-
Plastics are being increasingly recognized as a massive environmental pollutant[1]. Plastic debris is widely found in air, ocean, soil, and surface water[2]. Plastic debris can be categorized as macroplastics (> 25 mm), mesoplastics (5−25 mm), large microplastics (1−5 mm), small microplastics (1 μm − 1 mm), and nanoplastics (< 1 μm)[3]. The common types of microplastics include ethylene-vinyl acetate copolymer (EVA), linear low-density polyethylene (LLDPE), high-density polyethylene (PEHD), polymethylpropenate (PMMA), polyethylene (PE) plastic microspheres, polystyrene (PS), polyamide (PA), polyester (PES) fibers, and polyethylene terephthalate (PET). There are two possible sources of micro/nanoplastics in the natural environment. Micro/nanoplastics can be released directly from manufactured products[4] or can be derived from different environmental processes, such as UV-mediated photooxidation, temperature-mediated thermal oxidation, mechanical forces, biodegradation and hydrolysis, which break down large plastic fragments into increasingly smaller micro/nanoplastics[5].
Although micro/nanoplastics are ubiquitous in the natural environment[4], only a few studies have focused on their soil distributions. It is estimated that 8 million tons of plastic waste are released into the ocean each year, and this amount is expected to double by 2030[6]. Horton et al.[7] also reported that microplastic concentrations in terrestrial systems are 4−23 times higher than those in the ocean. Moreover, desalination technology is booming and is being used for high-value crop cultivation in Spain, Saudi Arabia, Italy, Qatar, the USA, and Israel. Countries such as Chile and China are now applying desalination technology in agricultural practice[8]. This technology can be further applied to remove nanoplastics from the ocean and bring them back to land.
Various surveys have established that worldwide nanoplastic and microplastic levels are steadily increasing[9]. A recent study showed that vegetable farms in the suburban areas of Wuhan, China, are commonly contaminated with microplastics, and these plastic residues in vegetables are a potential threat[10]. However, micro/nanoplastic abundance in soil systems is poorly understood due to technical limitations in detecting small nanoparticles. In this review, we adequately summarize the current methods and tools used to detect microplastics in the environment and in organisms.
The toxicological effects of micro/nanoplastics may be related to their small size, dose, chemical additive leaching and adsorption of other toxins. Micro/nanoplastics affect plant health through direct internalization and plant molecule interactions by altering soil physicochemical properties or by acting as carriers of persistent organic/inorganic pollutants[11]. Many studies have demonstrated microplastic bioaccumulation and toxic effects in fish and other aquatic organisms[5,12−15]. However, to date, we know little about the adverse effects of micro/nanoplastics on plants. In this review, we will discuss this point in more detail.
-
Direct sources of micro/nanoplastics in agricultural production include mulching films, plastic greenhouses, small tunnels, nets, twine, protective nets, irrigation pipes, and coated fertilizers and pesticides, specifically the plastic polymers that the fertilizer industry uses as coating agents for slow-release fertilizers and pesticides[16−18] (Fig. 1). Notably, the use of plastic mulch to improve crop productivity and environmental stress resistance[19,20] has become an essential part of agricultural production. Thus, mulch film and greenhouse film contribute most of the micro/nanoplastics in agricultural soil. During 2015, at least 1.5 million tons of plastic mulch covered more than 20 million hectares of arable land in China. At the end of production, the mulch cannot be completely removed from the soil[21]. Some small pieces of plastic are left in the soil, which is known as 'white pollution'. With the widespread promotion of protected land cultivation, large amounts of plastic-related materials are being applied in the production process, which aggravates the current undesirable agroecological situation.
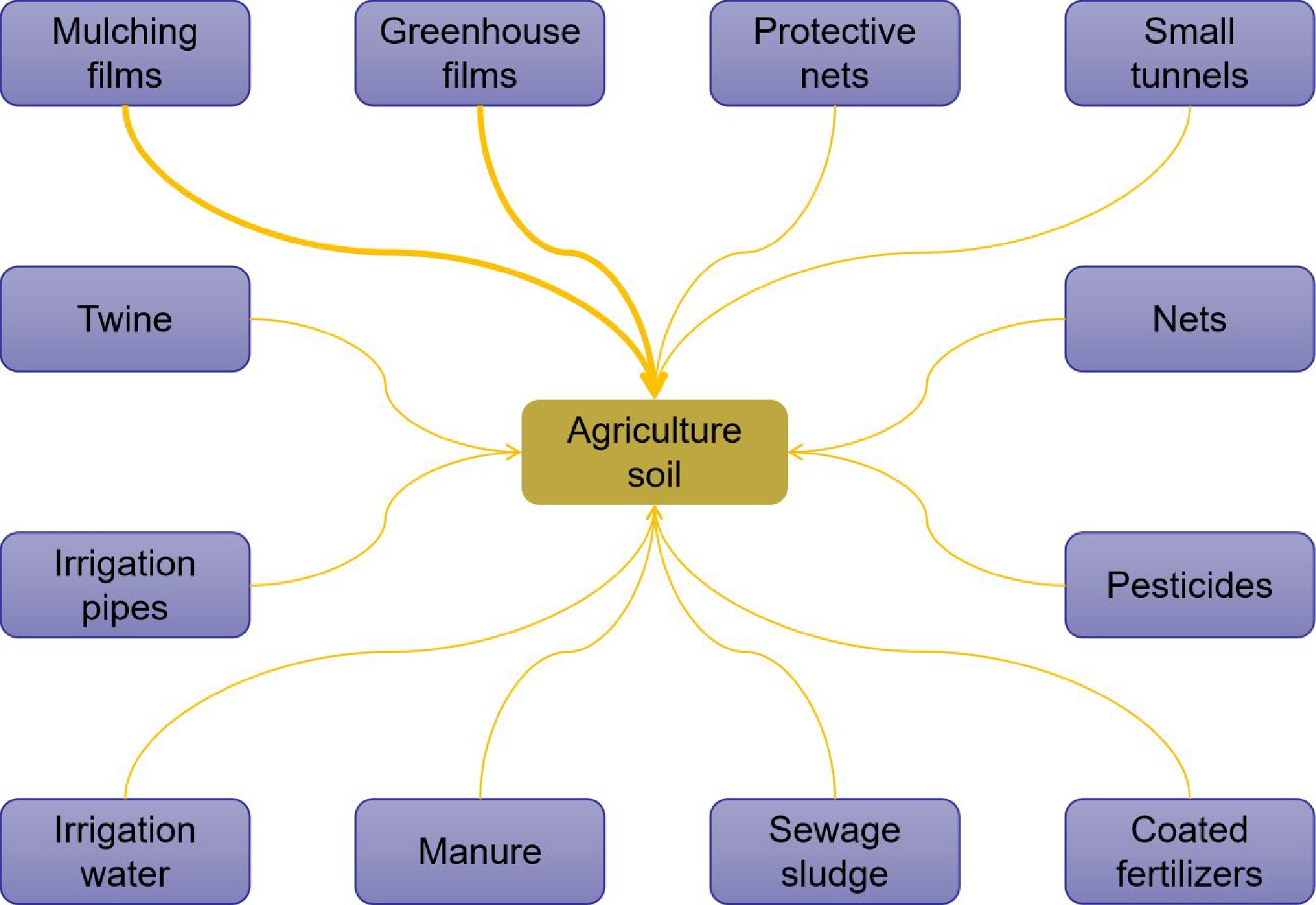
Figure 1.
Micro/nanoplastic sources in soils. The bold lines represent greater contributions of micro/nanoplastics.
Indirect sources of plastics are often overlooked in agricultural production and mainly include sewage sludge[22−24] and manure[25]. In many countries, sludge is used as a crop fertilizer, which inadvertently causes microplastics to be transported into agricultural fields, undoubtedly increasing agricultural safety risks. Various research teams in Chile, Spain and China have reported the presence of approximately 100−3,500 microplastic particles per kg in agricultural fields where sludge-based fertilizers are applied[22,26,27]. Crossman et al.[28] found that greater biosolid application resulted in higher microplastic accumulation in soil profiles.
-
Shruti & Kutralam-Muniasamy[29] compared the microplastic concentrations in different rivers around the world, finding the highest concentrations in the UK, Mexico and Germany, as well as in countries such as China, Canada and Portugal. Several studies have also reported the presence of microplastics in freshwater sources[30−32]. It is worth noting that microplastics in freshwater sources are very small and invisible to the naked eye[33]. Zhou et al.[21] conducted a comprehensive assessment of agricultural soils in Hangzhou Bay, China, and observed that irrigation water introduces microplastics into agricultural soils. In addition, studies have reported that the average densities of plastic particles in Lake Kusugul in Mongolia and Lake Taihu in China are 2.0 × 104 and 0.01−6.8 × 106 elements/km2, respectively[34,35]. In general, water sources used for agricultural irrigation, including rivers, lakes and oceans, contain microplastic elements. Treated wastewater still contains microplastics because water treatment processes are designed to remove impurities in the water, such as clay, metals or wood, and do not consider the removal of micro/nanoplastic particles[36].
-
To date, researchers have investigated a large number of methods, such as visual inspection[37], optical microscopy[38], scanning electron microscopy (SEM)[39,40], confocal laser scanning microscopy (CLSM)[41], thermal analysis[42], Raman spectroscopy (RS)[43], surface-enhanced Raman spectroscopy (SERS)[44], Fourier transform infrared spectroscopy (FTIR)[45], near-infrared spectroscopy (NIR)[46,47], hyperspectral imaging[48], pyrolysis coupled with gas chromatography/mass spectrometry (Py-GC/MS)[49], or a combination of these methods[24,50,51], for detecting micro/nanoplastics in seawater[52,53], beaches[54], freshwater[55], sewage[56,57], sediments[58], aquatic organisms[51], environment[59], soil[57], humans and animals[41,60,61]. These methods are suitable for different detection ranges, and no method is perfect (Table 1). Visual and microscopic observation methods are suitable for samples with large-diameter microplastic particles and relatively clean backgrounds, while RS, SERS, FTIR, NIR, Py-GC/MS and other methods are suitable for detecting microplastics with smaller particle sizes or even nanoplastics, but these methods have high sample requirements and require expensive equipment.
Table 1. Several micro/nanoplastic detection technologies.
Technology Microplastic scale Advantage(s) Disadvantage(s) Quantitative
(yes or no?)Used in crops Visual inspection 1 − 5 mm easy and quick only suitable for large-scale microplastics no Optical microscope observation 100 μm − 1 mm easy and quick large error no CLSM > 5 μm visual analysis limited applicability no wheat[54], Arabidopsis[55], lettuce[56], wheat[57], rice[58,59] SEM > 0.1 μm intuitive and clear images strong background interference and expensive equipment no lettuce[56,60], wheat[57] TEM < 100 nm intuitive and clear images Technical difficulty No Arabidopsis[61] Thermal analysis no limit quick destruction of microplastic structures yes RS > 10 μm wide range of applications, unaffected by water easily interfered with by fluorescent substances yes SERS 5 − 100 nm high sensitivity narrow detection range yes FTIR 10 − 300 µm fast and accurate samples need to be dried yes Py-GC/MS no limit quick not suitable for analyzing samples with complex matrices yes cucumber[62] XPS no limit nondestructive testing expensive and inconvenient yes ICP‒MS < 1 μm trace detection and visualization PS-Eu particles need to be labeled yes wheat[60], lettuce[60] Detection accuracy is closely related to the sample itself and the pretreatment method. Generally, nets (including a neuston net, plankton net, manta net, continuous net, and manual net) are used to detect microplastics in seawater, lake water, sewage and other water bodies[62−64]. Pumping systems and discrete sampling devices are also used to sample water[65−67]. Density centrifugation is often used for soils and sediments[57,58], and sometimes a combination of two methods is used[52]. Pretreatment is usually combined with chemical digestion[65,68] and enzymatic digestion[64,69] to remove organic matter in samples. Direct chemical digestion is usually used for the analysis of organisms[70]. However, in general, due to the interference of other substances in the sample, microplastic extraction is particularly difficult, which makes quantitative microplastic detection difficult.
The agricultural field is mainly focused on microplastic detection in farmland soil. Usually, density centrifugation combined with chemical digestion is used for extraction, and Raman spectroscopy, FTIR or energy-dispersive X-ray spectroscopy (EDS) is used to detect the microplastic content[23,71,72]. At present, the lack of effective methods makes it very difficult to extract nanoplastics from plants[73]. Recently, Li et al.[74] developed a Py-GC/MS analysis method to quantify nanoplastic uptake in cucumber (Cucumis sativus). Current research has mainly focused on the impact of microplastics on plant growth[75−77]. Fluorescently labeled microplastic particles were applied to plant roots, and after a period of time, the microplastic distribution and accumulation in the plant shoots were observed by CLSM or SEM[78] to reveal the absorption and transfer mechanisms of plant microplastics. For example, several researchers used fluorescent labeling observation and SEM methods and inferred that the roots of rice[79,80], lettuce[81] and Arabidopsis[82] seedlings can absorb nanoplastics (< 100 nm) and submicrometer plastics (< 1 µm) and translocate them to the aerial parts of the plants. Although medium-sized microplastics cannot be directly absorbed by plants, they were shown to enter the endodermis through the damaged root gaps of lettuce and wheat[83] and then be transported to the aerial parts of plants by transpiration. These methods provide a reference for the visualization of microplastics in samples, but they cannot be used to quantitatively detect micro/nanoplastics in plants. A new method based on using lanthanide chelates as organic-complex fluorescence labels can solve this problem. PS particles were doped with the europium chelate Eu–β-diketonate (PS-Eu), which was used to quantify PS-Eu particle uptake by wheat and lettuce using ICP‒MS[84]. This method overcomes the disadvantages of traditional fluorescent labeling methods, such as background fluorescence interference, easy dye leakage, and difficulty in simultaneous accurate quantification. However, at present, no detection methods can be used to monitor micro/nanoplastic absorption in the natural states of plants.
In general, there are still many problems with microplastic monitoring and detection technology, especially the extraction method and visual monitoring, that require further exploration.
-
Understanding microplastic fate and transport is important in pollution control[85]. However, there are few studies on the effects of plants on microplastic movement. An investigation revealed that giant reeds (Arundo donax) on the west coast of Italy act as sinks for plastic waste. Because of the ecological importance of the area where the reed grows, this plant cannot simply be removed[86]. A similar situation was found to occur in the Dongting Lake area of China, where sediment from reed farms contained significantly higher microplastic levels than that from other surrounding areas. Microplastics were also found to be adsorbed on the reed plant surface[85]. Plants are capable of intercepting and adsorbing microplastics in water. Moreover, this adsorption between negatively charged microplastics and root surfaces is very strong[87]. This raises concerns about whether aquatic vegetable crops may also cause contamination of their edible parts due to adsorption. Although there are no studies available, it is expected that this is inevitable.
Plants can adsorb microplastics from the atmosphere (Fig. 2). For example, both Pittosporum tobira and Camellia japonica plants were found to have microplastics attached to their surfaces; the microplastic abundance on the leaves of these two plants ranged from 0.07 to 0.19 items/cm2. It was estimated that a total of 0.13 trillion microplastics are adsorbed by plant leaves in 11 countries[88,89]. Microplastics have even been found in various edible fruits in supermarkets[90]. To date, there is still no effective way to remove microplastics from the natural environment[91]. Therefore, microplastics will remain with humans for a long time and will become a long-term ecological threat.
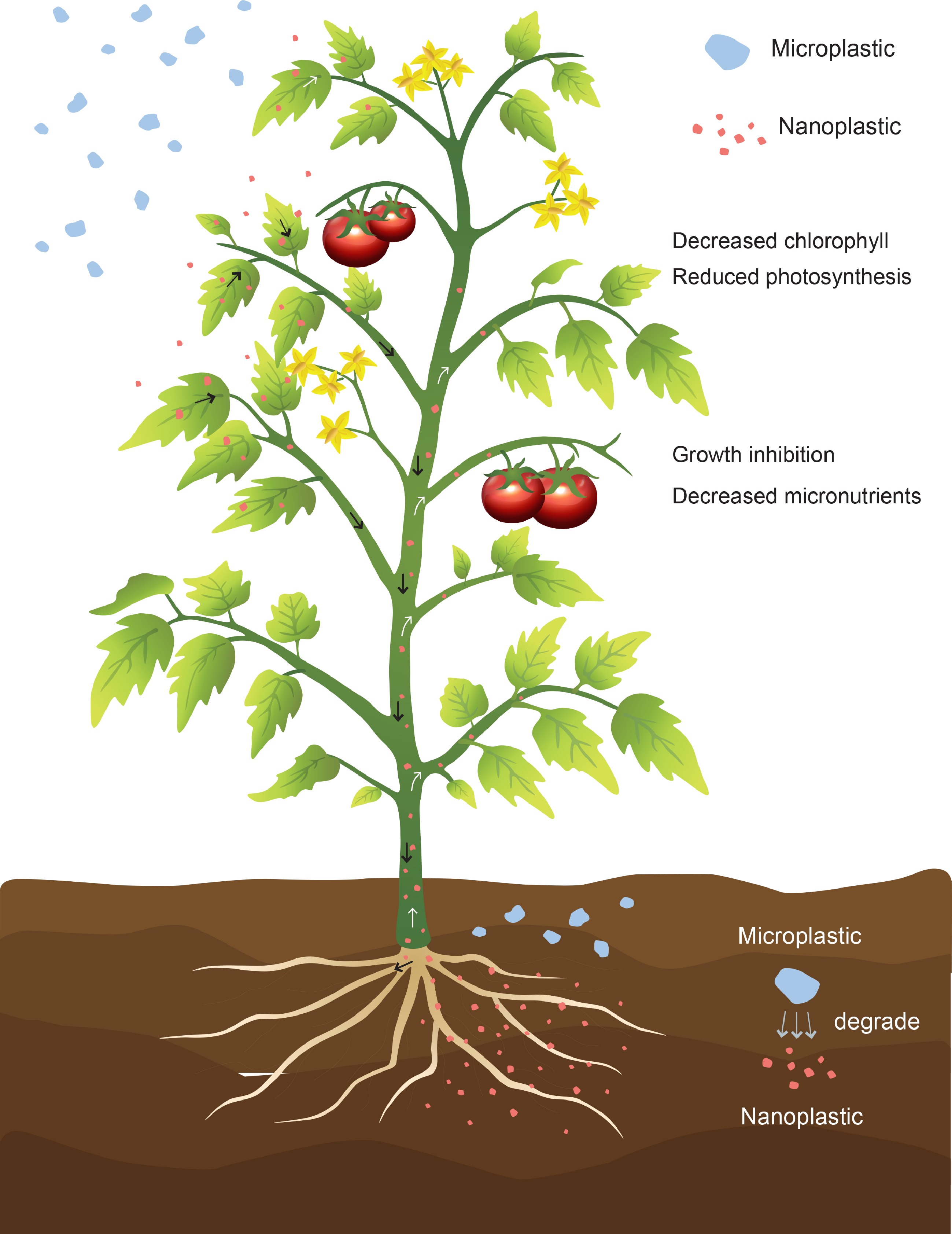
Figure 2.
Nanoplastic (pink particles) transport from roots to shoots in plants. Microplastics (blue gray particles) in soil are gradually degraded into nanoplastics by biotic and abiotic actions. Nanoplastics are absorbed by plant roots, enter vascular tissues and are transported to the shoot by transpiration (shown by white arrow). Plant leaves adsorb microplastics and absorb nanoplastics from the atmosphere (shown by black arrow). Airborne nanoplastics enter the plant through the stomata.
-
Studies have shown that micro/nanoplastics can be detected on the root surfaces of floating plants, such as Lemna minor and Spirodela polyrhiza. Microplastics do not seem to enter the root system[92,93]. However, larger microplastics can slowly degrade into nanoplastics[94], which are then absorbed into plants (Fig. 2). Recent studies have begun to address the root uptake of nanoplastics by terrestrial plants and nanoplastic transport pathways within plant tissues[1,82]. Nanoplastics can use the intercell-wall pathway (a lignified epidermis path) to enter plant tissues through the epidermal layer of roots[77]. Li et al.[78] reported that 2.0 and 0.2 μm PS particles can be absorbed by wheat and lettuce roots via crack entry at lateral root emergence sites (Fig. 3). However, the mechanism by which plastic passes through the cortex and casparian strip is not known[77].
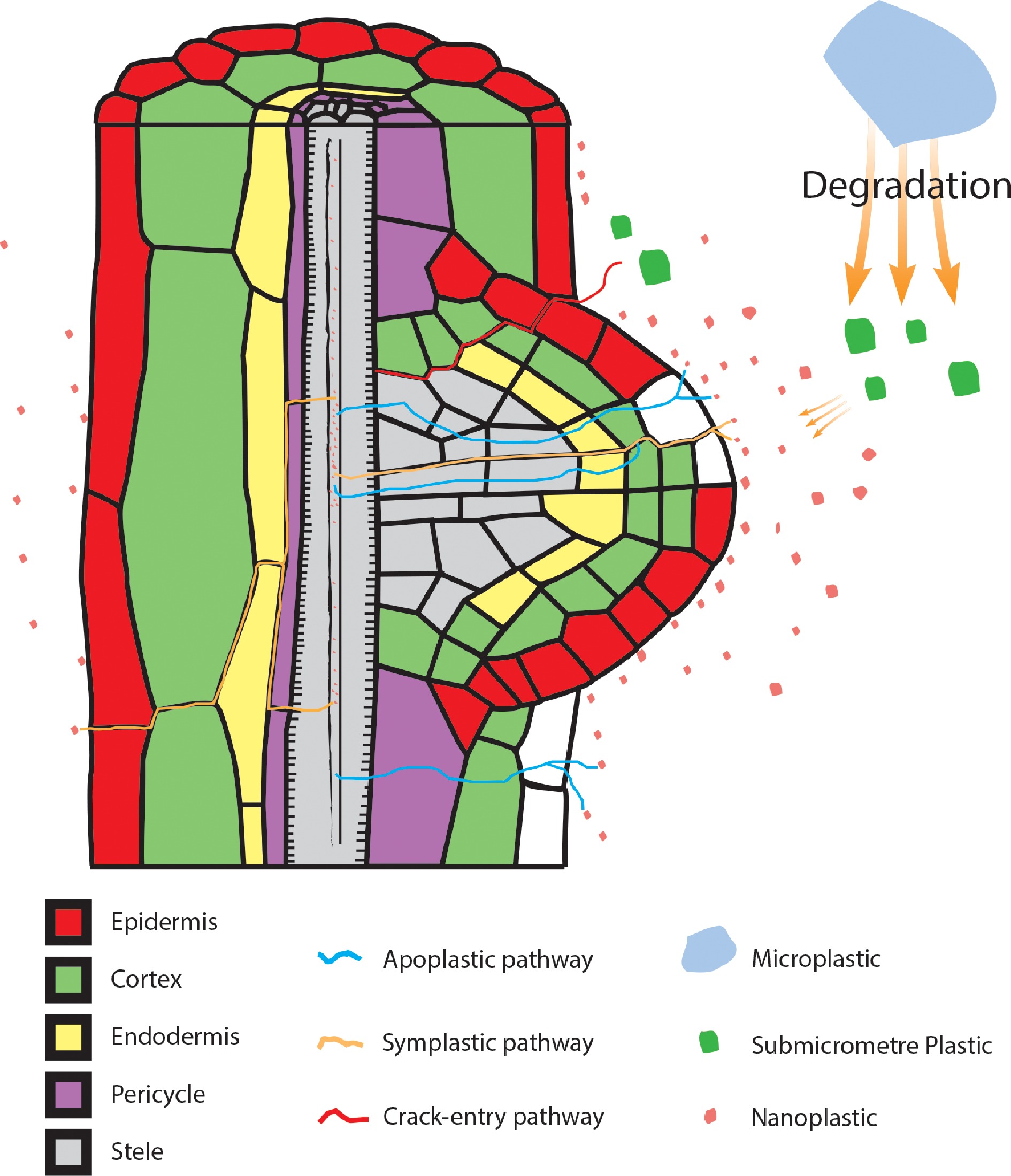
Figure 3.
Nanoplastic pathways in plant roots. Nanoplastics have been reported to enter plant root systems through two pathways, namely, the symplastic pathway and apoplastic pathway. Symplastic pathway: plants absorb nanoplastics around the root system through endocytosis or channels (e.g., aquaporins) in the cell membrane. This pathway is represented by the blue line. Apoplastic pathway: nanoplastics enter the root system and move through the intercellular space. This pathway is indicated by the orange line. Submicrometer plastics (green particles) are taken up by crop plants via a crack-entry mode that is indicated by the red line.
Plant roots absorb nanoplastics from the surrounding medium into their bodies[78,81,95], and these nanoplastics are then transported to the vascular bundles[81] and finally driven by transpiration into the aboveground plant parts[78] (Fig. 2). Li et al.[83] demonstrated the transfer of nanoplastics from below ground to above ground in wheat and lettuce. Zhou et al.[96] reported that nanoplastics absorbed by rice root systems are distributed in intercellular spaces, suggesting that nanoplastics are potentially transported via the apoplastic pathway. Moreover, they suggested that aquaporins played an important role in nanoplastic uptake by rice roots. Another research team reported that plastic particles enter cells through endocytosis[97] (Fig. 3). Giorgetti et al.[98] also observed polystyrene nanoplastic (PSNP) internalization in the cellular compartments of Allium cepa using a transmission electron microscope. A review by Maity et al.[11] thoroughly discusses the possible transport pathways of nanoplastics once they enter plants. In this review, we will not discuss this topic further.
Micro/nanoparticle foliar and root uptake are two well-known pathways that serve as entry points for plastics into the plant and thus into the food chain, threatening food safety and posing a risk to human health[99]. Plant leaves play an important role in intercepting some airborne microplastics due to their large and uneven surfaces and act as a major sink for microplastics[100]. According to a recent statistical study, plant foliage can indiscriminately retain large amounts of airborne microplastics[89]. Hence, some researchers have begun to shift the focus of nanoplastic uptake from the root to the shoot. Nanoplastics enter the leaf mainly through the stomata and then travel into the vascular system and down through the vascular bundle into the roots[101,102]. It is thus expected that the shoot-to-root nanoplastic transport mechanism must be different from the root-to-shoot nanoplastic transport mechanism. However, there is still a lack of research on these two mechanisms.
-
Studies have shown that micro/nanoplastics can affect the growth of plants, including phycophyta[103−105], Arabidopsis[82], and crops[95,106−111]. Microplastics are usually too large to enter plants, so microplastics can affect plant growth by altering the soil structure and water dynamics. For example, de Souza Machado et al.[107] demonstrated the effects of six different microplastics (PES fibers, PA beads, PE, PET, polypropylene (PP), and PS) on soil health and spring onion growth. In addition, microplastic chemical precipitates can also affect plant growth. Pignattelli et al.[109] showed that PVC was more toxic than PE and PP because PVC microplastics led to a 9-fold increase in H2O2 content and a more than 95% reduction in ascorbic acid levels in Lepidium sativum. In another study, biodegradable plastic residues showed stronger negative effects than PE[112]. These two examples show that it is not microplastics, but substances degraded or precipitated from microplastics that affect plant growth. Since microplastics affect crop growth, they must also have an impact on plant physiology. Gao et al.[108] reported that the photosynthesis-related parameters of lettuce decreased after application of PE microplastics to the roots. The contents of superoxide radicals and hydrogen peroxide in leaves and roots significantly increased.
Nanoplastic effects on plant growth
-
Nanoplastics can enter plants and usually show toxic effects on plants. An excellent study was conducted using Arabidopsis thaliana to demonstrate that nanoplastics with different surface charges accumulated in plants. Both of the differently charged nanoplastics resulted in an approximately 50% reduction in fresh weight. Interestingly, positively charged nanoplastics (PS-NH2) were less likely to enter the root system but had a more pronounced inhibitory effect on plants. Compared to negatively charged nanoplastics (PS-SO3H), PS-NH2 led to a 20% reduction in plant height and a 20% − 70% reduction in root length. More PS-SO3H than positively charged nanoplastics entered the apoplast and xylem[82]. Moreover, we assume that there are other factors besides surface charge that influence nanoparticle entry into plants. However, current technological conditions limit our exploration in this area.
In addition, foliar-applied polystyrene nanoplastics (PSNPs) showed negative effects on lettuce, which led to the onset of oxidative stress and a decline in micronutrients and essential amino acids[101]. An experiment in Arabidopsis showed that root application of nanoplastics significantly reduced chlorophyll in silique[82]. Similarly, 0.5% low-density polyethylene microplastics (LDPE-MPs) led to significantly lower chlorophyll content in leaves of common bean[111]. Li et al.[113] showed that 300-nm PS nanoplastics increased the malondialdehyde (MDA) and proline contents of cucumber roots. Some scholars have compared the toxicity of microplastics and nanoplastics under the same conditions. PS fluorescent nanoplastics with a size of 100 nm exhibited higher genotoxicity and oxidative damage to Vicia faba than 5 μm microplastics[95].
Molecular responses of plants to nanoplastics
-
Current research at the plant molecular level has focused on the molecular responses of plants to exogenous nanoplastics. These studies involved very limited crops, such as wheat[114], corn[115], rice[116,117] and Torreya grandis[118]. Transcriptome analysis showed that PSNPs significantly affected carbon metabolism, amino acid biosynthesis and plant hormone signaling pathways in wheat (Triticum aestivum L.)[114]. Similarly, a metabolomic study of corn exogenously treated with PSNPs demonstrated the enrichment of changed metabolites in the alanine, aspartate and glutamate metabolic pathways[115]. Wang et al.[117] demonstrated that the effects of PSNPs with different functional groups were distinct through transcriptome analysis. PS mainly affects RNA metabolic processes, while PS-COOH affects ion transport and PS-NH2 affects macromolecular protein synthesis, suggesting that PS functional groups play a crucial role in the interactions between nanoplastics and plants. A multiomics analysis of Torreya grandis indicated that PSNPs regulate terpenoid and flavonoid biosynthetic pathways, the latter being in the phenylpropanoid pathway, by regulating small-RNA transcription and protein expression[118]. Moreover, rice partially reduced nanoplastic toxicity by regulating phenylpropane biosynthesis[117]. These studies suggest that the phenylpropanoid metabolic pathway may be involved in the interactions between nanoplastics and plants. In rice roots, carbon metabolism was activated, whereas jasmonic acid and lignin biosynthesis were inhibited by PSNP treatment[119]. In addition, 100 nm PSNPs induced cytogenotoxicity through the induction of ROS generation and inhibition of cyclin-dependent kinase (cdc2) expression in Allium cepa L.[120].
-
Recently, microplastic degradation and removal technology has also become one of the most popular research topics because microplastics are special plastics originating from tiny particles that are highly difficult to further degrade in the environment. To address this issue and avoid persistent damage to agricultural production, researchers have suggested three main types of approaches: physical, chemical, and biological methods[121,122]. In addition, microplastics/nanoplastics removal techniques that rely on microrobots have been developed.
Membrane technology is a commonly used physical method for removing micro/nanoplastics[122,123]. To remove polyacrylonitrile (PAN) from wastewater, researchers synthesized reduced graphene oxide (rGO)-doped PAN as a membrane, which showed a removal efficiency of 82% and reusability[124]. Magnetic carbon nanotubes (M−CNTs) have also been synthesized as adsorbates to remove microplastics. The results showed that microplastics (5 g/L) were completely removed by 5 g/L M−CNTs within 300 min[125]. A filtration system is also an effective method for removing micro/nanoplastics. Zirconium metal-organic framework-based foam materials were filled in a filter and prewetted, achieving an efficiency of up to 95.5 ± 1.2%[126].
Because many common microplastics have polar groups, especially after oxidation and aging in the environment, chemical bonding is an effective method for removing microplastics. Hydrophilic bare Fe3O4 nanoaggregates can effectively remove the most common microplastics, including high-density PE, PP, PVC, PS and PE terephthalate, and hydrogen bonding is the main force in Fe3O4 adsorption[127]. In addition, CeO2 is an excellent adsorbent for removing abrasive microplastics by forming a complex with heavy metal ions[128]. Microplastics interact frequently with common dissolved organic matter (DOM) in the environment. Through analysis by electron paramagnetic resonance spectroscopy, high-performance liquid chromatography, FTIR and two-dimensional correlation spectroscopy analyses, DOM was found to promote electron transfer to produce reactive oxygen species (ROS) and accelerate the microplastic aging process[129]. Interestingly, microplastics can be degraded abnormally quickly in freezing environments. For example, the PS degradation rate (± 20 °C) in ice is surprisingly competitive compared with that induced by most artificial technologies[130].
In the environment, micro/nanoplastics are subject to biodegradation, which has also been a popular research topic in recent years. To date, microorganisms such as algae, fungi, and bacteria have attracted the attention of scientists as tools for microplastic treatment[131]. Some microorganisms produce specific enzymes that participate in micro/nanoplastic degradation. PP, pro-oxidant blended PP (MI-PP) and starch blended PPs (ST-PPs) can be degraded by two kinds of fungi, Phanerochaete chrysosporium NCIM 1170 (F1) and Engyodontium album MTP091 (F2), and the biodegradation inefficiency is accelerated by UV pretreatment[132]. Yoshida et al. isolated a novel bacterium named Ideonella sakaiensis 201-F6 that could use PET as a carbon source. When this bacterium grows on PET, it can produce two enzymes, PETase and MHETase, to hydrolyze PET into two environmentally benign monomers, terephthalic acid and ethylene glycol[133]. A naturally occurring fungus in the marine environment and present in Portuguese coastal waters, Zalerion maritimum, has the ability to degrade microplastics[134]. Recently, Yuan et al. isolated a kind of bacterium, Bacillus cereus CH6, from lake sediments and investigated its ability to degrade PS microplastics. Bacillus cereus CH6 has a short generation time, high activity, and fast substrate utilization. The microplastic weight loss was 10.7% after 50 d of Bacillus cereus CH6 incubation[135]. Furthermore, microalgae can be used as a potential biosolution to remove microplastics, as they easily interact and combine[136]. Researchers have shown that positively charged PS microplastics are more efficiently adsorbed on algae surfaces than are negatively charged microplastics[137]. The edible macroalga (seaweed) Fucus vesiculosus adsorbed fluorescent PS microplastics with a diameter of approximately 20 μm through an alginate compound released from the cell wall of its section area, and the algae exhibited a sorption efficiency of 94.6%[138].
Currently, a number of microrobots to remove microplastics/nanoplastics have been developed by Pumera’s team. The self-propulsion of microrobots produces a local stirring effect in an energy-efficient manner, allowing them to encounter more contaminants per unit of time[139]. An active photocatalytic degradation process based on an intelligent visible light-driven microrobot capable of 'on-the-fly' capturing and degrading microplastics was introduced. Photocatalytic robots can effectively degrade different synthetic microplastics, especially polylactic acid and polycaprolactone[140]. The authors prepared adhesive polydopamine (PDA)@Fe3O4 magnetic microbots (MagRobots) by simulating the basic characteristics of adhesive chemistry in marine mussels. The synthetic MagRobots are externally triggered by a transverse rotating magnetic field and have the ability to remove targeted microplastics due to their strong adhesive properties[141]. Recently, MXene-derived oxide microrobots have also been designed for trapping and detecting nanoplastics[142].
-
Large amounts of plastics are used in agriculture, industry and other human activities. In the natural environment, plastics are degraded into microplastics and nanoplastics, both of which are widely distributed in the soil. Microplastics are absorbed by crops and contaminate our food, such as edible plant fruits, leaves, and stems[143], and a mean amount of 132,740 particles/g was detected in fruits and vegetables[90]. Li et al.[78] reported that polystyrene nanoplastics accumulated in the roots of cucumber and were sequentially transferred to the stems, leaves, flowers and fruits. Dessì et al.[144] investigated plastics in store-bought rice in Australia, and reported that Australians may consume 3.7 mg of plastics per serve (100 g) if not washed and 2.8 mg if washed. A recent review discussed the varying impact of different shapes and sizes of plastics on food safety in an agricultural context. And it was noted that nanoplastics particularly affect the food safety of edible plant underground organs[145].
Recent studies reported small micro/nanoplastics in human feces[146]. He et al.[147] proposed a potential transfer pathway of micro/nanoplastics from soils to plants and human food. Several rodent tests have confirmed that microplastics can be absorbed by endocytosis[148−150]. Moreover, nanoplastics can penetrate deep into organs such as the liver, spleen, lungs and brain. The International Agency for Research on Cancer (IARC) has classified various types of plastics, derivatives and components as potential carcinogens, such as polyvinyl chloride (PVC), polystyrene (PS) and derivatives of phthalates[33]. And when microplastics entering to human body, it could disrupt immune function which might lead to autoimmune diseases or immunosuppression[151]. Therefore, people should be alert to the threat to food safety posed by microplastic enrichment through the food chain.
-
Due to the durability of plastic in the environment of hundreds of thousands of years, the impact of plastic pollution in the world is of increasing concern[151]. As micro/nanoplastics pose a potential risk to plants, animals and humans[147]. In the past few years, researchers have investigated the sources of micro/nanoplastics in agriculture and gained new insights into the effects of micro/nanoplastics on plants and plant responses to micro/nanoplastic stress (Fig. 4). Moreover, nanoplastic uptake by plants and their translocation in planta were initially studied. Micro/nanoplastics were absorb by plant roots from the surrounding medium (including soil and atmosphere), and then were transported to the vascular bundles and finally driven by transpiration into the aboveground plant parts (Fig. 2), which mean that our food chain was exposed to the dangers of micro/nanoplastics. In addition, methods for detection, degradation, and removal of micro/nanoplastics in the environment have been developed. In general, the key ways to degradation, and removal of micro/nanoplastics include physical, chemical, biological and microrobots methods. In the future, it is worthwhile to devote more effort to investigate the following aspects.
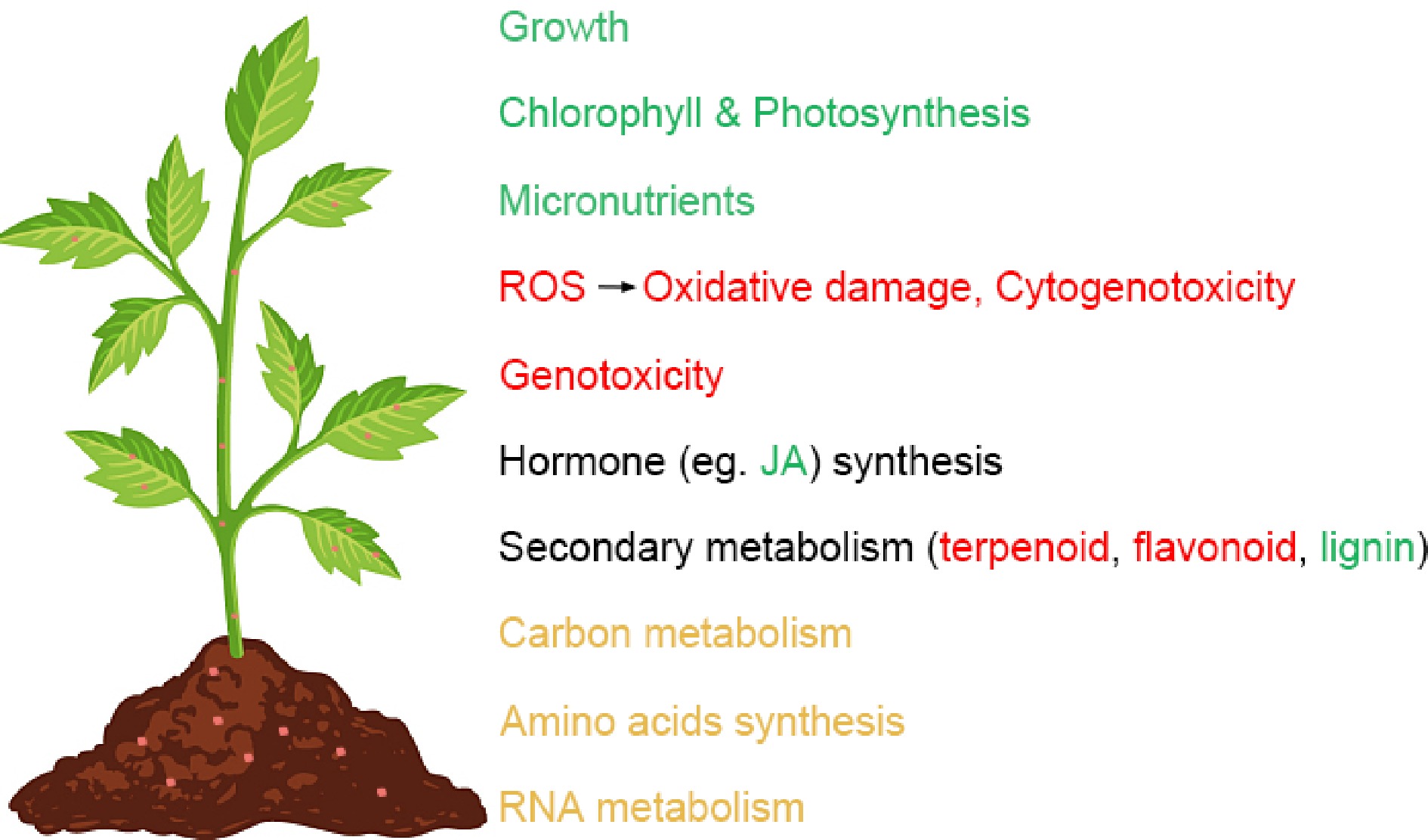
Figure 4.
Main effects of micro/nanoplastics on plants. Green indicates a decrease, red indicates an increase, and yellow indicates that the effect is not yet clear.
(1) Analyzing and blocking micro/nanoplastic sources in arable soils or developing plastic-free farming methods.
(2) Understanding nanoplastic uptake and transport mechanisms in plants and developing methods to interrupt these processes.
(3) Studying the response and tolerance mechanisms of plants to micro/nanoplastics.
(4) Developing a quantification method for analyzing nanoplastic contents in cultivated soils and plants to avoid cultivation and food safety risks.
(5) Developing effective methods for removing micro/nanoplastics from soil and water.
(6) Answering the following questions: Does biodegradable mulch produce more micro/nanoplastics? Is biodegradable mulch bad for the environment and crops? What is the future research direction of biodegradable mulch?
This work was supported by the National Key R&D Program of China (2018YFD1000803, 2019YFD1000300) and the Beijing Natural Science Foundation Project - Key Project of Science and Technology Plan of Beijing Education Commission (KZ202010020027).
-
The authors declare that they have no conflict of interest.
-
# These authors contributed equally: Lulu Sun, Xiaoyun Wang, Hanqing Zhao
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Sun L, Wang X, Zhao H, Wang Z, Zhao Y, et al. 2023. Micro/nanoplastics: a potential threat to crops. Vegetable Research 3:18 doi: 10.48130/VR-2023-0018
Micro/nanoplastics: a potential threat to crops
- Received: 11 December 2022
- Accepted: 11 May 2023
- Published online: 14 June 2023
Abstract: The distribution of micro/nanoplastics in soil and water environments is a potential agricultural threat. Since micro/nanoplastics are a new and highly concerning contaminant, in recent years research on micro/nanoplastics has rapidly increased. Here, we review recent scientific papers on micro/nanoplastics in agricultural systems, including micro/nanoplastic sources, microplastic adsorption, nanoplastic absorption, micro/nanoplastic effects on crops, and micro/nanoplastic detection and removal methods. There is very little information available concerning nanoplastic transport in planta; therefore, more research is needed to gain a better understanding of how micro/nanoplastic particles are transported. We also discuss the accumulation of micro/nanoplastics in crops as a potential threat to food safety. Finally, we propose future micro/nanoplastic research directions.
-
Key words:
- Micro/nanoplastics /
- Adsorption /
- Absorption /
- Toxicity /
- Detection /
- Removal












