-
Wetlands are critical terrestrial ecosystems that contain a huge global carbon (C) store (Gorham, 1991; Fourqurean et al., 2012; Mitsch et al., 2013) and may exert a significant impact on climate change (Zhang et al., 2002). Peat-accumulating wetlands currently hold approximately 390−455 Pg of terrestrial C, or nearly one-third of the world’s soil C stock (Jenkinson et al., 1991). C capture and sequestration within wetlands is a complicated process as it involves both aerobic and anaerobic processes (Kayranli et al., 2010). Additionally, a variety of substrates could potentially be used to capture and sequester CO2, such as microorganism, algae (Sayre, 2010). In contrast, wetlands also have a major part to play in C cycling, with high GHG emission, estimated at 0.65 Pg C per year in the form of CH4 (Bastviken et al., 2011), totalling up to 2.1 Pg C per year as CO2 (Aufdenkampe et al., 2011). Therefore, maintaining wetlands as a large carbon sequestration site is an essential way to balance the whole terrestrial ecosystem.
Wetland reclamation under human factors would generate many additional anthropogenic GHG emissions and removals, of which aquaculture could be a typical artificially managed freshwater wetland system (Pendleton et al., 2012; Tan et al., 2020; Yang et al., 2022b). Yang et al. (2022a) reported a large increase in CH4 emission following conversion of coastal marsh to aquaculture ponds. Zhang et al. (2022) reported that aquaculture-emissions in China offset about 7% of terrestrial carbon burial, and revealed that the growth and intensification of the aquaculture sector raised climate concerns. Seitzinger (2000) showed that one third of the global anthropogenic N2O emissions were from aquatic ecosystems, and Hu et al. (2012) reported that the global N2O emission from aquaculture would be up to 383 Gg comprising 5.72% of anthropogenic N2O emission by 2030 from 93 Gg in 2009 if the aquaculture industry kept its current annual growth rate of roughly 7.1%. Wetlands Supplement (IPCC, 2013) for aquaculture emission adopted the literature from Hu et al. (2012 & 2013), and mentioned the need for in-situ experiments on the measurement of GHGs emissions from aquaculture systems primarily for developing emission factors of different wetland systems (Hu et al., 2013). Furthermore, some of the emission factors of Wetlands Supplement Guidelines were based on changes in soil C stocks or lab experiments, rather than on the limited field measurements available in the literature (Zheng et al., 2014). Given the limited available data to estimate GHGs emissions from constructed wetland system (IPCC, 2013), more scientific data from in-situ GHGs emissions measurement could support aquatic wetland emission estimation and management.
In addition, the reclamation of paddy fields for aquaculture was also an important mode of aquatic wetland system in China, which caused more GHGs emissions. Liu et al. (2016) demonstrated that CH4 and N2O emissions reduced following conversion of rice paddies to inland crab-fish aquaculture. Particularly, N2O and CH4 emission from aquaculture had become violent and vital because a vast input of feeding forage enriched substrates for biological activities in water and sediment (Hu et al., 2013); and Fang et al. (2022) confirmed that ebullition dominated the pathways of CH4 emissions from inland aquaculture, which could be mainly influenced by water temperature and dissolved oxygen (Yang et al., 2023). Hence, the impact of natural and constructed wetlands for intensive aquatic culture on GHGs emissions should vary greatly due to differences in environment factors (water and sediment quality) and humanistic management practices (feed types, stocking density). Given this, the main purposes of this research were to (i) quantify the diurnal CHGs (including CO2, CH4 and N2O) fluxes and seasonal variations in different types of managed wetlands; (ii) ascertain the effect of human-based management and environmental factors on CHGs emissions; and (iii) determine the influence of wetland utilization change on CHGs emissions.
-
There was an abundance of aquaculture resources in Anhui province in China, with total water resources of 68 billion m3, 580,000 ha of inland water aquaculture, and an annual output of 2.3 Tg of aquatic products. Chizhou city was known for its aquatic products, with an annual output of 111 Gg, accounting for 11% of water resource in Anhui province. There were various types of aquaculture land with lakes accounting for 63% of the aquaculture area in inland waters, ponds for 20%, and paddy fields for 15%. Thus, Chizhou city (30°41' N, 117°34' E) was selected for this case study to measure GHGs emissions from different managed wetlands with a static floating gas-sampling chamber. The study area is close to the Yangtze River, and has a subtropical monsoon climate. Chizhou city has an annual mean temperature of 16.5 °C, an annual mean precipitation of 1,800 mm, a flat topography, dense network of rivers, and a suitable climate.
Experiment design
-
Four wetlands, including natural wetland (NW), enclosure wetland for intensive aquaculture (EWIA), constructed wetland for intensive aquaculture (CWIA) and constructed wetland for extensive aquaculture (CWEA), were selected for this study and they differ in terms of production and management. Of the four wetlands, one was natural wetland, while the other three were constructed. The information of the four wetlands are shown in Table 1.
Table 1. Basic information of the four experimental sites.
Sites Origin Area Water table Location Feed Forage content Yield (hm2) m Longitude Latitude Total C
(g kg−1)Total N
(g kg−1)C/N* t ha−1 year−1 NW A natural lake 1,000 3−9 30.67° 117.52° NA NA NA NA / EWIA Wetland shifted to aquatic production 53 2−4 30.64° 117.46° Fed on forage from March to October 127.12 ± 7.41 77.85 ± 2.03 1.6:1 11.32 CWEA Paddy converted constructed wetland with 2 1 30.73° 117.58° Fed on wheat grain or grass or fertilizer irregularly 5.00 CWIA Paddy converted constructed wetlands with intensive aquaculture 2 1−2 30.66° 117.50° Fed on forage from April to October 151.31 ± 2.80 72.39 ± 1.29 2.1:1 7.50 * C/N represented the carbon-nitrogen ratio. GHGs sampling and analysis
-
The static floating gas-sampling chamber was used for the experiments at four sites (Gao et al., 2014). The chambers were made of PVC and covered by a layer of foam and aluminum foil in order to insulating and reducing heat transmission and reflect light. Every chamber (30 cm inner diameter ×18 cm net height above the surface of the water after being dipped 5 cm into the water) had two holes on top, one for a sampling pipe with a three-way airtight valve and the other for a thermometer to monitor the temperature inside the chamber. All connections were keeping "air tight".
Diurnal dynamics monitoring
-
For monitoring the diurnal dynamics of GHGs under different wetland management, the campaigns were conducted for 3 d at each site during the months of September to December 2014. Each site contained three replicate points, each of which was a tripod constructed in advance from three bamboo sticks. On each sampling day, five gas samples were taken every 2 h from 8:00 AM to 4:00 PM. For each chamber run, gas samples were collected at 0, 10, 20 and 30 min following the chamber installation (with a wire attached to a tripod to hang over the water). A syringe was used to extract a 40 mL gas sample, which was then injected into a vacuum gas sampling battle (27 mL). At the end of each gas sample collection, the three-way airtight valve should be closed and the water, air and inside-chamber temperatures were noted. After four gas samples, floating chambers were raised and transported to the following sampling location. After collection, all samples were delivered to the lab to be measured over the course of two days.
Seasonal dynamics monitoring
-
The seasonal dynamic monitoring lasted 13 months, from June 2015 to June 2016 (Fig. 1) showed the temperature and precipitation during the experiments. The four sites of CWIA, CWEA, EWIA and NW had five, five, six and six replicate sampling points, respectively. On each sampling day, gas samples were taken between 9:00 to 12:00 AM. The same procedure as outlined above was applied to each chamber run. The seasonal monitoring frequency was once a month. Nevertheless, due to freezing temperatures, no data on GHG fluxes were available in January 2016.
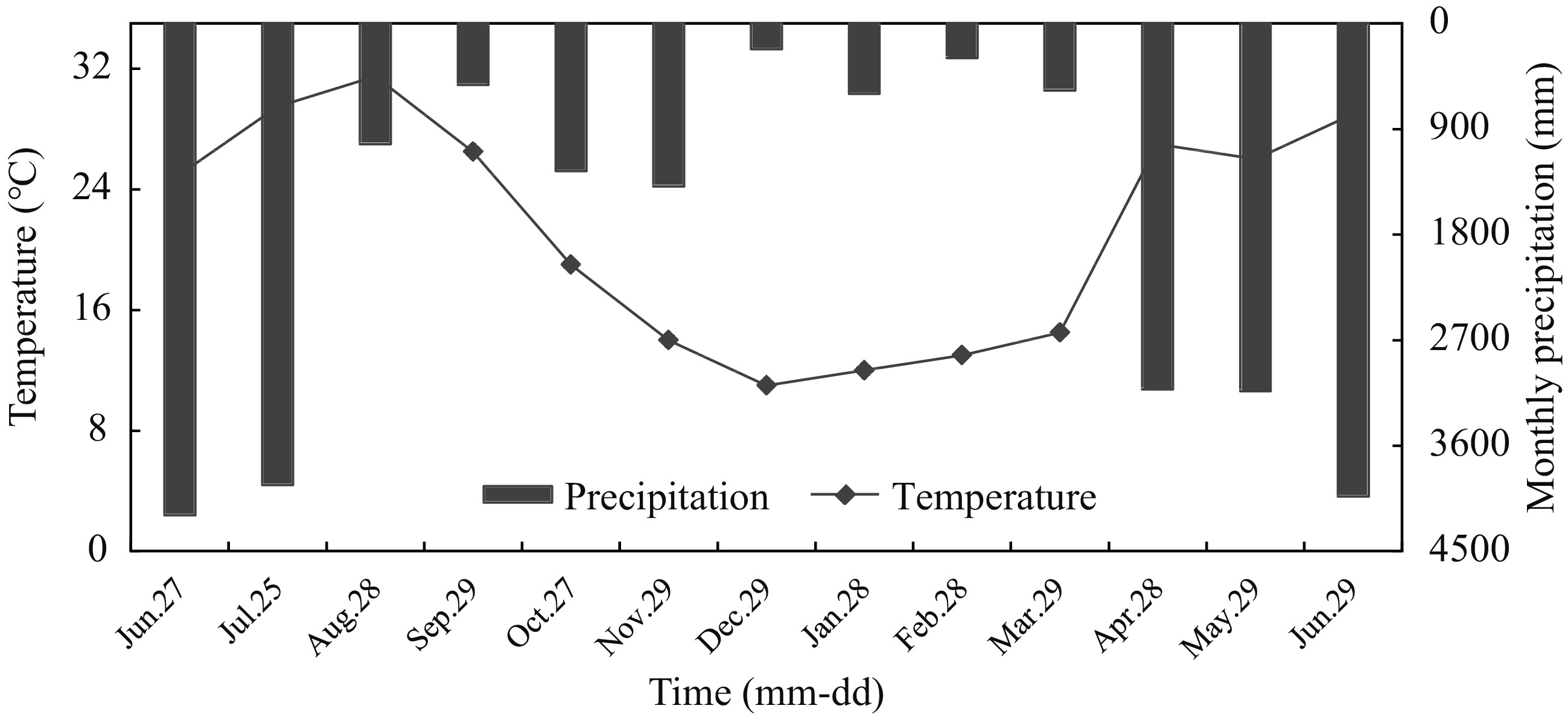
Figure 1.
Temporal variation of environmental temperature and precipitation at the experimental sites.
Gases flux calculation
-
The gas samples were analyzed by a gas chromatograph (Agilent 7890A GC, USA), and its measurement accuracy (reproducibility), which was the average relative standard deviation, was less than 1%. The detector used for CH4 and CO2 analysis was the FID with detection sensitivity of 0.5 and 1 ppm for CO2 and CH4, and detection limit ≤ 5.1 × 10−12 g CO2 s−1 and 1.9 × 10−12 g CH4 s−1, the column temperature was 80 °C, and the detector temperature was 200 °C; N2 as the carrier gas with the flow rate of 40 mL min−1; H2 as the fuel with the flow rate of 35 mL min−1; air as auxiliary fuel with the flow rate of 350 mL min−1. The detector for N2O was the ECD with detection sensitivity of 0.5 ppm for N2O and detection limit ≤ 4.4 × 10−15 g mL−1, the column temperature was 65 °C, and the detector temperature was 320 °C, argon methane gas as the carrier gas with a flow rate of 30 mL min−1. Gases flux rates were calculated (Healy et al., 1996; Altor & Mitsch, 2006) according to the following equation:
$ {F}_{G}=kV/A\times (dC/dT)\times \rho \times \left[\frac{{T}_{0}}{\left({T}_{0}+{T}_{C}\right)}\right]\times \frac{P}{{P}_{0}}\times 60 $ (1) Where,
$ {F}_{G} $ $ V $ $ A $ $ dC/dT $ $ \rho $ $ {T}_{C} $ $ {T}_{0} $ $ {P}_{0} $ $ k $ In general, the flux rate by different time intervals of 4 gas sample concentration by linear regression analysis (Zou et al., 2005). The daily and seasonal cumulative GHGs emissions could be obtained by average value and observation interval times.
Physiochemical analysis of sediment and water properties
-
At the beginning of the season's monitoring in June 2015, sediment samples at 0−15 cm depth were collected for four points by soil sample collectors and kept in plastic bags. From June 2015 to February 2016, water samples under the water's surface at a depth of 30 cm were taken monthly during the seasonal monitoring, and stored in 250 mL plastic bottles. Accordingly, no samples were collected in January 2016 due to the freezing temperatures. All the samples were stored in an ice-filled cooler box, and were delivered to the lab being stored in a 4 °C refrigerator, then indicator analysis was completed within 2 d.
In the laboratory, the soil samples were air-dried, and finely ground to pass a 2 mm sieve prior to analysis. A portion of soil was ground to pass a 0.15 mm sieve for physical and chemical analysis of pH, soil organic matter (SOM), ammonium-N (
${\text{NH}^+_4} $ ${\text{NO}^-_3} $ ${\text{NH}^+_4} $ ${\text{NO}^-_3} $ ${\text{NO}^-_2} $ Data analysis
-
Data processing was performed using Microsoft Office Excel 2013 and Origin 2016, and all statistical analyses were conducted using one-way ANOVA of SPSS and the least significant difference test were used to check the differences among the GHGs emissions of different aquaculture wetlands types.
-
The diurnal variations of GHGs fluxes for four different aquaculture wetlands varied greatly at both time and spatial scales, of which were measured for three days with different measurement dates and temperature. As shown in Fig. 2, there was no consistency in the daily variation dynamics of N2O-N, CH4-C and CO2-C emission fluxes among the four sites. For the three human-managed wetland systems of CWIA, CWEA and EWIA, the GWP values were mainly determined by the CH4 fluxes based on the similar trends. Particularly when the temperature dropped below 10 °C, the CH4 emissions and GWP values at the CWIA site significantly decreased (Fig. 2a). However, this was not the case at the NW site, where the GHG fluxes remained relatively stable despite the temperature dropping below 10 °C, and the GWP values largely following the same dynamics as CO2-C fluxes (Fig. 2d).
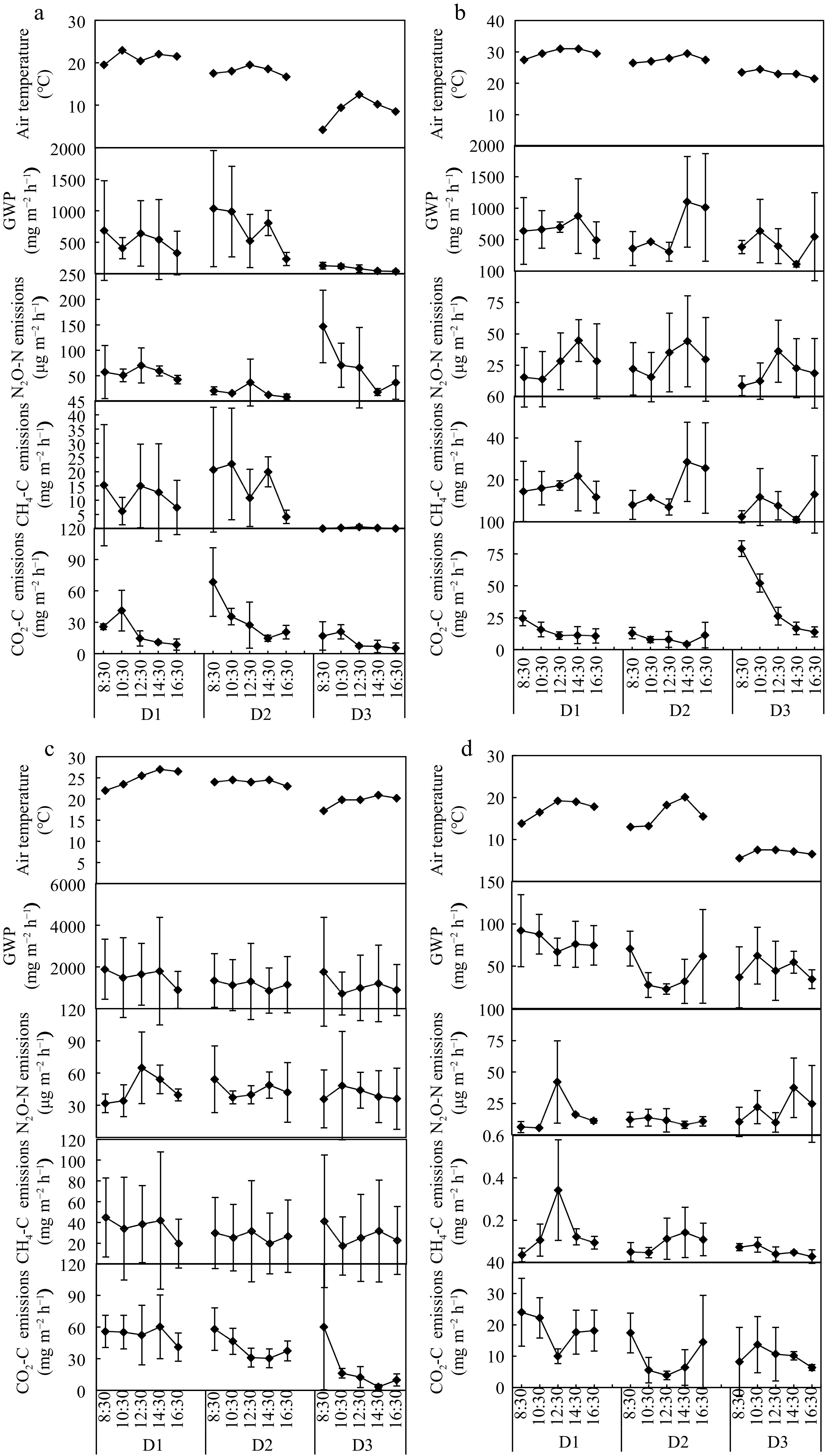
Figure 2.
Diurnal variation of CO2, CH4 and N2O fluxes for (a) CWIA, (b) CWEA, (c) EWIA, (d) NW. D1, D2 and D3 for CWIA were October 16, November 3 and December 9 with daily mean temperature ranging from 21.3 to 9.0 °C; D1, D2 and D3 for CWEA were September 4, September 7, and September 22 with temperatures ranging from 29.7 to 23.1 °C; D1, D2 and D3 for EWIA were September 21, September 22, and October 14 with temperatures ranging from 24.9 to 19.6 °C; D1, D2 and D3 for NW were November 4, November 20, and December 11 with temperatures ranging from 17.6 to 8.4 °C. GWP, the global warming potential.
Seasonal variation of GHGs fluxes
-
As shown in Fig. 3, the emissions of N2O-N, CH4-C and CO2-C exhibited pronounced seasonal variations, with the highest emission rates occurring in the summer months. Firstly, the N2O-N fluxes ranged from 1.90 to 41.74 μg m−2 h−1, 3.03 to 20.59 μg m−2 h−1, 4.16 to 51.33 μg m−2 h−1 and 3.25 to 24.82 μg m−2 h−1 for CWIA, CWEA, EWIA and NW, respectively (Fig. 3a). Secondly, the CH4-C fluxes from EWIA and CWIA were higher compared to CWEA and NW during the periods of June to September 2016 and May to June 2017. However, all sites showed low emission values ranging from 0.03 to 3.76 mg m−2 h−1 during October 2016 to March 2017 (Fig. 3b). The CO2-C fluxes varied across the sites, with values ranging from −9.71 to 67.24 mg m−2 h−1, −8.30 to 35.20 mg m−2 h−1, 4.14 to 55.08 mg m−2 h−1 and −7.05 to 29.40 mg m−2 h−1 for CWIA, CWEA, EWIA and NW, respectively (Fig. 3c). In summary, the GHGs fluxes were consistently positive, except for the negatives values observed in April 2017, indicating that the four aquaculture systems were net emitters of GHGs.
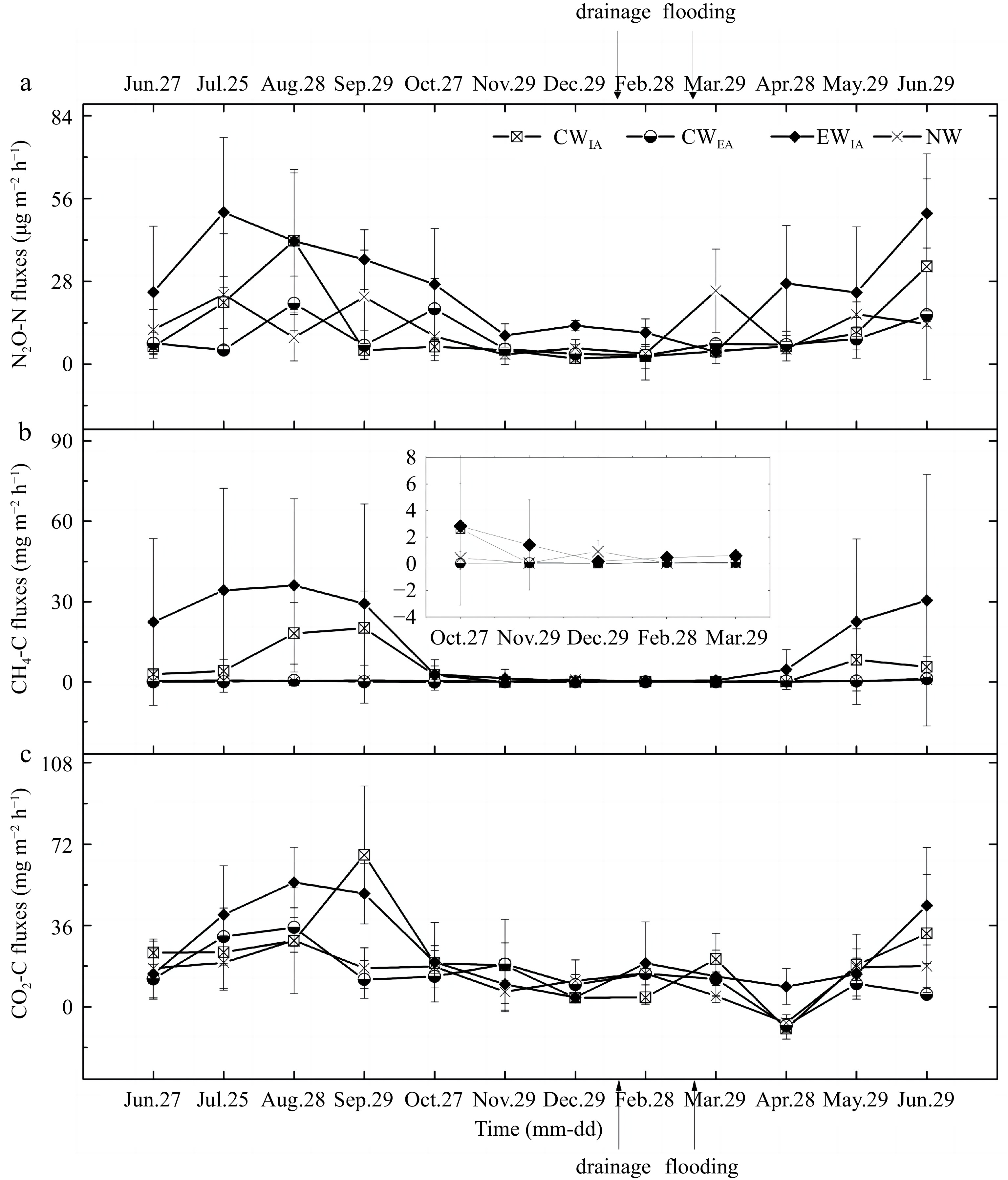
Figure 3.
Seasonal GHG emission dynamics of (a) N2O, (b) CH4 and (c) CO2 fluxes for different wetlands. The management practices of drainage and flooding occurred only to the sites of CWIA, CWEA and EWIA.
Regarding cumulative GHG emissions, EWIA had the highest cumulative N2O, CH4 and CO2 emissions with values of 2.43 kg N2O-N ha−1, 1,360.27 kg CH4-C ha−1, and 2,246.79 kg CH4-C ha−1 (Table 2). Additionally, the GHGs emission intensities per unit product at the yield-scaled ranged from 1.21 to 5.30 kg CO2-eq kg−1, and the highest N2O and CH4 emission factors of aquaculture production were both from EWIA, with the values of 0.34 g N2O kg−1 and 0.19 g CH4 kg−1, respectively (Table 2). In Table 3, the GWP of EWIA, with a value of 60.02 t CO2-eq ha−1 (the highest), was about 10 times higher than that of NW being 6.04 t CO2-eq ha−1. For intensive aquaculture wetlands, the biggest GWP contributions came from CH4 emission, with proportions of 85% and 70% for EWIA and CWIA, respectively. In contrast, for NW and CWEA, respectively, CO2 emissions made up the greatest quantities, at 72% and 80%.
Table 2. Cumulative CO2, CH4 and N2O fluxes and yield-scaled GHG emission intensity for different wetland systems.
Sites Cumulative emission GHG emission intensity per kg product N2O-N (kg ha−1) CH4-C (kg ha−1) CO2-C (kg ha−1) N2O (g kg−1) CH4 (kg kg−1) Total (kg CO2-eq kg−1) CWIA 1.06 ± 0.20 457.08 ± 161.20 1,877.04 ± 527.95 0.22 0.10 3.25 CWEA 0.81 ± 0.12 23.83 ± 7.80 1321.32 ± 91.11 0.25 0.01 1.21 EWIA 2.43 ± 0.20 1,360.27 ± 1552.98 2,246.79 ± 488.23 0.34 0.19 5.30 NW 1.12 ± 0.42 38.29 ± 11.99 1,305.48 ± 216.63 / / / /: represented no data. The values were presented as the mean ± standard deviation. Table 3. Global warming potentials and their contributions for different wetland systems.
Sites GWP Contributions (%) t CO2-eq ha−1 y−1 N2O CH4 CO2 CWIA 24.38 2% 70% 28% CWEA 6.04 6% 14% 80% EWIA 60.02 2% 85% 14% NW 6.67 7% 21% 72% Environmental properties
-
Table 4 shows the analysis of environmental indicators at four different sites. For water quality, the pH values were slightly alkaline with an average of 7.48, ranging from 7.39 to 7.57. The concentrations of
${\text{NH}^+_4}{\text {-N}} $ ${\text{NO}^-_3} $ Table 4. Water and sediment qualities at the four experimental sites.
Sites pH $ {\text{NH}^+_4}{\text {-N}}$ (mg L−1) ${\text{NO}^-_3}{\text {-N}} $ (mg L−1) ${\text{NO}^-_2}{\text {-N}} $ (mg L−1) TN (g L−1) DOC (mg L−1) Water quality CWIA 7.52 ± 0.14a 0.60 ± 0.35a 0.57 ± 0.26b 0.48 ± 0.51a 1.93 ± 0.69ab 24.99 ± 8.50ab CWEA 7.39 ± 0.48a 0.46 ± 0.33a 0.36 ± 0.12b 0.10 ± 0.16ab 1.30 ± 0.52bc 21.83 ± 7.67ab EWIA 7.50 ± 0.28a 0.66 ± 0.61a 1.36 ± 0.56a 0.36 ± 0.49ab 2.07 ± 0.88a 29.93 ± 8.92a NW 7.57 ± 0.29a 0.25 ± 0.20a 0.28 ± 0.08b 0.03 ± 0.03b 0.66 ± 0.23c 20.01 ± 7.71b Sites pH $ {\text{NH}^+_4}{\text {-N}}\;{\rm{ (mg\; kg^{−1})}} $ $ {\text{NO}^-_3}{\text {-N}}\;{\rm{ (mg\; kg^{−1})}} $ SOM (g kg−1) TN (g kg−1) C/N Sediment quality CWIA 6.81 ± 0.13a 36.67 ± 4.32b 273.70 ± 185.08a 39.69 ± 2.75ab 2.95 ± 0.18ab 7.81 ± 0.51ab CWEA 6.22 ± 0.18b 33.21 ± 6.39b 54.57 ± 20.31a 39.66 ± 4.16ab 2.68 ± 0.19b 8.58 ± 0.42a EWIA 6.59 ± 0.29a 203.22 ± 16.08a 251.03 ± 263.67a 44.00 ± 1.15a 3.34 ± 0.06a 7.46 ± 0.19b NW 6.58 ± 0.14a 27.86 ± 5.07b 21.23 ± 5.10a 35.78 ± 4.09b 2.91 ± 0.47ab 7.00 ± 0.73b TN, DOC, SOM and C/N represented total nitrogen, dissolved organic carbon, soil organic matter and carbon : nitrogen ratio, respectively. The sediment properties at all sites were weakly acidic, with pH values ranging from 6.0 to 6.9. Significantly, the pH value of CWEA was lower than that of the other three sites. The highest TN content was found at EWIA with a value of 3.34 g kg−1. The SOM contents showed a significant difference between NW and EWIA. The
${\text{NH}^+_4}{\text {-N}} $ ${\text{NO}^-_3}{\text {-N}} $ Correlation analysis between environmental and management factors and GHGs emissions
-
As shown in Table 5, there were strong positive correlations between
${\text{NH}^+_4}{\text {-N}} $ ${\text{NO}^-_3}{\text {-N}} $ Table 5. Correlation coefficients between GHGs emission and water parameters in all four wetlands.
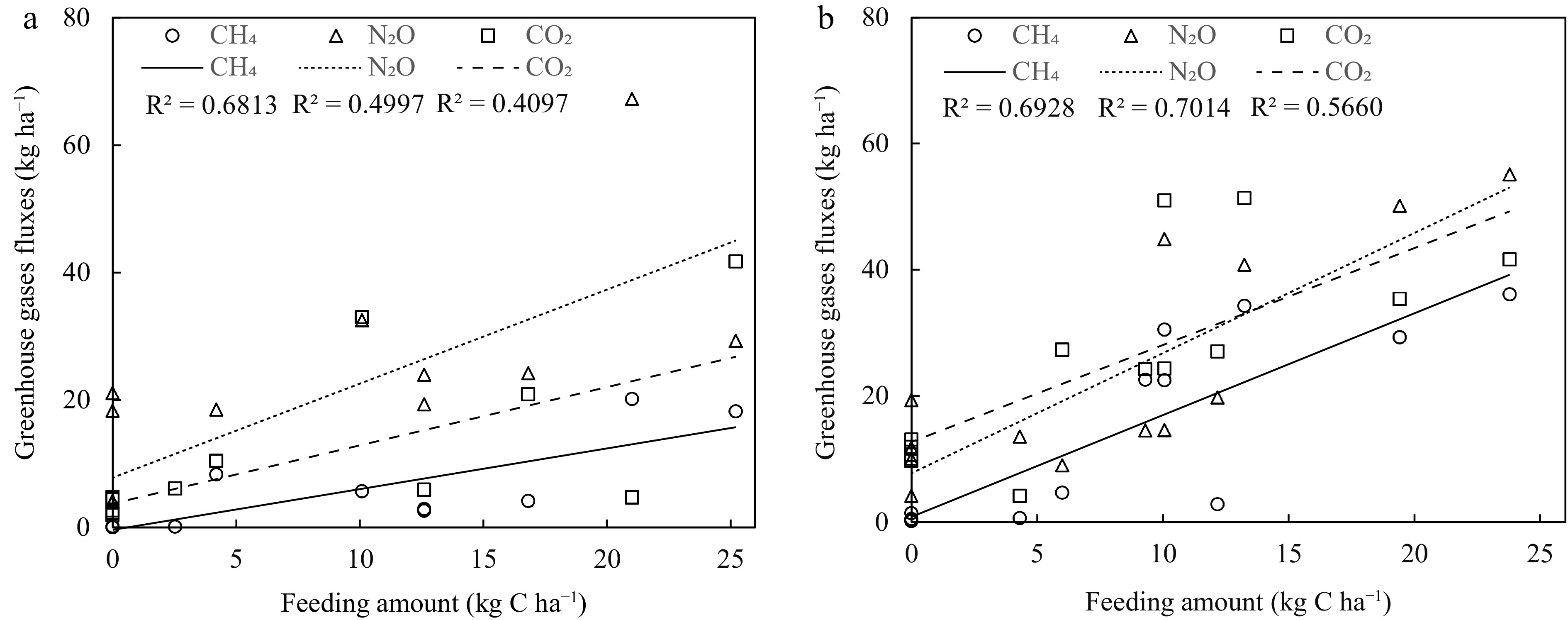
Items pH DOC ${\text{NH}^+_4}{\text {-N}} $ ${\text{NO}^-_3}{\text {-N}} $ ${\text{NO}^-_2}{\text {-N}} $ TN Water temperature Correlation CH4 0.11 0.09 0.60** 0.80** −0.17 0.13 0.46* N2O 0.31 0.20 0.37* 0.60** −0.22 0.10 0.55** CO2 0.28 −0.11 0.70** 0.54** −0.23 0.11 0.65** P value CH4 0.574 0.633 0.002 0.000 0.399 0.517 0.014 N2O 0.109 0.299 0.049 0.001 0.249 0.605 0.003 CO2 0.144 0.576 0.000 0.003 0.246 0.575 0.000 * Significant at P < 0.05; ** Significant at P < 0.01. Furthermore, upon exploring forage-fed impact of CWIA and EWIA (Fig. 4), it was found that there were positive correlations between forage feeding and the CH4 fluxes (R2 = 0.68), N2O fluxes (R2 = 0.50) and CO2 fluxes (R2 = 0.41) at the EWIA site; and there were positive correlations between forage feeding and the CH4 fluxes (R2 = 0.69), N2O fluxes (R2 = 0.70) and CO2 fluxes (R2 = 0.57) at the CWIA site.
-
This study tracked GHGs emissions for four different aquatic-wetland systems, partially reflecting the variations in how human management practices affect wetland GHG emissions. In the current study, the average CO2, CH4 and N2O emissions from nature wetland (NW) were 51.09 mg m−2 h−1, 0.55 mg m−2 h−1and 19.50 μg m−2 h−1, respectively, which were similar to those values ranging from 15.40 to 1082.00 mg CO2 m−2 h−1, 0.04 to 175.94 mg CH4 m−2 h−1 and 13.3 to 747 μg N2O m−2 h−1 shown in Table 6. The high fluxes of CH4 and CO2 at the canal were mostly caused by domestic waste pollution (Han et al., 2013). The N2O fluxes from aquatic-wetlands converted by agricultural land in the study were 14.08 μg m−2 h−1 (CWEA) and 18.65 μg m−2 h−1 (CWIA), which were higher than the values of 10.74 and 11.8 μg m−2 h−1 for the aquatic wetlands systems of shrimp and mixed shrimp-fish pond (Yang et al., 2015), but lower than that of the crab-fish aquatic system with a value of 48.1 μg m−2 h−1 (Liu et al., 2016). The CH4 fluxes of 0.35 mg m−2 h−1 at the CWEA site was the lowest among these inland aquaculture systems possibly due to the absence of amount forage feed.
Table 6. Comparison of greenhouse gases fluxes from different wetland types with previous studies in China and other regions.
Location Sources Time horizon Wetland types N2O fluxes CH4 fluxes CO2 fluxes μg m−2 h−1 mg m−2 h−1 mg m−2 h−1 China East China Current study 13 months Constructed wetland for intensive aquaculture 18.65 ± 20.48 6.92 ± 9.41 77.23 ± 68.95 Constructed wetland for extensive aquaculture 14.08 ± 9.58 0.35 ± 0.45 51.21 ± 40.97 Enclosure wetland for intensive aquaculture 41.89 ± 24.83 20.60 ± 19.90 90.34 ± 65.45 Natural wetland 19.50 ± 12.48 0.55 ± 0.41 51.09 ± 33.48 East China Liu et al. (2016) 12 months Rice paddies 110.5 0.71 Crab-fish aquaculture 48.1 0.37 Southeastern China Yang et al. (2015) 5 months Shrimp pond 10.74 19.95 20.78 4 months Shrimp-fish pond 11.8 1.65 −60.46 Lab simulation of aquatic system Hu et al. (2014) 2 months Control 5,504 ± 1,221 2,401 ± 343 Treatment 911 ± 488 4,590 ± 546 Overseas
of Nature wetlandsYangtze estuarine, China Wang et al. (2009) 12 months Marsh site 2.06 Bare tidal flat 0.04 East China Han et al. (2013) 3 months Open river 62.17 ± 2.13 16.41 ± 3.06 162.18 ± 10.55 Canal 111.74 ± 7.41 175.94 ± 18.42 1,082.00 ± 90.53 Reservoir 50.99 ± 2.68 1.70 ± 0.14 25.61 ± 4.08 Northeast China Yang et al. (2013) 3 months Freshwater marsh 13.3 8.92 394.5 India Selvam et al. (2014) 24 h/1−8 h Ponds 11.93 ± 12.33 123.02 ± 117.33 Rivers 4.13 ± 8.27 36.85 ± 70.58 Reservoirs 2.13 ± 2.33 15.40 ± 35.75 Colombia Dennis et al. (2014) 11 months Restored mangrove 162 ± 48 3.47 ± 1.52 97 ± 46 11.78 ± 10.54 747 ± 299 6.12 ± 9.69 Costa Rica Nahlik et al. (2011) 29 months Forested marsh 5.05 Rainforest swamp 33.39 Alluvial marsh 39.94 Australia Allen et al. (2011) 6 months Mangrove 30.00 0.30 Orinoco River Floodplain, Venezuela Smith et al. (2000) 17 months Open river 1.33 Flooded forest 4.67 Macrophyte mats 1.33 The estimates of global wetland emissions were still subject to severe data limitations and distributional biases, especially for the emission under human intervention (Selvam et al., 2014). The 2006 IPCC Guidelines, which was limited to peatlands that had been drained and managed for peat extraction, provided methods for estimating national anthropogenic emissions (IPCC, 2006). The 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories was published to reduce uncertainties of wetland emissions estimates. It included information on inland organic soils, wetlands on mineral soils, coastal wetlands, as well as constructed wetlands for wastewater treatment (IPCC, 2013). However, the evaluation methods for artificially managed aquatic wetlands still needed to be improved. Hence, based on more reliable observational data, scientists can simulate GHGs emissions through modeling and analyze human impacts on the GHG balance of wetland ecosystems (Petrescu et al., 2015).
Effect of human management and environmental factors on GHG emissions
-
For aquatic wetland system, human factors such as C and N inputs, water level control, fish type and fish intensity could affect the intensity of GHGs emission (Yang et al., 2013; Zheng et al., 2014; Yang et al., 2023). Forage feeding rate directly affected ammonia concentrations in the aqueous phase, which then affected the fate or release rate of CH4 and N2O from the sediment-water interface to the atmosphere (Liu et al., 2016). Beaulieu et al. (2011) reported that approximately 1.00% of N input may be transformed to N2O for rivers and estuaries, and Seitzinger & Kroeze (1998) discovered that roughly 0.75 % of N for streams was released to the atmosphere as N2O. Hu et al. (2013) also revealed that about 1.3% of the nitrogen input was emitted as N2O gas in laboratory-scaled aquaculture system. While this study found that approximately 0.2%−0.3% of N input became N2O emissions. In addition, changes in water levels (such as drainage or irrigation) were common management practices in aquaculture, which could significantly affect CH4 emissions (Friborg et al., 2000; Jackowicz‐Korczyński et al., 2010).
Environmental factors were closely related to the input of C and N, directly affecting GHGs emissions. Numerous research had demonstrated that the CH4 and N2O fluxes were associated with environmental factors, such as air and soil temperature, dissolved oxygen and DOC in water, and the availability of C and N substrate in sediment (Bhattacharyya et al., 2013; Hu et al., 2016). According to this study, both the
${\text{NH}^+_4}{\text {-N}} $ ${\text{NO}^-_3}{\text {-N}} $ Among various forms of aquatic management, GHGs emission factors on the scale of area and yield varied significantly. This study found a positive correlation between cumulative GHG emissions intensity and yield in aquatic wetlands. Generally speaking, the production of CWEA, CWIA and EWIA increased sequentially, reaching 5.00, 7.50 and 11.32 t ha−1, respectively. The corresponding annual cumulative emissions also increased sequentially, reaching 0.81, 1.06 and 2.43 kg N2O-N ha−1, 23.83, 1,877.04 and 2,246.79 kg CH4-C ha−1, 1,321.32, 1,877.04 and 2,246.79 CO2-C kg ha−1. Petrescu et al. (2015) reported that management intensity strongly influenced the net climate footprint of wetlands. The highest N2O emission factor of aquaculture production from the intensive aquaculture type of EWIA was 0.34 g kg−1, which was still much lower than the values of 2.65 and 2.57 g kg−1 reported by Hu et al. (2012) and Liu et al. (2016) using lab simulation experiments. In terms of GWP, aquatic system with high forage feeding intensity had significantly higher annual GWP compared to the wetland systems with no or low forage feeding intensity. After the intensive aquaculture management of wetlands, the main GHG emissions of the system shifted from CO2 to CH4, which greatly increased the GWP. For instance, the largest contributions to GWP for the intensive aquaculture of EWIA and CWIA was CH4 emission, while for natural aquatic-wetland and exclusive aquaculture, the main contributor turned out to be CO2 emission (Table 3). Therefore, scientific management of aquaculture density, forage feeding intensity, and water level in aquatic wetland systems would play a crucial role for the trade-off between CH4 and CO2 emission, as well as GHG mitigation (Petrescu et al., 2015).
Uncertainties analysis
-
The GHGs emission fluxes found in this investigation were usually characterized by high standard deviations (Figs 2 and 3), which were in agreement with the results from Nahlik et al. (2011) and Dennis et al. (2014). The large variances indicated significant differences in emissions between parallel points at the same site, which might be caused by changes in water level, temperature, ebullition, and environmental disturbance (Liu et al., 2016; Petrescu et al., 2015; Fang et al., 2022; Yang et al., 2022b). For example, the sediment and water quality in the areas near the feeding machines were complicated, and the breeding density in this region was frequently high, both of which greatly affected the GHGs emission intensity (Chen et al., 2013; Hou et al., 2013).
Besides, the sampling points were uniformly set at different locations of each site, and the process of sailing for sampling could also cause some interference to the water body, like creating some surface waves, thereby affecting the detection results and causing uncertainties to the results (Gao et al., 2014). More importantly, the bamboo poles used for fixation were more or less influenced by ships, causing disturbance to the sediment and generating bubbles, further affecting the fate or release rate of GHGs through the sediment-water interface system (Hirota et al., 2004; Bastviken et al., 2008). Therefore, future work should improve the equipment technology for monitoring GHGs emission in wetlands. Finally, diurnal dynamic measurements were carried out at the four sites for eight hours due to the safety issues, night time monitoring was excluded. This may consequently lead to the overestimation of the GHGs fluxes because of lower night time temperatures. So, it is necessary to monitor the emission characteristics throughout the whole day as much as possible.
-
For the four aquatic-wetlands, the extensive aquaculture system from reclaimed paddy land (CWEA) had the lowest GHG emission intensity with 0.81 kg N2O-N ha−1, 23.83 kg CH4-C ha−1 and 1,321.32 kg CO2-C ha−1, while the intensive aquaculture system from wetland enclosure (EWIA) had the highest cumulative GHG emission with the values of 2.43 kg N2O-N ha−1, 1360.27 kg CH4-C ha−1, 2,246.79 kg CO2-C ha−1, respectively. And the GHG emission intensity per unit of production ranged from 1.21 (CWEA)−5.30 (EWIA) kg CO2-eq kg−1. The current study supported a valuable reference for N2O and CH4 emission factors in constructed wetlands and demonstrated that the intensive aquaculture ultimately led to the high area-scaled and yield-scaled GHGs emission intensity. These measurement data served as a crucial support for the follow-up model simulation and GHGs emission prediction on a large scale.
This work was financially supported by Natural Science Foundation of China under a grant number 41907073 and 41877546.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Yue Q, Cheng K, Sheng J, Wang L, Ji C, et al. 2023. Greenhouse gas emissions from managed freshwater wetlands under intensified aquaculture. Soil Science and Environment 2:3 doi: 10.48130/SSE-2023-0003
Greenhouse gas emissions from managed freshwater wetlands under intensified aquaculture
- Received: 15 January 2023
- Accepted: 19 April 2023
- Published online: 11 May 2023
Abstract: Wetlands are important niches for aquatic organisms and biodiversity as well as for organic carbon sequestration. However, wetlands are frequently managed for aquaculture production, which jeopardized their ability to act as a natural carbon sink. In this experiment, greenhouse gases (GHG) including CO2, CH4 and N2O fluxes at the water-air interface, were monitored on a daily and monthly basis in four aquaculture wetlands: a natural wetland (NW), an enclosure wetland for intensive aquaculture (EWIA), a constructed wetland made from reclaimed paddy land for intensive aquaculture (CWIA), and a constructed wetland made from reclaimed paddy land for extensive aquaculture (CWEA). As a result, annual GHG fluxes for CWEA, CWIA, EWIA and NW wetlands averaged 0.81, 1.06, 2.43 and 1.12 kg N2O-N ha−1, 23.83, 457.08, 1,360.27 and 38.29 kg CH4-C ha−1, 1,321.32, 1,877.04, 2,246.79 and 1,305.48 kg CO2-C ha−1, respectively. Also, the highest yearly global warming potential (GWP) for EWIA, which was 60.02 t CO2-eq ha−1, was roughly ten times greater than that of NW, which was 6.04 t CO2-eq ha−1. Furthermore, the GHG emission intensity per unit of production ranged from 1.21 (CWEA) −5.30 (EWIA) kg CO2-eq kg−1, with EWIA having the highest N2O and CH4 emission factors per unit of aquaculture output at 0.34 g N2O kg−1 and 0.19 kg CH4 kg−1. The study also revealed that feed quantity and water parameters positively linked with GHG emissions, suggesting that improving human management and water environmental quality could achieve large mitigation for both natural and artificial aquaculture wetlands. Given the high heterogeneity of GHGs emissions in aquaculture wetlands, future research should conduct long-term continuous automatic monitoring of aquaculture wetlands to understand the patterns of GHGs emissions and their responses and mechanisms to human management.
-
Key words:
- Wetlands /
- Aquaculture /
- Human management /
- Greenhouse gases