-
In recent years there has been an increasing demand to use natural pigments for food colouring because of their positive health effects and also their non-toxicity and eco-friendly characteristics. A variety of compounds like anthocyanins, betalains, carminic acid, carotenoids and chlorophylls could easily be obtained from nature. Anthocyanins are the most important natural pigments synthesized by several plants (corn, cabbage, carrot, sweet potato, berry, grape, among others) that are cyaniding–based and are responsible for the red, purple, pink, orange and blue colours of certain fruits, flowers and vegetables. They are also hydrophilic watersoluble pigments that belong to bioactive flavonoid compounds. Beside their intense colour attributes they have several beneficial effects like antidiabetic, antibacterial, anticarcinogenic and antioxidant activities[1−6]. Therefore, in the present study, we aimed to develop an aqueous two-phase extraction (ATPE) system for the extraction and purification of anthocyanins which could be an alternative for the food industry as a natural colourant. In recent years, red cabbage anthocyanins are gaining popularity, especially in the food industry, because of their features.
Anthocyanins can be obtained from plant sources by numerous extraction, isolation and purification methods like solvent extraction, microwave-assisted extraction, ultrasonic extraction, supercritical fluid extraction, macroporous resin adsorbtion, high-performance liquid chromatography, alcohol/salt aqueous two-phase systems (ATPS) and so on[1, 2, 6, 7−13]. The solvent extraction using different organic solvents such as methanol, ethanol and acetone is traditionally used techniques for the extraction of anthocyanins[14, 15]. As the most efficient extraction solvent, methanol is not generally preferred because of its toxicity. Instead of methanol, especially in the food industry, ethanol is used for this purpose as it is less toxic in comparison. Nowadays due to limitations in use of large amounts of organic solvents, special equipment and the high energy need, time consuming and high costs[5, 12, 16] aqueous two-phase extraction (ATPE) systems replaced the traditional and also modern techniques as mentioned above. Salt/alcohol ATPE systems are powerful and valuable techniques that offer many advantages like low cost, low viscosity, easy mixing, recycling and reuse of solvent, safe and environmentally sound process[16, 17]. However, to provide the quality and to utilize the anthocyanin extracts in food applications, it is necessary to develop ethanol/salt based ATPE systems that may have enormous potential for the separation and purification of anthocyanins.
ATPE is a liquid-liquid extraction technique and is a simple single step operation that concentrates the target biomolecule into the one phase. It provides a mild and non-toxic medium for biological activities because of its high water content. New ATPE systems prepared with short-chain alcohol (ethanol, methanol, 1-propanol, 2-propanol etc.) and inorganic salt (phosphate, sulfate etc.) have been used to purify various biomolecules like enzymes, proteins, natural compounds etc. with higher yields, capacity and efficiency[11−13, 17, 18]. Because of the salting-out effect of the salt and the low solubility of the salt in alcohol it is possible to establish both stable and optimized systems. In these systems, two liquid phases occur: the top alcohol rich phase and the bottom salt rich phase[5, 12]. There are several reports for extracted and purified anthocyanins with ATPE systems[5, 11, 13, 16, 19]. These systems selectively partitioned the anthocyanins to the upper alcohol phase while the contaminants like proteins, enzymes, sugars etc. to the salt phase. In these systems, besides the extraction and purification of the anthocyanins, it could also be concentrated into the one phase. The anthocyanins from grape juice[16], purple sweet potatoes[12], mulberry[5], Hibiscus sabdariffa[19], Garcinia indica[11] are extracted and purified by ATPE systems with high yields by removing the majority of sugars effectively.
In the present study, an ATPE system containing ethanol and ammonium sulfate is used for the extraction, concentration and preliminary purification of anthocyanins from red cabbage for the first time. The red cabbage is known as an excellent and promising source of anthocyanin that is also contain several micronutrients and phytochemicals. Beside of this, it is a cheap source, widely available and rich in anthocyanins. Due to having many biological activities it is very popular, especially in the food industry[20]. The partition behavior of anthocyanins and sugars in the ATPE system have been characterized. In order to develop an efficient ATPE system for the extraction and purification of red cabbage anthocyanins, the influence of several factors such as the concentration of ethanol and ammonium sulfate, the extract amount and the system pH on anthocyanin partitioning and recovery is investigated. The optimal conditions of anthocyanin extraction are determined to achieve high recoveries. A reproducibility study of anthocyanin extraction with optimal ATPE system is also carried out. The anthocyanins are very sensitive to temperature and pH. These environmental factors are important especially for food applications that affect the colour and nutritional properties of anthocyanins. Therefore, the effect of temperature and pH on the stability of anthocyanins obtained from an optimized ATPE system is investigated in terms of temperature (40−70 °C) and pH (3−6).
-
The chemicals were purchased from several companies, NaNO2, AlCl3, C6H5OH, Na2CO3 and sodium acetate from Riedel de Haan (Germany), NaOH from Merck (Germany), glucose, H2SO4 and catechin from Fluka (Switzerland), ammonium sulfate, gallic acid and KCl from Sigma Chem. Co. (St. Louis, MO, USA). All other chemicals are of the highest available purity and used as purchased. The red cabbage and others are bought from a market (İzmir, Turkey) and used as an anthocyanin source.
Extraction of anthocyanins from plants
Extraction of anthocyanins from different sources
-
In order to determine the best anthocyanin source, five different plant sources (red cabbage, grape, strawberry, blackberry and cherry) were used. After removing the surface dirt with water, all were washed with distilled water. In method A, 25 g of plant was first chopped with a blender and then homogenized by the addition of 50 mL of distilled water. After filtration, the extract is centrifuged at 4,000× g for 15 min at room temperature and the centrifugate is separated and analyzed for its anthocyanin content. In method B, after homogenization as mentioned above the homogenate is incubated and rinsed at room temperature for 1 h and the same steps are applied as mentioned for Method A. In method C, the homogenate is first warmed up to 100 °C for 2 min and then immediately cooled. The sample is rinsed at room temperature for 1 h and then the same steps are applied as in Method A. After analysis of anthocyanin content of plants, the red cabbage is determined as the best source and used in the following studies.
Extraction of red cabbage anthocyanins
-
For the productive extraction of red cabbage anthocyanins, three different extraction paths were followed. In the first path, Method A is applied as mentioned above and used as control. In the second, after homogenization the pH of the medium is first adjusted to pH 2.5, 3.5 and 4.5 and then warmed up to 100 °C for 2, 5 and 10 min. After that, the extract is rinsed at room temperature for 1 h and the same procedure is applied as mentioned for Method A. Thirdly, the homogenate is warmed up to 65 °C for 10, 15, and 20 min and then immediately cooled. Then rinsed at room temperature for 1 h and the same procedure is applied as mentioned for Method A. According to anthocyanin assay results the third path (15 min incubation at 65 °C) gave the highest anthocyanin content and so this is used as the pre-extraction condition. The extract is immediately used in ATPE systems for further partitioning and purification studies.
Aqueous two-phase extraction of red cabbage anthocyanins
-
In order to determine the optimal conditions for the extraction and partitioning of red cabbage anthocyanins in ATPE system, a single factor experiment is performed. For this aim, the effect of ammonium sulfate concentration (16%, 18%, 20%, 22% and 24%, w/v), ethanol concentration (24%, 25%, 26%, 27% and 28%, v/v), pH (2, 3, 4, 5, 6 and 7) and extract amount (1%, 2.5%, 5%, 10%, 15% and 20%, w/v) is investigated.
For the extraction and purification of red cabbage anthocyanins the desired amount of solid ammonium sulfate is dissolved with distilled water and then the ethanol is added to this solution in a 10 mL centrifuge tube. The red cabbage extract is put into the system and vortexed gently to form a homogenous phase and then the tubes allowed to stand for 20 min at room temperature. Afterwards it is centrifuged at 4,500× g for 10 min to facilitate the phase separation. The upper and bottom phases are separately collected, the volumes are noted and analyzed for its anthocyanin, phenolic, flavonoid and sugar content. In order to remove ethanol and to concentrate and dry the anthocyanins evaporation under vacuum is made using a rotary evaporator at 35 °C with 60 mbar pressure. The concentrated extract is stored in a freezer (−20 °C) until biochemical characterisation studies.
The best conditions which resulted in maximum recovery was used as standard extraction procedure for red cabbage anthocyanins. All the experiments were carried out in triplicate and the difference in the readings in triplicate are less than ± 5%.
Analytical assays
Total anthocyanin
-
The total monomeric anthocyanin amount is determined by the spectrophotometric pH-differential method[18, 21]. Two dilutions are prepared, one for pH 1.0 using potassium chloride buffer (0.025 M) and the other for pH 4.5 using sodium acetate buffer (0.4 M). The absorbance of the mixture is measured against distilled water as a blank at 520 and 700 nm after 20 min incubation. The total anthocyanin content (mg·L−1) is calculated as cyanidin-3-glucoside equivalents by using the equation (Eqn. 1) given below:
$ { Anthocyanin\; concentration }\;(mg/L)=\frac{A \times M_w \times DF}{\epsilon \times L} $ (1) where A =
$ \left[ {\left( {A_{520} - A_{700}} \right)_{pH1.0} - \left( {A_{520} - A_{700}} \right)_{pH4.5}} \right] $ All the measurements are performed in triplicate.
Total phenolics
-
The total phenolic content of red cabbage raw extract and both the top and bottom phases of ATPE system are determined by the Folin-Ciocalteu colorimetric method with slight modifications[22, 23]. This method involves the oxidation of phenols using a molybdotungstate reagent to yield a coloured product. Briefly, 1 mL of extract (1 mg·mL−1) or gallic acid standard is mixed with 9 mL of distilled water and then 2.5 mL of Folin-Ciocalteu reagent (1:10, v/v) is added. After 5 min 10 mL of Na2CO3 (7.5%, w/v) is added and then the volume is made up with distilled water. The mixture is allowed to stand at room temperature for 90 min in dark. The absorbance is measured at 760 nm. The gallic acid (5−200 μg·L−1) is used as standard[24]. All determinations and readings are performed in triplicate.
Total flavonoids
-
The total flavonoid content of samples is determined with the aluminium chloride colorimetric method[23, 25]. After the addition of 4 mL of distilled water on to the 1 mL of extract it is mixed with 0.3 mL of 5% (w/v) NaNO2 and stand at room temperature for 5 min. 0.3 mL of 10% (w/v) AlCl3 is added to this solution. The mixture is neutralized with 2 mL of 1 M NaOH for 5 min and the last volume is made up to 10 mL with distilled water. The absorbance is measured spectrophotometrically at 510 nm. Catechin (1.0−100 μg·L−1) is used as standard and the total flavonoid amount is expressed as mg of catechin equivalent per mL of extract. The total recovery is the ratio of the flavones partitioned in the top phase to the total amount of flavones. All determinations and readings are performed in triplicate.
Total sugars
-
The total sugar content of samples is determined with phenol-sulphuric acid method[26, 27]. For a blank system, 0.5 mL of 5% (w/v) phenol is first mixed with 0.5 mL of distilled water and then 2.5 mL of sulphuric acid (96%) is added. 0.5 mL of glucose standard and the samples are first mixed with 0.5 mL of 5% phenol (w/v) and then with 2.5 mL of sulphuric acid (96%). The samples are incubated at 30 °C for 20 min at first and then the sugar amounts are measured spectrophotometrically at 490 nm against the blank. The removal rate yield of sugars are calculated[12, 13]. The total recovery is the ratio of the sugars partitioned in the bottom phase to the total amount of sugars. Glucose solution (10−90 μg·mL−1) is used as standard. All determinations and readings are performed in triplicate.
Stability of red cabbage anthocyanins
Effect of temperature on the stability of anthocyanins
-
The effect of temperature on anthocyanin extract is determined following the given methodology. Equal amounts of anthocyanin extracts are put into cleanscrew capped amber coloured bottles and subjected to heating at 40, 60, 70 and 80 °C in a waterbath for 1, 2, 4, 6, 10 and 12 h. After incubation they were cooled to room temperature and the anthocyanin amounts are analyzed according to the standard assay method. Percent pigment retention was calculated on an hourly basis for 12 h as percentage of the absorbance before heat treatment. Analyses and measurements are performed in triplicate.
Effect of pH on the stability of anthocyanins
-
The stability of anthocyanins is also evaluated at different pH values (pH 3.0, 4.0, 5.0 and 6.0). The same amount of anthocyanin extract is put into cleanscrew capped amber coloured bottles and the pH is adjusted to the required pH values. After that the extracts are incubated at room temperature in the dark for 1, 2, 4 and 6 h. The amount of anthocyanin is assayed with the standard assay method. Percent pigment retention is calculated on an hourly basis for 6 h as percentage of the absorbance before pH adjusment. Analyses and measurements are performed in triplicate.
-
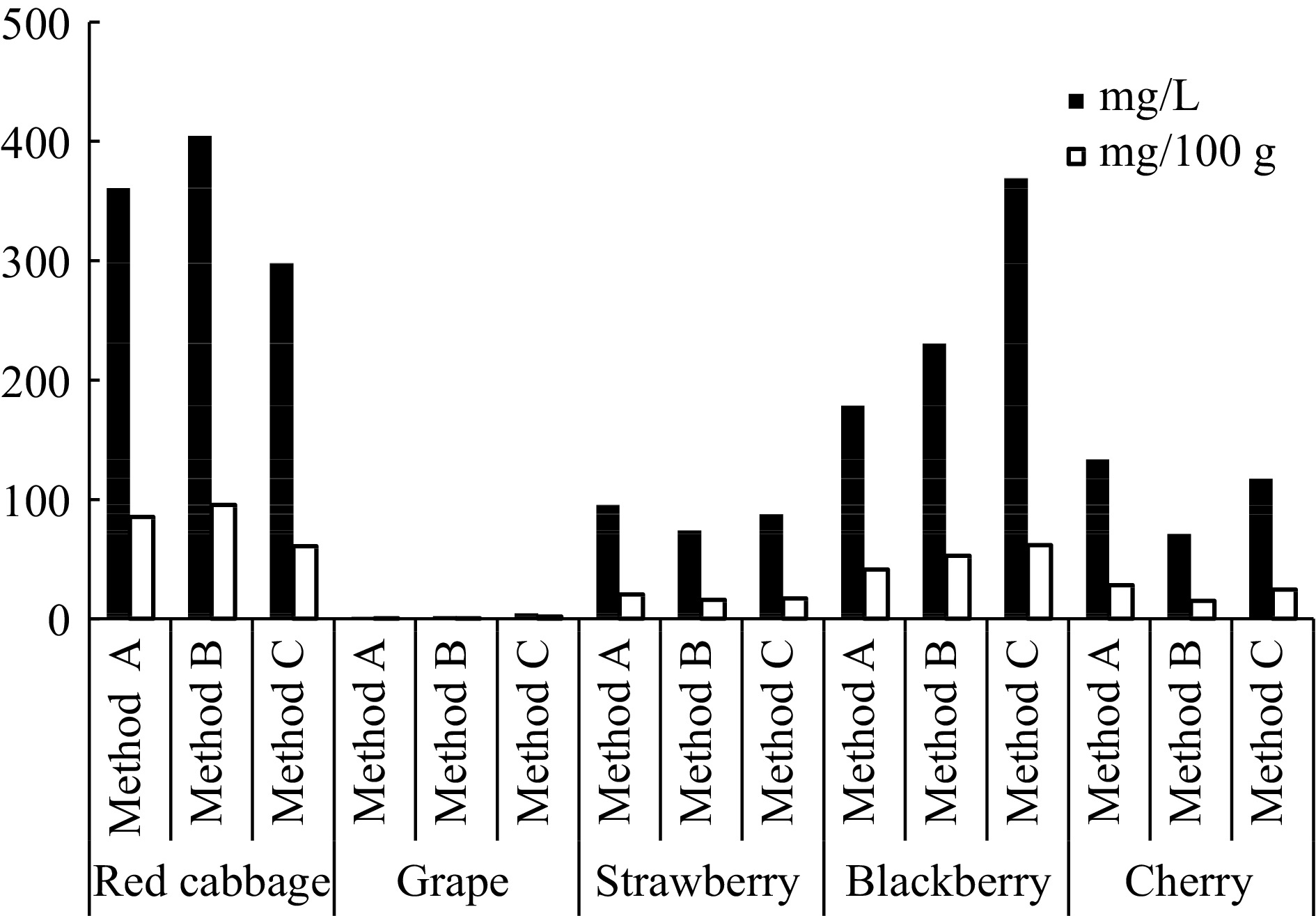
As natural colourants, anthocyanins have become more attractive in recent years. Various plant sources are compared for their anthocyanin content to determine the best pigment source. The most crucial step to obtain an anthocyanin-rich extract is to determine a suitable extraction process. For this purpose, red cabbage, grape, strawberry, blackberry and cherry are used as an anthocyanin source and the anthocyanins are pre-extracted by using three methods (Method A, Method B and Method C). As can be seen from Fig. 1, red cabbage anthocyanins are pre-extracted more efficiently and productively by using all three methods. The anthocyanin contents are determined as 361, 404.6 and 298 mg·L−1 for Method A, B and C, respectively. Therefore, the red cabbage is choosen as the best anthocyanin source and used in the subsequent studies. According to the results, the most effective method is determined as Method B.
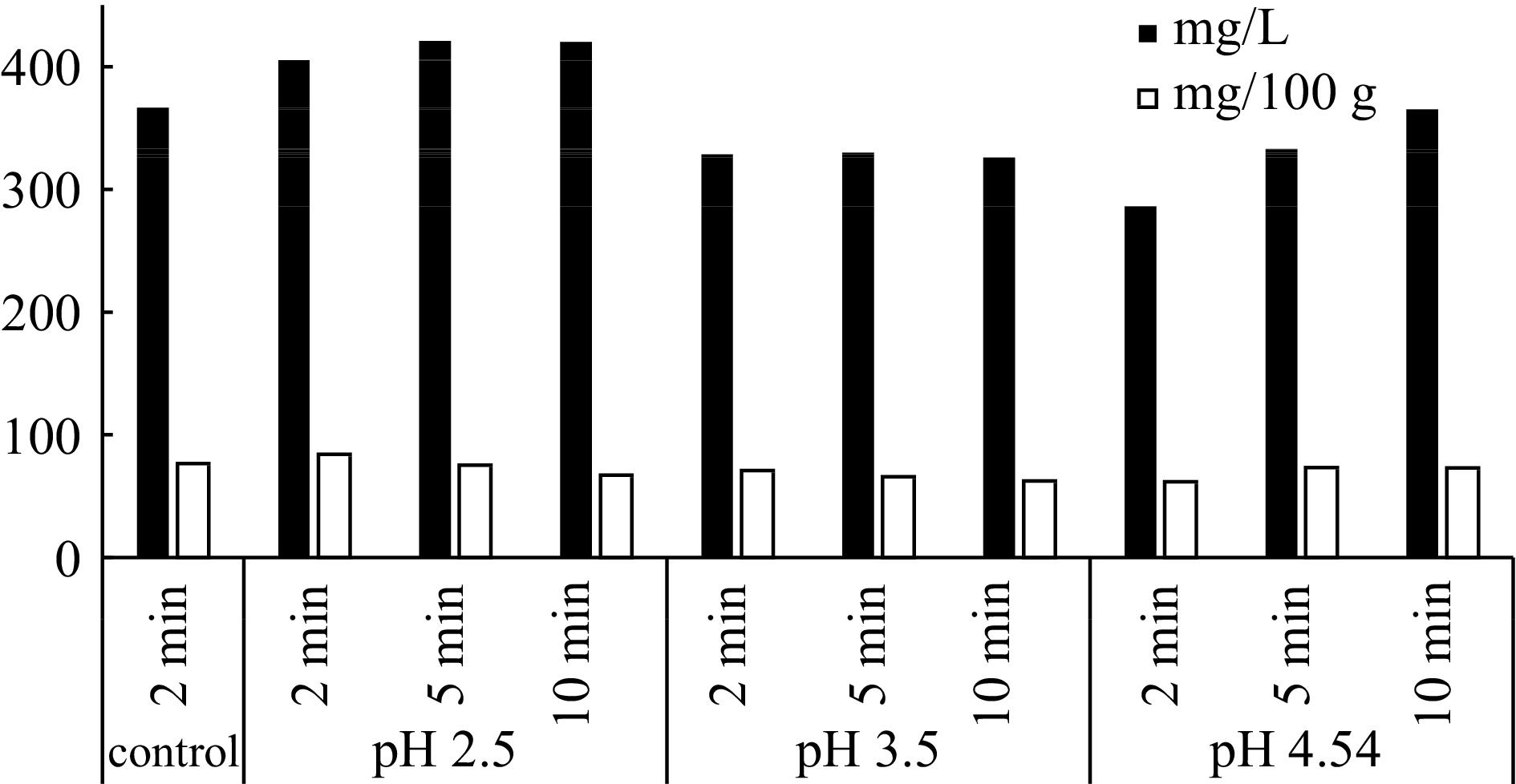
In order to fix the optimal pre-extraction conditions for red cabbage anthocyanins three different extraction sets are applied. In these sets, the effect of pH, incubation time and temperature is searched. As shown in Fig. 2, the pH and heat treatment is very important for extraction of anthocyanins. At pH 2.5, 2, 5 and 10 min incubation at 100 °C gave the best pigment content amounts as 405, 421 and 420 mg·L−1, respectively.
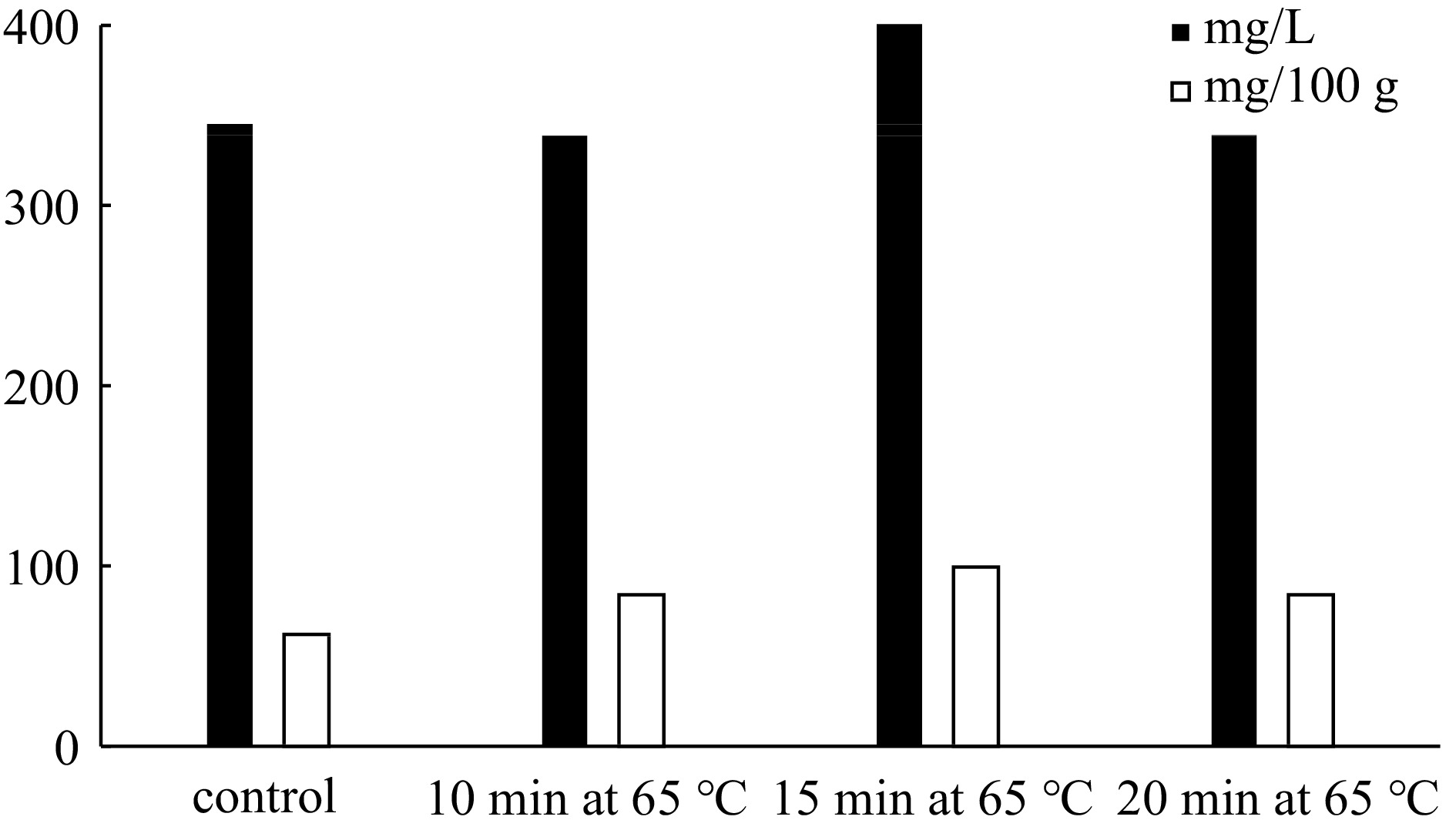
The influence of the temperature (65 °C) and incubation time (10, 15, 20 min) on anthocyanin extraction is also studied (Fig. 3). At 65°C, 338.8, 440 and 339 mg·L−1 anthocyanin amounts are obtained for 10, 15 and 20 min incubation, respectively. The results showed that the heat treatment of anthocyanin extract (pH 4.54) at 65 °C for 15 min is the best extraction strategy. The red cabbage anthocyanins prepared by this way are used in aqueous two-phase extraction systems for partitioning and concentration of anthocyanins. According to Chandrasekhar et al. the aqueous extract of anthocyanins from red cabbage is changed between the range of 230−308 mg·L−1[7].
Aqueous two-phase extraction and partial purification of red cabbage anthocyanins
Effect of ammonium sulfate concentration on ATPE of anthocyanins
-
For efficient partitioning of the target biomolecule the phase forming salt concentration is an important factor that affects the extraction performance. So, several ATPE systems are set up to determine the salt effect on extraction of red cabbage anthocyanins. Ammonium sulfate is selected because of the high stability of the anthocyanins in it. The salt concentration is changed between 16%−24 % (w/v) by keeping the ethanol concentration constant (26%, v/v) and extract amount (10%, w/v) without pH adjustment. The anthocyanins are partitioned to the upper ethanol phase in all systems and the majority of sugars and other contaminants partitioned to the lower phase. This behavior allows the final anthocyanin product to be purified very quickly in one step. Wu et al. have designed an ATPE system for grape juice anthocyanins and found that the greater part of sugars transferred to the bottom phase of the system[16]. The anthocyanin amounts and extraction yields are increased from 16% (w/w) to 22% (w/w) ammonium sulfate concentrations. Above this point there is not any notable increase (Fig. 4). At 22% (w/w) of ammonium sulfate concentration the total anthocyanin amount and extraction yield is determined as 0.48 mg·L−1 and 61.5%, respectively. Therefore, 22% (w/w) of ammonium sulfate concentration is chosen and used in the following experimental steps.
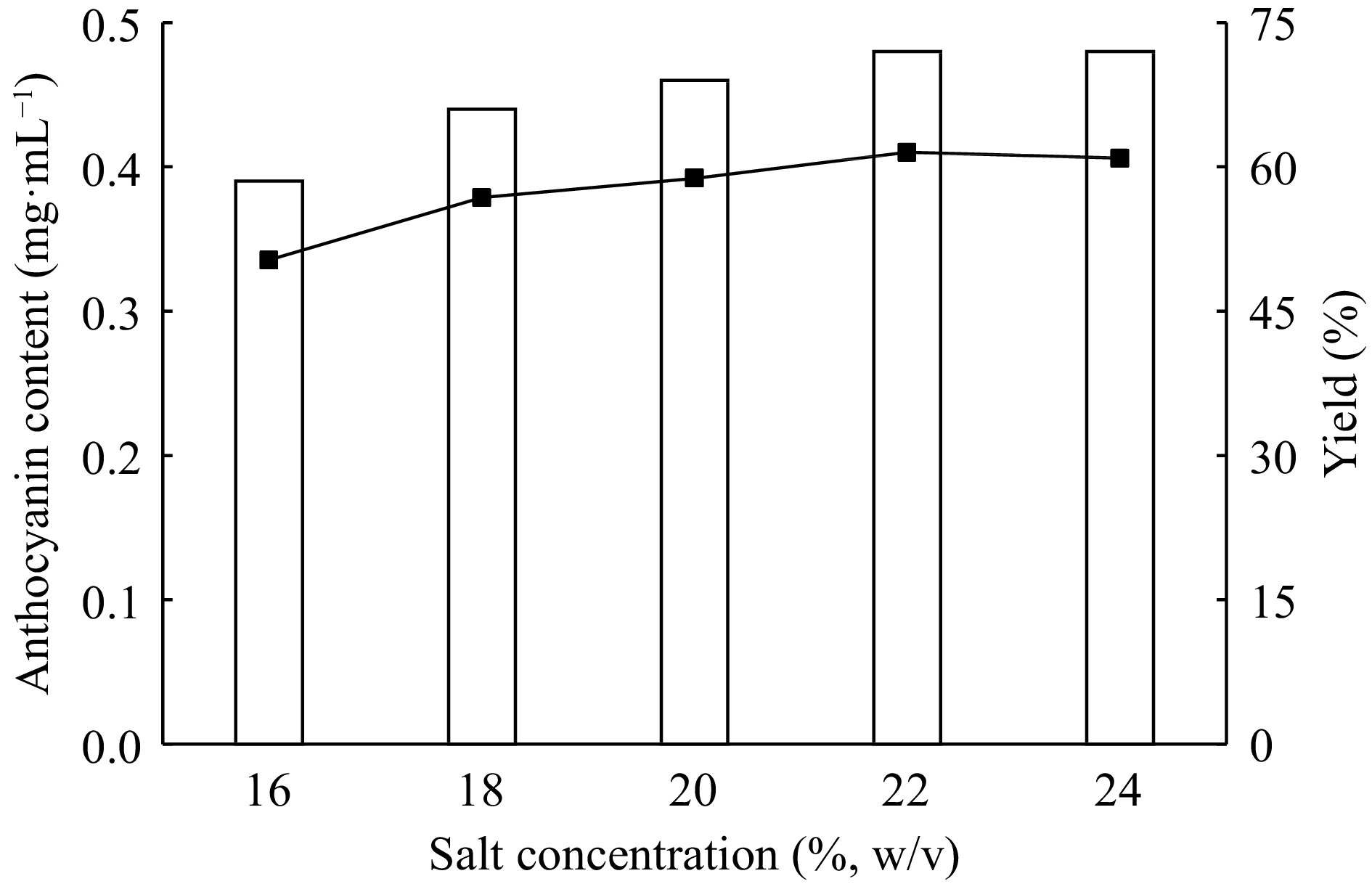
Figure 4.
Effect of salt concentration on extraction and purification of red cabbage anthocyanins by ATPE.
Effect of ethanol concentration on ATPE of anthocyanins
-
Partitioning behavior of biomolecules also influenced by phase forming alcohol type and concentration. Because of its advantages like nontoxicity, ease of availability in comparison to other alcohols, ethanol is used as a phase forming alcohol. Therefore, the five ATPE system is carried out for determination of the best ethanol concentration by using 22% (w/w) ammonium sulfate, 10% (w/w) extract and 24%−28% (v/v) ethanol concentration. The results are given in Fig. 5.
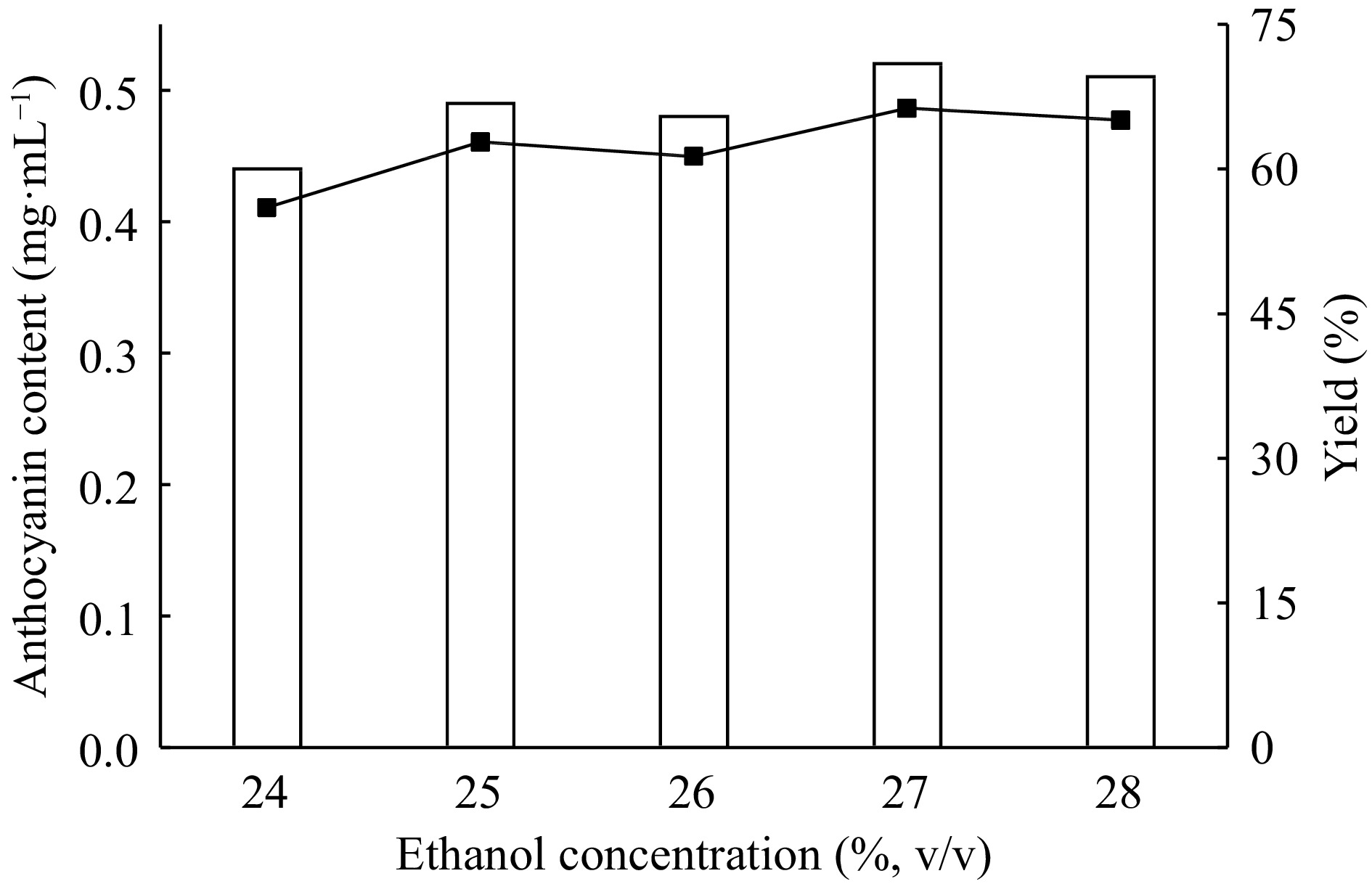
Figure 5.
Effect of ethanol concentration on the extraction and purification of red cabbage anthocyanins by ATPE.
The results indicated that, as the ethanol concentration is increases from 24% to 27% (v/v) the anthocyanin contents are also increased. With 22% (w/w) ammonium sulfate and 27% (w/w) ammonium sulfate and 27% (v/v) ethanol concentrations the anthocyanin content and extraction yield are found as 0.52 mg·mL−1 and 66.3 mg·mL−1, respectively. So, 27% (v/v) ethanol concentration is used later. With these conditions the sugars also significantly partitioned into the lower aqueous phase.
Effect of pH on extraction of anthocyanins
-
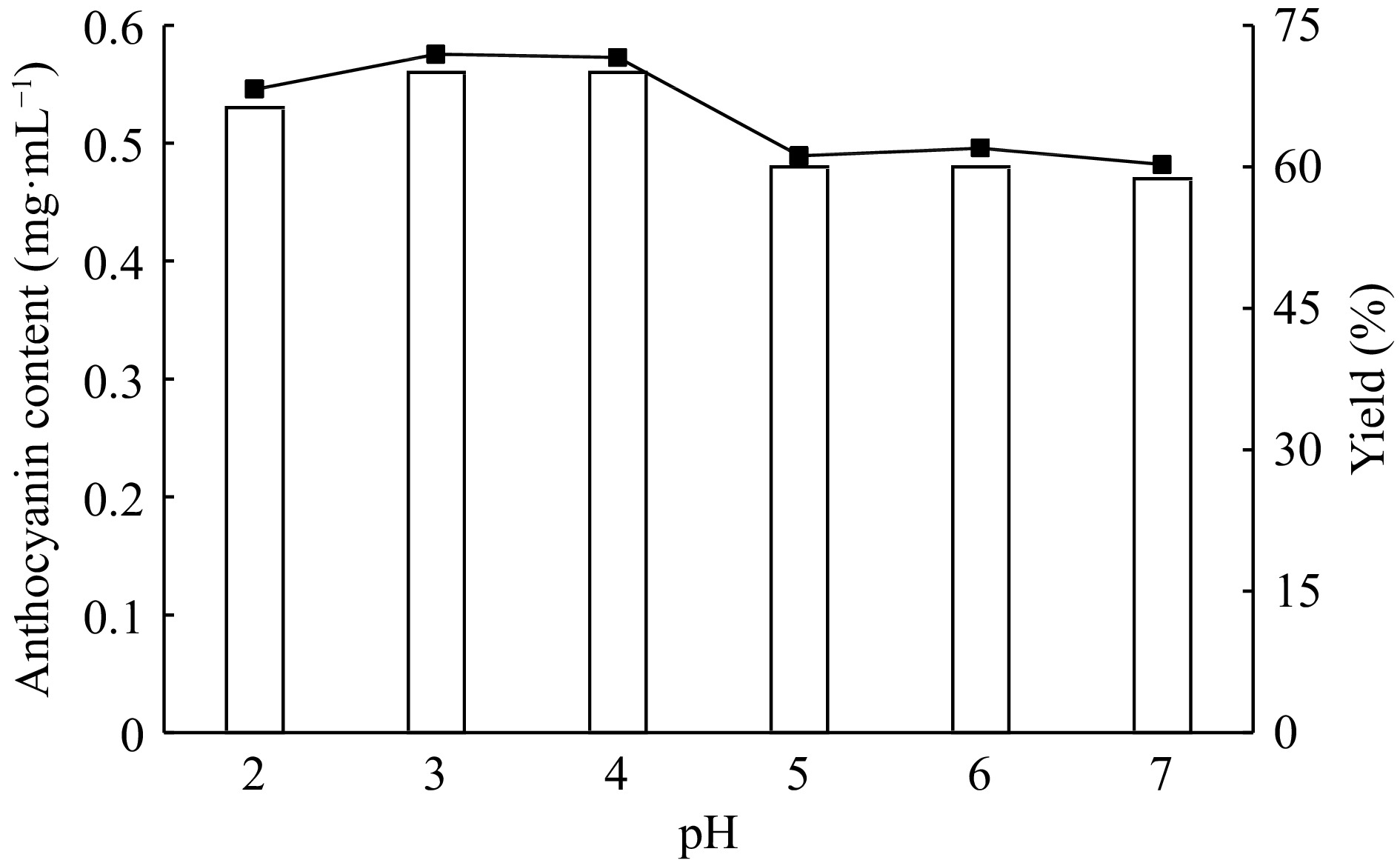
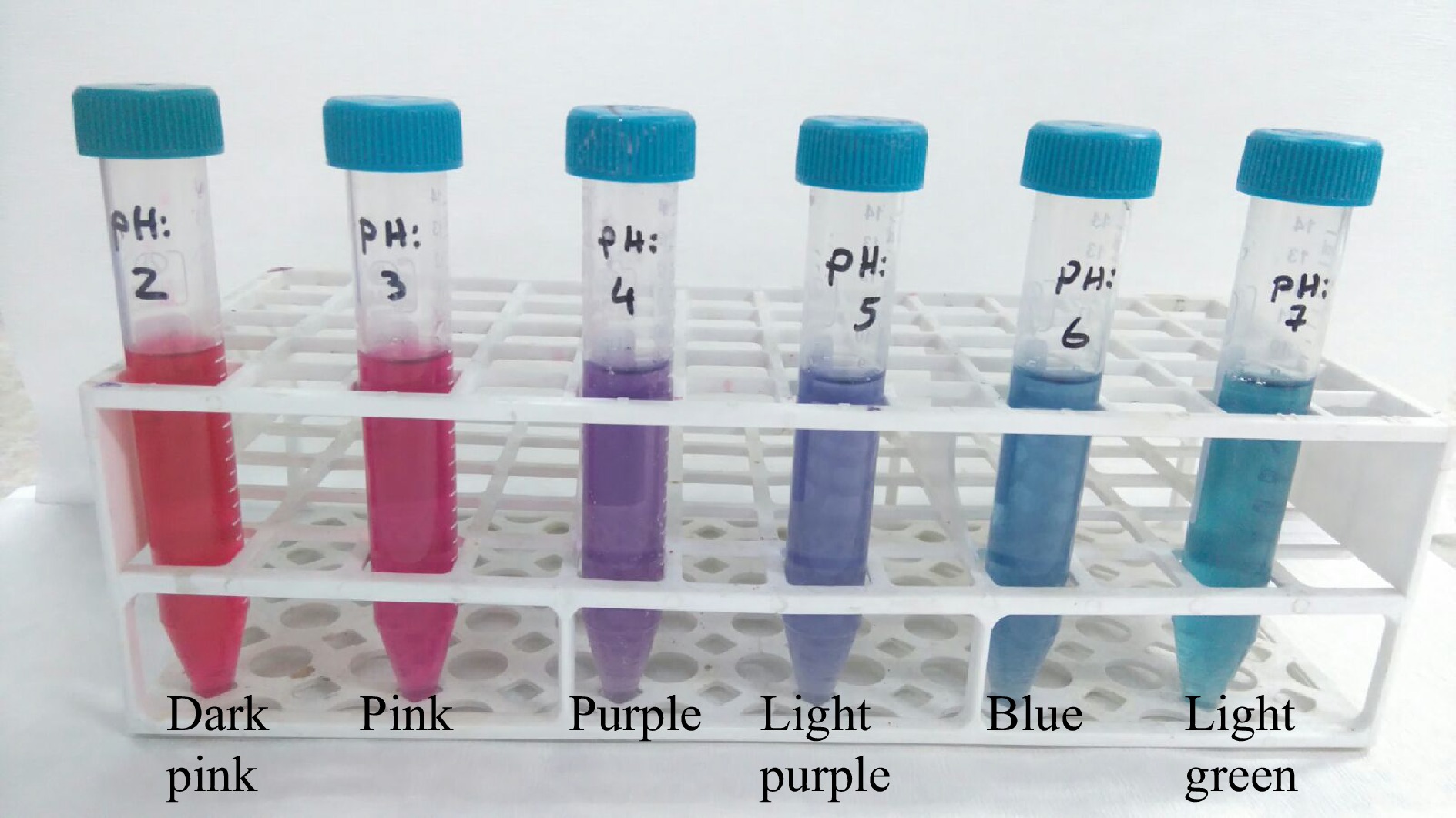
By using 22% (w/w) ammonium sulfate and 27% (v/v) ethanol concentrations six different ATPE systems are prepared and the pH of the systems are adjusted to pH 2.0−7.0 range to determine the effect of pH on partition behavior of anthocyanins and sugars. As is seen from the Fig. 6, above pH 4.0 both the anthocyanin contents and extraction yields are decreased. At pH 3.0 and pH 4.0 very similar results are obtained. At these pHs the anthocyanin contents and extraction yields are calculated as 0.56 mg·mL−1, 0.558 mg·mL−1 and 71.9%, 71.6% for pH 3.0 and pH 4.0, respectively. So, the system pH is choosen as pH 3. As shown in Fig. 7, the colour of red cabbage anthocyanins are changing according to pH value as dark pink (pH 2.0), pink (pH 3.0), purple (pH 4.0), blue (pH 5.0) and light green (pH 6.0).
According to the literature the anthocyanins derived from different sources are very sensitive to pH and show colour changes according to pH levels[15, 20]. This feature and responsivity is very attractive especially in the food industry as a natural colour indicator[15]. The colour dependent on pH is the result of anthocyanin components and structures. At lower pHs, anthocyanins are generally exists in the form of flavylium cation that contributes to pink-red and purple colour. Our results are in agreement with several previous studies[10, 28].
Effect of extract amounts on extraction of anthocyanins
-
For the influence of anthocyanin extract amount on the ATPE system the ammonium sulfate concentration (22%, w/w), ethanol concentration (27%, v/v) and pH (3.0) are kept constant and the extract amount is changed between 1%−20% (w/w). The maximum extraction yield (83.8%) is obtained by using 2.5% (v/v) extract amount (Fig. 8).
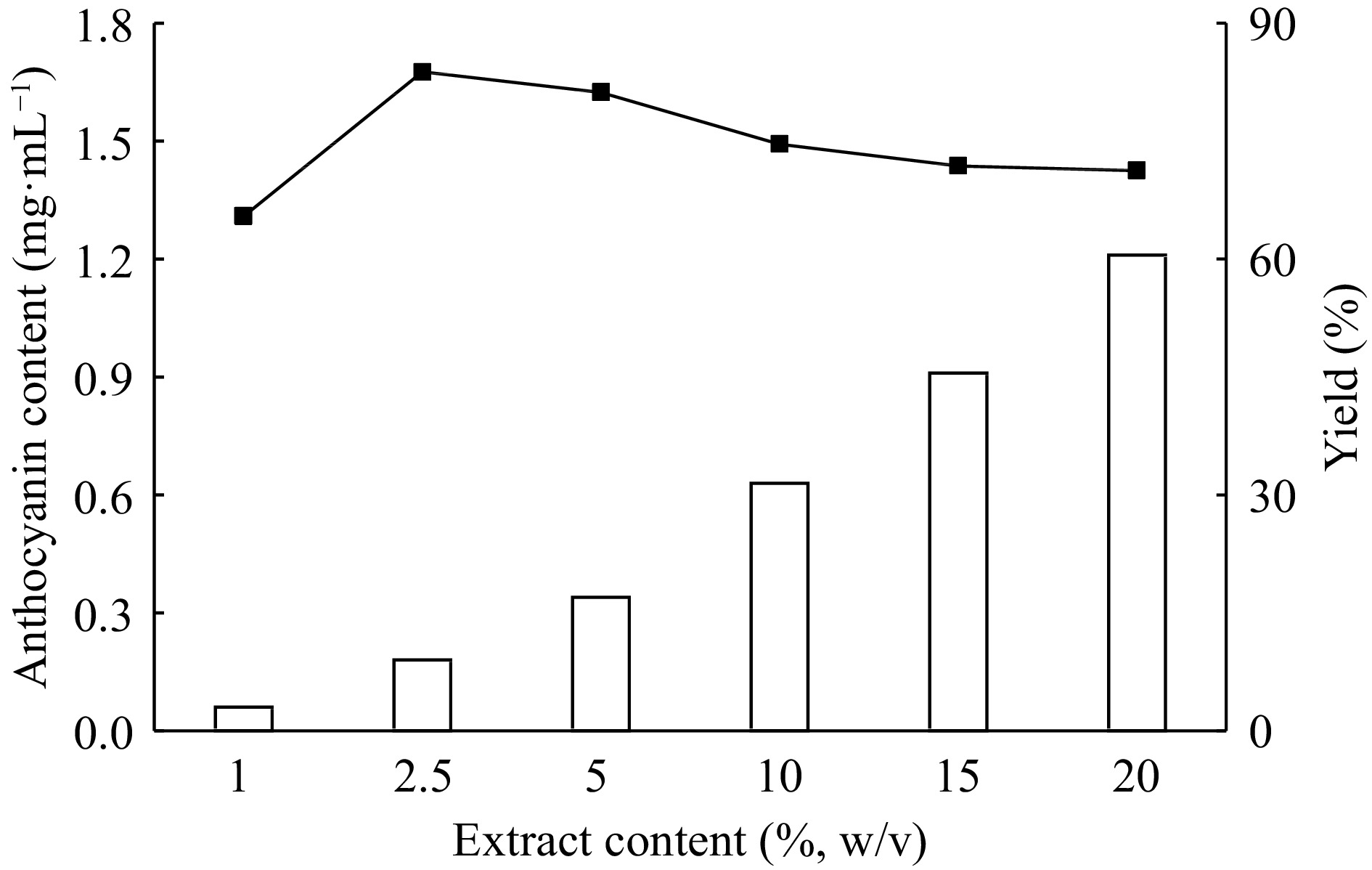
Figure 8.
The effect of extract content on the extraction and purification of red cabbage anthocyanins by ATPE.
The overall purification of red cabbage anthocyanins
-
Under optimal conditions the red cabbage anthocyanins are extracted and purified with developed ATPE system containing 22% (w/v) ammonium sulfate, 27% (v/v) ethanol, 2.5% (v/v) anthocyanin extract at pH 3.0 with 83.7% extraction yield (Table 1).
Table 1. Overall purification of anthocyanins from red cabbage by aqueous two-phase extra action.*
Step Total anthocyanin
(mg/L)Yield
(%)Pre-extract 0.215 100 ATPE-top phase 0.180 83.7 ATPE-bottom phase 0.030 16.2 * Ammonium sulfate (22%, w/v) was added to the anthocyanin extract (2.5%, w/v) of red cabbage and after then the pH was adjusted to pH 3.0. Afterwards, ethanol (27%, v/v) was added to the extract and two phases were watched clearly. The upper phase and the lower aqueous phase were separated and tested for anthocyanin amount. Each experiment was carried out in triplicate and the difference in the readings was less than ± 5%. The repeability of the ATPE system was also studied. Under determined optimal conditions, four separate systems are prepared. The extraction yields of these systems are determined as 80.85%, 81.38%, 84.84% and 84.16% and the anthocyanin amounts are calculated as 0.152, 0.153, 0.153 and 0.16 mg·L−1, respectively (Fig. 9).
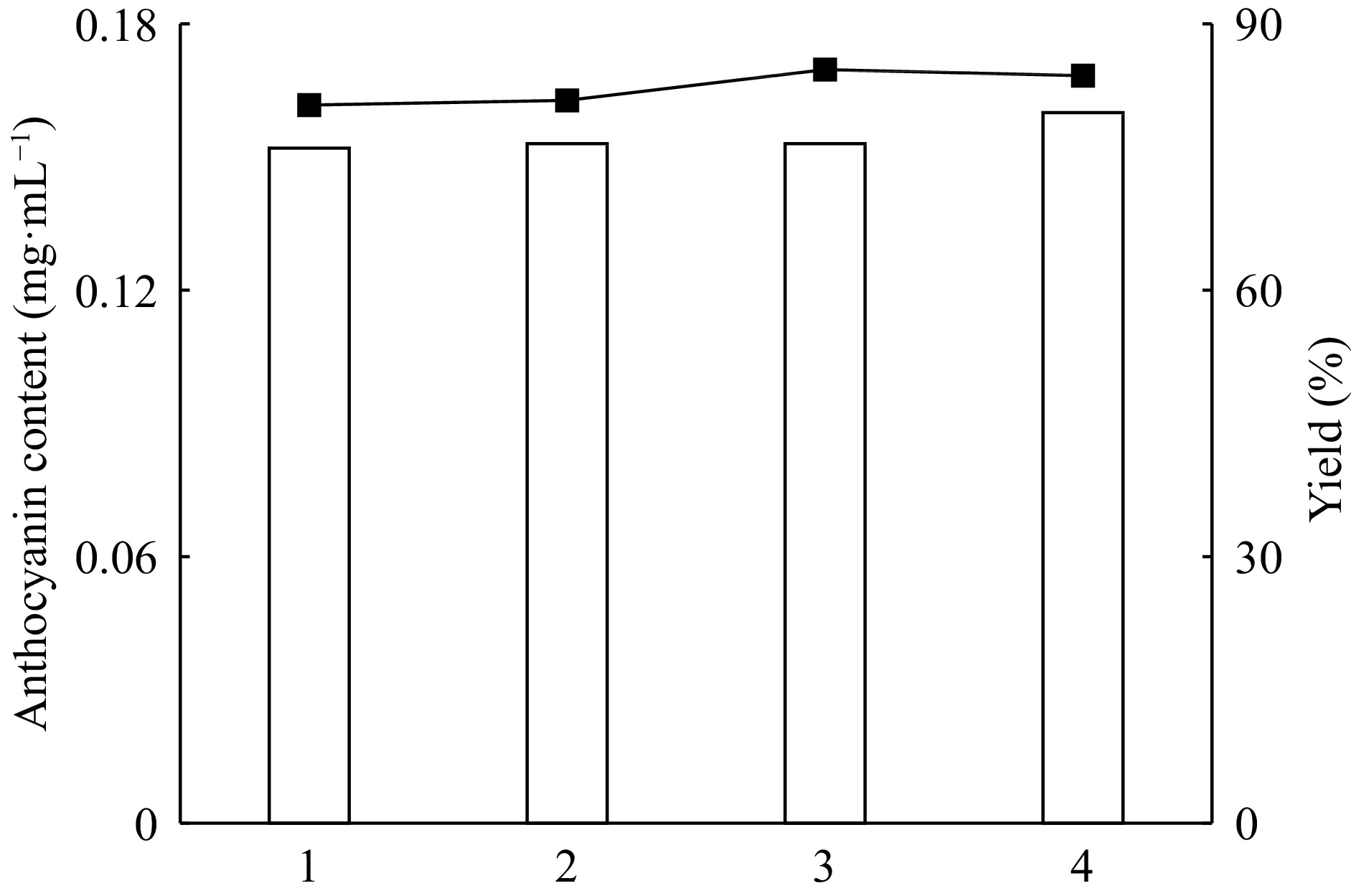
Figure 9.
Reproducibility of red cabbage anthocyanins with ATPE systems (reproducibility numbers; 1, 2, 3, 4).
Researchers have extracted anthocyanins from many plant sources by using ATPE systems which are good alternatives for their extraction. To determine the optimal extraction conditions, several parameters affecting the partitioning of anthocyanins are investigated. The grape juice anthocyanins are extracted using an optimal ATPE system (sodiumdihydrogen phosphate: 28% (w/w), ethanol: 25% (w/w), 1 mL of extract) with 99.35% relative recovery in one step. 75.08% of the sugars are also removed to the bottom phase of the system[16]. Wu et al. have also studied to determine the optimal ATPE system for mulberry anthocyanins. Under optimum conditions (20% (w/w) ammonium sulfate; 30% (w/w) ethanol; 10% (w/w) extract and 40% (w/w) water at pH 4.5) 85.1% of anthocyanins partitioned to the top phase and nearly 89.5% of sugar are transferred to the bottom phase of the system[5]. Liu et al. investigated the extraction of purple sweet potato anthocyanins using the ATPE systems using response surface methodology (RSM). According to obtained extraction results they suggested this system may be a potential for industrial purposes[12].
The sugar, flavonoid and phenolic compound amounts of red cabbage pre-extract, the bottom and top phase of ATPE system are given in Table 2. As is seen from Table 2 the sugars are concentrated in the bottom phase of the ATPE system with 79.5% yield. The flavonoids and phenolics are partitioned and concentrated into the top phase with a yield of 78% and 82.5%, respectively.
Table 2. Analysis of total sugar, flavonoid and phenolic compound content in red cabbage raw extract, ATPE system of bottom and top phases.
Sample Sugar content
(mg)Sugar yield
(%)Flavonoid content
(mg)Flavanoid yield
(%)Phenolic content
(mg)Phenolic yield
(%)Red cabbage (pre-extract) 21.9 100 0.44 100 1.10 100 ATPE-system (bottom phase) 17.4 79.5 0.10 23.5 0.32 29.02 ATPE-system (top phase) 4.4 20 0.34 78 0.91 82.5 Stability of red cabbage anthocyanins
-
pH and temperature stand as the most referred factors that affect the stability of anthocyanins. Anthocyanins are very sensitive to temperature and pH that are destructive to the colour and nutritional properties of anthocyanins. This also restricts the use of them in the food industry. The stability of anthocyanins is dependent on thermal treatment conditions (temperature, time) and environmental factors (pH, light, oxygen). Anthocyanins are known to be thermally stable below pH 3.0 and unstable at neutral pH. So, improving thermal stability of anthocyanins at neutral and weakly acidic pHs may expand their application[29]. For that reason, it is necessary to research red cabbage anthocyanin stability.
Temperature stability
-
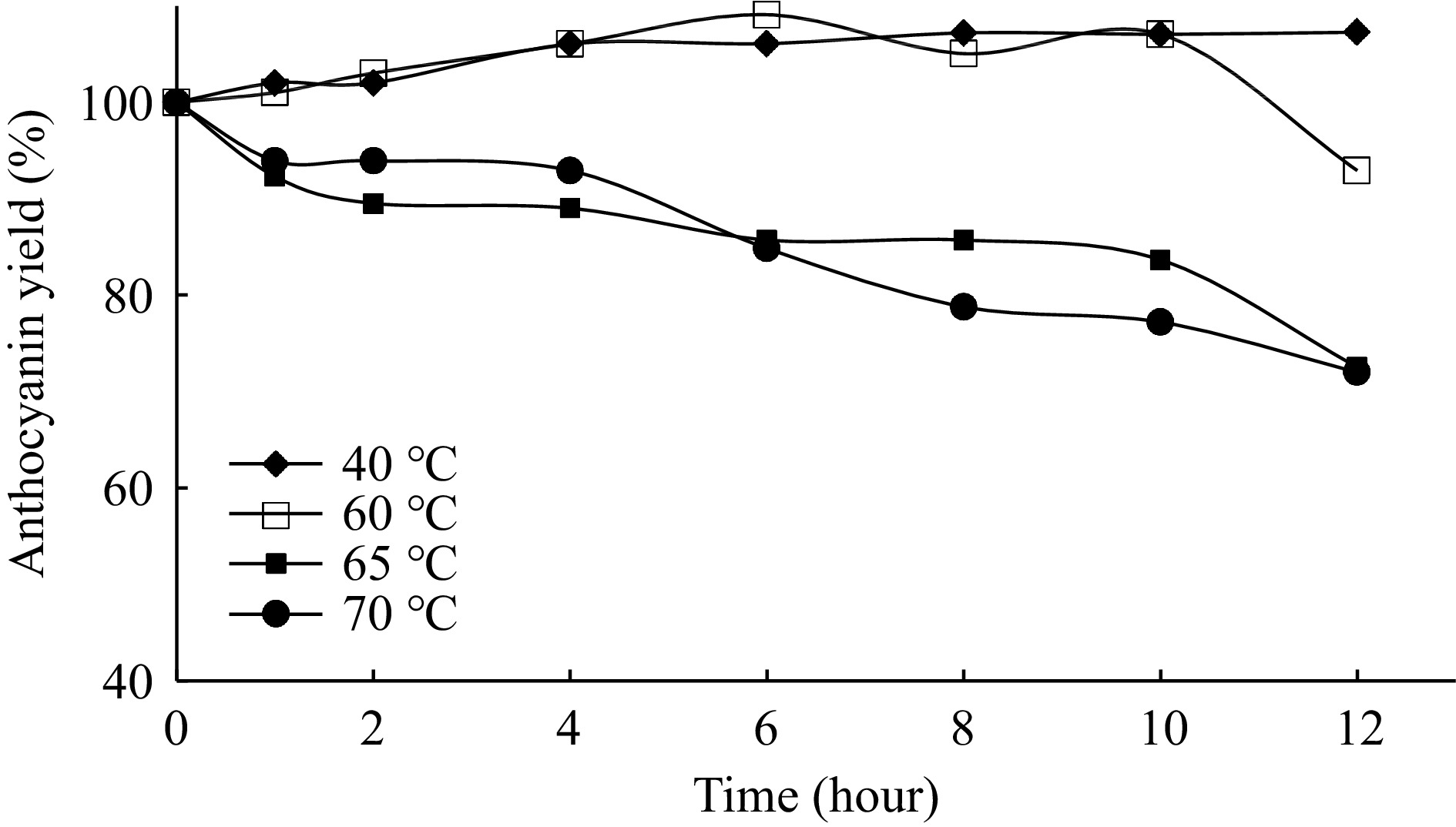
Generally, it is shown that, the thermal treatment at several temperatures multiple times resulted in loss of total anthocyanins and with the increase in temperature the anthocyanins are considerably degraded[13]. The time-dependent temperature stability of anthocyanins is determined at the temperature range of 40−70 °C (Fig. 10).
As shown in the Fig. 10, the thermal stability is changing significantly according to both the temperature and incubation time. The red cabbage anthocyanins are found to be very stable at these temperatures at the end of the 12 h. After treatment at 40−70 °C for 12 h, more than 75% of anthocyanin yields are obtained. The results showed that after 12 h treatment at 40, 60, 70 and 80 °C, 102%, 92.9%, 72.5% and 72.1% of the anthocyanin pigment is retained in the extract, respectively. Similar results are obtained by several researchers that this high thermal stability is based on the presence of acylated anthocyanins[30−33]. In general, the stability of anthocyanins is also affected by the number and position of hydroxyl groups, degree of methylation, acylation and also glycosylation[34, 35]. Dryby et al. have also reported that the red cabbage anthocyanins are very stable in the temperature range of 25−80 °C that is attributed to the complex sugar residues[31].
pH stability
-
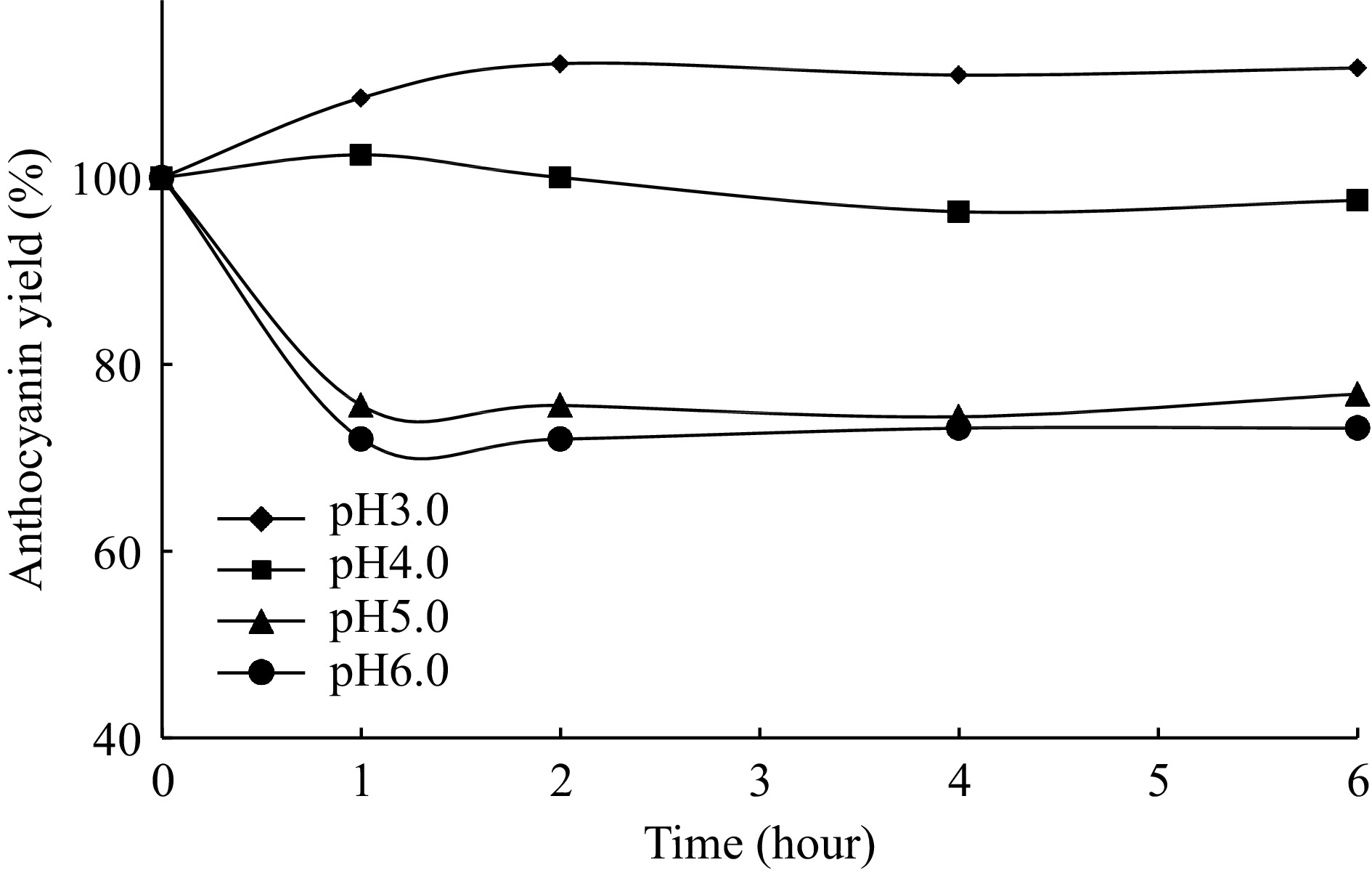
pH is an important factor that affect to the colour and stability of anthocyanins. The pH-stability of red cabbage anthocyanins is also researched and the results are given in Fig. 11. As is can be seen from the figure, the red cabbage anthocyanins showed the highest pH stability at pH 3.0−4.0. At pH 5.0 and pH 6.0, it showed very similar stability results at the end of 6 h. All of them at these pH values have more than 70% of anthocyanin yields. At pH 3.0, 4.0, 5.0 and 6.0, 113%, 97.6%, 76.8%, 73.2% of the anthocyanin pigment is maintained in extract after 6 h incubation, respectively.
This colour stability may be attributed to the composition and structure of red cabbage anthocyanins. It is known that anthocyanins are more stable under acidic pHs[10, 35]. Several researchers also reported similar pH stability results for different anthocyanins[10,30−33,36]. Krithika et al. reported that the anthocyanins extracted from onion peel are more stable in the pH range of 1−3[37].
-
Natural colorants have attention due to their considerable potential application in various industrial areas. Therefore, for better extraction yields and higher purification degrees there is a need to develop more effective methods and analytical techniques as novel technologies which can replace classical approaches. Anthocyanins are water soluble pigments and bioactive flavonoid compounds that are used in food products and also have protective and promoting effects on human health. Because of their popularity, there is a need for a novel extraction method to obtain more stable red cabbage anthocyanins.
Aqueous two-phase extraction (ATPE) is generally used as a primary step to recover, concentrate and purify the biological components in a single step. In the present work, an alcohol/salt ATPE system composed of ethanol and ammoniumsulfate is developed for the extraction of anthocyanins from red cabbage for the first time. This system is considered as GRAS and the extracts obtained from this system are safe and compatible in food and personal care products. The optimal extraction conditions are determined that is very necessary, valuable both commercially and scientifically. An ATPE system composed of 27% (v/v) ethanol, 22% (w/v) ammonium sulfate, 2.5% (v/v) anthocyanin extract, pH 3.0 is found as the best condition for the extraction of red cabbage anthocyanins. Under this condition, the anthocyanins are concentrated at the top phase of the system and the extraction yield is calculated as 83.7%. The sugars are also effectively partitioned into the bottom phase of the system with 79.5% yield. Anthocyanins are significantly affected by environmental conditions like temperature and pH. So, the stability studies in terms of temperature (40−70 °C) and pH (3.0−6.0) with different incubation times are also investigated. The stability results are very satisfying that at 70 °C and pH 6.0 more than 70% of anthocyanin yield is obtained. However, in future there is a need to improve the stability of red cabbage anthocyanins by using copigmentation and encapsulation which is present in our future plan.
Overall, the results indicated that, ATPE of anthocyanins can be used as an alternative and effective extraction method. Besides this, anthocyanins can be used as natural colorant due to their bright colours in food, pharmaceuticals and the cosmetic industries.
-
The authors confirm contribution to the paper as follows: investigation, formal analysis: Abdoulaye A; concept, resources, original draft, writing, reviewing, editing: Önal S. Both of authors read and approved the final manuscript.
-
The authors confirm that the data supporting the findings of this study are available within the article.
-
The authors declare that they have no conflict of interest.
-
These authors contributed equally: Adam Abdoulaye, Seçil Önal
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Abdoulaye A, Önal S. 2023. An aqueous-two phase extraction system for partitioning of anthocyanins from red cabbage. Food Materials Research 3:28 doi: 10.48130/FMR-2023-0028
An aqueous-two phase extraction system for partitioning of anthocyanins from red cabbage
- Received: 11 July 2023
- Accepted: 28 August 2023
- Published online: 02 November 2023
Abstract: Due to their aesthetic value and positive health effects, anthocyanins as natural colorants have become very attractive in recent years. There is a need to develop simple and effective methods for the separation and purification of these natural pigments. An aqueous two-phase extraction (ATPE) system based on ethanol/ammonium sulfate is employed for the extraction and preliminary purification of anthocyanins from red cabbage. The influence of ethanol, ammonium sulfate, red cabbage amount and pH on the extraction of anthocyanins is investigated. The optimal extraction conditions (27% (v/v) ethanol, 22% (v/v) ammonium sulfate, 2.5% (v/v) anthocaynin extract, and pH 3.0) gave 83.7% of extraction yield for anthocyanins. The flavonoids and phenolics are concentrated at the top phase of ATPE with a yield of 78% and 82.5%, respectively. The sugars also partitioned and concentrated at the bottom phase with a yield of 79.5%. The stability studies showed that anthocyanins are very stable between the temperature range of 40−70 °C and the pH range of 3.0−6.0, respectively. At a temperature of 70 °C and at pH 6.0 nearly more than 70% of anthocyanin yield is obtained. The results indicated that ATPE technique have a great promise for the efficient recovery of anthocyanins and also removing of the impurities such as sugars.