-
Tree peony (Paeonia suffruticosa Andr.) is a unique aromatic ornamental plant in China, famous for its flowers' huge size, bright colors, and strong fragrance, and which has high ornamental, medicinal, and edible value[1,2]. Flower shape, color, and fragrance are the most important apparent characteristics and the main indicators relied upon to evaluate the ornamental value of the flowers. Previously, people paid more attention to flower shape and color. But nowadays, flower fragrance is gradually emerging as a key factor greatly affecting customer choice in domestic and international flower markets[3].
The fragrance released by flowers is a volatile with low molecular weight mixture emitted by plants. Almost all higher plants can emit volatile substances, which might form initially as a lipophilic liquid that passes through the cell membrane in epidermal cells for release into the local environment, thereby producing the flower fragrance[4,5]. Aromatic components mainly include alkanes, terpenes, alcohols, aldehydes, ketones, ethers, esters, and benzene ring compounds; according to their biosynthetic pathways, these were mainly divided into the terpenoids, the alkaloids, and the phenylpropanoids and allied phenolic compounds[6]. Being an excellent source of natural antioxidants, volatile substances emitted by tree peony flowers mainly include terpenes, alcohols, esters, aldehydes, ketones and other fragrance substances[7−9]. These compounds can reduce the harmful effects of reactive oxygen species[7,10]. Recently, mounting attention has been paid to characteristics of tree peony's flower fragrance, leading to numerous aromatic products being developed, such as scented tea, essential oil, flower cake, etc., underscoring the need for systematic research to elucidate the flower fragrance synthesis mechanism of tree peony.
Research to date on tree peony has mainly focused on variety research, potential germplasm resources, introduction and cultivation[11]. Flower fragrance breeding and cultivation is now an important trend in tree peony breeding. A total of 81 scent components in the petals of 30 tree peony cultivars have been detected and classified into five fragrance patterns: a woody scent, a rose scent, a lily of the valley scent, a phenolic scent, and an unidentified scent[8]. Cis-ocimene, D-citronellol, linalool, 1,3,5-trimethoxybenzene (TMB), and pentadecane are their major floral fragrances, respectively. Evaluation of the potential utilization of seven tree peony cultivars demonstrated that 'High Noon' might harbor a significantly high fragrance content useful for fragrant cultivar breeding[8]. Additionally, 124 volatile components in petals of nine wild tree peony species were identified and clustered into five major chemical classes: terpenoids, alkanes, alcohols, aldehydes, and ketones, yet the main components and sensory evaluation differed starkly among species[9]. P. ostii had herbal and waxy attributes, mainly dominated by hexanal and pentadecane; P. rockii, P. qiui, P. jishanensis, and P. decomposita featured a sweet attribute, which was positively correlated with geraniol and citronellol; P. delavayi, P. lutea, P. ludlowii, and P. potanini were characterized by an intense floral attribute dominated by linalool and trans-linalool oxide. Furthermore, that study also revealed the phylogenetic relationships of nine wild tree peony species according to the volatile components that they emitted[9].
Studies on the genetic population construction and phenotypic trait investigation of hybrids in strawberry[12,13], pepper[14,15], maize[16], and wheat[17] have been carried out recently, which could help to lay a theoretical foundation for QTL mapping of phenotypic traits and molecular marker-assisted breeding. Most genetic populations of plants in use presently are the F2 generation, largely because it is conducive for detecting the trait separation phenomenon according to Mendel's law of inheritance. Regarding tree peony, it is a self-incompatible plant with a highly heterozygous genome; hence, its phenotypic traits could be separated in the F1 generation. Thus, the F1 generation of tree peony could be considered as the hybrid F2 generation of homozygous parents, and F1 populations of tree peony have been constructed and used for genetic analysis based on their common phenotypic traits. For example, Li[18] constructed an F1 population via mixed pollination and statistically analyzed three continuous traits and six discrete traits. Correlation analysis and mixed genetic analysis of 20 phenotypic traits (such as plant height, and crown width) of the F1 population of P. ostii 'Fengdan' and P. suffruticosa 'Xinriyuejin' uncovered 20 phenotypic traits that presented hybrid superiority and transgressive segregation, with some traits showing genetic effects controlled by major genes[19]. However, to our knowledge, no study has yet reported on the aroma traits of F1 genetic population in tree peony.
In this work, an F1 segregating population was established using P. ostii 'Fengdan' (light fragrance type) as the female parent and P. suffruticosa 'Chunguihuawu' (strong fragrance type) as the male parent. Then, the flower aromatic components of this F1 population, for both parents and progeny, were identified and analyzed. This is the first report to analyze the genetic mechanism of flower fragrance in an F1 genetic population of tree peony. This study's findings may provide valuable information for clarifying the genetic mechanism of flower fragrance formation and help to lay a theoretical basis for flower fragrance breeding of tree peony.
-
Floral volatiles samples were collected from the flowers at three different flowering stages (initial flowering stage, half-opening stage, and full blooming stage) of the light fragrance-type cultivar P. ostii 'Fengdan' and the strong fragrance-type cultivar P. suffruticosa 'Chunguihuawu', as well as the half-opening stage flowers of 109 F1 progeny. All these plants were grown at the National Tree Peony Genebank (Luoyang City, Henan Province, China) under consistent management practices and favorable growing conditions.
Floral volatiles' collection
-
Collection of floral volatile components was performed using the dynamic headspace sampling technique applied to tree peony flowers of moderate size at noontime on sunny and windless days in April 2020. Each sample was placed into a sampling bag connected to a Tenax TA adsorption tube and an active charcoal pump (QC-1S, Beijing Municipal Institute of Labor Protection, Xicheng District, Beijing, China); samples were collected for 3 h at a flow rate of 400 mL min-1. In the experiment, headspace collection from ambient air served as a negative control.
After sampling, the adsorption tube was sealed with fresh-keeping film and wrapped with tin foil, then placed in an icebox and brought to the laboratory. When the collection was complete, 1,000 μL of n-hexane solution was used to elute the aroma adsorbed by the Tenax TA tube into a clean, brown injection bottle. Each injection bottle was sealed with sealing film and stored in a refrigerator at –20 °C until the samples were analyzed with the instruments below.
Floral volatiles analysis
-
The analysis was carried out using a gas chromatograph-mass spectrometer (Trace GC Ultra-ISQ, Thermo Fisher Scientific, 81 Wyman Street, Waltham, MA, USA) fitted with a TM-5MS capillary column (30 m × 0.25 mm, 0.25 μm). Helium was the carrier gas, delivered at a flow rate of 1.0 mL min−1. The initial oven temperature was maintained at 70 °C for 1 min and raised to 142 °C at a rate of 6 °C min−1, then raised to 148 °C at a rate of 1 °C min−1, then raised to 180 °C at a rate of 2 °C min−1, and finally raised to 250 °C at a rate of 10 °C min−1 at which it was held isothermally for 20 min. The temperature of the injector, transfer line, ion source, and quadrupole were set to 250 °C, 260 °C, 230 °C, and 150 °C, respectively. The ionization potential of the mass selective detector was 70 eV and its scan range was 29–386 amu.
Data analysis
-
Compounds were identified by comparing their mass spectra to the NIST 2011 database. In order to fairly compare the released amounts of volatile components, 69.32 mg L−1 of decanoic acid, ethyl ester served as an internal standard, with 0.4 μL of this internal standard solution added to every 80 μL of a given sample. The calculation formula is as follows:
$ C = \frac{{S_v}}{{S_i}} \times \frac{{m_i}}{{m_s}} \times f $ where C is the amount of the volatile component; Sv is the peak area of the volatile compound; Si is the peak area of the internal standard; mi is the quality of the internal standard; ms is the quality of the sample; and f is a correction factor of each component for the internal standard.
Statistical Program for Social Sciences 21.0 software(SPSS, Inc., Chicago, IL, USA) was used for variation analysis, Gaussian distribution test, and X2 test. Origin 8.0 software (OriginLab Corporation, Northampton, MA, USA) was used to draw the figures.
-
Figure 1 shows the chromatographic profiles of two different tree peony cultivars in three different flowering stages, for which a significant difference was found. In total, 67 volatile compounds were identified as emitted from the flowers of these tree peonies, of which 43 volatile compounds were shared by them. These 67 volatile compounds belonged to six categories: alcohols, esters, aldehydes, terpenes, benzenes, and hydrocarbons. However, among them, 35 hydrocarbons were found, far more than in any other category; esters were another important category with 13 compounds identified; six alcohols and five aldehydes were found in the volatiles, along with benzenes and terpenes (four kinds each).
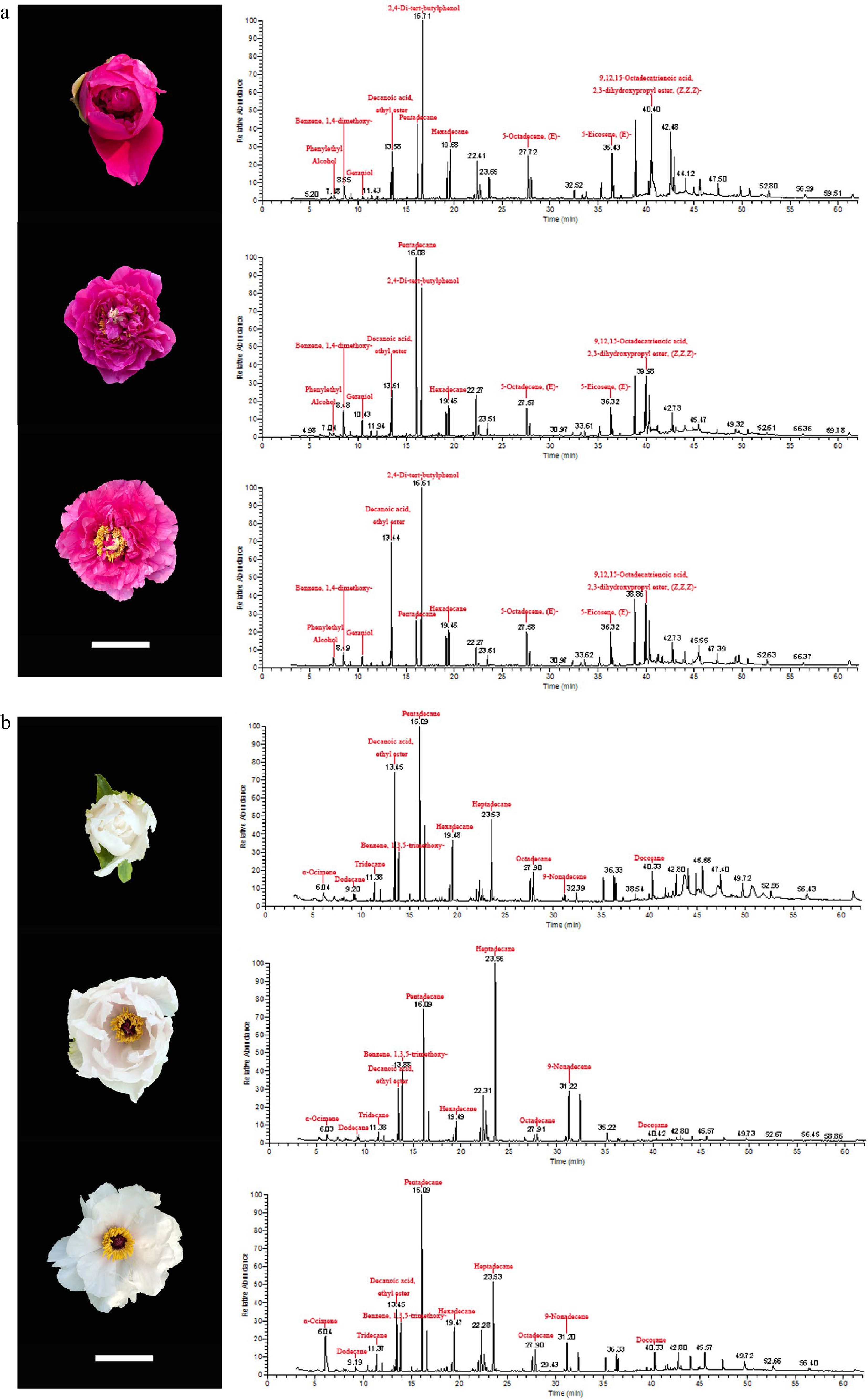
Figure 1.
Chromatographic profiles of two tree peony cultivars at three flowering stages. (a) P. suffruticosa 'Chunguihuawu', (b) P. ostii 'Fengdan'. From top to bottom are initial flowering stage, half-opening stage, and full blooming stage. Bar = 5 cm.
Volatile components of P. suffruticosa 'Chunguihuawu' at different flowering stages
-
The content of each volatile component in P. suffruticosa 'Chunguihuawu' and P. ostii 'Fengdan' are shown in Table 1. A total of 56 volatile compounds were detected in three different stages of P. suffruticosa 'Chunguihuawu', consisting of three alcohols, 11 esters, three aldehydes, three terpenes, three benzenes, and 33 hydrocarbons. There were 51, 53, and 46 volatile compounds detected in the initial flowering stage, half-opening stage, and full blooming stage, respectively. At the initial flowering stage, the specific volatile components were 2-hexen-1-ol,acetate,(E)-, decanal, and (Z,E)-alpha-farnesene, while the contents of nonanal, glycerol linolenate, and nonadecane were much higher than those in the other two stages. At the half-opening stage, only α-ocimene, trans-α-ocimene, nerol, and farnesal were detected. In addition, the contents of benzene,1,4-dimethoxy-, geraniol, and pentadecane at the initial opening stage surpassed those at the other two stages, indicating these compounds may be characteristic volatile compounds at this stage. Moreover, 2,4-di-tert-butylphenol was detected at all three stages, its content exceeding that of other volatile compounds at every stage.
Table 1. Volatile components and content (μg g−1) of two tree peony cultivars at different flowering stages.
Number RT (min) Compound P. suffruticosa 'Chunguihuawu'
(male parent, ♂)P. ostii 'Fengdan' (female parent, ♀) Initial flowering Half-opening Full blooming Initial flowering Half-opening Full blooming 1 5.23 2-hexen-1-ol,acetate,(E)- 0.0513 − − − 0.1823 − 2 6.04 α-ocimene − 0.0648 − 0.1076 0.2547 1.0646 3 6.21 Trans-α-ocimene − 0.0305 − 0.0355 − − 4 7.06 Undecane 0.0308 0.0877 0.0163 − 0.0112 − 5 7.17 Nonanal 0.1436 0.0734 0.0232 0.0738 0.1248 − 6 7.44 Phenylethyl alcohol 0.1607 0.1351 0.1192 − − − 7 7.97 Myroxide − − − − 0.0615 0.0477 8 8.50 Benzene, 1,4-dimethoxy− 0.4856 0.9253 0.1587 − − − 9 9.02 2-dodecene, (E)- 0.0308 0.0218 0.0094 − − − 10 9.19 Dodecane 0.1265 0.0955 0.0403 0.0538 0.0731 0.0561 11 9.34 Decanal 0.0171 − − 0.0392 0.1116 0.0505 12 9.89 Nerol − 0.0280 − − − − 13 9.99 3-phenylpropanol − − − − 0.0236 − 14 10.44 Geraniol 0.0992 0.3784 0.0798 − 0.0133 0.1290 15 10.65 2-propen-1-ol, 3-phenyl − − − − 0.0213 − 16 11.21 6-tridecene − − − − 0.0113 − 17 11.37 Tridecane 0.0923 0.0889 0.0292 0.1148 0.1072 0.1936 18 11.65 2-propen-1-ol, 3-phenyl-, (E)- − − − − 0.0194 − 19 12.56 Benzeneacetic acid, à-hydroxy-,
ethyl ester, (R)0.0684 0.0185 0.0395 − − − 20 12.79 2-undecenal − − − − 0.0173 − 21 13.00 3-phenyl-1-propanol, acetate − − − − 0.0164 0.0295 22 13.20 Geranyl acetate 0.0239 0.0243 0.0120 − − 0.0673 23 13.35 5-tetradecene, (E)- 0.2325 0.1871 0.0635 0.0574 0.0709 0.1080 13.44 Decanoic acid, ethyl ester 0.5232 0.5232 0.5232 0.5232 0.5232 0.5232 24 13.52 Tetradecane 0.6018 0.6233 0.1621 0.2415 0.2714 0.3815 25 13.87 Benzene, 1,3,5-trimethoxy- − − − 0.2479 0.8071 0.4741 26 16.08 Pentadecane 1.1558 2.8816 0.2350 0.8495 1.5199 1.7575 27 16.45 (Z,E)-α-farnesene 0.0308 − − − − − 28 16.61 2,4-di-tert-butylphenol 2.9236 2.6407 0.9589 0.3992 0.3514 0.4516 29 17.53 Farnesal − 0.0128 − − − − 30 17.76 1-decanol, 2-hexyl- 0.0479 0.0326 0.0146 0.0465 0.0096 0.0491 31 19.19 Cetene 0.7386 0.5136 0.2007 0.0993 0.1049 0.1262 32 19.47 Hexadecane 0.9916 0.6651 0.2427 0.3937 0.3089 0.6494 33 20.02 Hexadecanal − − − − 0.0091 − 34 21.28 Hexadecane, 7-methyl- 0.0342 0.0210 0.0086 − 0.0057 − 35 21.41 Pentadecane, 2,6,10-trimethyl- 0.0923 0.0539 0.0240 0.0574 0.0108 0.0757 36 21.98 Hexadecane, 2-methyl- 0.1026 0.0905 0.0180 0.0866 0.2203 0.1557 37 22.57 8-heptadecene 0.4240 0.3443 0.0377 0.1440 0.6727 0.3815 38 23.52 Heptadecane 0.4958 0.2969 0.0806 0.5870 2.8875 1.3086 39 26.59 Heptadecane, 3-methyl- 0.0684 0.0330 0.0172 − 0.0753 0.0814 40 27.61 5-octadecene, (E)- 1.0463 0.7361 0.2805 0.1723 0.1007 0.2314 41 27.92 Octadecane 0.5163 0.3099 0.1132 0.2689 0.1351 0.3997 42 29.14 Isopropyl myristate 0.0342 0.0149 0.0069 − − − 43 31.22 9-nonadecene 0.0376 0.0051 − 0.0602 1.0123 0.5021 44 32.38 Nonadecane 0.2394 0.0912 0.0480 0.0747 0.9132 0.3296 45 33.61 Hexadecanoic acid, methyl ester 0.1846 0.1466 0.0583 − − − 46 35.57 Nonadecane, 3-methyl- − 0.0200 0.0086 − 0.0161 − 47 36.36 5-eicosene, (E)- 0.8719 0.6104 0.2178 0.1568 0.0461 0.2272 48 36.41 Hexadecanoic acid, ethyl ester 0.1436 0.1202 0.0437 − − − 49 36.55 Eicosane 0.2291 0.1309 0.0472 0.1167 0.0405 0.1767 50 37.26 Isopropyl palmitate 0.0855 0.0502 0.0214 0.0264 − − 51 38.53 10-heneicosene 0.0547 0.0185 0.0060 − 0.0089 − 52 38.75 Methyl linoleate 0.3351 0.2929 0.1046 − − − 53 38.82 Heneicosane − − − − 0.0273 − 54 38.87 6,9,12-octadecatrienoic acid,
methyl ester0.9848 0.8387 0.3019 − − − 55 39.88 Ethyl linoleate 0.2906 0.2341 0.0849 − − − 56 39.96 2-Propenoic acid,
3-(4-methoxyphenyl)-,
2-ethylhexyl ester− − − 0.0474 0.0158 0.0435 57 39.98 9,12,15-octadecatrienoic acid,
2,3-dihydroxypropyl ester, (Z,Z,Z)-1.3199 0.7741 0.2796 − − − 58 40.41 Docosane 0.4548 0.3641 0.0978 0.0948 0.0222 0.1262 59 41.66 Tricosane 0.0752 0.0417 0.1535 0.0693 0.0220 0.0701 60 42.80 Tetracosane 0.2257 0.0968 0.0583 0.1094 0.0461 0.1725 61 44.06 Pentacosane 0.2052 0.0810 0.0652 0.1139 0.0647 0.1865 62 45.56 Hexacosane 0.3009 0.1577 0.1338 0.2242 0.0819 0.3044 63 47.40 Heptacosane 0.3009 0.0846 0.0832 0.2005 0.0609 0.2244 64 49.71 Octacosane 0.3351 0.1001 0.1278 0.1622 0.0659 0.2230 65 52.64 Nonacosane 0.2325 0.0681 0.0669 0.1130 0.0511 0.1459 66 56.41 Triacontane 0.2052 0.0641 0.0549 0.0875 0.0391 0.1164 67 61.26 Hentriacontane 0.2359 0.1227 0.0986 0.1805 0.0484 − As shown in Fig. 2a, the six compounds were ranked in content as follows: hydrocarbons > benzenes > esters > alcohols > aldehydes > terpenes. With the opening of flowers, the total content of different compounds featured different trends. The total content of hydrocarbons and esters decreased gradually, that is, peaking at the initial flowering stage. Except for hydrocarbons and esters, the content of other volatile components in P. suffruticosa 'Chunguihuawu' reached their maximum levels at the half-opening stage.
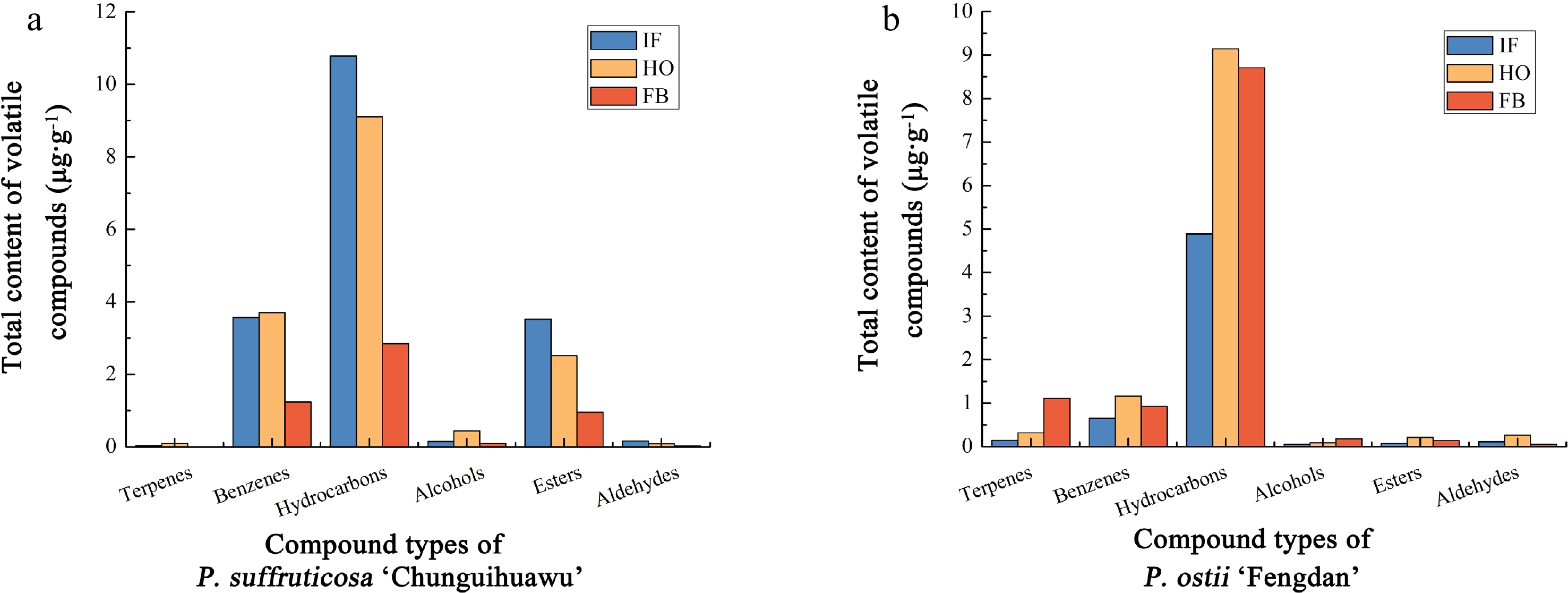
Figure 2.
Classification and content of volatile compounds of parents of the F1 population in different flowering stages. (a) P. suffruticosa 'Chunguihuawu', (b) P. ostii 'Fengdan'. IF: initial flowering stage. HO: half-opening stage. FB: full blooming stage.
Volatile components of P. ostii 'Fengdan' at different flowering stages
-
A total of 53 volatile compounds were detected from all three flowering stages of P. ostii 'Fengdan', consisting of five kinds of alcohols, five esters, four aldehydes, three terpenes, two benzenes, and 34 hydrocarbons. Specific volatile components were detected at different flowering stages: 30 species at the initial flowering stage, 43 species at the half-opening stage, and 31 species at the full blooming stage. The unique volatile components at the initial flowering stage were trans-α-ocimene and isopropyl palmitate, whereas those at the half-opening stage were 2-hexen-1-ol,acetate,(E)-, 3-phenylpropanol, cinnamyl alcohol, cis-cinnamyl alcohol, 2-undecenal and hexadecanal, while geranyl acetate was detected only at the full blooming stage.
As seen in Fig. 2b, the content of hydrocarbons was always the greatest at all three stages, followed by benzenes and terpenes, being least for the other three compound categories. The content of terpenes and alcohols during various flower stages was ranked as follows: full blooming stage > half-opening stage > initial flowering stage; for aldehydes, the ranking differed, as follows: half-opening stage > initial flowering stage > full blooming stage; the same ranking was found for other three compounds: half-opening stage > full blooming stage > initial flowering stage. Across all three stages, α-ocimene, 1,3,5-trimethoxybenzene, pentadecane, and heptadecane were detected, whose contents were much higher than those of other volatile compounds. The total relative content of those four compounds was 19.66%, 38.81%, and 32.83% respectively at the three stages, showing a trend of increasing initially and then decreasing. Our experimental results show that these four compounds are the main volatile components of P. ostii 'Fengdan'.
Comparing the volatile components of P. suffruticosa 'Chunguihuawu' and P. ostii 'Fengdan'
-
The two tree peony cultivars had 43 components in common, mainly 2,4-di-tert-butylphenol and various hydrocarbons such as dodecane, tetradecane, and pentadecane. It was worth noting that 14 volatile components were exclusive to P. suffruticosa 'Chunguihuawu', most of them being ester volatile oils. Similarly, 11 kinds of compounds, such as myroxide, 1,3,5-trimethoxybenzene and octyl p-methoxycinnamate, were only detected in the flowers of P. ostii 'Fengdan', but most of these were oxygenated derivatives of hydrocarbons. These results confirmed there were significant differences in volatile components between the two cultivars studied here.
Analysis of volatile components in the F1 population
Types and content of volatile components in F1 population
-
Due to differences in flower color, flower type, and floral fragrance between the parents, the hybrids exhibited clear segregation for these traits. After the volatile components of hybrids were counted, it was found that the hybrids had more complex profiles of volatiles than did their corresponding parent accessions. For 109 progeny, they featured 73 volatile compounds, namely six alcohols, 18 esters, five aldehydes, six terpenes, four benzenes, and 35 hydrocarbons, while their parents harbored only 56 and 53 volatiles, respectively. Shared by both parents and progeny were 22 compounds, such as pentadecane, 2,4-di-tert-butylphenol, cetene, hexadecane, 8-heptadecene, and heptadecane, but most of them were hydrocarbons. However, seven compounds—α-pinene, alloocimene, phenylethyl acetate, citronellyl acetate, neryl acetate, (Z,E)-α-farnesene and tetradecanoic acid—were detected only in progeny (i.e., absent from parents altogether). After analyzing the data, α-ocimene and benzene,1,3,5-trimethoxy were found present in 104 and 69 progeny, these compounds attaining their highest content for progeny F1-1, at 37.39 μg g−1 and 10.81 μg g−1, respectively. Nerol existed in 104 progeny, and its content was the highest for progeny F1-2 and F1-6. Finally, 2,4-di-tert-butylphenol was the only compound that presented in all progeny, whose content was maximal for progeny F1-8 and F1-10.
Genetic variation analysis of volatile components in the F1 population
-
The inheritance of these 73 components can be broadly divided into two cases: (i) 50 components separated in the progeny, which showed the inheritance of quality traits, and (ii) 23 components not segregated in the progeny, which showed the inheritance of quantitative traits. Variation analysis was performed for these 23 components, whose statistical results are shown in Table 2. The CV (coefficient of variation) of the 23 components in the F1 population ranged from 0.618% to 3.207%, indicating small differences. The CV of octadecane was the lowest, and that of of tridecane the highest. Distribution testing of these 23 components showed that only cetene, hexadecane, octadecane, and docosane conformed to a Gaussian (normal) distribution. The frequency distribution analysis of these four components (Fig. 3) showed they had relatively prominent unimodal distributions, thus conforming to the characteristics of quantitative traits. However, the absolute values of skewness and kurtosis of the four components were greater than 1 or close to 1, demonstrating the presence of positive skewness and extremeness.
Table 2. Statistics of traits in the F1 population from the cross of P. ostii ‘Fengdan’ and P. suffruticosa ‘Chunguihuawu’.
Compound (μg g−1) Minimum Maximum Range Fm SD CV (%) Skewness Kurtosis Tridecane 0.007 19.459 19.452 0.625 2.003 3.207 8.171 74.442 5-tetradecene, (E)- 0.020 2.360 2.340 0.268 0.318 1.187 4.180 22.175 Tetradecane 0.057 19.184 19.127 1.225 2.049 1.673 6.828 56.189 Pentadecane 0.003 68.622 68.619 8.336 11.083 1.330 2.652 9.002 2,4-di-tert-butylphenol 0.057 9.050 8.992 0.870 1.342 1.543 4.072 19.137 Cetene 0.021 2.097 2.076 0.483 0.343 0.709 1.915 5.375 Hexadecane 0.096 4.448 4.352 1.162 0.758 0.652 1.701 3.914 8-heptadecene 0.014 22.622 22.608 2.318 2.996 1.292 3.777 20.159 Heptadecane 0.057 20.504 20.447 3.336 3.646 1.093 2.598 7.966 5-octadecene, (E)- 0.027 2.862 2.834 0.663 0.465 0.702 1.823 5.009 Octadecane 0.034 1.610 1.576 0.505 0.312 0.618 1.006 0.866 5-eicosene, (E)- 0.017 2.822 2.805 0.454 0.372 0.820 3.117 15.673 Eicosane 0.011 0.747 0.736 0.207 0.136 0.660 1.033 1.342 Docosane 0.017 0.925 0.907 0.164 0.138 0.838 3.066 12.963 Tricosane 0.010 0.979 0.969 0.118 0.145 1.238 3.844 17.306 Tetracosane 0.012 1.934 1.921 0.268 0.287 1.072 3.455 15.065 Pentacosane 0.004 2.521 2.517 0.309 0.380 1.229 3.765 17.557 Hexacosane 0.016 3.440 3.425 0.557 0.541 0.972 3.102 12.482 Heptacosane 0.008 3.166 3.157 0.373 0.472 1.264 3.730 17.515 Octacosane 0.014 3.009 2.995 0.380 0.445 1.170 3.565 16.462 Nonacosane 0.007 2.215 2.209 0.257 0.320 1.246 3.685 17.671 Triacontane 0.006 1.615 1.608 0.187 0.227 1.214 3.550 17.157 Hentriacontane 0.003 0.996 0.993 0.184 0.166 0.901 2.109 6.168 Fm: The mean value of a component in progeny. SD: standard deviation. CV: coefficient of variation. 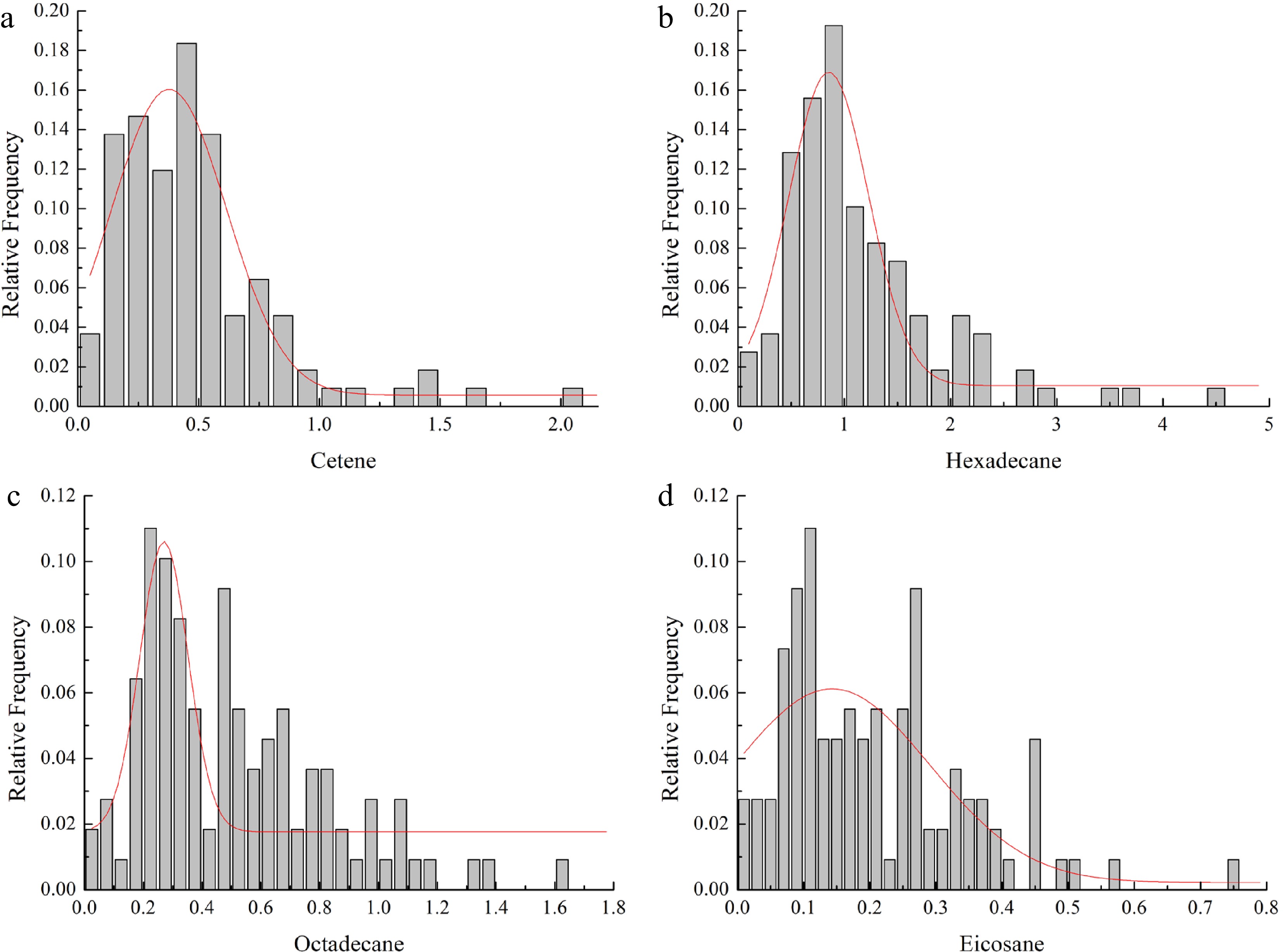
Figure 3.
Histograms (frequency distribution) for traits of the F1 population from the cross of P. ostii 'Fengdan' and P. suffruticosa 'Chunguihuawu'. (a) Histogram for cetene, (b) histogram for hexadecane, (c) histogram for octadecane, (d) histogram for eicosane.
Genetic analysis of 29 aromatic components in the F1 population
-
Most terpenes, alcohols, esters, aldehydes, and benzenes have special odor characteristics. Among the 73 kinds of volatile compounds detected in the genetic population, 29 were aroma compounds. Their genetic analysis revealed that four of these 29 were shared by the parents, while another 18 were belonged to a single parent, with another seven kinds not found in either parent.
Genetic analysis of aromatic components shared by parents but separated in progeny
-
Among the four aromatic components shared by the parents, three got separated in progeny. The X2 test results showed that this agreed with theoretical separation ratio of 1:1, 1:7, or 15:1 according to Mendelian's law of genetic separation (Table 3). This suggested the three components were controlled by single or multiple genes. Notably, there was a generally higher content of α-ocimene in progeny than the parent, exhibiting transgressive inheritance.
Table 3. Inheritance of the aromatic components shared by parents and separated in progeny.
Compound Male parent Female parent Mid-parent value Separate Separation ratio Progeny average value Progeny
distributionP value X2 value Yes No α-ocimene 0.0648 0.2547 0.1597 104 5 15:1 1.8694 0.0074~37.3901 0.473 0.514 Nonanal 0.0734 0.1248 0.0991 54 55 1:1 0.0939 0.0089~0.3674 0.924 0.009 Geraniol 0.3784 0.0133 0.1959 100 9 1:7 0.3875 0.0053~3.9761 0.180 1.794 Genetic analysis of aromatic components shared by parents and not separated in progeny
-
Among the four aromatic components shared by the parents, only one was not separated in the progeny, in that 2,4-di-tert-butylphenol was detected in all progeny (Table 4). It was the characteristic aromatic component of this tree peony genetic population and had the potential for stable inheritance. According to the relevant parameters of the progeny distribution, the average value was less than mid-parent value and the variation was continuous, demonstrating typical quantitative trait inheritance.
Table 4. Inheritance of aromatic components shared by parents but not separated in progeny.
Compound Male parent Female parent Mid-parent
valueProgeny average
valueCoefficient of
variationProgeny distribution 2,4-di-tert-butylphenol 2.6407 0.3514 1.4960 0.8696 1.5291 0.0575~9.0498 Genetic analysis of aromatic components in one of the parents
-
There were 18 kinds of components in line with this phenomenon, mainly including esters, terpenes, alcohols, and so on (Table 5). Among them, 14 components agreed with the theoretical separation ratio by the X2 test. In the case of separation ratios of 1:1 and 3:1, the components could be controlled by one pair of dominant genes; while a separation ratio of 1:7 indicated possible control by two pairs of recessive genes; however, the separation ratio of 15:1 suggested they might be controlled by multiple pairs of dominant genes. These correspond to the inheritance of quality traits. On the contrary, for trans-α-ocimene, benzene,1,4-dimethoxy-, nerol, and geranyl acetate, the X2 test was significant (P < 0.05), which did not obey the test. Phenylethyl alcohol, benzene,1,4-dimethoxy-, nerol, and geranyl acetate were the characteristic aromatic components of this tree peony genetic population, these inherited from the male parent P. suffruticosa 'Chunguihuawu'.
Table 5. Inheritance of aromatic components in one of the parents.
Compound Male
parentFemale parent Mid-parent value Separated Separation ratio Progeny
average valueProgeny distribution P value X2
valueYes No Trans-α-ocimene 0.0305 − 0.0153 44 65 1:1 0.0400 0.0119~0.1216 0.044 4.046 Phenylethyl alcohol 0.1351 − 0.0675 79 30 3:1 0.1433 0.0039~1.5277 0.543 0.370 Myroxide − 0.0615 0.0307 57 52 1:1 0.2028 0.0083~1.8797 0.632 0.229 Benzene, 1,4-dimethoxy− 0.9253 − 0.4626 44 65 1:1 0.5051 0.0266~1.8513 0.044 4.046 Decanal − 0.1116 0.0558 79 30 3:1 0.0462 0.0036~0.2198 0.543 0.370 Nerol 0.0280 − 0.0140 104 5 15:1 0.4760 0.0060~5.9410 0.473 0.514 2-propen-1-ol, 3-phenyl − 0.0213 0.0106 13 96 1:7 0.0426 0.0045~0.1141 0.856 0.033 2-propen-1-ol, 3-phenyl-, (E)− − 0.0194 0.0097 18 91 1:7 0.0446 0.0050~0.1175 0.205 1.606 2-undecenal − 0.0173 0.0087 21 88 1:3 0.0206 0.0031~0.0601 0.167 1.911 Geranyl acetate 0.0243 − 0.0122 80 29 3:1 0.1534 0.0023~1.2838 0.699 0.150 Benzene, 1,3,5-trimethoxy− − 0.8071 0.4035 69 40 1:1 0.5456 0.0037~10.8091 0.005 7.716 Farnesal 0.0128 − 0.0064 14 95 1:7 0.0213 0.0096~0.0952 0.914 0.012 Hexadecanal − 0.0091 0.0045 18 91 1:7 0.0297 0.0102~0.0636 0.205 1.606 Isopropyl myristate 0.0149 − 0.0075 55 54 1:1 0.0202 0.0041~0.0887 0.924 0.009 Hexadecanoic acid, methyl ester 0.1466 − 0.0733 40 69 1:1 0.0559 0.0035~0.4906 0.005 7.716 Hexadecanoic acid, ethyl ester 0.1202 − 0.0601 9 100 1:7 0.1089 0.0163~0.3005 0.180 1.794 Methyl linoleate 0.2929 − 0.1464 14 95 1:7 0.2208 0.0121~0.9635 0.914 0.012 Ethyl linoleate 0.2341 − 0.1170 15 94 1:7 0.1836 0.0099~0.8226 0.690 0.159 Genetic analysis of de novo aroma compounds
-
Some de novo aroma compounds (those present in the hybrid, but absent in the parents, such as α-pinene, neryl acetate, etc.) were detected exclusively in the progeny. The capacity to produce these compounds might have been inherited from the parents in an additive genetic fashion. Seven kinds of components were line with this phenomenon, all of which were terpenes and esters, and they got separated in the progeny. The first four components in Table 6, namely α-pinene, alloocimene, phenylethyl acetate, and citronellyl acetate, agreed with the theoretical separation ratio of 1:3 or 1:15 according to the X2 test; hence, we speculated they could be respectively controlled by a pair, or multiple pairs, of recessive genes, showing the inheritance of quality traits. Nevertheless, for neryl acetate, (Z,E)-α-farnesene, and tetradecanoic acid, ethyl ester, the X2 tests were significant (P < 0.05), which did not obey the test.
Table 6. Inheritance of aromatic components absent from both parents.
Compound Male parent Female parent Mid-parent value Separate Separation ratio Progeny average value Progeny distribution P value X2 value Yes No α-pinene − − − 34 75 1:3 0.1279 0.0061~0.4870 0.135 2.290 Alloocimene − − − 19 90 1:3 0.1271 0.0162~0.5828 0.068 3.330 Phenylethyl acetate − − − 24 85 1:3 0.1544 0.0181~0.5714 0.472 0.517 Citronellyl acetate − − − 4 105 1:15 0.0202 0.0077~0.0395 0.266 1.239 Neryl acetate − − − 66 43 1:1 0.0693 0.0044~0.4162 0.028 4.853 (Z,E)-α-farnesene − − − 66 43 1:1 0.0680 0.0013~0.4992 0.028 4.853 Tetradecanoic acid − − − 1 108 1:15 0.0122 0.0000~0.0122 0.021 5.290 -
The release of a flower's aroma occurs with the unfolding of its petals. The formation of aromatic components is a dynamic process during the development of flowers from the initial flowering stage to the full blooming stage[20]. According to the analysis of volatile components of two peony cultivars at different flowering stages, it can be seen that the types of volatile compounds showed a trend of first increasing and then decreasing, that is, they peaked at the half-opening stage. However, the trends of the six volatile compounds were quite different between the two cultivars. The total content of volatile compounds in P. suffruticosa 'Chunguihuawu' reached its maximum at the initial flowering stage, and then decreased gradually, and vice versa for P. ostii 'Fengdan'. When tree peony flowers transition from the half-opening stage to the full blooming stage, their pollen sac will burst open, which can interfere with detection of volatile components in living plants. Hence, when studying the volatile components of different tree peony cultivars (or varieties) at the same flowering stage, the flowers at the half-opening stage are the best choice. This is because the types of volatile compounds are most abundant and their total content is relatively moderate during this pivotal stage.
Our results for the analyzed volatile components of P. suffruticosa 'Chunguihuawu' and P. ostii 'Fengdan' at different flowering stages showed that the types of n-alkanes were the most common and basically consistent, with the relative content of n-alkanes being the highest found, a result is in line with findings reported by Zhou et al.[21]. Another study found that the main components of plant leaf wax are n-alkanes[22]. Plant wax will not only appear on the leaves, but may also appear on the surface of other vegetative organs, such as flowers and fruits[23]. Therefore, we speculated that the flower surface of the two tree peony cultivars studied here may be covered with a wax layer. Moreover, pentadecane accounted for many peak areas in P. suffruticosa 'Chunguihuawu' and the content of heptadecane in the n-alkanes detected for P. ostii 'Fengdan' was the highest overall. Zhou et al.[24] indicated that n-alkanes had a high threshold value and thus their contribution to floral fragrance was limited. Whether n-alkanes constitute floral compounds is still an open question, but it is not in doubt that a high level of n-alkanes may lighten the fragrance of a flower. Further, P. suffruticosa 'Chunguihuawu' is a purplish red-flowered cultivar, and P. ostii 'Fengdan' is a white-flowered cultivar. The aromatic components and contents of different tree peony cultivars are directly related to flower color, which may partly explain the pronounced differences in floral fragrance and fragrance pattern between these two cultivars in this study. In addition, benzene,1,3,5-trimethoxy-, phenylethyl alcohol, benzene,1,4-dimethoxy-, and geraniol are the common characteristic aromatic components of tree peony flowers; the contents of these four compounds in either cultivar are high, which may be closely linked to the strong scent of tree peony when it blooms. Benzene,1,3,5-trimethoxy- has high medicinal value, phenylethyl alcohol can make derived food products such as tree peony tea or tree peony wine taste better[25], benzene,1,4-dimethoxy- can be used as a fragrance-fixing agent, and geraniol is often used as essence. Therefore, studying the aromatic components of different tree peony varieties provides a certain theoretical basis for improving the economic value of tree peony.
The inheritance of traits in hybrids is not entirely an additive process, and some volatile compounds can be overexpressed or underexpressed in hybrids[26]. The hybrids in this experiment basically displayed intermediate or transgressive inheritance for the trait of floral fragrance. Likewise, both propensities were also documented in Capsicum by Moreno et al.[14]. A portion of the progeny presented volatiles whose amount and content was in between the range of the corresponding parents, as expected, but another portion of the progeny surpassed either parent in certain volatiles. This suggests that hybridization may help to enhance the scent of a particular cultivar or developing hybrids with a more diverse spectrum of volatiles than that possessed by their parents. In addition, some progeny produced volatiles that did not appear in the parents, indicating that the two genomes may interact during hybridization, leading to the convergence of genetic factors that are silenced in both parents, thereby increasing the overall complexity and potential of the volatile profiles in progeny. This view is consistent with the findings of Moreno et al.[14] as well as Delort et al.[26].
-
Crosses of P. suffruticosa 'Chunguihuawu' × P. ostii 'Fengdan' could be used to obtain novel aroma types with more intense combinations of floral and fruity aromas. Hybrids of this population presented the following: (i) higher levels of α-ocimene than the parents; (ii) more fruity esters, including the combination of traces of alloocimene, phenylethyl acetate, and citronellyl acetate, which the parents did not present; and (iii) α-pinene (increased woody aromas), also lacking in the parents. Genetic analysis of the aromatic components demonstrated that the volatiles differed among hybrids, which might provide fundamental information for a better understanding of tree peony's genetics and its aroma improvement. Future crosses using ideal volatile-complementary parental material could be selected based on hybrids having the best scents.
This work was supported by the National Key R&D Program of China (under grant number 2018YFD1000406); the National Natural Science Foundation (under grant number U1804233); and the Central Plains Academics of Henan Province (under grant number 212101510003).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Niu T, Zhang C, Wang C, Xue X, Zhang Y, et al. 2022. Identification of floral fragrance components in an F1 population derived from Paeonia ostii 'Fengdan' × P. suffruticosa 'Chunguihuawu' Cross. Ornamental Plant Research 2:20 doi: 10.48130/OPR-2022-0020
Identification of floral fragrance components in an F1 population derived from Paeonia ostii 'Fengdan' × P. suffruticosa 'Chunguihuawu' Cross
- Received: 17 August 2022
- Accepted: 19 September 2022
- Published online: 29 November 2022
Abstract: Tree peony (Paeonia suffruticosa Andr.) is a unique aromatic plant famous for its huge flowers, bright colors and strong fragrance, having high ornamental, medicinal, and edible value. Research on tree peony's flower fragrance has mainly focused on the comparative analysis among its varieties, leaving the inheritance of aroma compounds in this plant an overlooked area of study. Here, the volatile components of flowers at three different flowering stages of the light fragrance-type cultivar P. ostii 'Fengdan' and the strong fragrance-type cultivar P. suffruticosa 'Chunguihuawu', as well as the half-opening stage flowers of 109 F1 progeny, were collected and characterized in-depth by dynamic headspace sampling technique combined with gas chromatography-mass spectrometry (GC-MS). Diverse profiles of volatiles that included alcohols, esters, aldehydes, terpenes, benzenes, and hydrocarbons were identified from the evaluated accessions. These results revealed that the volatile components and content of parents were significantly different, and that hybridization generated more complex volatile components. Most volatile compounds in the hybrids, especially the main aromatic components, existed in at least one of the parents, being characterized by intermediate or transgressive inheritance for the floral trait; this demonstrated that volatile compounds can be inherited from parents to progeny. Further, seven de novo aroma compounds—those present in progeny yet absent in their parents—were found in progeny. This study preliminarily clarified the segregation performance of aroma traits in tree peony hybrids, which might provide a theoretical basis for selecting breeding parents and the breeding of new varieties for aroma traits.
-
Key words:
- F1 Genetic Population /
- Floral Fragrance Components /
- Tree Peony












