC-Gran samples within 28-d of storage (P > 0.05). There was a slow decrease in heat stability of Gran-LDL-CMC, but it still had an enhanced heat stability compared to FY. Overall, LDL-CMC, DC-Gran and LDL mayonnaises demonstrated the best heat stability (> 90%) by the end of the 28-d refrigeration storage. This indicates that oil droplet size plays an important role in the texture and stability of mayonnaise, where small particle size may contribute to a more stable and firmer texture over extended storage.
Other than mayonnaises, the two unique yolk-based products, LDL-CMC and DC-Gran, can be utilized in preparation of other emulsions. For example, DC-Gran might be able to structure edible oil into solid-like fats as potential alternatives for partially hydrogenated oils (PHOs) by the formation of Pickering emulsions[61]. In addition, these two products might be used in preparations of high internal phase emulsions (HIPEs) such as delivery systems for nutraceuticals or bioactives[62] and templates for highly porous materials (polyHIPEs)[63]. Demonstrating the superior functionalities of the fractionated yolk products, the industrial IgY recovery process can be modified to use food grade processing aids or standards, so the co-products of LDL and granular fractions can be made into high-performing food ingredients.
-
Mayonnaises prepared using different egg yolk co-products derived from IgY separation demonstrate an excellent heat stability and improved rheological properties compared to the mayonnaise made from fresh yolk. Calcium reduction treatment of egg granules leads to an increased solubility of the granular proteins under acidic conditions, resulting in an increased accessibility of granular components for providing emulsification function. The use of CMC for complexing with LDL enhances the emulsifying property of LDL, resulting in mayonnaise with the smallest particle size. Overall, LDL, LDL-CMC and DC-Gran demonstrate the ability to produce mayonnaises with higher firmness and stability compared to mayonnaises made from fresh yolk.
This work was supported by the USDA National Institute of Food and Agriculture, Hatch/Multi-state project (accession number: 1023982), and the Egg Industry Center located at Iowa State University, USA.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wan Z, Fei T, Wang T. 2022. Mayonnaise formulated with novel egg yolk ingredients has enhanced thermal and rheological properties. Food Materials Research 2:11 doi: 10.48130/FMR-2022-0011
Mayonnaise formulated with novel egg yolk ingredients has enhanced thermal and rheological properties
- Received: 11 May 2022
- Accepted: 13 June 2022
- Published online: 15 July 2022
Abstract: Egg yolk is a good emulsifier used in many food applications and is required for mayonnaise. This study aimed to evaluate the function of fractionated yolk as co-products of obtaining IgY, the plasma fraction which is rich in low-density lipoprotein (LDL) and granules consisting of mainly high-density lipoprotein (HDL), in preparation of mayonnaise. Particularly, a novel modification was performed to remove the divalent ions, i.e., calcium, in the granular fraction that forms phosphocalcic bridges that hold the HDL particles together, and are responsible for the low solubility and functionality. The removal of 55% calcium (of the total ion) increased the solubility of de-calcium granules (DC-Gran) under acidic conditions of pH 4.0, 42.9% compared to 4.2% of the untreated Gran. The mayonnaise made with the LDL-based products and DC-Gran had a high heat stability (greater than 90%) and small oil droplet sizes of 4.59 and 5.02 μm. Higher interaction strength, firmness, and low polydispersity index (PDI) were obtained in mayonnaise prepared by DC-Gran compared to the Gran counterpart. This is the first report of using such modified granular fraction to produce mayonnaise, with improved functionality and demonstrating the high-value application of yolk fractionation co-products.
-
Key words:
- Mayonnaise /
- Egg yolk /
- Granular modification /
- Co-products /
- Rheology /
- Confocal microscopy













